Abstract
Objective
Neonatal-onset multisystem inflammatory disease (NOMID; also known as chronic infantile neurologic, cutaneous, articular [CINCA] syndrome) is characterized by fever, chronic meningitis, uveitis, sensorineural hearing loss, urticarial skin rash, and a characteristic deforming arthropathy. We investigated whether patients with this disorder have mutations in CIAS1, the gene which causes Muckle-Wells syndrome and familial cold autoinflammatory syndrome, two dominantly inherited disorders with some similarities to NOMID/CINCA syndrome.
Methods
Genomic DNA from 13 patients with classic manifestations of NOMID/CINCA syndrome and their available parents was screened for CIAS1 mutations by automated DNA sequencing. Cytokine messenger RNA (mRNA) levels were assessed by real-time polymerase chain reaction on peripheral blood leukocyte mRNA, and serum cytokine levels were assayed by enzyme-linked immunosorbent assay. Protein expression was assessed by Western blotting of lysates from plastic-adherent peripheral blood mononuclear cells.
Results
In 6 of the 13 patients, we found 6 heterozygous missense substitutions in CIAS1. Five of the 6 mutations are novel. None of these sequence changes was observed in a panel of >900 chromosomes from healthy controls. Two distinct nucleotide changes in a single codon in unrelated patients resulted in the same amino acid change. In 4 mutation-positive children whose parental DNA was available, no mutation was found in the parental DNA, supporting the conclusion that the mutations arose de novo. Consistent with the recently discovered role of CIAS1 in the regulation of interleukin-1 (IL-1), we found evidence of increased IL-1β, as well as tumor necrosis factor, IL-3, IL-5, and IL-6, but not transforming growth factor β, in a mutation-positive patient compared with normal controls.
Conclusion
Our data increase the total number of known germline mutations in CIAS1 to 20, causing a spectrum of diseases ranging from familial cold autoinflammatory syndrome to Muckle-Wells syndrome to NOMID/CINCA syndrome. Mutations in CIAS1 were only found in ~50% of the cases identified clinically as NOMID/CINCA syndrome, which raises the possibility of genetic heterogeneity. IL-1 regulation by CIAS1 suggests that IL-1 receptor blockade may constitute a rational approach to the treatment of NOMID/CINCA syndrome.
The systemic autoinflammatory diseases are a group of disorders characterized by episodic or fluctuating degrees of inflammation without evidence of hightiter autoantibodies or antigen-specific T cells (1–3). Within the last 5 years, the genetic basis of several autoinflammatory diseases with Mendelian inheritance, including familial Mediterranean fever (FMF) (4,5), familial Hibernian fever (now renamed the tumor necrosis factor receptor–associated periodic syndrome [TRAPS]) (1), the hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) (6–8), Muckle-Wells syndrome (MWS) (9), familial cold autoinflammatory syndrome (FCAS; formerly known as familial cold urticaria) (9), and Blau syndrome (10), has been identified.
At the outset of the current studies, the molecular basis of another likely autoinflammatory syndrome, known in North America as neonatal (or infantile)–onset multisystem inflammatory disease (NOMID) (11–13) and in Europe as chronic infantile neurologic, cutaneous, and articular (CINCA) syndrome (14,15), had not been elucidated. Patients with NOMID/CINCA syndrome present with a severe but unexplained phenotype, including dermatologic, arthropathic, and neurologic symptoms (for review, see refs. 16 and 17). An urticaria-like skin rash is often present from birth. A highly characteristic arthropathy, with distinctive radiographic findings of premature patellar and epiphyseal long bone ossification and resultant osseous overgrowth (18,19), often develops early in life and leads to severe contractures and disability. Neurologic manifestations, including chronic aseptic meningitis, cerebral ventricular dilation, cerebral atrophy, uveitis, optic disc edema, high-frequency hearing loss, and mental retardation, are present in various subsets of patients. Short episodes of recurrent fevers are frequently noted. Approximately 20% of patients die before reaching young adulthood (16).
Findings suggestive of an ongoing inflammatory process include lymphadenopathy, splenomegaly, a prolonged erythrocyte sedimentation rate, leukocytosis, eosinophilia, and hyperglobulinemia, but autoantibodies are generally not present. A few families with more than one affected member have been described (20,21), and unrelated patients often have a sibling-like resemblance, but genetic studies have been hampered by the fact that most cases are sporadic.
With the recent identification of the genes that cause other autoinflammatory syndromes, we considered the possibility that abnormalities in one of these newly discovered genes might also cause NOMID/CINCA syndrome. CIAS1 (9), the gene underlying FCAS and MWS, was a particularly attractive candidate because of clinical similarities between these syndromes, including fever and urticarial rash, with dermal polymorphonuclear leukocytes seen in FCAS (22), MWS (23), and NOMID/CINCA syndrome, and with lymphadenopathy and sensorineuronal hearing loss seen in MWS (23) and NOMID/CINCA syndrome. CIAS1 is located on chromosome 1 and belongs to a newly recognized family of genes that encodes proteins that share a structural motif with pyrin (or marenostrin), the FMF protein. The amino terminus of pyrin encodes an ~90–amino acid motif (the PYRIN domain) found in a number of regulators of the inflammatory response and apoptosis (3,24–30). The PYRIN domain bears 3-dimensional structural similarities to death domains, death-effector domains, and caspase-recruitment domains, and is thought to be important in homotypic protein–protein interactions. The product of CIAS1, cryopyrin (also called PYPAF1 or NALP3), has been shown to activate nuclear factor κB (NF-κB) (31), and it may also regulate interleukin-1 (IL-1) processing through PYRIN domain–mediated caspase 1 signaling (32).
