Abstract
Background
Preterm neonates are highly vulnerable to infection.
Objectives
To investigate the developmental contribution of prematurity, chorioamnionitis and antenatal corticosteroids (ANS) on the maturation of neonatal microbial pathogen recognition responses.
Methods
Using standardized protocols, we assayed multiple inflammatory cytokine responses (IL-1β, IL-6, TNF-α and IL-12/23p40) to three prototypic Toll-like receptor (TLR) agonists: TLR4 (LPS), TLR5 (flagellin) and TLR7/8 (R848), and to the non-TLR retinoic acid-inducible gene I (RIG-I)-like receptor agonist, in cord blood mononuclear cells from neonates born before 33 weeks of gestation and at term.
Results
TLR responses develop asynchronously in preterm neonates, whereas responses to TLR7/8 were more mature and followed by the development of TLR4 responses, which were also heterogeneous. Responses to TLR5 were weakest and most immature. Maturity in TLR responses was not influenced by sex. Overall, we detected no significant contribution of ANS and chorioamnionitis to the developmental attenuation of either TLR or RIG-I responses.
Conclusions
The maturation of anti-microbial responses in neonates born early in gestation follows an asynchronous developmental hierarchy independently of an exposure to chorioamnionitis and ANS. Our data provide an immunological basis for the predominance of specific microbial infections in this age group.
Keywords: Neonate, infection, Toll-like receptor, antenatal corticosteroids, chorioamnionitis
INTRODUCTION
Preterm neonates are vulnerable to morbidity and mortality from infection. This is partly attributable to attenuated immune responses [1]. Immune cells recognize pathogens through a series of germ-line encoded Pattern Recognition Receptors (PRR), such as the Toll-like receptors (TLR); the carbohydrate-binding C-type lectin receptors (CLR); the retinoic acid-inducible gene I (RIG-I)-like receptors that primarily detect non-self, double-stranded RNA (dsRNA); and the nucleotide-binding oligomerization domain-containing (NOD)-like receptors (NLR) [2]. TLR responses have been best studied and are markedly attenuated in preterm neonates [3,4]. However, this could result from a number of factors in utero, which may also broadly suppress innate immune responses. Specifically, chorioamnionitis, commonly associated with prematurity, may induce a state of immune tolerance [5]. The use of antenatal corticosteroids (ANS) in mothers of preterm infants delivered before 34 weeks of gestation is standard practice in perinatal medicine [6]. Significant immune attenuation can also be observed in mononuclear cells exposed in vitro to corticosteroid equivalents comparable to levels measured in serum of pregnant women after a standard ANS treatment [7–10]. When examining infants exposed to ANS, however, no effects were detected on cord blood IL-6 responses [9]. One major limitation of previous studies is the lack of rigorous definitions of chorioamnionitis and of the timing of ANS exposure, which can vary substantially among subjects. Moreover, we have recently shown that RIG-I responses are also attenuated in human preterm neonates, indicating a more global immaturity [11]. However, it is unclear to what extent exogenous perinatal factors may contribute to such global attenuation of innate immune responses in neonates born early in gestation.
Here, we applied robust experimental procedures to determine the contribution of prematurity, ANS and chorioamnionitis to the extent of innate immune attenuation observed in preterm neonates born early in gestation.
MATERIAL AND METHODS
Study population
After written consent, cord blood samples were collected in sodium heparin-anticoagulated Vacutainer tubes (BD Bioscience) from 43 preterm neonates born before 33 weeks of gestation, and from 20 healthy neonates born at term by Caesarean section delivery at the Children’s & Women’s (C&W) Health Centre of British Columbia between July 2009 and July 2012. Clinical characteristics of preterm neonates are shown in table 1. Exposure to ANS was defined according to the timing of the last maternal dose received. Chorioamnionitis was determined by a blind histological examination of at least 5 micro-dissection slides by a medical pathologist (CS), and defined as maternal stage 1 or greater according to validated criteria [5]. Based on i) the half-lives for serum levels and receptor occupancy of acetate betamethasone of up to 9.0+/−2.7 and 14 hours, respectively, in pregnant women [12–14], and on ii) an estimated fetal-to-maternal serum drug ratio at delivery between 0.3 and 0.5 [12–14], and on data in preterm neonates confirming virtual plasmatic drug clearance beyond 48 hours [15], the direct effect of a short-term exposure to ANS was assessed by comparing four subgroups: exposed to ANS for less than 12 hours, 12 to 72 hours or more than 72 hours. Our study was approved by the C&W Research Ethics Board (protocol #H05-70519).
