Abstract
The caudal part of the cerebellar fastigial nucleus (CFN) influences the horizontal component of saccades. Previous reports show that activity in the CFN contralateral to saccade direction aids saccade acceleration and that activity in the ipsilateral CFN aids saccade deceleration. Here we refine this description by characterizing how blocking CFN activity changes the distance that the eye rotates during each of 4 phases of saccades, the increasing and decreasing saccade acceleration (phases 1 & 2) and deceleration (3 and 4). We found that unilateral CFN inactivation increases total eye rotation to ~1.8X normal. This resulted from rotation increases in all four phases of ipsiversive saccades. Rotation during phases 1 and 2 increases slightly, more during phase 3, and most during phase 4, to ~4.4X normal. Thus, the ipsilateral CFN normally reduces eye rotation throughout a saccade but reduces it the most near saccade end. After unilateral CFN inactivation, rotation during contraversive saccades was ~0.8X normal. This resulted from decreased rotation during phases 1–3, to ~0.7X normal, and then normal rotation during phase 4. Thus the CFN contraversive to saccade direction normally increases eye rotation during acceleration and the first phase of deceleration. These data indicate that the influences of the CFNs on saccades overlap extensively and that there is a smooth shift from predominance of the contralateral CFN early in a saccade to the ipsilateral CFN later. The pathway from the CFN to contralateral IBNs and then to the abducens nucleus can account for these effects.
Keywords: saccades, caudal fastigial nucleus, muscimol, monkey, fastigial oculomotor region
1. INTRODUCTION
The cerebellum affects the horizontal component of saccades via the output of the caudal part of the medial, or fastigial nucleus, an area we call the caudal fastigial nucleus (CFN), also called the fastigial oculomotor region or FOR. CFN output makes saccades the right size, fast, and, consistent, i.e., saccades to the same target locations exhibit very similar sizes and velocities. When we block activity in the CFN on one side of the cerebellum saccades are dysmetric, overshooting toward the inactivated side and undershooting away from it. Saccades of a particular size are also often slower than normal (Goffart et al., 2004; Robinson et al., 1993). Blocking CFN output bilaterally makes both leftward and rightward saccades too large. No previous proposal explains why hypermetria predominates over hypometria when both CFNs are silent.
CFN neurons fire a burst of action potentials for every saccade. Bursts for contraversive saccades usually start within ~10 ms before saccade start, while bursts for ipsiversive saccades start a bit later, within ~10 ms after movement onset. A previous model (Fuchs et al., 1993; Lefevre et al., 1998; Ohtsuka & Noda, 1991; Optican & Quaia, 2002; Quaia et al., 1999) proposes that the later CFN bursts for ipsiversive saccades help stop these saccades. In this model activity in the right CFN, for example, causes off-direction spikes after saccade start in left inhibitory burst neurons (IBNs). This late IBN activity on the left inhibits right abducens neurons late in the saccade “choking off” the abducens burst at the end of the saccade and stopping rightward eye rotation. In this view, off-direction IBN activity ends near the end of the saccade so that it does not impair the tonic abducens firing rate that holds the eye in its post-saccade position.
This model is consistent with several findings. The CFN projects to contralateral IBNs (Noda et al., 1990; Sugita & Noda, 1991). Via this connection CFN activity almost certainly drives some, and maybe all, off-direction activity in contralateral IBNs. We infer this from the following findings. About 50% of IBNs fire off-direction spikes for contraversive saccades (Scudder et al., 1988). Nearly all of these IBNs that fire off-direction spikes fire more of them after saccade adaptation that decreases the size of off-direction saccades (Kojima et al., 2008). For example, in that study, 14/42 IBNs fired off-direction spikes consistently and 15/42 fired off-direction spikes occasionally. Adaptation increased the average number of off-direction spikes/burst fired by the consistent group from 7.1 to 8.3, a 14% increase. It increased average number for the occasional group from 1.4 to 3.1, a 55% raise. Increased IBN activity inhibits agonist abducens neurons more, decreasing saccade size by contracting the agonist muscle less. The CFN neurons that project to and excite IBNs are almost certainly the origin of adaptation-related increase in off-direction IBN activity because these CFN neurons also increase their activity during the same adaptation (Inaba et al., 2003).
Finally, both CFN activity for ipsiversive saccades, and the off-direction spikes in contralateral IBNs that the CFN putatively drives, begin after saccade onset. Given this, CFN activity probably affects the later parts of saccades. Indeed CFN inactivation impairs the deceleration of ipsiversive saccades while having much less, if any, effect on saccade acceleration (Goffart et al., 2004; Robinson et al., 1993).
Goffart et al., (2004) show that CFN activity affects ipsiversive saccades and contraversive saccades almost exclusively during deceleration and acceleration, respectively. Here we measure at higher resolution the periods during which CFN activity affects horizontal saccades. We compare saccades before and after CFN inactivation during each of four phases, 1) increasing and 2) decreasing acceleration and 3) increasing and 4) decreasing deceleration. We found that CFN activity affects ipsiversive saccades most strongly during phase 4, falling deceleration, and affects contralateral saccades about equally during phases 1–3, i.e., acceleration and the first phase of deceleration. These data provide the clearest current picture of when signals from both sides of the cerebellum affect horizontal saccades.
2. RESULTS
We made 11 unilateral injections into the CFNs of 5 monkeys and 3 bilateral injections into the CFNs of 3 monkeys (one bilateral inactivation in each monkey). We restricted our analysis to horizontal saccades to targets 10° to the left or right. After CFN inactivation previously described deficits were evident: dysmetria, and a fixation offset toward the side of the injection (Goffart et al., 2004; Robinson et al., 1993).
In these experiments the monkey tracked targets that were continuously illuminated so we inspected the velocity profiles of post-injection saccades to be sure that they did not have several peaks indicating visual feedback. Post-inactivation saccades did not have multiple velocity peaks. Below we describe the dysmetria after CFN inactivation and focus on the size and variability of three attributes that we measured.