We hypothesized that mutations in cryopyrin might have a profound proinflammatory effect, as is seen in NOMID/CINCA syndrome. To test the hypothesis that CIAS1 mutations cause NOMID/CINCA syndrome, we sequenced this gene in 13 patients. While in the process of preparing a manuscript describing our findings, Feldmann and colleagues (33) reported 7 different CIAS1 mutations in 7 unrelated cases of NOMID/CINCA syndrome. One of the mutations identified in our screen is the same as 1 of the 7 mutations reported by Feldmann et al; the other 5 mutations we identified are novel. We also examine the issue of genetic heterogeneity in NOMID/CINCA syndrome and the possible impact of CIAS1 mutations on cytokine signaling.
PATIENTS AND METHODS
Patients
The present study included 12 Caucasian patients and 1 African American patient referred to the National Institute of Arthritis and Musculoskeletal and Skin Diseases for molecular evaluation of unexplained systemic inflammatory disease. The study was approved by the institutional review board at the National Institutes of Health, and written informed consent was obtained from the subjects or their parents. All of the patients included in this study had been clinically evaluated by physicians experienced in the diagnosis of NOMID/CINCA syndrome (BSA, PD, DG, DJL, TLM, RR, or KS). Five patients included in this study have been subjects of previous studies (11,19,34).
Mutation detection
Genomic DNA was extracted from whole blood using a commercially available kit (Puregene; Gentra Systems, Minneapolis, MN). Mutation detection was performed by bidirectional fluorescence sequencing with dye-primer chemistry (Amersham, Piscataway, NJ) on an ABI 377 automated sequencer (Applied Biosystems, Foster City, CA) as previously described (1).
Mutation screening in controls
A panel of Caucasian control DNA samples was tested for the presence of the 6 CIAS1 mutations that were identified in NOMID/CINCA syndrome patients. All of the detected mutations were found in the Caucasian children. For the D303N mutation, we developed a Taq I restriction endonuclease assay using the following primers: forward 5′-GAC-CTG-ATC-ATG-AGC-TGC-TGC-3′ and reverse 5′-GCT-CGT-CAA-AGG-CAC-CTT-GCA-GCT-CAT-3′. The presence of the mutation abolishes a Taq I restriction site. All 6 of the mutations were screened by multiple-base extension and mass spectroscopy (MassArray Genotyping System; Sequenom, San Diego, CA).
Immunoblotting
Peripheral blood mononuclear cells (PBMCs) were isolated from 40 ml of heparinized blood and cultured with or without 1 µg/ml of Escherichia coli O127:B7 lipopolysaccharide (Sigma, St. Louis, MO). The cells were then lysed in buffer containing 0.5% Triton X-100, 300 mM NaCl, 50 mM Tris HCl (pH 7.5), 2 mM EDTA, and complete proteinase inhibitor (Roche, Gipf-Oberfrick, Switzerland). The total protein concentrations of the cell lysates were measured by bicinchoninic acid reagent (Pierce, Rockford, IL). A total of 12 µg of protein was loaded onto 16% Tris glycine gels (Invitrogen, Carlsbad, CA) for sodium dodecyl sulfate–polyacrylamide gel electrophoresis and analyzed by Western blotting with goat polyclonal anti-human IL-1β (0.5 µg/ml; R&D Systems, Minneapolis, MN).
Quantitative real-time polymerase chain reaction (PCR) of cytokine mRNA expression
Total RNA was isolated from PBMCs using an RNeasy Mini kit (Qiagen, Valencia, CA). For real-time PCR experiments, complementary DNA was reverse-transcribed from 1 µg of total RNA by using a first-strand complementary DNA synthesis kit (Roche) and analyzed with the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Commercially available IL-1β, IL-3, IL-5, IL-6, transforming growth factor β1 (TGFβ1), and GAPDH probe and primer sets (Applied Biosystems) were used to determine cytokine mRNA expression by PBMCs. All samples were normalized to the GAPDH internal positive control. The relative cytokine levels are presented as the percentage of control PBMC values.
Serum cytokine concentrations
Concentrations of the following serum cytokines were determined using a sandwich enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems): tumor necrosis factor (TNF), IL-1 receptor antagonist (IL-1Ra), IL-6, and TGFβ1.
