Abstract
This study examined vocal coordination during mother-infant interactions in the infant siblings (high risk infants; HR) of children with autism spectrum disorder (ASD), a population at heightened risk for developing language delays. Vocal coordination between mothers and HR infants was compared to a group of low risk (LR; no first- or second-degree relative with ASD) dyads, and used to predict later language development. Nine-month-old infants were videotaped at home playing with their mothers, and interactions were coded for the frequency and timing of vocalizations. Percent infant simultaneous speech was predictive of later language delay (LD), and dyads with LD infants were less coordinated with one another in average latency to respond than dyads with non-delayed (ND) infants. The degree of coordination between mothers and infants on this variable predicted a continuous measure of language development in the third year. This research underscores the importance of understanding early development in the context of interaction.
Keywords: Parent-infant interaction, language development, high risk siblings, coordination
From early in life, parent-infant interactions are a coordinated and bidirectional experience characterized by a mutual synchronization of body, voice, and gaze (Condon & Sander, 1974; Crown, Feldstein, Jasnow, Beebe, & Jaffe, 2002; Jasnow & Feldstein, 1986; Kato, Takahashi, Sawada, Kobayashi, Watanabe & Ishii, 1983). One of the best-studied examples of this coordination occurs in the vocal behavior of caregivers and infants. Like adults, typically developing (TD) infants and their mothers coordinate the timing of their vocalizations with that of their conversational partner (Jaffe, Beebe, Feldstein, Crown, & Jasnow, 2001). The timing of infant and caregiver vocalizations in a dyadic interaction has been shown to relate to several aspects of development. For example, caregivers’ prompt and contingent responses to their infants’ vocalizations can have both short- and long-term positive effects on children’s language abilities (Goldstein & Schwade, 2008; Tamis-LeMonda, Bornstein, & Baumwell, 2001). Furthermore, coordination of the duration of vocalizations and pauses in parent-infant interactions is related to future cognitive ability as well as to attachment style (Jaffe et al. 2001). These findings are consistent with the view that infants learn through a dyadic process that involves both their own behavior and the input they receive from adults around them (Sameroff, 2009). A disruption in this dyadic process could therefore have cascading effects in multiple modalities, making social interactions an ideal context in which to understand both typical and atypical development.
The present study examines vocal coordination during early parent-infant interactions in the younger siblings (High risk siblings; HR1) of individuals with autism spectrum disorder (ASD). The younger siblings of children with ASD have recently become the subject of a wide range of prospective longitudinal studies due to their heightened genetic risk for developing the disorder (Ozonoff et al., 2011). While these studies were largely developed to identify early markers of ASD specifically, this work revealed that even those HR siblings who do not go on to develop ASD follow heterogeneous trajectories of development, ranging from completely typical to significantly delayed (Presmanes, Walden, Stone, & Yoder, 2007; Yirmiya et al., 2006). In particular, language development among HR siblings is variable, with many infants exhibiting language delays in the second and third year of life (Gamliel, Yirmiya, & Sigman, 2007; Stone, McMahon, Yoder, & Walden, 2007; Toth, Dawson, Meltzoff, Greenson, & Fein, 2007).
Despite the prevalence of early language delays among HR siblings who do and do not go on to have ASD, few studies have focused specifically on predicting language outcomes in HR infants and relatively little is known about mechanisms that may underlie these delays. The presence of notable developmental heterogeneity in this population, particularly in the domain of language, provides an opportunity to examine the impact of early experience on language development. Thus, the primary aim of the present study was to explore the relationship between characteristics of early parent-infant vocal interactions and later language development in a sample of infants at high and low risk for ASD in order to understand how subtle differences in behavior during dyadic interactions may impact later development.
Vocal Coordination in Typical Development
A large body of research amassed over several decades has detailed the development and characteristics of what is often called “coordinated interpersonal timing” (CIT), or the mutual influence of the temporal patterning of behavior (typically vocal) of two participants in an interaction (Jaffe et al., 2001). When two individuals engage in conversation, they coordinate the timing of periods of sound and silence with their interlocutors (Capella, 1981; Jaffe et al., 2001; Jaffe & Feldstein, 1970). Jaffe and Feldstein (1970) characterized the temporal patterning of dyadic vocal interactions using a description of conversational states: vocalizations, intrapersonal pauses (silences between two vocalizations of the same speaker), switching pauses (silences occurring between two speakers), and simultaneous speech. These conversational states occur within alternating “turns”, which begin the moment one individual begins speaking alone, and end at the moment their conversational partner speaks alone (Jaffe et al., 2001).
The characteristics of these states, as well as their temporal coordination, have implications for both the individual and the dyad. Speakers who produce fewer vocalizations will provide their interlocutors fewer opportunities to follow those vocalizations with contingent responses, thereby reducing the flow and coordination of the conversation. In addition to producing a sufficient number of vocalizations, speakers must also time these vocalizations properly. For example, individuals must leave sufficient time after speaking before they speak again in order to give a partner the opportunity to respond, even if their partner does not respond vocally (intrapersonal pause). The duration of switching pauses, or the pauses between when one person stops speaking and their partner begins, has been consistently implicated as one of the most informative aspects of vocal coordination. Research on a variety of dyads, from infant-adult to adult-adult, has repeatedly shown that individuals match their switching-pause durations to those of their partners on a global level (i.e., averaged across an entire interaction; Beebe, Alson, Jaffe, Feldstein, & Crown, 1988; Crown, 1991; Jaffe et al., 2001; Jaffe & Feldstein, 1970). Matching switching pause durations on a global level indicates a mutual regulation of the verbal give-and-take, such that each partner pauses for a similar amount of time before the other takes a turn (Beebe et al., 1988). In conversations between adults, the degree of congruence of switching-pause durations has been associated with individual perception of, liking of, and empathy for conversation partners (Crown, 1991; Feldstein & Welkowitz, 1978).
Finally, individuals tend to inhibit their vocalizations while other speakers are talking, so that instances of simultaneous speech are rare in typical adult conversation (Feldstein & Welkowitz, 1978; Jaffe et al., 2001; Jaffe & Feldstein, 1970). The frequency of simultaneous speech during the course of a conversation is therefore often used as a proxy for the degree to which partners are engaging in vocal turn-taking (Jaffe et al., 2001).
Outside of the research focused specifically on CIT, the time between when one individual ends a vocalization and the next individual begins speaking (i.e. the switching pause duration) is attributed to the speaker who breaks the pause and is often referred to as “response time” or “latency to respond”. Latency to respond has been implicated as an important factor in infant learning and development. Research has shown not only that mothers are quite good at providing prompt and contingent responses—responding within 2 seconds of their infants’ pre-linguistic vocalizations over 70% of the time—but also that infants are able to recognize this contingent behavior from a young age (Gros-Louis, West, Goldstein, & King, 2006; Millar & Watson, 1979). Moreover, characteristics of response timing in parent-infant interactions relate to caregiver mood. Specifically, depressed mothers have longer and more variable latencies to respond when interacting with their infants than do non-depressed mothers (Bettes, 1988; Zlochower & Cohn, 1996).