2.1 Unilateral Muscimol Inactivation
2.1.1 Ipsiversive Saccades
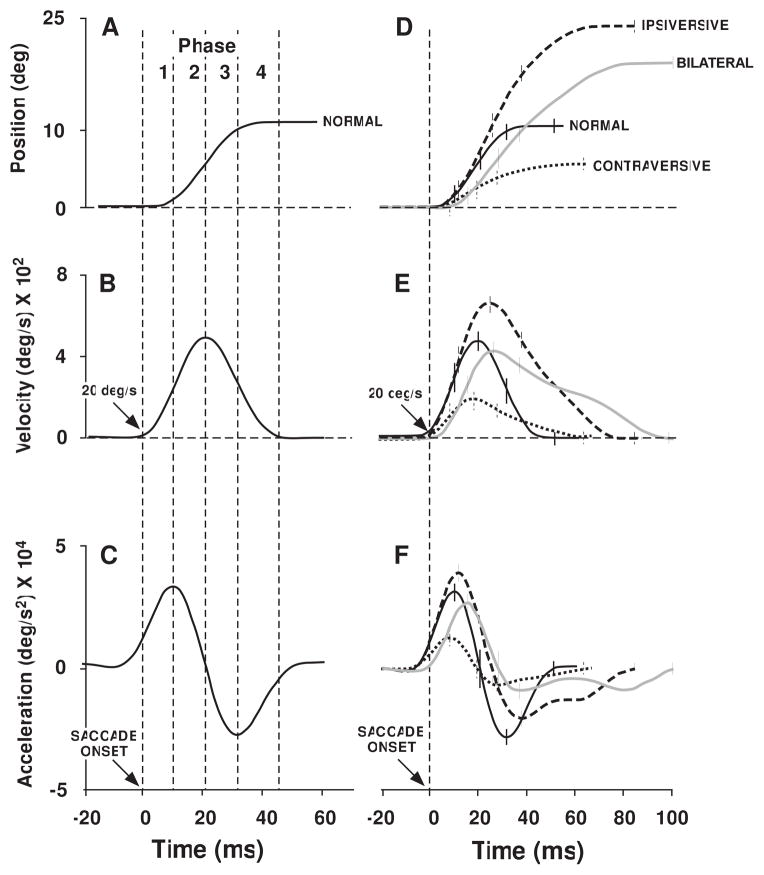
We analyzed 835 normal saccades and 2492 saccades ipsiversive to an inactivated CFN. Figure 1 shows the phases into which we divided each saccade. Figures 1A, B, and C show eye position, velocity, and acceleration, respectively, during a normal saccade and the borders between each phase. Figures 1D, E, and F show the same information for examples of each of the four types of saccades that we characterized, 1) normal saccades and saccades after unilateral inactivation of the 2) ipsilateral and 3) contralateral CFN and after 4) bilateral inactivation.
Figure 1. Average position, velocity and acceleration profiles after unilateral and bilateral CFN inactivation and phases of saccades.
A. position; B. velocity; C. acceleration traces of normal saccades. Phase 1: saccade onset to peak acceleration, phase 2: peak acceleration to peak velocity (0 acceleration), phase 3: peak velocity to peak deceleration, phase 4: peak deceleration to saccade end. D–F. Average of ten representative saccades for normal (black solid), post-injection contraversive (small dashes), ipsiversive (large dashes) and bilateral (gray solid) saccades. Vertical tick marks show borders between phases. D. position (offset not visible because we superimposed saccade starting positions); E. velocity; F. acceleration. Saccade onset: 20 deg/s on velocity traces.
2.1.1.1 Dysmetria
In Figure 1D the trace with large dashes shows the average eye position during 10 example saccades to targets displaced 10° in the direction ipsiversive to an inactivated CFN. Small arrows in Figure 2 (described below) indicate the injection from which we took the saccades averaged in Figure 1. The short vertical marks on each trace in Figures 1D–F show the borders between phases. As evident in Figure 1D ipsiversive saccades were hypermetric and overshot their targets. The common mean gain of ipsiversive saccades was 2.06±0.04. This is 202% of normal since normal saccade gain was 1.01.
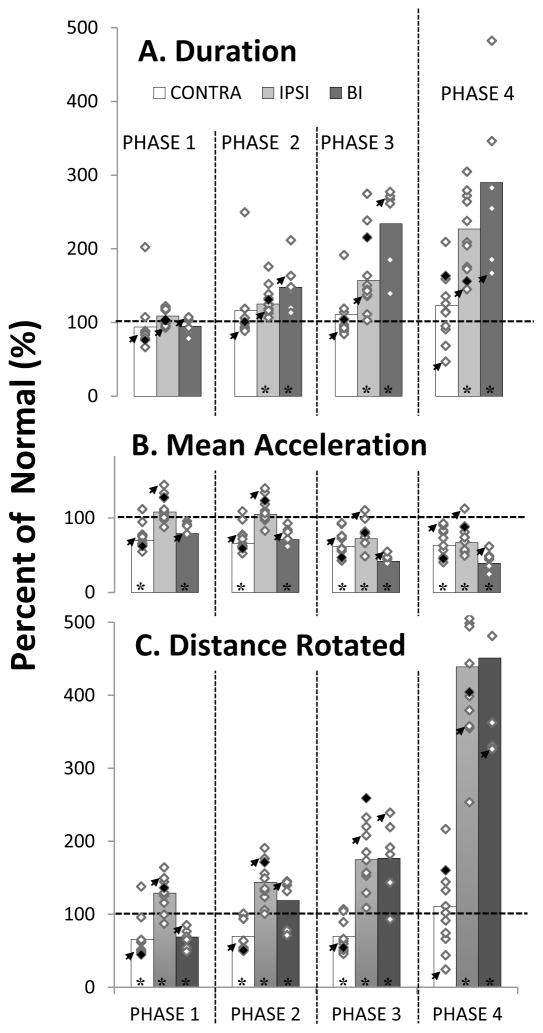
Figure 2. Duration, mean acceleration and distance the eye rotated for contraversive, ipsiversive and bilateral saccades.
Histogram bar height represents the percent of normal values of saccades after inactivation of the contralateral (white bars) or ipsilateral (light gray bars) CFN, or after inactivation of both CFNs (dark gray bars). The dashed line at 100% marks the value of normal saccades. The diamonds superimposed on each bar are the mean value of each CFN inactivation. The filled black diamond on each bar is the mean value recorded after the largest muscimol injection. The small arrows indicate the data from the inactivation from which we used saccades for Figure 1. Significance is marked by an asterisk p≤ 0.05. A. duration of each phase; B. the mean acceleration (phases 1 and 2) or deceleration (phases 3 & 4); C. the distance that the eye rotated.