RESULTS
Characteristics of the patient cohort
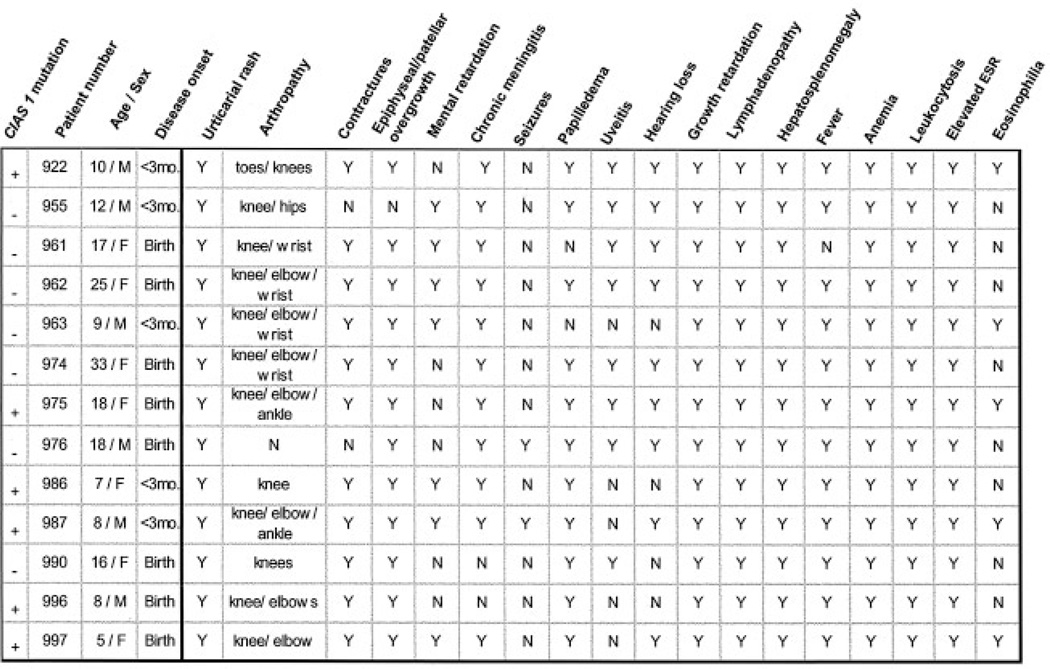
A total of 13 NOMID/CINCA syndrome patients (6 males, 7 females), ranging in age from 5 to 33 years, were evaluated for mutations in the CIAS1 gene (Figure 1). All 13 were noted to have an urticarial rash within the first 3 months of life; two-thirds of the patients had urticarial rash within the first few hours of life. All 13 patients had arthropathy or patellar/epiphyseal overgrowth, and 11 of the 13 patients had both findings. All of the patients had neurologic involvement, including one or more of the following: mental retardation, chronic meningitis, seizures, papilledema, uveitis, and hearing loss. Papilledema was noted in 11. Lymphadenopathy, hepatosplenomegaly, growth retardation, anemia, neutrophilic leukocytosis, and an elevated erythrocyte sedimentation rate were observed in all 13 patients.
Figure 1.
Characteristics of the 13 study patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, and articular syndrome. CIAS1 mutations were found in 6 of the 13 patients. ESR = erythrocyte sedimentation rate.
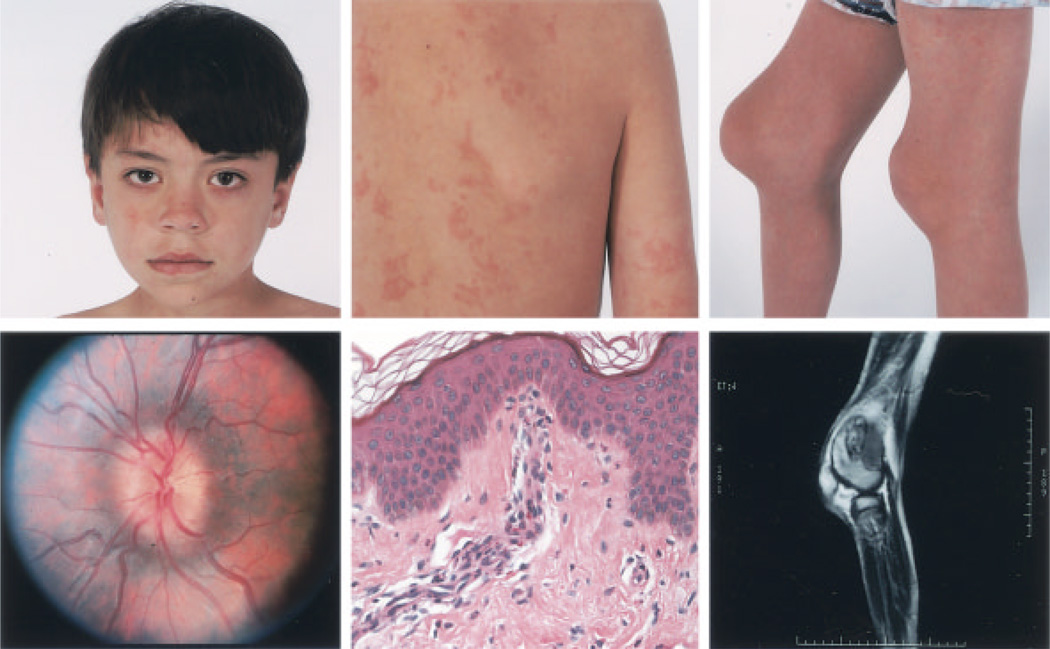
Figure 2 illustrates several of the clinical features of NOMID/CINCA syndrome, including the facial characteristics, papilledema, the urticarial rash with neutrophilic perivascular infiltrates, and severe contractures caused by overgrowth of the femoral epiphysis and patella. There was no evidence of synovitis on the contrast-enhanced magnetic resonance image shown for patient 922.
Figure 2.