Table 1.
Clinical characteristics of preterm neonates
| Clinical characteristics | Preterm neonates (n = 43) | |
|---|---|---|
| Gestational age, average ± SD (weeks) | 29 ± 3.0 | |
| Birth weight, average ± SD (g) | 1182 ± 480 | |
| Male gender (%) | 63 | |
| Histological chorioamnionitis† (%) | 28 | |
| Antenatal corticosteroid exposure (n) | <12 hours | 8 |
| 12 – 72 hours | 8 | |
| >72 hours | 25 | |
| None | 2 | |
| Caesarean deliveries (%) | 70 | |
| Labour at delivery (%) | 58 | |
| Post-natal infection (%)* | 35 | |
Defined as blood culture-positive infection, pneumonia or necrotizing enterocolitis; all of the infections occurred beyond 72 hours of life. None of the infants had early-onset (<72 hours) sepsis.
Cord blood processing, stimulation and cytokine measures
We used strict procedures for processing of blood samples, TLR stimulation and cytokine measures, as described [3]. To minimize experimental variability, blood was processed within 2 hours of collection. Cord mononuclear cells (CMBC) were stimulated in 96-well, round-bottom plates containing pre-mixed concentrations of TLR4 (LPS, Invivogen #tlrl-3pelps), TLR5 (flagellin, Invivogen #tlrl-pstfla) and TLR7/8 (R848, Invivogen #tlrl-r848) agonists, for 24 hours in a 37°C incubator, at 5% CO2. Pre-made plates containing batched dilutions of TLR agonists were stored at −80°C for a maximum of 6 months. For standardization, significant batch-to-batch variability over a relatively long study period was excluded by ensuring IL-6 and IL-1β responses in term neonates remained within one standard deviation of each other (supplementary figure 1). In addition, an equivalent proportion of preterm versus term samples were tested within each batches. Finally, responses from each batch of plates were benchmarked on a reference group of healthy adult subjects (data not shown).
RIG-I responses were determined following stimulation of CBMC from a subgroup of neonates born between 28 and 33 weeks of gestation (n = 13–20 per group) in RPMI-1640 medium supplemented with 10% FBS using 1 μg/mL of the synthetic RIG-I agonist 5′ppp-dsRNA delivered into the cytosol by transfection (LyoVec). To control for RIG-I independent responses, we transfected cells with a similar dsRNA-19mer, which lacks the 5′tri-phosphate group (ctl-dsRNA) required for RIG-I activation [16]. After stimulation, cell culture supernatants were stored at −80°C and cytokines (IL-1β, IL-6, TNF-α, IL-12/23p40) were measured using enzyme-linked immunosorbent assays (ELISA) [3].
Statistical analyses
Based on our previous studies, we estimated that a minimum of 6 subjects per chorioamnionitis or ANS exposure subgroup would provide 80% power to detect differences in either IL-1β, IL-6 or IL-12/23p40 LPS responses between preterm and term neonates (p<0.01) [3]. In order to obtain this number of subjects in each subgroup, we projected to recruit 42 preterm neonates. Relationships between non-transformed cytokine responses were first examined graphically. In subsequent analyses, peak responses and area under the dose-response curve (AUC) yielded similar results. Therefore, only the AUC-based data are presented. Differences in TLR responses between term neonates and preterm neonates according to subgroups of ANS and chorioamnionitis exposure were assessed using a Mann-Whitney U test. Differences between TLR/cytokine dose-responses were determined using mixed-model ANOVA. Sex-related differences in responses were determined using an ANOVA.