Coordinated interpersonal timing: HR infants
Studies of HR infants reveal that, as a group, these infants show delays in a number of areas throughout infancy and toddlerhood (Presmanes et al., 2007; Yirmiya et al., 2006). Given the predictive value of characteristics of infant-caregiver vocal coordination and contingency in typical development, there is reason to believe that delays in HR infants may be indicative of disruptions in interactive processes important for learning. While there has been very little research specifically examining vocal interactions between caregivers and their HR infants, research on communicative development, attention, and parent-infant interactions in this population provide evidence for potential disruptions in vocal coordination.
Several studies have found delays in spontaneous social communication in HR infants (Goldberg et al., 2005; Paul, Fuerst, Ramsay, Chawarska, & Klin, 2011; Toth et al., 2007). For example, Yirmiya et al. (2006) reported differences in spontaneous non-verbal communication at 14 months, with HR infants producing fewer higher-level requesting behaviors during the Early Social Communication Scales (ESCS; Mundy, Sigman & Kasari, 1990), a semi-structured assessment. Cassel, Messinger, Ibanez, Haltigan, Acosta, and Buchman (2007) also reported lower rates of initiated joint attention in HR infants at 15 months using the same assessment. These findings provide evidence that as a group, HR infants are delayed in non-verbal communication important for engaging with an interactive partner. While this behavior is not specifically vocal, verbal and non-verbal communication are closely intertwined in such a way that delays in non-verbal communication could have important implications for parent-infant interactions in multiple domains.
Differences in where infants allocate their attention could also have implications for parent-infant interactions in dyads with HR siblings. From the moment TD infants are born, they show a preference for attending to their mother’s voice, for human faces, and for human biological motion (DeCasper & Fifer, 1980; Simion, Regolin, & Bulf, 2008; Valenza, Simion, Cassia, & Umilta, 1996). These types of early preferences have implications for coordination in parent-infant interactions, which requires attention to one’s interactive partner and the complementary disregard of distractions. While research has yet to examine newborn orienting to social stimuli in HR infants, a study by Nadig et al. (2007) suggests there may be some differences in attentional patterns in this population by 6 months of age. At this age, HR infants showed a marginally weaker preference for infant directed speech over adult directed speech than their low risk peers. In addition, a recent study by Droucker, Curtin, and Vouloumanos (2013) provides evidence that HR siblings’ preference for faces is not as strong as that of LR infants, and that attentional preferences in HR infant siblings relate to later language development. Specifically, this group found that attention to faces over checkerboards between 6 and 12 months was predictive of 18-month expressive vocabulary. It is important to note that while both of these studies found that HR infants still allocated preferential attention to infant-directed speech and faces over adult-directed speech and checkerboards respectively, the preference was less strong than that shown by LR infants. Nonetheless, even subtle differences in attentional preferences could have cascading effects on how infants interact and communicate with others.
A few studies have reported differences specifically in parent-infant interactions in infancy among HR infants and their mothers. Yirmiya et al. (2006) found that at 6 months, HR dyads were less synchronous during periods of infant-led play—as measured by the time-series correlation of phases of caregiver and infant engagement (e.g., avert, object attend, social attend)—than LR dyads. A more recent study by Wan et al. (2013) also reported differences in mother and infant behavior during a toy play interaction. Specifically, this study reported lower mother “nondirectiveness” and lower infant “liveliness” at 6 months in HR mother-infant dyads as compared to LR mother-infant dyads. At 12 months, differences in interactive behavior were specific only to the group of HR infants who went on to have an ASD diagnosis (HR-ASD) and their mothers, and no differences were observed between dyads with HR No-ASD infants and LR dyads.
Finally, Leezenbaum, Campbell, Butler, & Iverson (2014) investigated maternal responses to HR infants’ gestures and vocalizations at 13 and 18 months and found that overall, mothers of HR infants were similarly responsive to their infant’s communicative behaviors as mothers of LR infants. Intriguingly, this study did find a difference in how maternal responsiveness to infant vocalizations changed over time. Specifically, mothers of LR infants increased their responsiveness to infant non-word vocalizations from 13 to 18 months, while mothers of HR infants did not. While none of the studies described here looked at vocal coordination specifically, collectively they suggest that HR dyads may structure their interactions differently than LR dyads, a finding that could extend to vocal coordination.
The Current Study
Despite the clear conceptual link between early vocal behavior and the development of language capabilities, to date little research has examined the relationship between parent-infant vocal coordination and later language development. As noted above, the younger siblings of children with ASD display heterogeneous development in a number of areas relevant to language and communication, and, furthermore, are at heightened risk for developing language delays in toddlerhood. To our knowledge, there is no work examining vocal coordination in HR infants and their mothers. Here we aim to fill both of these gaps by examining HR and LR mother and infant vocal behavior during a toy play interaction when infants are 9 months old, and relating this early vocal behavior to later language outcomes. The current study was designed to answer two primary questions: (a) do HR infant-mother dyads differ from LR infant-mother dyads in characteristics of vocal interactions?; and (b) are aspects of vocal interactions at 9 months predictive of language outcome in toddlerhood? Specifically, we examine whether characteristics of vocal interactions are predictive of the presence of language delay, as well as whether these variables are predictive of individual differences in language ability in toddlerhood.
Methods
Participants
Participants included 35 mother-infant dyads drawn from two larger longitudinal studies investigating language and motor development over the first years of life. Twenty-five infants (13 male) were high risk (HR; have an older sibling with a confirmed diagnosis of ASD), while 10 (5 male) were low risk (LR; have no first- or second-degree relatives diagnosed with ASD). Families in the HR group were recruited between 2007 and 2009 through a university-based Autism Research Program, parent support organizations, and local agencies and schools serving families of children with ASD. Prior to infant enrollment, the Autism Diagnostic Observation Schedule was administered to all older siblings (ADOS; Lord et al., 2000) by a trained clinician to confirm their diagnosis. Families in the LR group were recruited between 2002 and 2004 from two separate sites, a small Midwestern city and a Northeastern city, through local newspaper birth announcements and word of mouth.
