Abstract
Background and Purpose
15-Lipoxygenase (15-LOX) activity is associated with inflammation and immune regulation. The objectives of the present study were to investigate the expression of 15-LOX-1 and 15-LOX-2 and evaluate the enzymes’ roles in the polarization of human lung macrophages (LMs) in response to LPS and Th2 cytokines (IL-4/-13).
Experimental Approach
LMs were isolated from patients undergoing surgery for carcinoma. The cells were cultured with a 15-LOX inhibitor (PD146176 or ML351), a COX inhibitor (indomethacin), a 5-LOX inhibitor (MK886) or vehicle and then stimulated with LPS (10 ng·mL−1), IL-4 (10 ng·mL−1) or IL-13 (50 ng·mL−1) for 24 h. Levels of ALOX15 (15-LOX-1) and ALOX15B (15-LOX-2) transcripts were determined by real-time quantitative PCR. Immunoassays were used to measure levels of LPS-induced cytokines (TNF-α, CCL2, CCL3, CCL4, CXCL1, CXCL8 and CXCL10) and Th2 cytokine-induced chemokines (CCL13, CCL18 and CCL22) in the culture supernatant.
Key Results
Stimulation of LMs with LPS was associated with increased expression of ALOX15B, whereas stimulation with IL-4/IL-13 induced the expression of ALOX15. PD146176 and ML351 (10 μM) reduced the release of the chemokines induced by LPS and Th2 cytokines. The effects of these 15-LOX inhibitors were maintained in the presence of indomethacin and MK886. Furthermore, indomethacin revealed the inhibitory effect of PD146176 on TNF-α release.
Conclusions and Implications
Inhibition of the 15-LOX pathways is involved in the down-regulation of the in vitro production of chemokines in LMs. Our results suggest that the 15-LOX pathways have a role in the pathogenesis of inflammatory lung disorders and may thus constitute a potential drug target.
Tables of Links
| LIGANDS | ||
|---|---|---|
| 13-HODE | CCL13 | IL-4 |
| 15-HETE | CCL18 | IL-13 |
| Arachidonic acid | CCL22 | Indomethacin |
| CCL2 | CXCL1 | LPS |
| CCL3 | CXCL8 | MK886 |
| CCL4 | CXCL10 | TNF-α |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14
Alexander et al., 2013a,b,).
Introduction
Human lipoxygenases (LOXs) catalyse the stereoselective dioxygenation of 1,4-cis, cis-pentadiene-containing polyunsaturated fatty acids (such as arachidonic acid) to form hydroperoxyfatty acids. The human LOXs include 5-LOX, two 12-LOXs and two 15-LOXs (15-LOX-1 and 15-LOX-2, respectively, encoded by the ALOX15 and ALOX15B genes) (Dobrian et al., 2011). In the lung, the different products resulting from the enzymatic activity of 15-LOXs can stimulate inflammation and mucus secretion (as is the case for 15-HETE and 13-HODE) or resolve inflammation and stimulate tissue repair (as is the case for lipoxin, resolvins and protectins) (Back et al., 2014; Levy and Serhan, 2014).
Increased activity of the 15-LOX inflammatory pathway in both childhood and adult asthma has been evidenced by high levels of 15-LOX transcripts and 15-LOX metabolites in exhaled breath condensates (Sachs-Olsen et al., 2010), bronchoalveolar lavage fluids (Chu et al., 2002; Ono et al., 2009; Lundstrom et al., 2012) and induced sputum (Profita et al., 2000; Thomson et al., 2014).
15-LOXs are strongly expressed in the bronchial epithelium – especially in severe asthmatics (Bradding et al., 1995; Zhao et al., 2009; Gras et al., 2012; Mabalirajan et al., 2013). In epithelial cells, the Th2 cytokines IL-4 and IL-13 are potent inducers of 15-LOX expression (Jayawickreme et al., 1999; Profita et al., 1999; Brown et al., 2001; Chaitidis et al., 2005; Kuhn and O’Donnell, 2006; Jakiela et al., 2013). In the human alveolar epithelial cell line A549, the overexpression of 15-LOX-1 is associated with enhanced chemokine release (Liu et al., 2009). 15-LOX-1 is also expressed in eosinophils and macrophages from asthmatic patients (Bradding et al., 1995; Profita et al., 2000; Chu et al., 2002; Mabalirajan et al., 2013).