Clinical features of neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, and articular syndrome in patient 922. Top left, Facial features. Top middle, Urticarial rash, which persisted throughout a week-long hospital visit. Top right, Contractures of the knees. Bottom left, Funduscopic image, demonstrating papilledema in the left eye (present bilaterally). Bottom middle, Skin biopsy sample, showing a mild perivascular leukocytic infiltrate, with some eosinophils but no epidermal changes. Bottom right, Magnetic resonance image of the right knee, demonstrating the absence of significant synovial enhancement and the presence of epiphyseal and patellar bony overgrowth.
Findings of mutation analysis
All 13 patients were screened for CIAS1 mutations by automated bidirectional sequencing of PCR-amplified genomic DNA for each of the 9 exons and the exon–intron junctions. In 6 patients, we identified single-nucleotide substitutions, all of which were in exon 3, that resulted in missense amino acid substitutions. Table 1 summarizes the CIAS1 mutations that were found in the 13 NOMID/CINCA syndrome patients and lists the number of control chromosomes from healthy unrelated individuals tested for the respective mutations.
Table 1.
Summary of the CIAS1 mutations identified*
| Patient | CIAS1 mutations |
CIAS1 mutations in the parents |
Frequency of CIAS1 mutations in controls |
||
|---|---|---|---|---|---|
| Exon | Nucleotide | Amino acid | |||
| 997 | 3 | 791 T→A | L264H | ND | 0/916 |
| 922 | 3 | 907 G→A | D303N | Negative | 0/1,126 |
| 986 | 3 | 1121 C→A | A374N | Negative | 0/972 |
| 987 | 3 | 1709 A→G | Y570C | ND | 0/936 |
| 975 | 3 | 1569 C→A | F523L | Negative | 0/936 |
| 996 | 3 | 1569 C→G | F523L | Negative | 0/936 |
A total of 6 CIAS1 mutations were identified in the 13 study patients. The control frequency represents the number of chromosomes examined in healthy unrelated control subjects. ND = not done.
In the 4 cases in which parental DNA was available, both parents were found to be negative for the substitution. Microsatellite analysis confirmed the parental relationships. Thus, the findings strongly indicate that the substitution arose de novo in the child. One of the mutations, D303N, has been reported as a de novo change in a French patient with MWS (35). The same mutation was also present in a father–daughter pair in a rare case of familial NOMID/CINCA syndrome (33). In 2 unrelated patients, one Canadian (patient 975) and the other Argentinian (patient 996), 2 distinct substitutions at nucleotide 1569 (C→A and C→G, respectively) resulted in the substitution of leucine for phenylalanine at residue 523 (F523L).
In 7 patients, bidirectional genomic sequencing failed to identify any changes. There were no discernible differences in the clinical features of patients for whom we found mutations and those for whom we did not find mutations (Figure 1).
Findings of cytokine studies in a NOMID/CINCA syndrome patient with a CIAS1 mutation
Patient 922, who was found to harbor the D303N CIAS1 mutation, has received followup care at the Warren Grant Magnuson Clinical Center of the National Institutes of Health. Since CIAS1 is highly expressed in monocytes and granulocytes and has recently been implicated in both NF-κB signaling and IL-1 production and processing (31,32), we compared cytokine profiles in this patient with those in controls. At the time the patient was studied, his erythrocyte sedimentation rate was 92 mm/hour and his C-reactive protein level was 7.11 mg/dl.
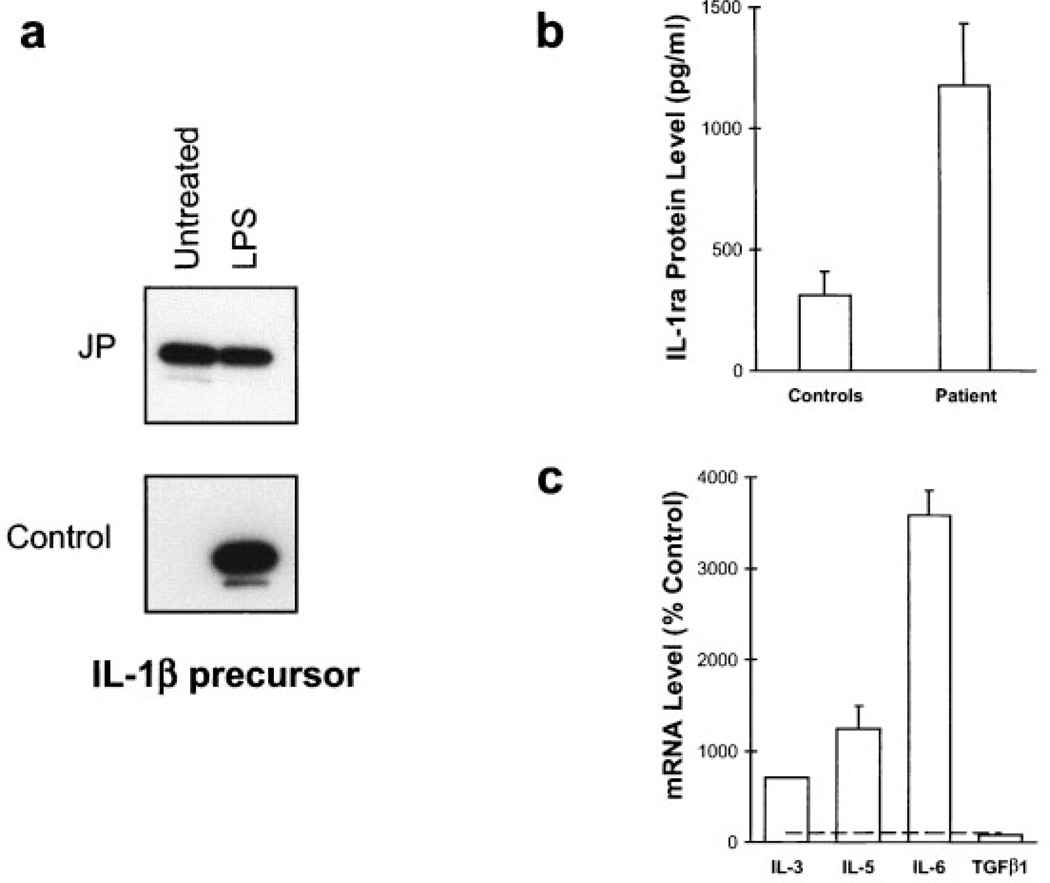