All infant participants in both samples were full-term, from uncomplicated pregnancies and deliveries, and came from English-speaking homes. Infants were included in the current study if they had an uninterrupted 5-minute toy play session with their mothers at their 9 month visit (see procedures below). Thirty (20 HR infants, 10 LR infants) were Caucasian, 4 (all HR infants) were Hispanic, and 1 HR infant was Asian American. Although information on family income was unavailable, parental occupations were identified for the purpose of providing a general index of social class. Because many of the mothers were home raising their children, Nakao-Treas occupational prestige scores (Nakao & Treas, 1994) were calculated for fathers’ occupation. For 7 cases (5 HR; 2 LR), it was impossible to identify the father’s occupation with enough precision to assign a prestige score. Results from the remaining families indicated that the mean prestige scores did not differ between groups (MHR = 55.94, SD = 15.99; MLR = 51.53, SD = 14.15). Mean maternal (MHR = 33.64, SD = 4.00; MLR = 31.50, SD = 3.72) and paternal (MHR =35.64, SD = 4.80; MLR = 35.30, SD = 3.30) ages also did not differ significantly by group. A categorical variable was created for maternal education level (0 = high school only; 1 = some college or college degree; 2 = graduate or professional school). A chi-square analysis revealed a marginally significant difference between mothers of HR and LR infants in educational attainment, Χ2 (2, N = 35) = 5.47, p = .065. Specifically, 70% of LR mothers and 28% of HR mothers had attended graduate or professional school. Thirty percent of LR mothers and 64% of HR mothers had some college or a college degree. Only two mothers (both HR) had completed high school only. Due to these risk status differences in maternal education, this variable was added as a covariate in all analyses.
Procedure
As part of a larger longitudinal study, HR infants were visited monthly at home between the ages of 5 and 14 months and at 18, 24, and 36 months. LR infants were visited once every two weeks from 2 to 19 months of age. For both HR and LR infants, mother and infant were videotaped at each visit for approximately 45 minutes in various structured and unstructured activities (for further details describing the procedures employed in the two larger studies, see Iverson & Wozniak, 2007).
For purposes of the present study, a 5-minute period of unstructured, naturalistic toy play during the 9-month visit was coded. During this time, infants and mothers were seated on the floor and mothers were asked to play together with their infant and some favorite familiar toys. To enhance the audio component of the recordings, infants wore a small wireless microphone clipped to a cloth vest worn over their clothing during the session.
Measures
Two primary language measures were administered in this study. First, parents of LR children completed the Words and Sentences Form of the MacArthur-Bates Communicative Development Inventory (CDI-II; Fenson et al., 1993) at 18 and 19 months. Parents of HR children completed the CDI-II at 18 and 24 months and the CDI-III at 36 months. The CDI is a widely used measure of expressive and receptive vocabulary and grammar in both general and high risk samples (e.g. Zwaigenbaum et al. 2005; Hudry et al, 2014). It has excellent internal consistency and test-retest reliability, as well as concurrent validity with tester-administered measures (Fenson et al., 1993). For the present study, the vocabulary checklist (Words Produced) was utilized from these assessments.
Second, children in the HR group were also administered the Mullen Scales of Early Learning (MSEL; Mullen, 1995) at 18, 24, and 36 months. The MSEL is a normed, standardized developmental assessment of language, cognitive, and motor functioning. For the purposes of the current study, only the Receptive (RL) and Expressive (EL) Language subscales were utilized.
For HR infants, a standardized language composite was created using scores from CDI and MSEL at 24 and 36 months to generate a continuous measure of language in toddlerhood. This was done by standardizing into z-scores and then averaging the 24- and 36-month CDI percentile scores and the 24- and 36-month RL and EL MSEL t-scores. CDI percentile scores were used because parents filled out two different forms at these time points—the CDI-II at 24 months and the CDI-III at 36 months. Level of internal consistency for the composite was more than adequate (Cronbach’s α = .858). Table 1 displays the correlation coefficients for these items. As can be seen in the table, inter-item correlations were all significant excepting two marginal correlations (p = .08, .06 respectively) and Pearson’s rs ranged from 0.40-0.85.
Table 1.
Correlations between language measures used in Language Composite.
| Measure | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. CDI WP Percentile 24 m | — | .46* | .73** | .40° | .44* | .56** |
| 2. Mullen RL t-score 24 m | — | .54** | .53* | .77** | .78** | |
| 3. Mullen EL t-score 24 m | — | .41° | .59** | .78** | ||
| 4. CDI WP Percentile 36 m | — | .45* | .52* | |||
| 5. Mullen RL t-score 36 m | — | .85** | ||||
| 6. Mullen EL t-score 36 m | — |
Note. WP = Words Produced; RL = Receptive Language; EL = Expressive Language; m = month;
p <.10;
p<.05;
p<.01
Outcome Classification
Among HR infants, language delay was assessed using a combination of the CDI and Mullen at 18, 24, and 36 months. Infants were categorized as language delayed (LD) if either of the following criteria were met (Parlade & Iverson, 2015):
Standardized scores on the CDI-II and CDI-III at or below the 10th percentile at more than one time point between 18 and 36 months (e.g., Gershkoff-Stowe, Thal, Smith, & Namy, 1997; Heilmann, Weismer, Evans, & Hollar, 2005; Robertson & Weismer, 1999; Weismer & Evans, 2002)
Standardized scores on the CDI-III at or below the 10th percentile and standardized scores on the Receptive and/or Expressive subscales of the MSEL equal to or greater than 1.5 standard deviations below the mean at 36 months (e.g., Landa, Holman, & Garrett-Mayer, 2007; Ozonoff et al., 2011).
Thirteen HR infants (6 male) met the above criteria for language delay (LD). Three of these infants also met criteria for ASD at 36 months (based on the ADOS and clinical judgment). Given the relatively small groups of LD and ASD infants, the current study focuses on the commonality of language delay among these participants. The remaining 12 HR infants (7 male) were classified as not delayed (HR-ND).
LR infants were followed to 19 months of age. No developmental concerns were ever reported by parent or examiners for any of these infants during the course of their involvement in the study. Furthermore, the second author has remained in contact with these families, and no children have subsequently received a diagnosis of a developmental disorder of any sort (e.g., ASD, language impairment). One infant had CDI scores below the 10th percentile at 18 and 19 months and was therefore excluded from analyses focusing on Outcome. The remaining 9 LR infants (4 male) had CDI scores at or above the 15th percentile at 19 months, and were therefore classified as not delayed (ND).
Coding
Mother-infant interactions were coded offline by four independent coders blind to infant Risk Status and Outcome using a time-locked annotation program (ELAN; Brugman & Russel, 2004) that allows coders to annotate and characterize data from an audio and video source on a moment-to-moment basis. Waveform files of the audio were created from each video to provide an additional visual component for identifying durations of sounds and pauses. The following conversational state variables were coded (see Figure 1 for a visual representation of conversational state variables):
Figure 1.