In pulmonary inflammatory diseases (such as asthma and chronic obstructive pulmonary disease), macrophages orchestrate inflammatory reactions by releasing chemokines and cytokines (Barnes, 2008). Macrophages are essential regulators of both innate and adaptive immune responses and the resolution of inflammation. Upon exposure to microbial products such as bacterial LPS, macrophages undergo M1 polarization (the classical activation state, associated with microbicidal and tumouricidal activities) (Mantovani et al., 2004; Benoit et al., 2008). In contrast, Th2 cytokines induce polarization towards a different activation status (referred to as M2a polarization), which is associated with immunoregulation, the dampening of inflammation and the promotion of tissue remodelling (Benoit et al., 2008; Van Dyken and Locksley, 2013). In line with recent guidelines on macrophage activation and polarization (Murray et al., 2014), we shall henceforth use the terms ‘LPS-induced polarization’ and ‘IL-4/IL-13 (Th2)-induced polarization’ (rather than the M1-M2 terminology). These two activation states involve the production of different sets of cytokines and (especially) chemokines (Martinez et al., 2006; Sica and Mantovani, 2012).
In vitro, ALOX15B expression increases as monocytes differentiate into macrophages. In monocyte-derived macrophages (MDMs), ALOX15 expression increases in response to Th2 cytokines and ALOX15B expression increases in response to Th2 cytokines and LPS (Wuest et al., 2012). In human MDMs, the overexpression of 15-LOX-2 results in the enhanced secretion of two chemokines (CCL2 and CXCL10) (Danielsson et al., 2008). However, the expression levels and functions of 15-LOXs in human LMs have yet to be characterized in detail. The objective of the present study was to assess the respective effects of LPS, IL-4 and IL-13 on the expression of 15-LOX-1 and 15-LOX-2 in human LMs and to investigate the role of 15-LOXs in the production of chemokines induced by LPS- and Th2 cytokines.
Methods
Isolation and culture of human LMs
Experiments with human tissues were approved by the regional independent ethics committee (Comité de Protection des Personnes Île de France VIII, Boulogne-Billancourt, France). Lung tissues were obtained from 39 patients [mean ± SEM age: 65 ± 2 years; gender (M : F): 29:10; mean ± SEM FEV1/FVC ratio: 0.82 ± 0.04; non-smokers: 7; smokers and ex-smokers: 12 and 20; pack years: 46 ± 4] undergoing surgical resection for lung carcinoma and who had not received chemotherapy or radiotherapy. The patients had not received any specific medications other than the anaesthetics required for the surgery and (in just four patients) the antibiotics cefazoline or cefamandole. The LMs were isolated from macroscopically normal lung parenchyma obtained from sites far from the tumour, as previously described (Buenestado et al., 2010; 2012,) (see Supporting Information). In culture, the mean ± SEM number of adherent macrophages was 194 ± 13 × 103 cells per well for 24-well plates and 482 ± 35 × 103 cells per well for 12-well plates. More than 95% of the adherent cells were macrophages, as determined by May–Grünwald–Giemsa staining and CD68 immunocytochemistry. Cell viability exceeded 90%, as assessed by trypan blue dye exclusion. On the day after isolation from lung parenchyma, macrophages were washed twice with RPMI medium. Next, 1 mL of RPMI medium supplemented with 1% FCS was added to each well.