Table 1 shows average measurements of entire saccades before (PRE) and after (POST) each CFN inactivation. Table 1A contains values for ipsiversive saccades. As the first two data columns in Table 1A show, every CFN inactivation made saccades hypermetric. For every inactivation, hypermetric ipsiversive saccades had durations that were longer than normal.
Table 1.
Mean values for each CFN inactivation of saccade gain, duration, and peak velocity before (PRE) and after (POST) CFN inactivation. Section 1 provides information for saccades ipsiversive to the inactivated CFN, section B for contraversive saccades and section C for saccades after we inactivated both CFNs are. We inactivated the CFN bilaterally three times. In section C we give separate measurements for saccades in each direction. The first column in each section identifies each inactivation in monkeys A, H, M, R, and W. The next two columns give the side and volume of the muscimol injection. In the PRE and POST columns bold numbers indicate values significantly different from normal (p ≤ 0.05). Numbers in the rightmost column under the GAIN, DURATION, and PEAK ACCELERATION sectors show the ratio POST/PRE coefficient of variation (CV). Values greater than 1 are bold and indicate more variability after CFN inactivation.
| A. IPSILATERAL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| GAIN | DURATION (ms) | PEAK VELOCITY (°/s) | |||||||||
|
|
|||||||||||
| INJ | INJ SIDE | VOL (μL) | PRE | POST | POST/PRE CV | PRE | POST | POST/PRE CV | PRE | POST | POST/PRE CV |
|
|
|||||||||||
| A6 | L | 0.5 | 1 | 1.92 | 59% | 40 | 60 | 97% | 470 | 593 | 73% |
| A7 | R | 0.7 | 1.01 | 1.46 | 76% | 45 | 56 | 65% | 476 | 484 | 57% |
| A8 | L | 0.7 | 1.09 | 2.31 | 95% | 45 | 78 | 113% | 501 | 544 | 120% |
| H1 | L | 3.0 | 1.03 | 2.15 | 293% | 52 | 88 | 209% | 368 | 536 | 89% |
| M3 | R | 1.0 | 1.10 | 2.16 | 71% | 58 | 109 | 158% | 399 | 450 | 89% |
| M4 | L | 1.5 | 1.01 | 1.95 | 103% | 54 | 96 | 149% | 421 | 480 | 99% |
| R1 | L | 0.7 | 1.00 | 2.23 | 350% | 45 | 57 | 81% | 448 | 670 | 130% |
| R2 | L | 1.0 | 1.00 | 2.55 | 279% | 45 | 82 | 168% | 448 | 620 | 129% |
| R3 | R | 1.0 | 0.95 | 2.26 | 236% | 48 | 74 | 206% | 404 | 551 | 112% |
| W1 | R | 1.0 | 0.94 | 2.27 | 490% | 48 | 94 | 378% | 411 | 460 | 147% |
| W5 | R | 1.0 | 0.99 | 1.45 | 420% | 43 | 74 | 250% | 435 | 380 | 121% |
|
|
|||||||||||
| AVG | 1.01 | 2.06 | 225% | 48 | 79 | 170% | 435 | 524 | 106% | ||
| B. CONTRALATERAL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| GAIN | DURATION (ms) | PEAK VELOCITY (°/s) | |||||||||
|
|
|||||||||||
| INJ | INJ SIDE | VOL (μL) | PRE | POST | POST/PRE CV % | PRE | POST | POST/PRE CV % | PRE | POST | POST/PRE CV % |
|
|
|||||||||||
| A6 | L | 0.5 | 1.15 | 0.72 | 76% | 43 | 41 | 81% | 636 | 335 | 84% |
| A7 | R | 0.7 | 1.09 | 0.81 | 143% | 45 | 46 | 86% | 501 | 352 | 148% |
| A8 | L | 0.7 | 1.01 | 0.6 | 182% | 45 | 48 | 120% | 476 | 270 | 97% |
| H1 | L | 3.0 | 1.00 | 1.13 | 1105% | 59 | 114 | 339% | 355 | 210 | 67% |
| M3 | R | 1.0 | 1.01 | 0.99 | 173% | 55 | 58 | 114% | 379 | 374 | 107% |
| M4 | L | 1.5 | 1.09 | 1.05 | 240% | 58 | 62 | 123% | 376 | 351 | 114% |
| R1 | L | 0.7 | 0.95 | 0.51 | 324% | 48 | 36 | 83% | 404 | 249 | 116% |
| R2 | L | 1.0 | 0.95 | 0.64 | 1321% | 48 | 50 | 374% | 404 | 236 | 101% |
| R3 | R | 1.0 | 1.00 | 0.66 | 394% | 45 | 51 | 119% | 448 | 243 | 107% |
| W1 | R | 1.0 | 1.04 | 0.49 | 983% | 46 | 79 | 205% | 411 | 236 | 118% |
| W5 | R | 1.0 | 1.08 | 0.78 | 325% | 46 | 58 | 92% | 446 | 254 | 95% |
|
|
|||||||||||
| AVG | 1.03 | 0.76 | 479% | 49 | 58 | 158% | 440 | 283 | 105% | ||
| C. BILATERAL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| GAIN | DURATION (ms) | PEAK VELOCITY (°/s) | |||||||||
|
|
|||||||||||
| INJ | INJ SIDE | VOL (μL) | PRE | POST | POST/PRE CV % | PRE | POST | POST/PRE CV % | PRE | POST | POST/PRE CV % |
|
|
|||||||||||
| B1 | L | 1.0 | 1.00 | 1.33 | 263% | 46 | 111 | 215% | 367 | 260 | 165% |
| B1 | R | 1.0 | 1.05 | 1.73 | 368% | 59 | 122 | 179% | 413 | 278 | 109% |
| M1 | L | 0.8 | 0.96 | 1.76 | 422% | 50 | 101 | 268% | 356 | 340 | 166% |
| M1 | R | 0.8 | 1.03 | 2.36 | 219% | 55 | 120 | 192% | 382 | 400 | 116% |
| R1 | L | 0.7 | 1.00 | 1.69 | 267% | 45 | 85 | 179% | 448 | 355 | 156% |
| R1 | R | 0.7 | 0.95 | 1.96 | 508% | 48 | 87 | 313% | 404 | 395 | 176% |
| AVG | 1.00 | 1.81 | 341% | 51 | 104 | 225% | 395 | 338 | 148% | ||
2.1.1.2 Phase duration
In the example saccade in Figures 1E and 1F, it is apparent that at least the later phases of ipsiversive saccades are longer after CFN inactivation. Figure 2 compares post-inactivation saccade duration (2A), acceleration (2B), and eye rotation (2C) to normal values. In Figure 2A the middle gray bar in each phase represents the duration of ipsiversive saccades. The diamond symbols superimposed on each bar are the average values from each individual inactivation. Asterisks at the bottom of each bar show values significantly different from normal (p<0.05). Figure 2A shows that during ipsiversive saccades the duration of phase 1 was not significantly different from normal while the durations of all subsequent phases were significantly longer than normal. Each later phase is more different from normal than the preceding one. Table 2 shows the average values in each phase of saccade duration, acceleration, and rotation. The top row of Table 2A shows that the durations of phases 2, 3, and 4 are 125%, 157%, and 227% of normal, for respectively.