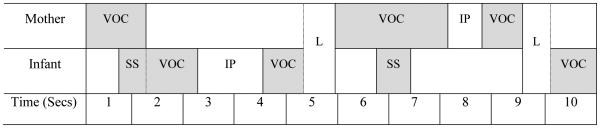
Visual representation of conversational state variables. Shaded areas are periods of sound. VOC = Vocalization; SS = Simultaneous Speech; IP = Intrapersonal Pause; L = Latency to Respond
Vocalizations
All sounds made by the mother and the infant were coded and attributed to the individual who produced them (the speaker). Sounds were categorized as either voluntary (e.g. babbles, words, raspberries etc.) or involuntary (e.g. sneezes, coughs, etc.). For analysis, only the frequencies of mother and infant voluntary vocalizations were calculated.
Intrapersonal pauses
An intrapersonal pause was coded as a period of silence between the end of a speaker’s voluntary vocalization and the beginning of another voluntary vocalization produced by the same speaker (e.g. Jasnow & Feldstein, 1986). It is attributed to the speaker who speaks before and after the pause. For analysis, we calculated the average durations (in seconds) of mother and infant intrapersonal pauses.
Latency to Respond
Latency to respond was coded as a period of silence between when one speaker stops a voluntary vocalization and the other speaker begins a voluntary vocalization (e.g. Bettes, 1988; Zlochower & Cohn, 1996), and is attributed to the speaker who breaks the pause. For analysis we calculated both the average durations (in seconds) and the coefficients of variance (CV; standard deviation divided by the mean) of mother and infant latency to respond. The CV indicates the variability of latency to respond with the effect of mean differences removed.
Simultaneous speech
Simultaneous speech was coded when both speakers were vocalizing at the same time. Both speakers’ vocalizations had to be voluntary (i.e., not a cough, sneeze, etc.) for simultaneous speech to be coded. Simultaneous speech is attributed to the “interrupting” speaker, or the speaker who chimes in. Thus, an instance of infant simultaneous speech occurs when a mother is speaking and the infant begins speaking during the mother’s vocalization. For analysis, we calculated the percentage of vocalizations (sum of infant and mother vocalization frequency) that included infant simultaneous speech and the percentage of vocalizations that included mother simultaneous speech. We used percent simultaneous speech in order to control for the possibility that differences in the frequency of infant and mother simultaneous speech may reflect variation in the total amount of speech occurring in the entire interaction (i.e., mothers and infants who vocalize more would be expected to have more simultaneous speech than those who vocalize sparingly).
Reliability
Coders were trained until they reached at least 80% reliability on all variables with a master coder (JBN) on three consecutive videos. To assess inter-coder reliability, approximately 30% of the videotaped data were scored by two separate coders (N = 10 sessions). Sessions were chosen at random with the constraint that all infant groups were equally represented. In order for identification agreement to occur, the start and end times of each vocalization had to occur within a quarter of a second (250 ms) of one another. Using this procedure, mean percent agreement for identification of vocalizations was 85% (range: 80-89%). Mean Cohen’s Kappa statistic for identification of vocalization type (voluntary vs. involuntary) was .94 (range: .90-.96). Pearson’s statistics for average durations of pauses ranged from .77 (for infant intrapersonal pauses) to .85 (for caregiver and infant latency to respond).
Results
The present study was designed to address two main research questions: a) Do characteristics of mother-infant vocal interactions differ between LR and HR dyads?; and b) how do characteristics of vocal interactions at 9 months relate to later language development? Results relevant to each study aim will be presented in turn. All analyses were conducted using version 20.0 of SPSS for Windows (IBM Corp., 2012). Due to differences in LR and HR maternal education levels reported above, all regression analyses included maternal education as a predictor in order to control for this variable.
The effect of risk status on characteristics of vocal interactions
Our first set of analyses examined Risk Status (LR vs. HR) differences in conversational state variables2. Group differences in frequencies of mother and infant vocalizations, mean durations of mother and infant intrapersonal pauses and latencies to respond, variability of mother and infant latencies to respond, and percent mother and infant simultaneous speech were examined using linear regressions. Table 2 displays the means, standard deviations, and results of statistical analysis for these conversational state variables in relation to Risk Status (LR vs. HR). As is evident in the table, Risk Status was not significant predictor of any of the conversational state variables.
Table 2.
Means, standard deviations, and results of statistical analyses for mother and infant vocal behavior in low risk (LR) and high risk (HR) dyads.
| Mother |
Infant |
|||||
|---|---|---|---|---|---|---|
| LR M (SD) |
HR M (SD) |
t (33) | LR M SD) |
HR M (SD) |
t (33) | |
| Vocalization Freq. | 77.30 (28.67) | 68.24 (19.15) | −0.96 | 26.90 (14.67) | 32.12 (23.43) | 1.12 |
| Intrapersonal Pause Dur. | 2.68 (2.01) | 2.81 (1.37) | −0.01 | 2.00 (2.01) | 1.80 (1.37) | −0.77 |
| Latency to Respond Dur. | 1.75 (1.26) | 2.20 (1.54) | 0.50 | 2.10 (1.55) | 2.55 (1.76) | −0.03 |
| Latency to Respond Var. | 1.17 (0.26) | 1.43 (0.36) | 0.12 | 1.10 (0.66) | 1.95 (3.49) | 0.05 |
| Perc. Simultaneous Speech | 3.48 (3.26) | 4.28 (3.52) | 0.27 | 4.70 (2.85) | 5.61 (3.20) | 1.30 |
Note. Freq. = Frequency; Dur = Duration (seconds); Var. = Coefficient of variance; Perc. = Percent
To examine coordination of latency to respond, we ran a linear regression predicting mother latency to respond duration with infant latency to respond duration, Risk Status, and an infant latency to respond duration-by-Risk Status interaction term. The interaction term allowed us to determine whether Risk Status had a moderating effect on the coordination between mother and infant latency to respond. While infant latency to respond duration was a significant predictor of mother latency to respond duration overall (B = .53, t (32) = 2.78, p =.009), Risk Status did not moderate this coordination.
Predicting language delay
Our next set of analyses examined whether characteristics of vocal interactions at 9 months predicted language delay in toddlerhood3. In order to determine whether conversational state variables were predictive of language delay above and beyond the effect of Risk Status, Risk Status was also included as a covariate in these models. Table 3 displays the means and standard deviations for each conversational state variable for dyads with infants who went on to be LD and dyads with infants who did not (LR and HR-ND infants). As can be seen in the table, LD infants were more likely to speak when their mothers were vocalizing than were HR-ND and LR infants (MLD = 6.73, SD = 3.40; MHR-ND = 4.41, SD = 2.58; MLR = 4.07, SD = 2.14). Linear probability models revealed that percent infant simultaneous speech was a significant predictor of language delay (b = .057, t(30) = 2.74, p = .01). These results indicate that for each 1 percent increase in infant simultaneous speech at 9 months, there was a 5.7 percentage point increase in the likelihood of the infant being language delayed in toddlerhood. A similar trend was observed for mother simultaneous speech (MLD = 5.36, SD = 3.77; MHR-ND = 3.12, SD = 2.95; MLR = 2.71, SD = 2.29), but it did not reach significance (b = .04, t(30) = 1.72, p = .096. No other variables were significant predictors of language delay.