Comparisons between ligand- and cytokine- specific maturation effects were adjusted for multiple comparisons, as described [17]. The effect of gestational age, birth weight and mode of delivery on TLR responses within the preterm group was assessed using correlations. Contributions of prematurity, ANS and maternal/fetal chorioamnionitis were determined separately, using adjusted correlations with responses in a linear regression model for each TLR/cytokine response (using AUC) as predictors, and 95% confidence intervals calculated using the bootstrap. ANS exposure was analyzed as a continuous variable (hours) after transformation to log(1/ANChours) to reduce skewness, as described [18]. For neonates with no ANC exposure ANC, Hours was set to the maximum of observed values + K, with K chosen to normalize the resultant distribution on the log scale.
RESULTS
Hierarchical, gestational age-dependent maturation in TLR responses
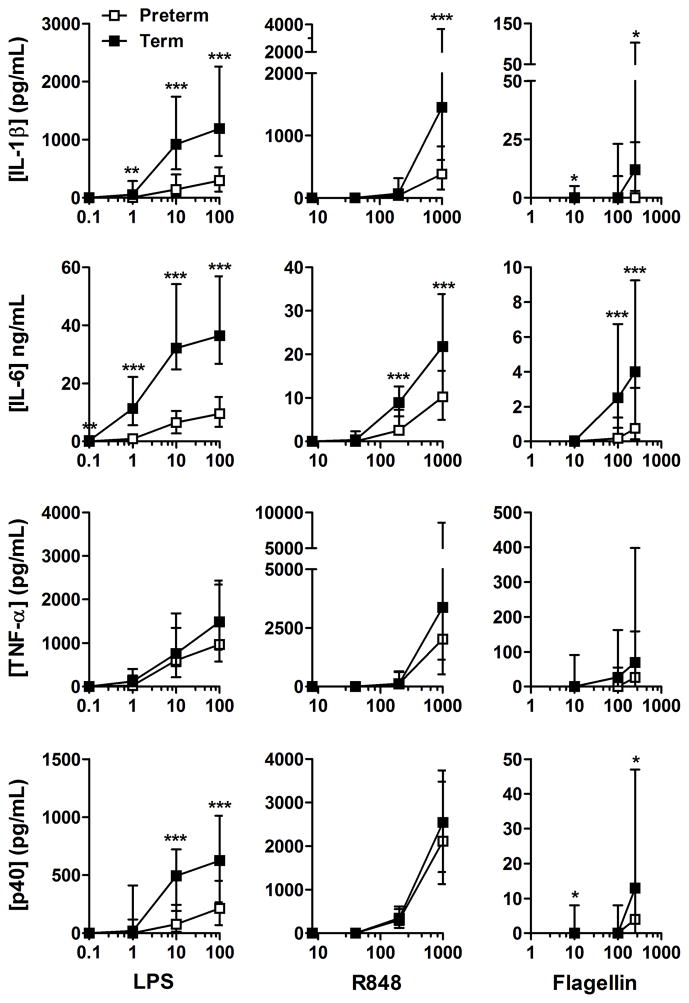
To assess the maturation of TLR responses at low gestation, we first compared dose-responses between preterm and term neonates. Most TLR responses were diminished in preterm neonates compared with term neonates (figure 1). Generally, responses to the TLR7/8 agonist R848 were also stronger, except for IL-6, followed by responses to the TLR4 agonist LPS; responses to the TLR5 agonist flagellin were weakest. In all neonates, sex did not significantly affect responses using an analysis of variance and in group comparisons (p>0.1; data not shown). Moreover, within the group of preterm neonates, no significant effect of gestational age, birth weight and mode of delivery was detected on TLR responses (data not shown).
Figure 1. TLR cytokine dose-responses.