Table 2.
Common mean values of saccade for duration, mean acceleration/deceleration, and the distance that the eye rotated before (Control) after (CFN Inactivated) unilateral and bilateral CFN inactivations during each of the 4 saccade phases. Control numbers are measured values. Numbers for measurements after CFN inactivation are the percent of the control represented by that measurement. Bold numbers indicate values significantly different from normal (p≤ 0.05).
| Unilateral Injections Common Mean | PHASE 1 | PHASE 2 | PHASE 3 | PHASE 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Control | CFN Inactivated | Control | CFN Inactivated | Control | CFN Inactivated | Control | CFN Inactivated | |
| Value | % of Control | Value | % of Control | Value | % of Control | Value | % of Control | |
| A. Ipsilateral | ||||||||
| DURATION (ms) | 10.6 | 109% | 9.72 | 125%* ↑ | 11.98 | 157%* ↑ | 15.14 | 227%* ↑ |
| MEAN ACCELERATION (deg/s2) | 20734 | 108% | 19981 | 105% | 14062 | 72%* ↓ | 17631 | 67%* ↓ |
| DISTANCE ROTATED (deg) | 1.36 | 129%* ↑ | 3.91 | 144%* ↑ | 4.03 | 175%* ↑ | 1.16 | 439%* ↑ |
| B. Contralateral | ||||||||
| DURATION (ms) | 10.92 | 94% | 9.43 | 116% | 11.96 | 111% | 15.66 | 123% |
| MEAN ACCELERATION (deg/s2) | 20172 | 70%* ↓ | 19342 | 66%* ↓ | 13257 | 62%* ↓ | 16342 | 63%* ↓ |
| DISTANCE ROTATED (deg) | 1.35 | 66%* ↓ | 3.76 | 70%* ↓ | 3.97 | 70%* ↓ | 1.20 | 111% |
| Bilateral Injections Common Mean | ||||||||
| C. Bilateral | ||||||||
| DURATION (ms) | 10.31 | 95% | 9.91 | 148%* ↑ | 11.95 | 234%* ↑ | 17.28 | 290%* ↑ |
| MEAN ACCELERATION (deg/s2) | 18286 | 79%* ↓ | 17622 | 71%* ↓ | 11951 | 42%* ↓ | 14340 | 39%* ↓ |
| DISTANCE ROTATED (deg) | 1.23 | 69%* ↓ | 3.59 | 119% | 3.71 | 177%* ↑ | 1.30 | 451%* ↑ |
2.1.1.3 Phase mean acceleration
The light gray bars in Figure 2B show the common mean acceleration or deceleration in each phase for ipsiversive saccades. As apparent in Figure 2B and the middle row of Table 2A, averaged across all inactivations, ipsiversive saccades accelerated at normal rates in phases 1 and 2 but decelerated more slowly than normal in phases 3 (72% of normal) and 4 (67%).
2.1.1.4 Phase distance rotated
Figure 2C shows the common mean of the distance that the eyes rotated during each phase. The eyes rotate significantly farther than normal in all four phases with each later phase more abnormal than the previous one, i.e., 129%, 144%, 175 % and 439% of normal, for phases 1, 2, 3, and 4, respectively (last row in Table 2A).
2.1.1.5 Variability
After most unilateral CFN inactivations ipsiversive saccades are more variable. In Table 2 the rightmost column in each division, i.e., GAIN, DURATION, and PEAK VELOCITY, shows the percent of the normal coefficients of variation (CV) represented by the post-inactivation CV. Values greater than 100% indicate more variability after inactivation and are bold. Although some saccade attributes after some inactivations are not more variable than normal, most are. For ipsiversive saccades, after CFN inactivation the average CVs for gain, saccade duration, and peak velocity were 225%, 170%, and 106% of normal, respectively (bottom row Table 1A). CVs for phase duration, mean acceleration/deceleration, and distance rotated were 228%, 163%, and 188% of normal, respectively.
2.1.2 Contraversive Saccades
We measured 742 normal saccades and 2404 saccades contraversive to an inactivated CFN.
2.1.2.1 Dysmetria
In Figure 1D the trace with small dashes shows the average eye position during 10 example saccades to 10° targets contraversive to an inactivated CFN. As apparent in Figure 2D, contraversive saccades fell short of their targets. The common mean gain of these saccades was 0.76 ± 0.02. This represents 75% of normal because the gain of normal saccades to targets 10° to the left or right was 1.01. As evident in the first two data columns Table 1B, 8 of our 11 CFN inactivations made contraversive saccades hypometric. For each of these 8 inactivations, hypometric contraversive saccades had average durations that were shorter than normal.
2.1.2.2 Phase duration
In Figure 2A the white histogram bars show the common mean duration for each phase of contraversive saccades. As Figure 2A and the top row in Table 2B show, the average duration of each phase did not change significantly after CFN inactivation.
2.1.2.3 Phase mean acceleration
In Figure 2B the white histogram bars show mean acceleration or deceleration in each phase of saccades contraversive to an inactivated CFN. As Figure 2B and Table 2B show, the average acceleration/deceleration of every saccade phase was significantly lower than normal, averaging 65% of normal.
2.1.2.4 Phase distance rotated
In Figure 2C the white histogram bars show the distance that the eyes rotated during each phase. As Figure 2C and the bottom row of Table 2B show, the distance that the eyes rotated was ~69% of normal in phases 1–3 but was normal in phase 4.