Table 3.
Means and standard deviations for mother and infant vocal behavior in low risk (LR), high risk-no delay (HR-ND), and language delay (LD) dyads.
| Mother | Infant | |||||
|---|---|---|---|---|---|---|
| LR M (SD) |
HR-ND M (SD) |
LD M (SD) |
LR M (SD) |
HR-ND M (SD) |
LD M (SD) |
|
| Vocalization Freq. | 74.78 (29.23) | 65.83 (20.96) | 70.46 (17.88) | 25.00 (14.20) | 29.83 (25.45) | 34.23 (22.23) |
| Intrapersonal Pause Dur. | 2.82 (2.08) | 2.92 (1.38) | 2.71 (1.41) | 2.06 (2.12) | 2.01 (1.68) | 1.60 (1.02) |
| Latency to Respond Dur. | 1.86 (1.28) | 2.65 (1.64) | 1.78 (1.38) | 2.21 (1.61) | 2.94 (2.03) | 2.19 (1.46) |
| Latency to Respond Var. | 1.14 (0.26) | 1.48 (0.37) | 1.38 (0.35) | 1.05 (0.24) | 0.98 (0.39) | 1.19 (0.44) |
| Perc. Simultaneous Speech | 2.71 (2.29) | 3.12 (2.95) | 5.36 (3.77) | 4.07 (2.14) | 4.41 (2.58) | 6.73 (3.40) |
Note. Freq. = Frequency; Dur = Duration; Var. = Coefficient of variance; Perc. = Percent.
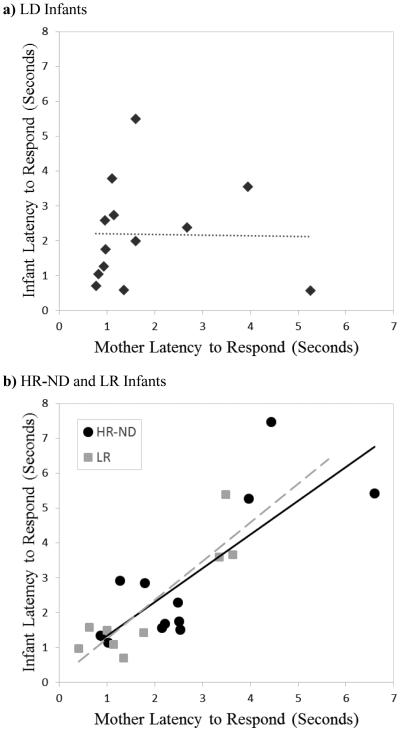
We next examined coordination of latency to respond. In order to determine whether there were differences between dyads with LD and ND infants, we conducted three separate regressions predicting average durations of mother latency to respond with infant latency to respond for dyads with LD, HR-ND, and LR infants. Figure 2 displays scatterplots of these relationships. As is apparent in the figure, HR-ND and LR infants and their mothers had latencies to respond that were strongly and positively related to one another (HR-ND: b = .61, t(11) = 3.68, p =.005; LR: b = .89, t(8) = 5.63, p = .001), while LD infants and their mothers did not exhibit this pattern of coordination (b = −.19, t(12) = −.52, p = .61) 4.
Figure 2.
Correlations between mother and infant mean latency to respond for a) Language Delay (LD) and b) High Risk-No Diagnosis (HR-ND) and Low Risk (LR) infants and mothers.
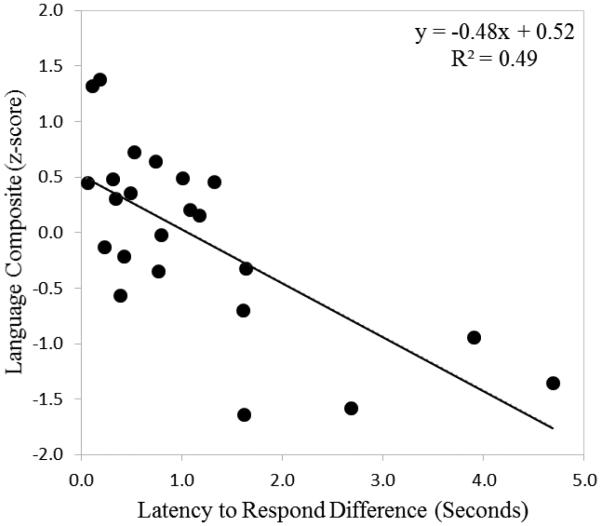
Given the increased frequencies of infant simultaneous speech and reduced coordination of latencies to respond in dyads with LD infants, we examined the relationship between these conversational state variables and language ability in the third year (i.e., the composite score combining 24- and 36-month CDI and MSEL language scores) among HR infants. For these analyses, we calculated an individual measure of latency to respond coordination for each dyad by taking the absolute value of the difference between mother and infant mean latency to respond (see Beebe et al., 1988). This value indicates how close to “perfect coordination” each mother-infant dyad is, with a difference score of zero indicating perfect coordination (e.g. both mother and infant have a mean latency to respond of exactly two seconds). A linear regression analysis was conducted predicting language composite scores with percent infant simultaneous speech, latency to respond difference score, and maternal education. The results of this analysis are displayed in Table 4. As can be seen in the table, the overall model was significant and explained 58% of the variance in language composite scores. Only latency to respond difference score was a uniquely significant predictor of language composite, although percent infant simultaneous speech was a marginally significant predictor. In other words, larger differences in latency to respond between a mother and her infant were predictive of lower language composite scores in the third year. Results indicated that for every one unit increase in latency to respond difference score, there was a corresponding .53 unit decrease in language composite z-score. The relationship between these two variables is displayed in Figure 3.
Table 4.
Linear regression predicting Language Composite.
| DV: Language Composite | |||
|---|---|---|---|
| Independent Variable | B | SE B | β |
| Percent Infant SS | −.06° | .03 | −.32 |
| Latency to Respond Difference Score | −.53*** | .12 | −.77 |
| Maternal Education | .18 | .23 | .13 |
| Constant | .74 | .41 | |
| R2 | .58 | ||
| F | 8.67*** | ||
Note.
p<.10,
p<.001, SS = Simultaneous Speech
Figure 3.
Relationship between latency to respond difference at 9 months and language composite z-score in toddlerhood. Note: Lower latency to respond differences indicate greater coordination.
In order to clarify whether this relationship was driven by a particular measure or age point, we ran follow-up bivariate correlations examining the relationship between latency to respond difference score and each of the six language scores that comprised the language composite (24- and 36-month CDI percentile scores and 24- and 36-month RL and EL MSEL t-scores). The results of these analyses are displayed in Table 5. As can be seen in the table, the latency to respond difference score was negatively and significantly correlated (r’s = −0.42 - −0.68) with all but one (CDI at 36 months, r = −0.32, p = .15) of the six individual language measures.