Median LPS (TLR4), R848 (TLR7/8) and flagellin (TLR5) responses were compared between preterm (white symbols) and term neonates (gray symbols). The X-axis shows ligand concentrations (ng/mL). Bars represent interquartile range; *p<0.05; **p<0.01; ***p<0.001.
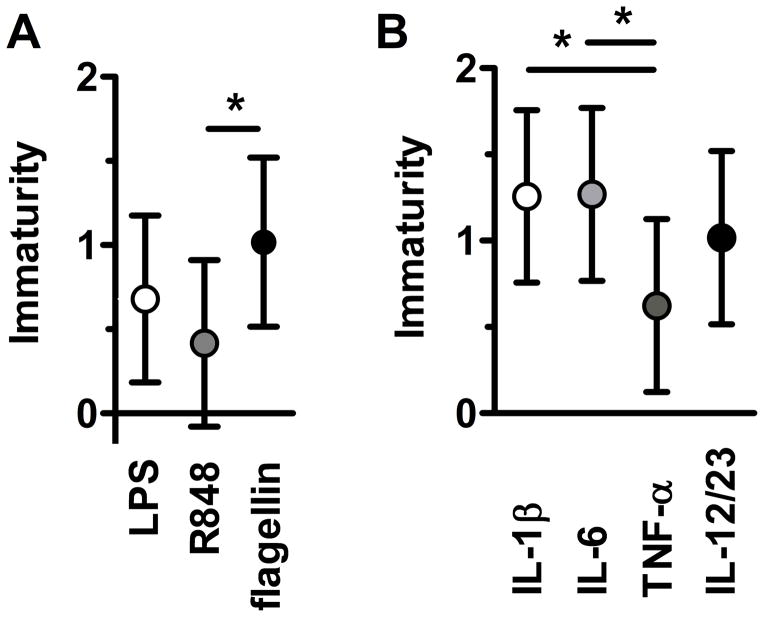
When the contribution of prematurity was considered, we observed a distinct developmental hierarchy (figure 2). When comparing individual responses according to each TLR ligand, R848 cytokine responses were more mature or “term-like”, followed by LPS responses and by flagellin responses. Likewise, when comparing cytokine responses, TNF-α responses were significantly more mature compared with IL-1β and IL-6 responses. Together, these data indicate that neonatal TLR responses follow a distinctive, asynchronous development before 33 weeks of gestation.
Figure 2. Developmental Hierarchy in neonatal TLR responses maturation.
Degree of immaturity (expressed as the difference in mean [term – preterm] area under the curve of each responses on the log(x + 1) scale) in (A) TLR ligand-specific or (B) cytokine-specific responses between term and preterm neonates with 95% confidence intervals (bars); *p<0.05 for maturation effects after adjusting for multiple comparisons (see methods for details).