2.1.2.5 Variability
After most unilateral CFN inactivations contraversive saccades are more variable. As with ipsiversive saccades, some attributes of contraversive saccades after some inactivations are less variable but most attributes after most injections are more variable. For contraversive saccades, after CFN inactivation the average CVs for gain, saccade duration, and peak velocity were 479%, 158%, and 105% of normal, respectively (Table 1B). Like ipsiversive post-injection saccades, contraversive saccades were also abnormally variable. The CVs for phase duration, acceleration/deceleration, and distance rotated were 352%, 149%, was and, 287% of normal.
2.2 Bilateral Muscimol Inactivation
We inactivated the CFN bilaterally once in each of the 3 monkeys. One-way ANOVAs of all attributes across all injections found no significant difference in saccades ipsiversive and contraversive to the first CFN injected. We therefore separated these saccades into ipsiversive and contraversive relative to the first CFN injected. Table 1C gives the mean gain, duration, and peak velocity of saccades in each direction. We compared 148 normal to 2266 post-injection saccades.
2.2.1 Dysmetria
As apparent in Figure 1D, bilateral CFN inactivation made saccade overshoot their targets. The second data column in Table 1C shows that every bilateral inactivation made saccades in both directions hypermetric. The common mean gain for these saccades was 1.8±0.07 or 181% of normal. Normal gain was 1.00. As Table 2 shows, the durations of hypermetric saccades after bilateral inactivations are longer than normal.
2.2.2 Phase duration
Figure 2A shows that after bilateral inactivation the duration of phase 1 was normal but the durations of phases 2, 3 and 4 were significantly longer than normal. As Figure 2A and the top row of Table 1C show, each subsequent phase was more abnormal than the preceding phase, i.e., 148%, 234%, and 290% of normal for phases 2, 3, and 4, respectively.
2.2.3 Phase mean acceleration
The dark gray bars in Figure 2B show the acceleration or deceleration of each saccade phase after bilateral inactivation. As Figure 2B and the middle row in Table 2C show, bilateral inactivation makes saccades accelerate and decelerate more slowly than normal in every phase. Phases 1 and 2 accelerated at 79% and 71% of normal, respectively. Phases 3 and 4, decelerated even more slowly than did either contraversive or the ipsiversive saccades, i.e., 42% and 39% of normal, respectively.
2.2.4 Phase distance rotated
As Figure 2C and the bottom row in Table 2C show, bilateral inactivation caused a contraversive-like deficit in phase 1 rotation, i.e., reducing it to 69% of normal. In phase 2, saccades showed a mixture of contraversive small rotation and ipsiversive large rotation deficits. After unilateral CFN inactivation, eye rotation in phase 2 was 70% of normal for contraversive saccades and 144% of normal for ipsiversive saccades and saccades. After bilateral inactivation the eyes travelled 119% of normal rotation, near the mean of the contraversive and ipsiversive values. Eye rotation in phases 3 and 4 was nearly identical to that of ipsiversive saccades, i.e., 177% and 451% of normal, respectively.
2.2.5 Variability
After every bilateral CFN inactivations saccades in both directions were more variable. As Table 1C shows, the CVs for gain, duration, and peak velocity were all larger than normal after bilateral inactivation, i.e., 341%, 225%, and 148% of normal, respectively. The CVs for phase duration, acceleration/deceleration, and distance rotated were 228%, 175%, and 190% of normal respectively.
3. DISCUSSION
This work characterizes the effect of CFN activity on horizontal saccades during each of 4 phases. By measuring the effect of CFN inactivation on saccades we found that normal CFN activity significantly reduces eye rotation in all 4 saccade phases of ipsiversive saccades. Its effect on acceleration in phases, 1 and 2 is small. During phase 3, when deceleration increases, CFN activity reduces eye rotation more than during phases 1 and 2. During phase 4, when deceleration decreases, CFN activity reduces eye rotation the most of all phases. Without CFN activity to slow it, the eyes rotate ~4X normal during phase 4 (Figure 2). During contraversive saccades, CFN activity significantly increases eye rotation during phases 1 – 3, but not during phase 4.
The results of our measurements are consistent with, and extend, the previous descriptions of how CFN activity affects saccades. Previous CFN inactivation data indicate that CFN signals normally aid the deceleration of ipsiversive saccades and the acceleration of contraversive saccades (Goffart et al., 2004; Robinson et al., 1993). Goffart et al., account for deficits following CFN inactivation with CFN projections to contralateral IBNs and EBNs. They propose that these neurons must be outside the feedback loop since the burst generator does not compensate for missing signals from the cerebellum.
Our current data are consistent with this picture and refine it by demonstrating CFN influence in three periods during a saccade where previous work does not describe it. First, while CFN activity decreases the distance that the eye rotates during the deceleration of ipsiversive saccades, we show that it reduces rotation much more during the final, phase of deceleration, phase 4, than during phase 3. We conclude this because CFN inactivation increases eye rotation during phase 4 much more than during phase 3 of ipsiversive saccades. Increased rotation in both phases is a consequence of a significant increase in the duration of both phases and a decrease in the deceleration of both phases. Both of these changes are significantly larger in phase 4 than in phase 3.
The fact that CFN activity reduces rotation most strongly at the end of ipsiversive saccades is consistent with the fact that most CFN activity during ipsiversive saccades occurs after saccade start (Fuchs et al., 1993; Ohtsuka & Noda, 1991). We conclude that the largest part of the CFN’s “choking off” of agonist abducens activity proposed in the model of CFN influence (Lefevre et al., 1998; Optican & Quaia, 2002; Quaia et al., 1999) occurs at the end of saccade-related abducens bursts for ipsiversive saccades.
Our second addition to current work shows that, while CFN activity decreases the distance that the eye rotates during the deceleration of ipsiversive saccades, it also decreases eye rotation during acceleration. We conclude this because CFN inactivation causes a small, but significant increase in eye rotation during phases 1 and 2 of ipsiversive saccades. We interpret this to mean that normally CFN activity reduces eye rotation during phases 1 and 2. This reduction is probably a consequence of a small, sometimes statistically insignificant, increase in the duration and/or decreases in eye acceleration of phases 1 and 2 of ipsiversive saccades.