Table 5.
Bivariate correlations between Latency to Respond Difference Score and individual language measures at 24 and 36 months.
| Measure | Pearson Correlation (r) |
|---|---|
| CDI WP Percentile 24 m | −0.42* |
| Mullen RL t-score 24 m | −0.60** |
| Mullen EL t-score 24 m | −0.64** |
| CDI WP Percentile 36 m | −0.32 |
| Mullen RL t-score 36 m | −0.54** |
| Mullen EL t-score 36 m | −0.68** |
Note. WP = Words Produced; RL = Receptive Language; EL = Expressive Language; m = month;
p<.05;
p<.01
Discussion
This research was designed to examine characteristics of parent-infant vocal interactions between the younger siblings of children with ASD—a population at heightened risk for developing significant language delays—and their caregivers. Specifically, we evaluated how HR dyads differed from dyads with LR infants and how these characteristics of vocal interactions related to later language development. There were two main findings. First, Risk Status was not directly related to any conversational state variable. Second, individual behaviors (e.g., how often mothers and infants spoke; how long mothers and infants paused between vocalizations) did not predict language delays. However, two measures of dyadic coordination—percent infant simultaneous speech, and latency to respond coordination—were significant predictors of later delay. Specifically, LD infants were more likely to vocalize when their mothers were speaking, and dyads with LD infants were less coordinated in the average durations of their latencies to respond than dyads with ND infants. Furthermore, coordination of latency to respond was a unique predictor of individual differences in language development in toddlerhood. Below, we highlight potential mechanisms underlying some of these differences in coordination and their predictive value.
Vocal Coordination and Language Delay
Coordinating vocal behavior with a partner both facilitates and requires the development of a complex mix of selective attention, sophisticated vocal production, and social engagement. For example, in toy play interactions like those coded here, infants must allocate attention to toys and their social partner, produce communicative vocalizations, and use non-verbal social cues to reflect their engagement. Difficulty in any of these areas could affect parent-infant interactions in subtle ways, and the ability to employ these skills simultaneously on a moment-to-moment basis in the context of an interaction is particularly complex. For example, an infant who has difficulty flexibly allocating attention in an interaction may not attend to a mother’s speech as consistently. This reduced attention could then lead to mismatches in the timing of the infant’s vocalizations in the form of simultaneous speech or less coordinated latencies to respond.
In the present study, dyadic coordination of latency to respond was uniquely predictive of individual differences in language ability in the third year, indicating that this may be a particularly sensitive variable. Switching pause coordination is apparent in typical development by 4 months of age (Crown et al., 2002; Jaffe et al., 2001; Jasnow & Feldstein, 1986). Coordination between mothers and infants is likely a phenomenon that develops over time, through repeated experiences interacting together. Absence of coordination could be indicative of delays in skills such as engagement, vocal control, contingency detection, and/or differences in the predictability of either or both partners.
In addition to being a potential marker of delay, lack of coordination may also have cascading effects for future learning. Dyadic coordination of latency to respond is indicative of a well-regulated interaction in which each member of the dyad is waiting a similar amount of time before taking a turn. One possibility is that coordinating latency to respond makes responses in vocal interactions more predictable, and may consequently provide more frequent or higher quality learning experiences for infants. It is also likely that lack of coordination in this domain coincides with a lack of coordination in other domains (e.g. visual attention, affective states, engagement with toys) as well, and it is in the context of this broader asynchrony that delays emerge.
With regard to simultaneous speech, it may be informative to gather a more detailed description of these instances of overlap. Jaffe et al. (2001) distinguish between simultaneous speech that “interrupts” the speaker (i.e. the original speaker stops speaking once the joining speaker begins) and simultaneous speech that does not interrupt (i.e. the original speaker continues to speak and entirely overlaps the joining speakers vocalization). The current study did not make this distinction. To the extent that different types of simultaneous speech may influence mother-infant interactions in varied ways, describing the type of simultaneous speech that young LD infants are engaging in may provide additional insights into the nature of the differences observed here.
While there is no previous research to our knowledge examining the relationship between early parent-infant vocal coordination and later language development in HR samples, research on non-vocal behaviors in HR infants may provide further insight into the results reported here. As indicated above, HR infants as a group exhibit delays in non-verbal communication, show reduced attentional preferences for social stimuli, and are less “synchronous” with their mothers than are LR infants (e.g. Droucker, Curtin, & Vouloumanos, 2013; Goldberg et al., 2005; Yirmiya et al., 2006). While we did not find differences in coordination based on risk status alone, the research cited above did not specifically examine subsets of infants in the samples who went on to have language delays (i.e., LD infants). However, it is possible that the on-average group differences based on risk status reported in previous work could have been driven by these infants. Thus, for example, if LD infants are less likely to jointly engage with their mothers, the timing of their vocalizations may be less influenced by that of their mothers’ vocalizations, leading to increased simultaneous speech and reduced coordination of pause durations. Examination of other infant behavior during interactions (e.g., non-verbal communication, direction of infants’ visual attention during vocalizations) may help clarify whether this type of engagement is related to the frequency of simultaneous speech or coordination of pauses in dyads with LD infants.
Limitations
To our knowledge, this study is the first to examine the relationship between early vocal coordination in mother-infant interactions and later language development in the younger siblings of children with ASD, a group at heightened risk for developing language delays. While the novelty of this approach and the detailed micro-analytic coding of dyadic vocal behavior are strengths of this research, a note of caution regarding the interpretation of the findings is in order. First, our sample size was relatively small and consisted primarily of well-educated Caucasian families. It is therefore possible that some of the null findings we report are due to low power, and the results clearly merit replication with a larger and more diverse sample. Second, standardized language assessments were not available for our LR group beyond the age of 19 months. Although there were no parent or experimenter concerns about language development for any of the LR children and the available CDI data and our subsequent contact with families give us every confidence that these children were acquiring language in a typical fashion, the absence of these assessments makes it difficult to conclude that none of these children exhibited language delays after the age of 19 months.
In the future, we plan to replicate this study with a larger sample of infants, and, in particular, to include more infants who eventually receive an ASD diagnosis. We are also interested in taking a broader approach to studying behavior in mother-infant interaction, including visual attention, affective states, infant vocalization quality, and quality of maternal responses. Having more information about these fundamental interactions may further our understanding of the differences described here and provide greater insight into how delays emerge over time.
Conclusions and Clinical Implications
Taken together, the findings reported here suggest two general conclusions. First, studying HR infants who go on to have language delays reveals differences that may not be apparent when only Risk Status is considered. Previous research on HR infants has revealed a variety of group-level differences between this population and LR infants over the first years of life (e.g. Cassel et al., 2007; Goldberg et al., 2005; Toth et al., 2007); however, these on-average discrepancies may be driven by a subset of infants who go on to have language delays. Very little research to date has looked specifically at LD infants, but the wide heterogeneity among HR infants in developmental trajectories suggests that creating subgroups in this way may give researchers a more nuanced understanding of early development and delay in this population. The findings reported here support this notion: a majority of differences in vocal interactions were revealed in analyses focused on Outcome rather than on Risk Status.