Contribution of ANS and chorioamnionitis
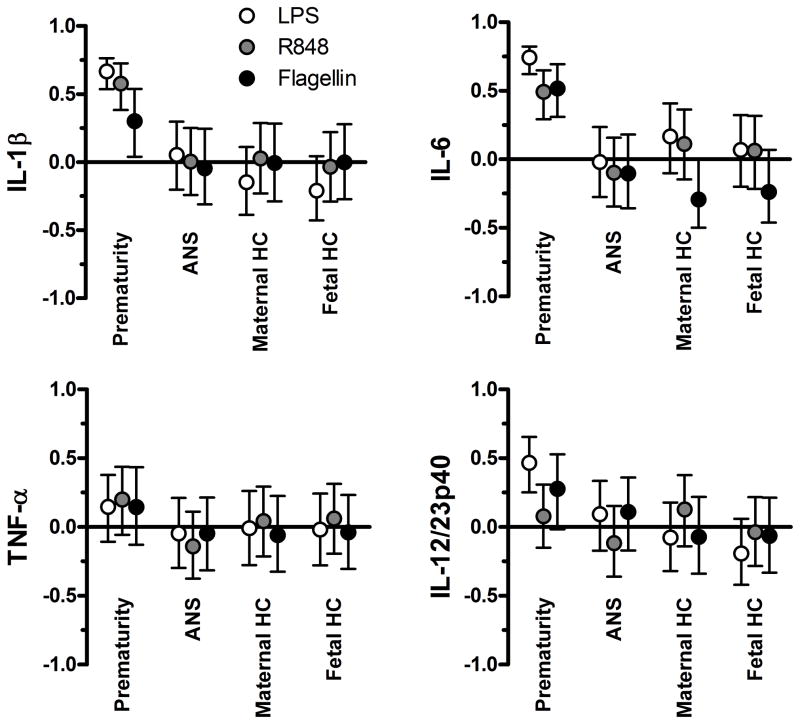
To determine the contribution of exogenous perinatal factors to the developmental attenuation in TLR responses, we stratified subgroups of preterm neonates according to rigorous exposure definitions for chorioamnionitis and ANS. Significant differences were observed in TLR responses between term neonates and each of the preterm ANS (supplementary figure 2) and chorioamnionitis (supplementary figure 3) exposure subgroups, whereas responses were comparable among preterm neonates. We used adjusted correlations to quantify the contribution of exposure to ANS, and maternal or fetal chorioamnionitis to each TLR/cytokine response within 95% confidence intervals. For IL-1β and IL-6, prematurity contributed significantly to all individual TLR responses, whereas, again, the contributions of ANS and either fetal or maternal histological chorioamnionitis were negligible (figure 3). For TNF-α responses as well as for the IL-12/23p40 response to R848, neither prematurity, ANS, nor maternal or fetal chorioamnionitis significantly contributed to the attenuation in preterm neonates. This finding is expected, considering that both TNF-α and R848-induced IL-12/23p40 responses are mature in preterm infants, resulting in very small variability. Overall, we detected a substantial contribution of prematurity whereas no separate contributions of ANS and chorioamnionitis were detected.
Figure 3. Contribution of prematurity, histological chorioamnionitis (maternal or fetal) and ANS to TLR responses.
Data show adjusted correlations (y-axis) with 95% confidence intervals (bars).
Attenuation in RIG-I-like receptor responses
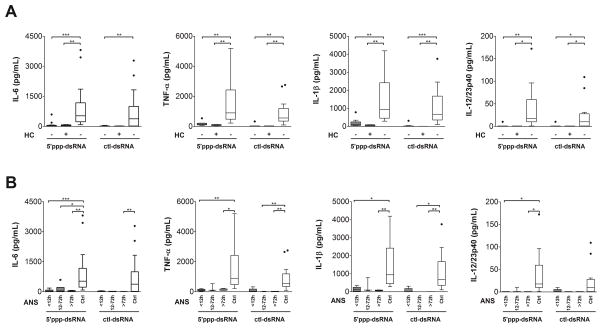
Next, we asked whether non-TLR responses were also affected by an exposure to ANS or chorioamnionitis. To this end, we compared cytokine responses to cytosolic delivery of a synthetic ligand of RIG-I (5′ppp-dsRNA), and of a RIG-I-independent control ligand (ctl-dsRNA). We chose these ligands because their signalling pathways are TLR-independent [19]. The attenuation in RIG-I responses in preterm neonates was also largely unaffected by exposure to chorioamnionitis (figure 4A), or to ANS (figure 4B). Interestingly, IL-1β, IL-6 and TNF-α (figure 4) and IFN-α (data not shown) cytokine responses were even more attenuated than TLR responses despite preterm neonates’ being more mature in this analysis (mean GA+/−SD = 30+/−1.6 weeks).
Figure 4. Contribution of chorioamnionitis and prematurity to TLR-independent cytokine responses.
RIG-I-dependent (5′ppp-dsRNA) and control RIG-I-independent (ctl-dsRNA) responses (boxes and whisker graphs) in term (white bars) and preterm neonates (gray bars) (A) with or without histological chorioamnionitis (HC) and (B) exposed to different levels of antenatal corticosteroids (ANS); *p<0.05; **p<0.01; ***p<0.001.