Classically activated (M1-like) macrophages were obtained by exposure to LPS. Alternatively activated (M2a-like) macrophages were produced by exposing them to the canonical Th2 cytokines IL-4 and IL-13 in vitro. The LPS concentration (10 ng·mL−1) was selected as being suboptimal on the basis of previous data from time-response and concentration-response curves (Buenestado et al., 2012). The IL-4 concentration (10 ng·mL−1) was chosen on the basis of previous findings from human macrophage models (Joshi et al., 2010; Pechkovsky et al., 2010; Staples et al., 2012). The IL-13 concentration (50 ng·mL−1) was chosen on the basis of previous experiments in our laboratory (data not shown).
RT-qPCR
Lung macrophages were stimulated for 24 h with LPS, IL-4 or IL-13. Total RNA was prepared using TRIzol® reagent (Life Technologies, Saint Aubin, France), according to the manufacturer’s instructions. The RNAs’ intactness was determined by running an aliquot of each sample on an ExperionTM automated electrophoresis station (Bio-Rad, Marnes-la-Coquette, France). Next, 1 μg of total RNA was reverse-transcribed using SuperScript® III First-strand SuperMix kit (Life Technologies).
Specific TaqMan® arrays based on predesigned reagents (Life Technologies) were used for the analysis of LOX and COX transcripts: ALOX5 (5-LOX), ALOX5AP (5-LOX activating protein), ALOX12 (12-LOX), ALOX15 (15-LOX-1), ALOX15B (15-LOX-2), PTGS1 (COX-1) and PTGS2 (COX-2). Quantitative PCR was performed using Gene Expression Master Mix (Life Technologies) with 20 ng of cDNA in a StepOnePlus thermocycler (Life Technologies). The thermal cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The housekeeping gene coding for hypoxanthine phosphoribosyltransferase (HPRT1) was used for signal normalization.
Assay of 15-LOX activity
15-HETE concentrations in the culture supernatant were measured using a commercially available enzyme immunoassay kit (Cayman Chemical Europe, Tallinn, Estonia), according to the manufacturer’s instructions. No arachidonic acid was added to the preparation. The limit of detection was 170 pg·mL−1. 15-HETE concentrations were expressed in pg per 106 LMs.
Effect of the inhibition of 15-LOX, 5-LOX and COX on the LPS- and IL-4/13-induced production of cytokines
Lung macrophages were treated with specific 15-LOX inhibitors [such as PD146176 (1–10 μM; Gregus et al., 2013)] and ML351 (1–10 μM; Rai et al., 2010) or DMSO vehicle for 1 h before stimulation with LPS, IL-4 or IL-13 for 24 h.
In order to investigate the involvement of 15-LOXs in cytokine release, a non-selective COX inhibitor (indomethacin, 1 μM; Buenestado et al., 2012), a 5-LOX-activating protein inhibitor (MK886, 1 μM) or the corresponding vehicles were added to the culture 30 min before addition of the 15-LOX inhibitors (or vehicle) and exposure of the macrophages to LPS, IL-4 or IL-13 for 24 h. The concentrations of PD146176, ML351 and MK886 used were those reported in the literature as selectively inhibiting the target enzymes (Gillard et al., 1989; Menard et al., 1990; Sendobry et al., 1997; Rai et al., 2010).
Stock solutions (10 mM) of PD146176, ML351, MK886 and indomethacin were prepared in DMSO. All subsequent dilutions were prepared daily in complete medium. The DMSO concentration applied to cells in culture did not exceed 0.12% and did not alter the production of cytokines. The cytotoxicity of PD146176, indomethacin and MK886 was determined by measuring LDH activity in LM supernatants (LDH assay; Cayman Chemical Europe). Neither the vehicles nor the compounds used in this study altered the viability of LMs (Supporting Information Table S1). All wells were run in duplicate. After 24 h of incubation, LM supernatants were collected, stored at −20°C and subsequently assayed for cytokines.
Cytokine measurements
Levels of cytokine in the culture supernatant were measured with specific elisas (the Duoset Development System from R&D Systems), according to the manufacturer’s instructions. The supernatant was diluted with RPMI medium as appropriate and the optical density was determined at 450 nm with an MRX II microplate reader (Dynex Technologies, Saint-Cloud, France). Cytokine concentrations are expressed as ng per 106 LMs. The assays’ limits of detection were 4 pg·mL−1 for CCL3, CCL13, CCL18, CCL22, 8 pg·mL−1 for TNF-α, CCL2, CCL4, and 32 pg·mL−1 for CXCL1, CXCL8 and CXCL10.