As shown in Figure 3a, high levels of IL-1β protein were identified by Western blotting of lysates from unstimulated monocytes from patient 922, compared with the levels in 7 healthy unrelated control subjects, but the patient’s IL-1β response to LPS was blunted. Levels of IL-1β mRNA from the patient’s unstimulated PBMCs were also increased (data not shown), although not as markedly as was the protein in purified monocytes. In addition, 3 separate serum IL-1Ra levels were obtained over a period of 6 months, and were found to be significantly higher in the patient than in the healthy controls. This finding is consistent with observations that endogenously produced IL-1Ra is a homeostatic response to limit the proinflammatory effects of pathologically elevated IL-1 (36,37) (Figure 3b).
Figure 3.
a, Western blot analysis of interleukin-1β (IL-1β) expression in monocytes from patient 922 (JP) and from a healthy control subject. Monocytes were untreated or were stimulated with lipopolysaccharide (LPS) for 24 hours. IL-1β protein was constitutively expressed in the untreated cells from the patient but was undetectable in cells from the healthy controls (only 1 of 7 control samples tested is shown). b, Mean levels of IL-1 receptor antagonist (IL-1Ra) in samples obtained from patient 922 at 3 different visits were significantly higher compared with the mean level in samples from 4 healthy control subjects, as determined by enzyme-linked immunosorbent assay. c, Real-time polymerase chain reaction for the expression of IL-3, IL-5, IL-6, and transforming growth factor β1 (TGFβ1) mRNA in peripheral blood mononuclear cells obtained from patient 922. IL-3, IL-5, and IL-6 mRNA levels were significantly elevated compared with the levels in normal controls (horizontal line), and are expressed as relative cytokine levels. All samples were normalized to the internal GAPDH control.
Expression of mRNA for 4 other cytokines in PBMCs from this patient was examined by real-time PCR. IL-3 and IL-5 were of particular interest because of their role in stimulating eosinophilia (38,39), a known manifestation of NOMID/CINCA syndrome. As illustrated in Figure 3c, IL-3 and IL-5 mRNA levels were, respectively, 7 and 12 times higher than those in the healthy controls. IL-6 levels were also of interest because of the recent report of cyclical elevations of this cytokine in a patient with MWS (40). IL-6 message in PBMCs was found to be more than 35 times higher than that in the controls (Figure 3c). Consistent with this finding, serum IL-6 and TNFβ1 levels were found to be elevated when examined by ELISA (data not shown). However, as evidenced by the normal TGFβ1 mRNA levels (Figure 3c), cytokine production was not globally up-regulated in the PBMCs from this patient.
DISCUSSION
The findings of the present study add to the emerging body of data implicating CIAS1 mutations in a range of autoinflammatory diseases, including MWS, FCAS, and now, NOMID/CINCA syndrome. In 6 patients with classic manifestations of NOMID/CINCA syndrome, we identified 6 different CIAS1 missense mutations, 5 of which were novel, that were not observed in at least 900 control chromosomes examined. Moreover, in 2 unrelated NOMID/CINCA syndrome patients, distinct substitutions at the same CIAS1 nucleotide position gave rise to the same amino acid change (F523L), an occurrence that would be extremely unlikely if these sequence changes were not disease-associated. These data, together with the recent report by Feldmann et al (33), strongly implicate CIAS1 mutations as a cause of NOMID/CINCA syndrome. The identification of de novo cases both in the present series and in that reported by Feldmann and colleagues, coupled with the reduction in reproductive fitness observed in the more severely affected NOMID/CINCA syndrome patients, probably accounts for the largely sporadic nature of this illness.