DISCUSSION
In order to assess the developmental impact of chorioamnionitis and ANS on innate immune responses, we quantified multiple TLR responses in a sizeable cohort of clinically well-characterized preterm neonates born early in gestation, using highly-standardized experimental procedures and rigorous exposure definitions. Overall, we demonstrate that the maturation of TLR responses before 33 weeks of gestation does not follow a linear continuum. Rather, these responses mature asynchronously, according to a distinct developmental hierarchy. Moreover, we detect a predominant contribution of prematurity to the extent of developmental attenuation observed in TLR response, whereas the influence of chorioamnionitis and of a short-term exposure to ANS appears negligible. To our knowledge this is the largest dataset reporting on multiple TLR function in very preterm neonates. Our data have important implications for understanding of the specific vulnerabilities to infection observed in preterm neonates.
The hierarchy observed in the maturation of preterm TLR responses is reminiscent of the maturation of other immune functions during development. For example, the development of TLR functions also progresses asynchronously in early childhood [20]. Likewise, placental transfer of maternal antibodies occurs selectively during pregnancy [21]. This conserved pattern may reflect a high degree of functional specialization required in humans exposed to an evolving micro-organism environment throughout development. The recognition of pathogen using PRRs is a key event in the defence against infections. Consistent with our findings, we observed even greater attenuation in response to LPS in neonates born before 29 weeks of gestation, which may explain the high occurrence of gram-negative bacterial infections in this age group [3]. In contrast, the more mature R848 responses may indicate potential avenues to enhance immune responses at this early age [22].
To our knowledge, our study is the first examining TLR5 (flagellin) responses in preterm neonates. We recently also reported diminished RIG-I responses in preterm neonates born at 28 to 32 weeks of gestation [11]. Engagement of RIG-I triggers activation of transcription factor NF-κB, and of interferon regulatory factor 3 (IRF3) and IRF7 through a distinct, dsRNA-dependent protein kinase R pathway, independent of TLRs [23]. The more global attenuation in both TLR and TLR-independent PRR responses indicate a central regulation either at the level or more downstream of NF-κB.
The marginal influence of chorioamnionitis is consistent with other studies in humans showing that exposure to prolonged rupture of membranes did not affect LPS-induced TNF-α and IL-6 production [24]. In contrast, in preterm sheep models exposure to intra-amniotic infection increases inflammatory responses upon ex vivo re-stimulation of cord blood cells [25]. Likewise, a short-term exposure to ANS in this model profoundly reduced endotoxin-stimulated IL-6 responses [26]. Several reasons might explain the discrepancies between human studies and animal models, including differences in the type and chronicity of infection. Our study stresses the importance of obtaining data directly in human populations. These data are also important for interpretation of neonatal immunology studies that mainly use cord blood, as a less invasive source of biological sample from small preterm neonates. In vitro, corticosteroids directly affect the function of immune cells as a dexamethasone drug equivalent to the concentration of corticosteroids found in cord blood during maternal treatment with ANS can suppress TLR-induced cytokine responses [8]. In human preterm neonates, ANS have been associated with lower HLA-DR expression on monocytes [15]. However, according to our study such transient decrease in antigen presentation function in cord blood due to ANS exposure is unlikely to be accompanied by significant TLR hypo-responsiveness. The negligible effect of chorioamnionitis and ANS on cord blood responses also implies that exposure to these factors should not be a systematic exclusion in studies, since neonates represent a considerable proportion of the “normal” population.