Data analysis and statistical procedures
Data are expressed as the mean ± SEM from experiments performed with LMs obtained from n patients. The concentration–effect curves were analysed using GraphPad Prism® software (version 5.01; GraphPad Software Inc., San Diego, CA, USA). The relative expression of mRNAs was calculated according to the 2(−ΔCt) method (Livak and Schmittgen, 2001). Statistical analysis of mRNA expression levels was performed with a paired or unpaired t-test, as appropriate. Other analyses were performed with a one-way, repeated-measures anova, followed by Bonferroni’s post-test. The threshold for statistical significance was set to P < 0.05.
Materials
PD146176 (6,11-dihydrol[1]benzothiopyrano[4,3-b]indole) and MK886 were obtained from Tocris Biosciences (Bristol, UK). ML351 was obtained from Merck Chemicals (Nottingham, UK). RPMI 1640 medium and BSA were acquired from Eurobio Biotechnology (Les Ulis, France). Indomethacin, penicillin-streptomycin, DMSO, L-glutamine, trypan blue dye, fetal calf serum (FCS) and LPS (from Escherichia coli serotype 0111:B4) were purchased from Sigma Aldrich (St. Louis, MO, USA). Human recombinant IL-13 and IL-4 were obtained from R&D Systems (Lille, France).
Results
Expression of 15-LOX isoenzyme transcripts and other enzymes of the eicosanoid pathways in polarized human LMs
We investigated the expression and regulation of ALOX15 (15-LOX-1) and ALOX15B (15-LOX-2) transcripts in human LMs in the presence or absence of LPS, IL-4 or IL-13. In unstimulated macrophages, the ALOX15B transcript was expressed at a higher level than the ALOX15 transcript (Figure 1A).
Figure 1.
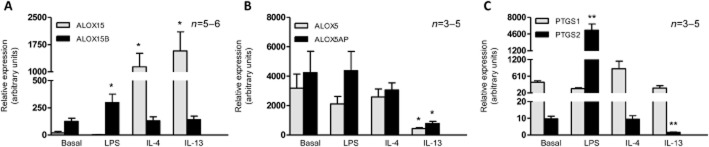
mRNA expression of ALOX15, ALOX15B, ALOX5, ALOX5AP, PTGS1 and PTGS2 in polarized human LMs. Lung macrophages were incubated with LPS (10 ng·mL−1), IL-4 (10 ng·mL−1) or IL-13 (50 ng·mL−1) for 24 h. Levels of (A) ALOX15 and ALOX15B; (B) ALOX5 and ALOX5AP; (C) PTGS1 and PTGS2 transcripts were determined by RT-qPCR and normalized against the expression of a housekeeping gene (HPRT1). Data correspond to the mean ± SEM of three to six independent experiments. *Indicates a significant difference relative to the baseline condition (*P < 0.05, **P < 0.01).
Quantitative analysis revealed that IL-4 and IL-13 increase the expression of ALOX15 transcripts but not ALOX15B transcripts. Conversely, incubation with LPS was associated with greater expression of ALOX15B transcripts but not ALOX15 transcripts (Figure 1A). A time-course experiment also showed an increase in ALOX15 expression 12 h after exposure to the Th2 cytokines (Supporting Information Fig. S1). Furthermore, the results of Western blotting experiments showed that 15-LOX-1 and 15-LOX-2 are expressed in unstimulated macrophages: marked inductions of 15-LOX-2 by LPS and of 15-LOX-1 were seen after 48 h (but not after just 24 h) of stimulation by IL-4 (Supporting Information Fig. S2).