The fact that CFN activity normally reduces eye rotation during ipsiversive rotation is consistent with the observation that some CFN neurons occasionally fire bursts early in ipsiversive saccades that can begin before saccade start (Fuchs et al., 1993; Kleine et al., 2003; Ohtsuka and Noda, 1991). This CFN activity early in ipsiversive saccades is small compared to later CFN firing. Still, our findings suggest that even this small, early CFN activity reduces eye rotation during the acceleration of ipsiversive saccades. Thus, our data indicate that, consistent with a proposal by Quinet & Goffart (2007), this early activity overlaps with, and acts as a small counterbalance to, the increase in eye rotation provided by the opposite CFN during phases 1 and 2. CFN activity early during ipsiversive saccades presumably increases early off-direction activity in contralateral IBNs which, in turn, slightly reduces the activity of agonist abducens neurons, reducing agonist contraction. Previous work describes relatively early off-direction IBN spikes (Cullen & Guitton, 1997; Kojima et al., 2008).
The third new contribution of this work shows that during contraversive saccades CFN activity not only normally increases eye rotation during saccade acceleration as previously described, but also increases rotation during the first phase of deceleration, phase 3. CFN inactivation significantly reduces rotation during phase 3. This is consistent with the fact that, though CFN bursts for contraversive saccades generally begin before contraversive saccades, they continue through a significant part of the saccade overlapping extensively with bursts in the contralateral CFN. Spikes late in CFN bursts for contraversive saccades presumably increase the on-direction activity of contralateral IBNs which, in turn, increase the depth and/or duration of the off-direction pause in antagonist abducens activity. We conclude that CFN activity extends the abducens pause long enough to increase rotation at the start of deceleration.
Goffart et al., (2004) observe that ipsilateral and contralateral CFN bursts overlap extensively. There is a smooth transition from the predominance of contralateral CFN influence early in a saccade to predominance of ipsilateral CFN influence at the end. We propose that the consequence of this overlap and transition are both the small influence of the ipsilateral CFN during saccade acceleration and of the contralateral CFN during deceleration.
The cerebellum may similarly shift the balance of early antagonist muscle inhibition to later agonist muscle inhibition to accelerate other movements and end them on target. For example, cerebellar patients exhibit abnormal agonist-antagonist muscle balance during arm and finger movements (Hore et al., 1991).
3.1 The CFN-IBN-abducens pathway accounts for dysmetria after CFN inactivation
3.1.1 Hypermetria of ipsiversive saccades & hypometria of contraversive saccades
Previous data and the proposed model of CFN influence on saccades account well for hypermetria of ipsiversive saccades after inactivation the CFN on one side. In summary, loss of activity in, for example, the right CFN makes rightward saccades overshoot because IBNs on the left do not fire enough off-direction spikes to appropriately reduce abducens firing, and eye rotation, at the end of the saccade. As Goffart et al. point out, the same pathway can account for the hypometria of contraversive saccades. Inactivation of the right CFN makes leftward saccades smaller by reducing the on-direction activity of IBNs on the left. This reduces the inhibition that these IBNs provide to the right, antagonist, abducens decreasing the depth and/or duration of the abducens off-direction pause. The smaller pause in antagonist abducens activity increases the contraction of the antagonist muscle and its resistance to the leftward saccade. The unimpaired CFN continues to provide its normal inhibition to agonist abducens neurons. The net result of increased antagonist activity and the normal reduction in agonist activity from the unimpaired CFN reduces eye rotation contraversive to the inactivated CFN.
3.1.2 Hypermetria of both left and rightward saccades after bilateral CFN inactivation
Inactivating both CFNs makes both leftward and rightward saccades hypermetric (Robinson et al., 1993; Table 1C). No previous work explains why hypermetria predominates when bilateral CFN inactivation would seem to simultaneously cause both hypermetria and hypometria.
We account for saccade hypermetria after bilateral CFN inactivation by proposing that the loss of CFN activity impairs off-direction IBN bursts more than it impairs on-direction bursts. CFN bursts are roughly the same size for contraversive and ipsiversive saccades (Fuchs et al., 1993; Ohtsuka and Noda, 1991). Thus, CFN activity adds about the same number of spikes to off-direction and on-direction IBN bursts. This number of added spikes is a very large proportion, maybe all, of off-direction IBN activity and a much smaller part of on-direction IBN activity.
For example, in Kojima et al., (2007) the off direction bursts of 79% (23/29) of the IBNs that fire off-direction bursts contained a median of ≤5 spikes for 10° saccades. In contrast, on-direction IBN bursts contained ≥18 spikes for 10° saccades. If we assume that CFN activity adds 5 spikes to IBN bursts for 10° saccades, then inactivating the CFN will completely eliminate off-direction bursts but will leave on-direction bursts at 72% of normal. Thus, bilateral CFN inactivation increases agonist muscle contraction more than it increases antagonist muscle contraction.
3.2 Limitations of the CFN-IBN pathway in explaining the consequences of CFN inactivation
Though the CFN-IBN-abducens pathway can explain saccade dysmetria after CFN inactivation, there are three eye movement deficits that this pathway does not explain. First, CFN inactivation usually strongly increased the variability of every attribute that we measured. There is nothing in the CFN-IBN pathway that explains why interfering with it increases saccade variability. Currently, the most straightforward conjecture is that CFN output normally varies saccade-to-saccade to compensate for variability from some other source (Quaia et al., 1999; Robinson, 1995).
Second, unilateral CFN inactivation causes a fixation offset toward the side of the inactivated CFN. After bilateral inactivation the offset remains to the side of the CFN inactivated first (Robinson et al., 1993). This post-inactivation offset could be the result of a combination of impaired saccade accuracy and an altered encoding of the foveal target position (Guerrasio et al., 2010). Whatever its cause, the offset is unlikely to result from a change in IBN activity because IBNs are silent during fixation.
Third, IBNs also do not fire during smooth pursuit. Therefore, it is unlikely that the CFN-IBN pathway can explain pursuit deficits after CFN inactivation (Robinson et al., 1997).
3.3 The potential role of the CFN-EBN-abducens pathway
CFN neurons project to contralateral excitatory burst neurons (EBNs). During saccades EBNs excite ipsilateral abducens neurons. This CFN-EBN-abducens pathway could help make ipsiversive saccades hypermetric after CFN inactivation (Goffart et al., 2004). In this view reducing late off-direction activity in these EBNs would reduce activity in the antagonist muscles late in a saccade. This would reduce or delay contraction of the antagonist lateral rectus muscle at the end of the saccade and let the saccade continue past its target. This mechanism for hypermetria after CFN inactivation is plausible. Consistent with it, Strassman et al. (1986) report off-direction bursts in some EBNs.