Our study has limitations. Despite its being the largest study of its kind in very preterm neonates, it was not designed to detect subtle effects of ANS or chorioamnionitis (or a major effect on a minority of preterm neonates) on innate immune responses. This undoubtedly requires considerably larger sample size, as our present dataset informs us. Furthermore, because only two neonates were not exposed to ANS as a comparison group, we were unable to assess the possibility of sustained effects of ANS exposure; for example, on the differentiation of immune cells, beyond the predicted plasmatic clearance of the drug [15,27]. However, the lack of major sustained effect of ANS on preterm immune functions is less likely, considering the results of clinical trials, which demonstrated a reduction (rather than a worsening) of the incidence of neonatal sepsis in neonates born from mothers who received ANS [6]. Finally in the clinical setting, a number of variables not captured in our analysis may also have affected the maturation of innate immune responses. Specifically, our preterm and term neonates differ substantially in the presence of maternal labour. Previous studies have reported differences in TLR responses with labour in term neonates [28,29]. However, no substantial effect was detectable in preterm neonates in our study beyond (data not shown). Severe growth restrictions may also affect innate immune responsiveness in a subset of preterm neonates over the attenuation already resulting from prematurity [30].
In conclusion, our study reveals an important developmental characteristic of the neonatal innate immune system. In preterm neonates, PRR responses mature asynchronously, according to a developmental hierarchy that is largely independent of perinatal exposures such as chorioamnionitis and ANS. This study represents a more explicitly defined step forward in our understanding of why human preterm neonates are so vulnerable to infection. Future studies are required to identify potential ways to promote the maturation of neonatal innate immune defences to prevent infections.
Supplementary Material
Acknowledgments
We thank the C&W Hospital staff for help with recruitment for this study; Nadine Lusney and Kristi Finlay for recruitment of research subjects; Yuan Shen Hu for assistance with stimulations using dsRNA ligands; Sonia Jeffries for help reviewing the ANS pharmacokinetic data; and Rosemary Delnavine for editorial assistance. This research was funded by Hospital for Sick Children Foundation (XG09-015R) and Canadian Institutes of Health Research (CIHR; MOP-110938) grants. AAS is supported by a Child & Family Research Institute (CFRI) Graduate Studentship and the CIHR/BC Transplantation Program. PML is support by Clinician-Scientist Awards from the CFRI and the Michael Smith Foundation for Health Research (MSFHR). SET holds the Aubrey J. Tingle Professorship in Pediatric Immunology and is a scholar of the MSFHR.
References
- 1.Sharma AA, Jen R, Butler A, Lavoie PM. The developing human preterm neonatal immune system: A case for more research in this area. Clin Immunol. 2012;145:61–68. doi: 10.1016/j.clim.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie PM, Huang Q, Jolette E, Whalen M, Nuyt AM, Audibert F, Speert DP, Lacaze-Masmonteil T, Soudeyns H, Kollmann TR. Profound lack of interleukin (il)-12/il-23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J Infect Dis. 2010;202:1754–1763. doi: 10.1086/657143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strunk T, Prosser A, Levy O, Philbin V, Simmer K, Doherty D, Charles A, Richmond P, Burgner D, Currie A. Human monocyte responsiveness to the commensal bacterium staphylococcus epidermidis develops late in gestation. Pediatr Res. 2012 doi: 10.1038/pr.2012.48. [DOI] [PubMed] [Google Scholar]
- 5.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: Nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 6.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Tsuei SE, Petersen MC, Ashley JJ, McBride WG, Moore RG. Disporition of synthetic glucocorticoids. Ii. Dexamethasone in parturient women. Clin Pharmacol Ther. 1980;28:88–98. doi: 10.1038/clpt.1980.136. [DOI] [PubMed] [Google Scholar]