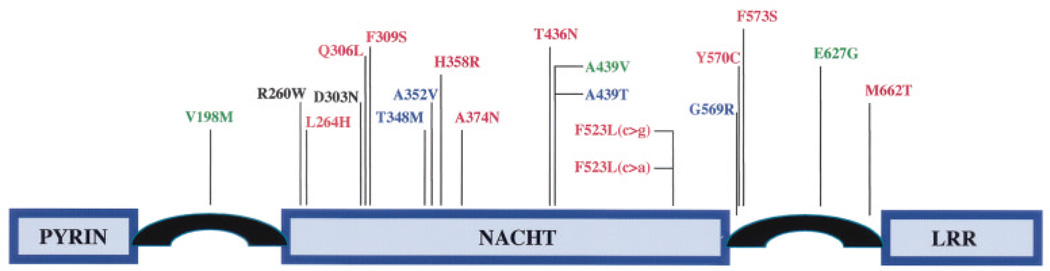
Figure 4 depicts all of the CIAS1 mutations, including those identified in the present study, that have been associated with autoinflammatory diseases (9,33,35). All 20 of these mutations fall within exon 3 of the CIAS1 genomic sequence, and 14 of the 20 mutations result in missense changes in a single domain of cryopyrin, the CIAS1 protein. The pertinent region, a nucleotide-binding site domain of the NACHT subfamily (an acronym for 4 proteins that define the subfamily: NAIP [neuronal apoptosis inhibitor protein], CIITA [class II major histocompatibility complex transcription activator], HET-E [incompatibility locus protein from Podospora anserina; a bacterial NTPase protein], and TP1 [telomerase-associated protein 1]), is found in a number of plant, animal, and bacterial proteins involved in apoptosis and inflammation, and is thought to be important in regulating their activity (41). Three of these mutations (D303N, Q306L, and D309S) are clustered near the Mg++ binding site of motif III of the NACHT domain, and there are 4 other clusters (R260W and L264H; T348M, A352V, and H358R; T436N, A439V, and A439T; G569R, Y570C, and F573S) that suggest less well-defined but perhaps equally important functional sites. It is noteworthy that all of the known CIAS1 mutations are missense changes, which suggests that more drastic truncating mutations may cause a different phenotype or may be incompatible with life.
Figure 4.
Protein structure of cryopyrin (9). All of the 20 mutations that cause familial cold autoinflammatory syndrome (FCAS), Muckle-Wells Syndrome (MWS), and/or neonatal-onset multisystem inflammatory disease (NOMID)/chronic infantile neurologic, cutaneous, and articular (CINCA) syndrome have been identified in exon 3 of CIAS1, which encodes the NACHT domain. Fourteen of the 20 mutations are located within the NACHT domain, and 6 mutations are found in the region of cryopyrin, which flanks the NACHT domain. Mutations shown in red cause NOMID/CINCA syndrome, those in blue cause MWS, those in green cause FCAS, and those in black are observed in more than one disease. The D303N mutation was identified in 2 unrelated NOMID/CINCA syndrome patients (ref. 33 and the present study) and has been reported in 1 MWS patient (35). The R260W mutation has been reported in 2 FCAS and 2 MWS families (35). LRR = leucine-rich repeat.
Mutations in CIAS1 cause MWS, FCAS, and NOMID/CINCA syndrome, and there is no apparent clustering of mutations associated with a particular illness (Figure 4). FCAS, MWS, and NOMID/CINCA syndrome appear to represent a spectrum of disease, with FCAS the mildest and NOMID/CINCA syndrome the most severe. It is therefore tempting to speculate that the phenotype determined by a specific mutation is related to its effect on cryopyrin function. However, this appears not to be the whole explanation, since two CIAS1 mutations have been associated with more than one disorder. R260W has been observed in both MWS and FCAS (35), while D303N has been documented in MWS (35) and in NOMID/CINCA syndrome (ref. 33 and the present study). These findings strongly suggest that additional modifier genes or environmental factors play a role in determining the disease phenotype.
No CIAS1 mutation has yet been identified in the N-terminal PYRIN domain of cryopyrin. This ~90– amino acid motif is also found in the protein of the same name (pyrin) encoded by the FMF gene. Manji et al (31) have recently shown that cryopyrin interacts with a second protein, ASC (apoptosis-associated speck-like protein with a caspase recruitment domain) through homotypic PYRIN domain interactions, leading to NF-κB activation. Feldmann and colleagues (33) have proposed that, through this pathway, CIAS1 mutations may have an antiapoptotic effect. In light of their demonstration that CIAS1 is expressed in chondrocytes, such a hypothesis could explain the peculiar arthropathy of NOMID/CINCA syndrome and is consistent with more general discussions of the etiology of autoinflammatory diseases (3). A defect in apoptosis might also explain the possible increased risk of malignancy in NOMID/CINCA syndrome (14,42).
CIAS1 is also expressed at high levels in leukocytes, predominantly monocytes, granulocytes, and T lymphocytes (9,31,33). In addition to the potential proinflammatory effect of alterations in NF-κB signaling in white blood cells, cryopyrin has recently been shown to regulate IL-1β production through a pathway involving first PYRIN domain interactions between cryopyrin and ASC, and then homotypic caspase recruitment domain interactions between ASC and caspase 1 (IL-1–converting enzyme) (32,43). Consistent with this pathway, we found markedly elevated IL-1β protein on Western analysis of monocyte lysates from a mutation-positive NOMID/CINCA syndrome patient. Moreover, serum IL-1Ra levels were also increased, possibly as a homeostatic response to IL-1 signaling (36,37). These data raise the possibility that the IL-1 pathway could be specifically targeted by further increasing IL-1Ra levels with the newly available therapeutic preparations of this biologic agent (44).