In addition, as Goffart et al. (2004) propose, inactivation of one CFN removes the counterbalance for the effect of the other, unimpaired CFN. For example, after inactivation of the right CFN, bursts in the normal left CFN for rightward saccades will drive right EBNs increasing the drive of agonist abducens neurons. This increased excitation of right abducens neurons is not offset as much as normal by inhibitory input from the left IBNs, which has been reduced by inactivating the right CFN.
3.4 Conclusion
During a horizontal saccade, activity in the CFN contralateral to saccade direction reduces antagonist muscle contraction thereby increasing eye rotation. It does so equally during the increasing and decreasing phases of saccade acceleration and during the increasing phase of deceleration. CFN activity has little effect on the last phase of saccades, i.e., during decreasing deceleration.
Activity in the ipsilateral CFN reduces agonist muscle contraction thereby decreasing eye rotation. CFN activity reduces rotation only a little during both phases of acceleration, more during the first phase of deceleration, and a great deal during the last phase of deceleration. The ipsilateral CFN has a small effect on saccades during acceleration and the contralateral CFN has a small effect on saccades during deceleration because ipsilateral and contralateral CFN bursts overlap extensively in time during a saccade.
4. EXPERIMENTAL PROCEDURE
All experiments were performed at the University of Washington in strict compliance with the animal Guide for the Care and Use of Laboratory Animals (Department of Health Education and Welfare Publication No. NIH85-23 1985) and recommendations from the Institute of Laboratory of Animal Resources and the American Association for Accreditation of Laboratory Animal Care International. Specific protocols were approved by the Institutional Animal Care and Use Committee at the University of Washington (ACC No. 2403–01).
4.1 Subjects
We used 6 juvenile rhesus monkeys (Macaca mulatta) ~4kg selected for their acceptance of humans and fondness for applesauce. Data from one of these monkeys, monkey A, appeared in a previous publication, Robinson et al., 1993. We digitized normal and post-inactivation saccades recorded from monkey A and reanalyzed them for this experiment.
4.2 Surgical procedures
4.2.1 Eye coil surgery
In an aseptic surgery we implanted a three-turn scleral search coil of Teflon-coated wire in one eye of each monkey and connected the ends to a plug attached to the top of the skull to monitor the eye position. We also implanted acrylic head-stabilizing hardware, securing it to the head with titanium screws, to hold the monkey’s head steady when we recorded eye movements. During surgery, we anesthetized the monkey with Isoflurane inhalation anesthesia, warmed it, and monitored its vital signs. After surgery the monkey recovered overnight in a heated, padded cage and then returned to its home cage for 7–10 days before training started.
4.2.2 Recording chamber surgery
After we trained each monkey to track the movement of a visual target with saccades, we implanted a recording chamber aimed at the caudal fastigial nuclei in the cerebellum. Typically, this chamber was positioned 8.0 mm posterior to ear bar 0 and aimed straight down. We used the same anesthesia, support, and monitoring, procedures as in the first surgery and the same post-surgery recovery.
4.3 Experimental procedures
4.3.1 Saccades
We measured head-fixed horizontal and vertical eye position with the search coil technique (Fuchs and Robinson, 1966; Robinson, 1963). We trained each monkey to make saccades to small (0.3°) laser spot targets projected on a screen 56 or 70 cm in front of the monkey’s eyes. A computer program set the position of the spot via two mirror galvanometers. Whenever the monkey aimed its eyes to within 2° of the target within 500 ms of target step and looked at the target for 0.8 –1s it received a dollop of applesauce through a feeding tube near its mouth. The target moved every 1–3 seconds. We tested 10° saccades to eccentric targets in 8 directions (along the horizontal, vertical meridians and oblique directions). We pooled centrifugal and centripetal saccades together. Typically the monkey made 2,500 saccades during each session. This paradigm was used to collect normal and post-injection saccades. The monkey and screen were in a light-tight, sound-attenuating booth that was dark or very dim except for the target spot. Before each session in which we inactivated the CFN we closely monitored the eye movements of each monkey to verify that there were no significant changes in normal saccade amplitude and gain potentially caused by previous electrode penetrations or injections.
4.3.2 Muscimol inactivation
We used the methods described earlier (Robinson et al., 1993). Briefly, prior to each muscimol injection we made electrode penetrations in the vicinity of the CFN seeking the omni-directional saccade-related activity characteristic of CFN neurons. Once we located the CFN we replaced the recording electrode with an injection pipette that was connected to a pressure source through solenoid valve (Pneumatic PicoPump, WPI PV830), by translucent tubing. We pressure-injected 1mg/ml muscimol dissolved in normal saline by using the valve to deliver brief (15–50 ms) air pressure pulses. We measured the volume of the injected muscimol solution as the displacement of the meniscus down the tubing. Table 2 provides the volumes of each injection.
As in the previous study (Robinson et al., 1993) we saw no difference in the deficits caused by small and large injections. For example, in Figure 2 measurements from our largest injection are identified with a filled black diamond. Note that the effects of the largest injection are not very different from the mean of all injections. We interpret this similarity to mean that even our small injections inactivated all of the CFN.
Injections took 5–20 minutes. We waited at least 5 minutes after the end of the injection before withdrawing the injection pipette. We then waited another 10–30 minutes for the full effect to manifest. After that the effects were stable for at least the following ~3 hours during which we recorded data. The muscimol effect faded within ~10 hours so saccades the next day were normal. Despite this, we waited at least 2 days before making another injection in the same animal.
For bilateral CFN inactivation we made electrode penetrations to locate the CFN on each side. Then we placed an injection pipette into one CFN and injected muscimol following the procedure for the unilateral injection described above. The first injection took 5–20 minutes. We waited 5 minutes before withdrawing the first pipette and placing a second into the other CFN. The second injection took from 5–20 minutes and after waiting 5 minutes we withdrew the second pipette. The total injection time for both injections was 20–50 minutes. We waited another 10–30 minutes for the second injection to take effect and recorded saccades after both injections had stable results (usually within 1.5 hours of beginning the first injection). Injection volumes ranged from 0.7 μL to 1 μL.
4.4 Data collection and analysis
We recorded normal saccades immediately before each injection and impaired saccades after we inactivated the CFN(s) with muscimol. We digitized voltages proportional to eye and target position at 1 kHz and recorded them on a computer hard drive with a CED Power 1401 laboratory interface. We used a custom-made Matlab program to search the eye position record for saccades by finding eye velocities greater than 200°/ s. The program marked saccade start and end when eye velocity rose or fell through 20°/s, respectively. We analyzed only eye movements > 2° and in the direction of the target. Here we report only on primary saccades to 10° horizontal targets. We used the Matlab fir1 filter to remove high frequency noise from our data. We created the velocity and acceleration traces by taking the first and second derivative of the position trace, respectively.