- 8.Bessler H, Kagazanov S, Punsky I, Sirota L. Effect of dexamethasone on il-10 and il-12p40 production in newborns and adults. Biol Neonate. 2001;80:262–266. doi: 10.1159/000047154. [DOI] [PubMed] [Google Scholar]
- 9.Kavelaars A, van der Pompe G, Bakker JM, van Hasselt PM, Cats B, Visser GH, Heijnen CJ. Altered immune function in human newborns after prenatal administration of betamethasone: Enhanced natural killer cell activity and decreased t cell proliferation in cord blood. Pediatr Res. 1999;45:306–312. doi: 10.1203/00006450-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Schultz C, Rott C, Temming P, Schlenke P, Moller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–322. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Marr N, Wang T-I, Hu YS, Sharma AA, Markowski J, Solimano A, Lavoie PM, Turvey SE. Attenuation of respiratory syncytial virus-induced and rig-i-dependent type i interferon responses in human neonates at birth. J Immunol. 2013 doi: 10.4049/jimmunol.1302007. in press. [DOI] [PubMed] [Google Scholar]
- 12.Subtil D, Tiberghien P, Devos P, Therby D, Leclerc G, Vaast P, Puech F. Immediate and delayed effects of antenatal corticosteroids on fetal heart rate: A randomized trial that compares betamethasone acetate and phosphate, betamethasone phosphate, and dexamethasone. Am J Obstet Gynecol. 2003;188:524–531. doi: 10.1067/mob.2003.136. [DOI] [PubMed] [Google Scholar]
- 13.Ballabh P, Lo ES, Kumari J, Cooper TB, Zervoudakis I, Auld PA, Krauss AN. Pharmacokinetics of betamethasone in twin and singleton pregnancy. Clin Pharmacol Ther. 2002;71:39–45. doi: 10.1067/mcp.2002.120250. [DOI] [PubMed] [Google Scholar]
- 14.Della Torre M, Hibbard JU, Jeong H, Fischer JH. Betamethasone in pregnancy: Influence of maternal body weight and multiple gestation on pharmacokinetics. Am J Obstet Gynecol. 2010;203:254 e251–212. doi: 10.1016/j.ajog.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palojarvi A, Andersson S, Turpeinen U, Janer C, Petaja J. Antenatal betamethasone associates with transient immunodepression in very low birth weight infants. Neonatology. 2013;104:275–282. doi: 10.1159/000353964. [DOI] [PubMed] [Google Scholar]
- 16.Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, Juranek S, Kato H, Kawai T, Poeck H, Fitzgerald KA, Takeuchi O, Akira S, Tuschl T, Latz E, Ludwig J, Hartmann G. Recognition of 5′ triphosphate by rig-i helicase requires short blunt double-stranded rna as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 18.Weisberg S. Applied linear regression. 3. Minneapolis, Minnesota US: Wiley; 2005. [Google Scholar]
- 19.Williams BR. Signal integration via pkr. Sci STKE. 2001;2001:re2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 20.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate tlr-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. Igg placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loo YM, Gale M., Jr Immune signaling by rig-i-like receptors. Immunity. 34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. 129:e967–974. doi: 10.1542/peds.2011-1579. [DOI] [PubMed] [Google Scholar]
- 25.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med. 2005;171:73–77. doi: 10.1164/rccm.200406-745OC. [DOI] [PubMed] [Google Scholar]
- 26.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res. 2004;55:764–768. doi: 10.1203/01.PDR.0000120678.72485.19. [DOI] [PubMed] [Google Scholar]
- 27.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;30:1807–1812. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Bessler H, Kuperman A, Beilin B, Klinger G, Gurary N, Mozes C, Sirota L. Labor affects cytokine production in newborns. Am J Reprod Immunol. 1998;39:27–32. doi: 10.1111/j.1600-0897.1998.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 29.Ly NP, Ruiz-Perez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, Laskey D, Delaney ML, DuBois AM, Levy H, Gold DR, Ryan LM, Weiss ST, Celedon JC. Mode of delivery and cord blood cytokines: A birth cohort study. Clin Mol Allergy. 2006;4:13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troger B, Muller T, Faust K, Bendiks M, Bohlmann MK, Thonnissen S, Herting E, Gopel W, Hartel C. Intrauterine growth restriction and the innate immune system in preterm infants of </=32 weeks gestation. Neonatology. 2013;103:199–204. doi: 10.1159/000343260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.