Second, these data emphasize the importance of understanding infant development in the context of social interaction. While much of the previous research seeking to identify early markers of delay in HR infants has involved the description of individual child behaviors (see Jones, Gliga, Bedford, Charman, & Johnson, 2014, for a review), the current study examined infant behavior in the context of dyadic interaction. The results call attention to the importance of studying development in this way, as it was only analyses of the coordination of vocal behavior that revealed subtle early differences between infants who went on to exhibit delays and those who did not, and only coordination that predicted the course of development. Measures of individual behaviors were not predictive of later communicative development. While describing the development of any particular skill (e.g. language development, selective attention, non-verbal communication) in isolation or outside the context of a social interaction may not reveal early delays, examining infants’ ability to coordinate with a social partner provides an opportunity to observe more complex and subtle differences in developmental trajectories.
We hope that these findings will be a starting point for identifying early markers of language delay and, subsequently, early targets for intervention within the context of parent-infant interaction. Although replication is necessary, this study indicates that coordination of vocal behavior could be used to identify infants at risk for delay. Early interventions focused specifically on developing and enhancing vocal coordination within the parent-infant dyad could then be developed in the hope that they might alter the infant’s developmental trajectory in positive ways.
Acknowledgments
This research was supported by grants from Autism Speaks and the National Institutes of Health (R01 HD41607 and R01 HD054979) to JMI. Additional support was provided by HD35469 and HD055748 to N.J. Minshew and by an Autism Science Foundation Predoctoral Fellowship to J. Northrup. We thank members of the Infant Communication Lab at the University of Pittsburgh for help with data collection and coding and Nancy Minshew and Diane Williams for valuable contributions at various stages of the project. Special thanks to Susan Campbell and Celia Brownell for insightful comments and suggestions and to the infants and their families, without whose enthusiastic and dedicated participation this study could not have been completed. This research was completed in partial fulfillment of the requirements for the degree of Master of Science at the University of Pittsburgh. Portions of these data were presented at the 2013 Biennial Meetings of the Society for Research in Child Development, Seattle, WA.
Footnotes
Throughout this manuscript, the term “high risk“ (HR) is used to refer specifically to the younger siblings of children with autism spectrum disorder (ASD).
Three variables (mother intrapersonal pause, infant intrapersonal pause, and frequency infant simultaneous speech) had outliers greater than 3.29 standard deviations above the mean and were transformed to one unit greater than the next highest value (see Tabachnick & Fidell, 2013). Results were unchanged with or without the use of transformations.
All predictive analyses were run with and without infants who went on receive an ASD diagnosis included and findings remained unchanged.
Mahalanobis distance was calculated to determine whether multivariate outliers were skewing the results for the LD mothers and infants. No outliers were detected. Visual inspection of the LD scatter plot (see Figure 2) led us to rerun the correlation with the data point on the far right of the plot removed. The correlation remained non-significant (b = .27, p = .34).
Contributor Information
Jessie B. Northrup, University of Pittsburgh, 3419 Sennott Square, 210 South Bouquet St. Pittsburgh, PA 15232
Jana M. Iverson, University of Pittsburgh
References
- Beebe B, Alson D, Jaffe J, Feldstein S, Crown C. Vocal congruence in mother-infant play. Journal of Psycholinguistic Research. 1988;17(3):245–259. doi: 10.1007/BF01686358. [DOI] [PubMed] [Google Scholar]
- Bettes BA. Maternal depression and motherese: Temporal and intonational features. Child development. 1988:1089–1096. doi: 10.1111/j.1467-8624.1988.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Brugman H, Russel A. Annotating multi-media/multi-modal resources with ELAN. Paper presented at the LREC. 2004 [Google Scholar]
- Capella JN. Mutual influence in expressive behavior: Adult-adult and infant-adult dyadic interaction. Psychological Bulletin. 1981;89(1):101–132. [PubMed] [Google Scholar]
- Cassel TD, Messinger DS, Ibanez LV, Haltigan JD, Acosta SI, Buchman AC. Early social and emotional communication in the infant siblings of children with autism spectrum disorders: An examination of the broad phenotype. Journal of Autism & Developmental Disorders. 2007;37(1):122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Condon WS, Sander LW. Neonate movement is synchronized with adult speech: Interactional participation and language acquisition. Science. 1974;183(4120):99–101. doi: 10.1126/science.183.4120.99. [DOI] [PubMed] [Google Scholar]
- Crown CL. Coordinated interpersonal timing of vision and voice as a function of interpersonal attraction. Journal of Language and Social Psychology. 1991;10(1):29–46. [Google Scholar]
- Crown CL, Feldstein S, Jasnow MD, Beebe B, Jaffe J. The cross-modal coordination of interpersonal timing: Six-week-olds infants' gaze with adults' vocal behavior. Journal of Psycholinguistic Research. 2002;31(1):1–23. doi: 10.1023/a:1014301303616. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers' voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Droucker Da., Curtin S, Vouloumanos A. Linking infant-directed speech and face preferences to language outcomes in infants at risk for autism spectrum disorder. Journal of Speech, Language and Hearing Research. 2013;56(2):567. doi: 10.1044/1092-4388(2012/11-0266). [DOI] [PubMed] [Google Scholar]
- Feldstein S, Welkowitz J. A chronography of conversation: In defense of an objective approach. In: Siegman AW, Feldstein S, editors. Nonverbal behavior and communication. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- Fenson L, Dale P, Reznick SJ, Thal DJ, Bates E, Hartung JP, Reilly JS. The macarthur communicative development inventories: User's guide and technical manual. Paul H. Brookes Publishing Company; Baltimore: 1993. [Google Scholar]
- Gamliel I, Yirmiya N, Sigman M. The development of young siblings of children with autism from 4 to 54 months. Journal of Autism and Developmental Disorders. 2007;37(1):171–183. doi: 10.1007/s10803-006-0341-5. [DOI] [PubMed] [Google Scholar]
- Gershkoff-Stowe L, Thal DJ, Smith LB, Namy LL. Categorization and its developmental relation to early language. Child Development. 1997;68(5):843–859. doi: 10.1111/j.1467-8624.1997.tb01966.x. [DOI] [PubMed] [Google Scholar]