We also investigated the mRNA expression of enzymes involved in other eicosanoid pathways. Unstimulated macrophages expressed transcripts of ALOX5, ALOX5AP, PTGS1 and PTGS2 (Figure 1B and C). Exposure to LPS was associated with greater mRNA expression of PTGS2 but did not alter the expression of ALOX5, ALOX5AP and PTGS1 (Figure 1B and C). Stimulation with IL-4 did not alter the expression of ALOX5, ALOX5AP, PTGS1 and PTGS2. However, exposure of LMs to IL-13 was associated with lower mRNA expression of ALOX5, ALOX5AP and PTGS2 but did not alter the expression of PTGS1 (Figure 1B and C). Transcripts of ALOX12 were not detected under any conditions (data not shown).
In order to assess the effects of LPS and IL-4 on the activity of the 15-LOXs, we measured the supernatant concentration of the arachidonic acid metabolite 15-HETE (produced by both 15-LOX-1 and 15-LOX-2). Exposure to LPS was associated with increased 15-HETE production by LMs. This relative increase was abolished in the presence of PD146176. Incubation with IL-4 was not associated with an increase in the production of 15-HETE (Table 2013a).
Table 1.
15-LOX activity in polarized human LMs
| Baseline | PD 10 μM | LPS only | LPS + PD 10 μM | IL-4 only | IL-4 + PD 10 μM | |
|---|---|---|---|---|---|---|
| 15-HETE (pg per 106 LMs) | 176 ± 47 | 167 ± 38 | 420 ± 95## | 191 ± 15** | 183 ± 34 | 222 ± 39 |
Lung macrophages were incubated for 1 h in the presence or absence of PD146176 before the addition of LPS (10 ng·mL−1) or IL-4 (10 ng·mL−1). 15-HETE levels were determined in the LM culture supernatant collected after 24 h of incubation. The data are reported as the mean ± SEM of seven independent experiments.
Indicates a significant difference relative to the baseline condition
P < 0.01).
Indicates a significant difference relative to the LPS condition
P < 0.01).
Inhibition of 15-LOX activity reduces the LPS- and IL-4/13-induced release of chemokines by human LMs
Effects of LPS, IL-4 and IL-13 on cytokine production by LMs
Incubation with LPS was associated with a marked increase in the production of CCL2, CCL3, CCL4, CXCL1, CXCL8 and CXCL10 (Table 2013b) but did not alter (or only weakly altered) the production of CCL13, CCL18 and CCL22 (Supporting Information Table S2).
Table 2.
Inhibitory effect of PD146176 on the LPS-induced release of chemokines
| Cytokine (ng per 106 LMs) | Baseline | LPS only | PD 1 μM + LPS | PD 10 μM + LPS |
|---|---|---|---|---|
| CCL2 | 1.50 ± 0.58 | 17.57 ± 3.49## | 21.05 ± 4.50 | 9.13 ± 3.66* |
| n = 8 | n = 8 | n = 8 | n = 8 | |
| CCL3 | 0.95 ± 0.40 | 233.38 ± 24.88### | 230.71 ± 27.45 | 164.45 ± 14.96* |
| n = 6 | n = 6 | n = 6 | n = 6 | |
| CCL4 | 1.67 ± 0.17 | 353.26 ± 42.85## | 319.16 ± 38.10 | 236.25 ± 48.50*** |
| n = 10 | n = 10 | n = 10 | n = 10 | |
| CXCL1 | 0.19 ± 0.06 | 208.17 ± 28.68### | 190.02 ± 21.01 | 95.20 ± 26.88** |
| n = 7 | n = 7 | n = 7 | n = 7 | |
| CXCL8 | 12.24 ± 2.77 | 920.67 ± 182.02## | 949.28 ± 162.56 | 491.97 ± 138.92** |
| n = 6 | n = 6 | n = 6 | n = 6 | |
| CXCL10 | 0 (LD) | 8.12 ± 2.61# | 2.55 ± 0.66$ | 3.10 ± 1.36* |
| n = 10 | n = 10 | n = 5 | n = 10 |
Human lung macrophages were incubated for 1 h in the presence of PD146176 (1–10 μM) or vehicle (the baseline condition) before being stimulated with LPS (10 ng·mL−1) for 24 h. The concentrations of the chemokines in the culture supernatant were measured using elisas. The data are reported as the mean ± SEM of 5–10 independent experiments.