Results of quantitative PCR and serum ELISA suggested a more general state of cytokine activation, with dramatic increases in the levels of IL-6 message and protein and TNFβ1 levels. There were also substantial increases in IL-3 and IL-5 message, which may account for the peripheral eosinophilia observed in some NOMID/CINCA syndrome patients. In a single previously published case of NOMID/CINCA syndrome without neurologic involvement (45), serum IL-1, IL-6, and TNF levels were reported to be normal. This underscores the need, especially in the light of the present findings, for a more systematic analysis of cytokine activation in NOMID/CINCA syndrome.
The data presented in this report also raise the possibility of genetic heterogeneity in NOMID/CINCA syndrome. In 7 of the 13 patients with typical features of this disorder, we failed to identify CIAS1 mutations by comprehensive sequencing of the 9-exon CIAS1 coding region, as well as the exon–intron boundaries. Feldmann and colleagues (33) also described a mutation-negative patient with skin and joint inflammation but without evidence of chronic meningitis. In our present series, there were no major clinical differences between mutation-positive and mutation-negative patients. The possibility remains that some of these patients may have mutations in the promoter or introns, or they may have large deletions that would not be detected by sequencing of genomic DNA. Alternatively, these patients may harbor mutations in a cryopyrin homolog or in a cryopyrin-binding protein.
Coupled with the recent report by Feldmann and colleagues, our findings establish NOMID/CINCA syndrome at the severe end of the spectrum of CIAS1-associated illnesses and further extend the list of autoinflammatory diseases caused by pyrin-related proteins. While there remain important unanswered questions with regard to genetic heterogeneity, pathogenesis, and treatment, discovery of the genetic basis of NOMID/CINCA syndrome should lay the foundation for significant advances in the care of patients who have this disease.
ACKNOWLEDGMENTS
The authors wish to thank the patients and their families for their participation in this study, Kim Morrison for help with preparing the manuscript, Dr. Balu Athreya for advice during the course of this work, and Dr. Peter Lipsky for providing helpful suggestions.
REFERENCES
- 1.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Aksentijevich I, McDermott MF, O’Shea JJ, Kastner DL. TNFRSF1A mutations and autoinflammatory syndromes. Curr Opin Immunol. 2000;12:479–486. doi: 10.1016/s0952-7915(00)00124-2. [DOI] [PubMed] [Google Scholar]
- 3.Kastner DL, O’Shea JJ. A fever gene comes in from the cold. Nat Genet. 2001;29:241–242. doi: 10.1038/ng1101-241. [DOI] [PubMed] [Google Scholar]
- 4.International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 5.French FMF Consortium. A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 6.Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerhof A, Romeijn GJ, et al. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat Genet. 1999;22:175–177. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- 7.Drenth JPH, Cuisset L, Grateau G, Vasseur C, van de Velde-Visser SD, de Jong JGN, et al. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. Nat Genet. 1999;22:178–181. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 8.Drenth JPH, van der Meer JWM. Hereditary periodic fever. N Engl J Med. 2001;345:1748–1757. doi: 10.1056/NEJMra010200. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miceli-Richard C, Lesage S, Rybojad M, Prieur A-M, Manouvrier-Hanu S, Häfner R, et al. CARD15 mutations in Blau syndrome. Nat Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 11.Hassink SG, Goldsmith DP. Neonatal onset multisystem inflammatory disease. Arthritis Rheum. 1983;26:668–673. doi: 10.1002/art.1780260515. [DOI] [PubMed] [Google Scholar]
- 12.Yarom A, Rennenbohm RM, Levinson JE. Infantile multisystem inflammatory disease: a specific syndrome? J Pediatr. 1985;106:390–396. doi: 10.1016/s0022-3476(85)80662-4. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith DP. The right stuff for a new syndrome. J Pediatr. 1985;106:441–443. doi: 10.1016/s0022-3476(85)80672-7. [DOI] [PubMed] [Google Scholar]
- 14.Prieur A-M, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. J Pediatr. 1981;99:79–83. doi: 10.1016/s0022-3476(81)80961-4. [DOI] [PubMed] [Google Scholar]
- 15.Prieur A-M, Griscelli C, Lampert F, Truckenbrodt H, Guggenheim MA, Lovell DJ, et al. A chronic, infantile, neurologic, cutaneous and articular (CINCA) syndrome: a specific entity analysed in 30 patients. Scand J Rheumatol Suppl. 1987;66:57–68. doi: 10.3109/03009748709102523. [DOI] [PubMed] [Google Scholar]
- 16.Hashkes PJ, Lovell DJ. Recognition of infantile-onset multisystem inflammatory disease as a unique entity. J Pediatr. 1997;130:513–515. [PubMed] [Google Scholar]
- 17.Prieur A-M. A recently recognized chronic inflammatory disease of early onset characterised by the triad of rash, central nervous system involvement and arthropathy. Clin Exp Rheumatol. 2001;19:103–106. [PubMed] [Google Scholar]