- Goldberg W, Jarvis K, Osann K, Laulhere T, Straub C, Thomas E, Spence M. Brief report: Early social communication behaviors in the younger siblings of children with autism. Journal of Autism and Developmental Disorders. 2005;35(5):657–664. doi: 10.1007/s10803-005-0009-6. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, Schwade JA. Social feedback to infants' babbling facilitates rapid phonological learning. Psychological Science. 2008;19(5):515–523. doi: 10.1111/j.1467-9280.2008.02117.x. [DOI] [PubMed] [Google Scholar]
- Gros-Louis J, West MJ, Goldstein MH, King AP. Mothers provide differential feedback to infants' prelinguistic sounds. International Journal of Behavioral Developmen. 2006;30(6):509–516. [Google Scholar]
- Heilmann J, Weismer SE, Evans J, Hollar C. Utility of the macarthur--bates communicative development inventory in identifying language abilities of late-talking and typically developing toddlers. American Journal of Speech-Language Pathology. 2005;14(1):40. doi: 10.1044/1058-0360(2005/006). [DOI] [PubMed] [Google Scholar]
- Hudry K, Chandler S, Bedford R, Pasco G, Gliga T, Elsabbagh M, & Charman T. Early Language Profiles in Infants at High-Risk for Autism Spectrum Disorders. Journal of autism and developmental disorders. 2014;44(1):154–167. doi: 10.1007/s10803-013-1861-4. [DOI] [PubMed] [Google Scholar]
- IBM, Corp. IBM SPSS Statistics for Windows (Version 21) IBM Corporation; Armonk, NY: 2012. [Google Scholar]
- Iverson JM, Wozniak RH. Variation in vocal-motor development in infant siblings of children with autism. Journal of Autism & Developmental Disorders. 2007;37(1):158–170. doi: 10.1007/s10803-006-0339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe J, Beebe B, Feldstein S, Crown CL, Jasnow MD, Rochat P, Stern DN. Rhythms of dialogue in infancy: coordinated timing in development. Monographs of the Society for Research in Child Development. 2001;66(2):i–149. [PubMed] [Google Scholar]
- Jaffe J, Feldstein S. Rhythms of dialogue. Academic Press; New York and London: 1970. [Google Scholar]
- Jasnow MD, Feldstein S. Adult-Like temporal characteristics of mother-infant vocal interactions. Child Development. 1986;57(3):754–761. [PubMed] [Google Scholar]
- Jones EJ, Gliga T, Bedford R, Charman T, Johnson MH. Developmental pathways to autism: A review of prospective studies of infants at risk. Neuroscience & Biobehavioral Reviews. 2014;39:1–33. doi: 10.1016/j.neubiorev.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Takahashi E, Sawada K, Kobayashi N, Watanabe T, Ishii T. A computer analysis of infant movements synchronized with adult speech. Pediatric Research. 1983;17(8):625–628. doi: 10.1203/00006450-198308000-00004. [DOI] [PubMed] [Google Scholar]
- Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64(7):853. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Leezenbaum NB, Campbell SB, Butler D, Iverson JM. Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism. 2014;18(6):694–703. doi: 10.1177/1362361313491327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28(2):355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Millar WS, Watson JS. The effect of delayed feedback on infant learning reexamined. Child Development. 1979;50:747–751. [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Pearson San Antonio, TX: 1995. [Google Scholar]
- Mundy P, Sigman M, Kasari C. A longitudinal study of joint attention and language development in autistic children. Journal of Autism and developmental Disorders. 1990;20(1):115–128. doi: 10.1007/BF02206861. [DOI] [PubMed] [Google Scholar]
- Nadig AS, Ozonoff S, Young GS, Rozga A, Sigman M, Rogers SJ. A prospective study of response to name in infants at risk for autism. Archives of Pediatric Adolescent Medicine. 2007;161(4):378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: How the new measures measure up. Sociological methodology. 1994;24:1–72. [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parladé M, Iverson J. The Development of Coordinated Communication in Infants at Heightened Risk for Autism Spectrum Disorder. Journal of Autism and Developmental Disorders. 2015:1–17. doi: 10.1007/s10803-015-2391-z. doi: 10.1007/s10803-015-2391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Fuerst Y, Ramsay G, Chawarska K, Klin A. Out of the mouths of babes: Vocal production in infant siblings of children with ASD. Journal of Child Psychology and Psychiatry. 2011;52(5):588–598. doi: 10.1111/j.1469-7610.2010.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presmanes AG, Walden TA, Stone WL, Yoder PJ. Effects of different attentional cues on responding to joint attention in younger siblings of children with autism spectrum disorders. Journal of Autism & Developmental Disorders. 2007;37(1):133–144. doi: 10.1007/s10803-006-0338-0. [DOI] [PubMed] [Google Scholar]
- Robertson SB, Weismer SE. Effects of treatment on linguistic and social skills in toddlers with delayed language development. Journal of Speech, Language and Hearing Research. 1999;42(5):1234. doi: 10.1044/jslhr.4205.1234. [DOI] [PubMed] [Google Scholar]
- Sameroff A. The transactional model of development: How children and contexts shape each other. American Psychological Association; Washington DC: 2009. [Google Scholar]
- Simion F, Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences. 2008;105(2):809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social-communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatrics & Adolescent Medicine. 2007;161(4):384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS, Osterlind SJ. Using multivariate statistics. Pearson; New Jersey: 2001. [Google Scholar]
- Tamis-LeMonda CS, Bornstein MH, Baumwell L. Maternal responsiveness and children's achievement of language milestones. Child Development. 2001;72(3):748. doi: 10.1111/1467-8624.00313. [DOI] [PubMed] [Google Scholar]
- Toth K, Dawson G, Meltzoff A, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. Journal of Autism and Developmental Disorders. 2007;37(1):145–157. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza E, Simion F, Cassia VM, Umilta C. Face preference at birth. Journal of Experimental Psychology: Human Perception & Performance. 1996;22(4):892–903. doi: 10.1037//0096-1523.22.4.892. [DOI] [PubMed] [Google Scholar]
- Wan MW, Green J, Elsabbagh M, Johnson M, Charman T, Plummer F. Quality of interaction between at-risk infants and caregiver at 12–15 months is associated with 3-year autism outcome. Journal of Child Psychology and Psychiatry. 2013;54(7):763–771. doi: 10.1111/jcpp.12032. [DOI] [PubMed] [Google Scholar]
- Weismer SE, Evans JL. The role of processing limitations in early identification of specific language impairment. Topics in Language Disorders. 2002;22(3):15–29. [Google Scholar]
- Yirmiya N, Gamliel I, Pilowsky T, Feldman R, Baron-Cohen S, Sigman M. The development of siblings of children with autism at 4 and 14 months: Social engagement, communication, and cognition. Journal of Child Psychology and Psychiatry. 2006;47(5):511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zlochower AJ, Cohn JF. Vocal timing in face-to-face interaction of clinically depressed and nondepressed mothers and their 4-month-old infants. Infant Behavior and Development. 1996;19(3):371–374. [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23(2-3):143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]