Indicates a significant difference relative to the baseline condition
P < 0.05
P < 0.01
P < 0.001).
Indicates a significant difference relative to LPS alone
P < 0.05
P < 0.01
P < 0.001). Data for the vehicle are not shown because 0.1% DMSO had no effect on cytokine production.
Not significant versus the five paired preparations of macrophages exposed to LPS (LPS: 4.34 ± 1.44 ng per 106 cells).
Conversely, incubation with IL-4 or IL-13 was associated with a significant increase in the production of CCL13 and CCL22, whereas the production of CCL18 remained unchanged (Table 2007).
Table 3.
Inhibitory effect of PD146176 on the IL-4- and IL-13-induced release of chemokines
| Cytokine (ng per 106 LMs) | Baseline | IL-4 only | PD 1 μM + IL-4 | PD 10 μM + IL-4 |
|---|---|---|---|---|
| CCL13 | 0.038 ± 0.011 | 0.23 ± 0.06# | 0.25 ± 0.08 | 0.09 ± 0.04** |
| CCL18 | 96.02 ± 22.41 | 101.70 ± 16.75 | 99.69 ± 22.78 | 59.32 ± 20.17* |
| CCL22 | 1.12 ± 0.30 | 4.73 ± 0.10## | 5.93 ± 1.22 | 2.27 ± 0.63* |
| Baseline | IL-13 only | PD 1 μM + IL-13 | PD 10 μM + IL-13 | |
|---|---|---|---|---|
| CCL13 | 0.04 ± 0.01 | 0.56 ± 0.14# | 0.40 ± 0.17 | 0.18 ± 0.11** |
| CCL18 | 91.67 ± 23.00 | 112.97 ± 19.05 | 118.31 ± 22.02 | 78.96 ± 22.83 |
| CCL22 | 1.12 ± 0.30 | 4.75 ± 1.18## | 5.42 ± 1.10 | 3.37 ± 0.88* |
Lung macrophages were incubated for 1 h in the presence or absence (the baseline condition) of PD146176 (1–10 μM) or vehicle before being stimulated with (IL-4 10 ng·mL−1) or IL-13 (50 ng·mL−1) for 24 h. The concentrations of chemokines in the culture supernatant were measured using elisas. The data are reported as the mean ± SEM of five to eight independent experiments.
Indicates a significant difference relative to the baseline condition
P < 0.05
P < 0.01).
Indicates a significant difference relative to IL-4 or IL-13
P < 0.05
P < 0.01).
Furthermore, the production of LPS-inducible chemokines was only weakly altered or not altered by Th2 cytokines (Supporting Information Table S3). These different effects of LPS and Th2 cytokines were also observed for mRNA expression of the cytokines (Supporting Information Table S4). The relative effects of LPS and IL-4/IL-13 on the production of TNF-α and the panels of chemokines indicate that (i) TNF-α, CCL2, CCL3, CCL4, CXCL1 and CXCL10 constitute an LPS-induced signature; and (ii) CCL13 and CCL22 constitute a Th2 signature.
Effect of inhibiting 15-LOX on the production of cytokines by LMs
As ALOX15 and ALOX15B were expressed in human LMs, we next assessed the effect of a 15-LOX inhibitor (PD146176, which inhibits both 15-LOX-1 and 15-LOX-2) on the production of the cytokines induced by LPS and IL-4/IL-13. At a concentration of 10 μM, PD146176 reduced the respective LPS- and IL-4/IL-13-induced increases in ALOX15 and ALOX15B gene expression but did not alter (or only weakly altered) the transcript expression of other genes involved in the eicosanoid pathways (Supporting Information Figs S3 and S4). Inhibition of 15-LOX isoenzymes by PD146176 was associated with a lower production of CCL2, CCL3, CCL4, CXCL1, CXCL8 and CXCL10 induced by LPS, with relative reductions of 48, 29, 33, 54, 46 and 56% respectively (Table 2013b). Similarly, the production of the chemokines CCL13, CCL22 and CCL18, induced by IL-4- and IL-13, was significantly lower in the presence of PD146176, with relative reductions of 58, 37 and 52% for IL-4 induction and 67, 28 and 29% for IL-13 induction respectively (Table 2007). Lastly, the production of TNF-α was not significantly reduced (relative reduction: 6%) after incubation with PD146176 (Table 2014).