Custom software divided each saccade into 4 phases. We determined phase boundaries based on the velocity and acceleration profile of each saccade. Phase 1 began at saccade onset and ended at peak acceleration, phase 2 was from peak acceleration to peak velocity, phase 3 began at peak velocity and ended at peak deceleration and phase 4 was from peak deceleration to saccade end. The left column of Figure1 shows the position (1A), velocity (1B), and acceleration (1C) of a normal saccade with the boundaries of phases 1–4. For normal saccades, the average durations of phases 1 and 2 were ~10 ms each. The average duration of phase 3 was 12 ms and of phase 4 was 15 ms. The right column of Figure 1 shows the position (1D), velocity (1E), and acceleration (1F) of a normal saccade as well as saccades ipsiversive and contraversive to an inactivated CFN and of a saccade after bilateral CFN inactivation. We measured three attributes of each phase: phase duration, mean acceleration or deceleration, and number of degrees of visual angle that the eyes rotate.
4.4.1 Common mean as the generalized least squares estimator
For this study we had to take into account both the within-monkey and between monkey variability when we calculated the common mean of all injections for different saccade attributes. The Mandel-Paule mean estimator (Savin, 2003) with the Kenward and Roger confidence interval (Kenward and Roger, 1997) allowed us to use both variabilities in the equation to make the best estimate of the common mean.
4.4.2 Coefficient of Variation
We calculated the coefficient of variation before and after CFN inactivation from the variances of the common mean for each attribute to compare variability before and after blocking cerebellar output. The formula for the coefficient of variation is: cv = σ/μ. We then averaged the variation of normal and post-injection saccades across all attributes to get a mean variability for normal, contraversive, ipsiversive and bilateral saccades.
Highlights.
We compared saccades after uni- or bilateral CFN inactivation with muscimol.
Ipsi- saccades rotated normally in P1 but increasingly farther in each later phase.
Contra- saccades rotated less than normal in phase 1–3 but normally in phase 4.
CFN influence on IBNs can account for most features of post-inactivation saccades.
Acknowledgments
We are grateful for the technical support of Christopher Noto and Jonathan Garlid, and the valuable comments of Chris Kaneko, Yoshiko Kojima, Leo Ling, Robijanto Soetedjo, and Avery Weiss on an earlier version of the manuscript.
GRANTS
Grants R01 EY018585 and RR-00166 from the National Institutes of Health supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. I. Relationship to eye movement dynamics during head-fixed saccades. J Neurophysiol. 1997;78:3259–3282. doi: 10.1152/jn.1997.78.6.3259. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–70. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol. 1993;70:1723–1740. doi: 10.1152/jn.1993.70.5.1723. [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol. 2004;92:3351–3367. doi: 10.1152/jn.01199.2003. [DOI] [PubMed] [Google Scholar]
- Guerrasio L, Quinet J, Büttner U, Goffart L. Fastigial oculomotor region and the control of foveation during fixation. J Neurophysiol. 2010;103:1988–2001. doi: 10.1152/jn.00771.2009. [DOI] [PubMed] [Google Scholar]
- Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist and fingers. J Neurophysiol. 1991;65:563–571. doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- Inaba N, Iwamoto Y, Yoshida K. Changes in cerebellar fastigial burst activity related to saccadic gain adaptation in the monkey. Neurosci Res. 2003;46:359–368. doi: 10.1016/s0168-0102(03)00098-1. [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–97. [PubMed] [Google Scholar]
- Kleine JF, Guan Y, Büttner U. Saccade-related neurons in the primate fastigial nucleus: what do they encode? J Neurophysiol. 2003;90:3137–54. doi: 10.1152/jn.00021.2003. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Robinson F, Noto C, Yoshida K. Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiology. 2008;99:220–230. doi: 10.1152/jn.00554.2007. [DOI] [PubMed] [Google Scholar]
- Lefevre P, Quaia C, Optican LM. Distributed model of control of saccades by superior colliculus and cerebellum. Neural Netw. 1998;11:1175–1190. doi: 10.1016/s0893-6080(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;8:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Noda H. Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J Neurophysiol. 1991;65:1422–1434. doi: 10.1152/jn.1991.65.6.1422. [DOI] [PubMed] [Google Scholar]
- Optican LM, Quaia C. Distributed model of collicular and cerebellar function during saccades. Ann N Y Acad Sci. 2002;956:164–77. doi: 10.1111/j.1749-6632.2002.tb02817.x. [DOI] [PubMed] [Google Scholar]
- Quaia C, Lefevre P, Optican LM. Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol. 1999;82:999–1018. doi: 10.1152/jn.1999.82.2.999. [DOI] [PubMed] [Google Scholar]
- Quinet J, Goffart L. Head-unrestrained gaze shifts after muscimol injection in the caudal fastigial nucleus of the monkey. J Neurophysiol. 2007;98:3269–83. doi: 10.1152/jn.00741.2007. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–45. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson FR. Role of the cerebellum in movement control and adaptation. Curr Opin Neurobiol. 1995;5:755–762. doi: 10.1016/0959-4388(95)80103-0. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol. 1993;70:1741–1758. doi: 10.1152/jn.1993.70.5.1741. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Participation of caudal fastigial nucleus in smooth pursuit eye movements. II. Effects of muscimol inactivation. J Neurophysiology. 1997;78:848–859. doi: 10.1152/jn.1997.78.2.848. [DOI] [PubMed] [Google Scholar]
- Savin AW, Gejza, Witkovsky Viktor. On Kenward-Roger confidence intervals for common mean in interlaboratory trials. Measurement Science Review. 2003;3:53–56. [Google Scholar]
- Scudder CA, Fuchs AF, Langer TP. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol. 1988;59:1430–1454. doi: 10.1152/jn.1988.59.5.1430. [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol. 1986;249:337–357. doi: 10.1002/cne.902490303. [DOI] [PubMed] [Google Scholar]
- Sugita S, Noda H. Pathways and terminations of axons arising in the fastigial oculomotor region of macaque monkeys. Neurosci Res. 1991;10:118–136. doi: 10.1016/0168-0102(91)90035-w. [DOI] [PubMed] [Google Scholar]