- 18.Kaufman RA, Lovell DJ. Infantile-onset multisystem inflammatory disease: radiologic findings. Radiology. 1986;160:741–746. doi: 10.1148/radiology.160.3.3737913. [DOI] [PubMed] [Google Scholar]
- 19.Torbiak RP, Dent PB, Cockshott WP. NOMID—a neonatal syndrome of multisystem inflammation. Skeletal Radiol. 1989;18:359–364. doi: 10.1007/BF00361425. [DOI] [PubMed] [Google Scholar]
- 20.Ansell MB, Bywaters EG, Elderkin FM. Familial arthropathy with rash, uveitis and mental retardation. Proc R Soc Med. 1975;68:584–585. [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsmith DP, Lasky AS, Prieur AM. CINCA/NOMID (CN): further clinical observations [abstract] Arthritis Rheum. 1996;39(Suppl 9):S236. [Google Scholar]
- 22.Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. J Allergy Clin Immunol. 2001;108:615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muckle TJ, Wells M. Urticaria, deafness, and amyloidosis: a new heredo-familial syndrome. QJM. 1962;31:235–248. [PubMed] [Google Scholar]
- 24.Bertin J, DiStefano PS. The PYRIN domain: a novel motif found in apoptosis and inflammation proteins. Cell Death Differ. 2000;7:1273–1274. doi: 10.1038/sj.cdd.4400774. [DOI] [PubMed] [Google Scholar]
- 25.Masumoto J, Taniguchi S, Sagara J. Pyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASC. Biochem Biophys Res Commun. 2001;280:652–655. doi: 10.1006/bbrc.2000.4190. [DOI] [PubMed] [Google Scholar]
- 26.Martinon F, Hofmann K, Tschopp J. The pyrin domain: a possible member of the death domain-fold family implicated in apoptosis and inflammation. Curr Biol. 2001;11:R118–R120. doi: 10.1016/s0960-9822(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 27.Staub E, Dahl E, Rosenthal A. The DAPIN family: a novel domain links apoptotic and interferon response proteins. Trends Biochem Sci. 2001;26:83–85. doi: 10.1016/s0968-0004(00)01717-5. [DOI] [PubMed] [Google Scholar]
- 28.Pawlowski K, Pio F, Chu Z, Reed JC, Godzik A. PAAD—a new protein domain associated with apoptosis, cancer and autoimmune diseases. Trends Biochem Sci. 2001;26:85–87. doi: 10.1016/s0968-0004(00)01729-1. [DOI] [PubMed] [Google Scholar]
- 29.Fairbrother WJ, Gordon NC, Humke EW, O’Rourke KM, Starovasnik MA, Yin J-P, et al. The PYRIN domain: a member of the death domain-fold superfamily. Protein Sci. 2001;10:1911–1918. doi: 10.1110/ps.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richards N, Schaner P, Diaz A, Stuckey J, Shelden E, Wadhwa A, et al. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J Biol Chem. 2001;276:39320–39329. doi: 10.1074/jbc.M104730200. [DOI] [PubMed] [Google Scholar]
- 31.Manji GA, Wang L, Geddes BJ, Brown M, Merriam S, Al-Garawi A, et al. PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-κB. J Biol Chem. 2002;277:11570–11575. doi: 10.1074/jbc.M112208200. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Manji GA, Grenier J, Al-Garawi A, Merriam S, Lora JM, et al. PYPAF7: a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J Biol Chem. 2002;277:29874–29880. doi: 10.1074/jbc.M203915200. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russo RAG, Katsicas MM. Chronic infantile neurological cutaneous and articular syndrome: two new cases with rare manifestations. Acta Paediatr. 2001;90:1076–1079. doi: 10.1080/080352501316978192. [DOI] [PubMed] [Google Scholar]
- 35.Dodé C, Le Dû N, Cuisset L, Letourneur F, Berthelot J-M, Vaudour G, et al. New mutations of CIAS1 that are responsible for Muckle-Wells syndrome and familial cold urticaria: a novel mutation underlies both syndromes. Am J Hum Genet. 2002;70:1498–1506. doi: 10.1086/340786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 37.Chomarat P, Vannier E, Dechanet J, Rissoan MC, Banchereau J, Dinarello CA, et al. Balance of IL-1 receptor antagonist/IL-1β in rheumatoid synovium and its regulation by IL-4 and IL-10. J Immunol. 1995;154:1432–1439. [PubMed] [Google Scholar]
- 38.Wong CK, Zhang J, Ip WK, Lam CW. Intracellular signal transduction in eosinophils and its clinical significance. Immunopharmacol Immunotoxicol. 2002;24:165–186. doi: 10.1081/iph-120003748. [DOI] [PubMed] [Google Scholar]
- 39.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 40.Gerbig AW, Dahinden CA, Mullis P, Hunziker T. Circadian elevation of IL-6 levels in Muckle-Wells syndrome: a disorder of the neuro-immune axis? QJM. 1998;91:489–492. doi: 10.1093/qjmed/91.7.489. [DOI] [PubMed] [Google Scholar]
- 41.Koonin EV, Aravind L. The NACHT family: a new group of predicted NTPases implicated in apoptosis and MHC transcription activation [letter] Trends Biochem Sci. 2000;25:223–224. doi: 10.1016/s0968-0004(00)01577-2. [DOI] [PubMed] [Google Scholar]
- 42.De Cunto CL, Liberatore DI, San Roman JL, Goldberg JC, Morandi AA, Feldman G. Infantile-onset multisystem inflammatory disease: a differential diagnosis of systemic juvenile rheumatoid arthritis. J Pediatr. 1997;130:551–556. doi: 10.1016/s0022-3476(97)70238-5. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–624. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 45.Huttenlocher A, Frieden IJ, Emery H. Neonatal onset multisystem inflammatory disease. J Rheumatol. 1995;22:1171–1173. [PubMed] [Google Scholar]