Table 4.
The inhibitory effect of PD146176, indomethacin and MK886 on the LPS-induced release of TNF-α in human LMs
| Cytokine (ng per 106 LMs) | LPS | LPS + PD 10 μM | + Indomethacin 1 μM | + MK 886 1 μM | + Indomethacin 1 μM + MK 886 1 μM | |||
|---|---|---|---|---|---|---|---|---|
| LPS | LPS + PD 10 μM | LPS | LPS + PD 10 μM | LPS | LPS + PD 10 μM | |||
| TNF-α | 19.7 ± 6.2## | 18.2 ± 5.5 | 31.1 ± 9.0++ | 18 ± 5.5** | 17.3 ± 5.6 | 20.1 ± 6.5 | 31.5 ± 9.3++ | 19.2 ± 4.5** |
Lung macrophages were exposed to medium alone (the baseline condition) or medium supplemented with indomethacin or MK886 or vehicles for 30 min before being pre-incubated for 1 h with PD146176 or vehicle before stimulation with LPS (10 ng·mL−1). Levels of TNF-α in the culture supernatant were determined after 24 h of incubation. Data are reported as the mean ± SEM of four independent experiments.
Indicates a significant difference relative to the baseline condition (0.2 ± 0.05 ng per 106 cells
P < 0.01).
Indicates a significant difference relative to LPS alone
P < 0.01).
Indicates a significant difference relative to the corresponding controls
P < 0.01).
Inhibition of 15-LOX-1 by ML351 (10 μM) was associated with significant inhibition of the LPS-induced production of CCL2 and CCL3 (relative reductions: 47 and 37% respectively) and the IL-4-induced production of CCL13 and CCL22 (relative reductions: 55 and 57% respectively) (Table 2008).
Table 5.
Inhibitory effect of ML351 on the LPS- and IL-4-induced release of chemokines in human LMs
| Cytokine (ng per 106 LMs) | With LPS | ||
|---|---|---|---|
| Baseline | ML351 1 μM | ML351 10 μM | |
| CCL2 | 48.27 ± 3.97 | 47.32 ± 5.41 | 23.37 ± 4.87** |
| n = 4 | n = 4 | n = 4 | |
| CCL3 | 184.96 ± 30.83 | 164.69 ± 23.54 | 117.04 ± 22.97** |
| n = 4 | n = 4 | n = 4 | |
| Cytokine | With IL-4 | ||
|---|---|---|---|
| Baseline | ML351 1 μM | ML351 10 μM | |
| CCL13 | 0.093 ± 0.02 | 0.10 ± 0.01 | 0.034 ± 0.006** |
| n = 5 | n = 5 | n = 5 | |
| CCL22 | 3.77 ± 1.34 | 4.06 ± 1.4 | 2.01 ± 0.79* |
| n = 5 | n = 5 | n = 5 | |
Lung macrophages were incubated for 1 h in the presence or absence (the baseline condition) of ML351 (1–10 μM) or vehicle before being stimulated with (LPS 10 ng·mL−1) or IL-4 (10 ng·mL−1) for 24 h. The concentrations of chemokines in the culture supernatant were measured using elisas. The data are reported as the mean ± SEM of four to five independent experiments.
Indicates a significant difference relative to LPS or IL-4
P < 0.05
P < 0.01). Data for the vehicle are not shown because 0.05% DMSO had no effect on cytokine production.
Confirmation of the specific involvement of the 15-LOX pathway in the LPS- and IL-4/IL-13-induced production of cytokines by LMs
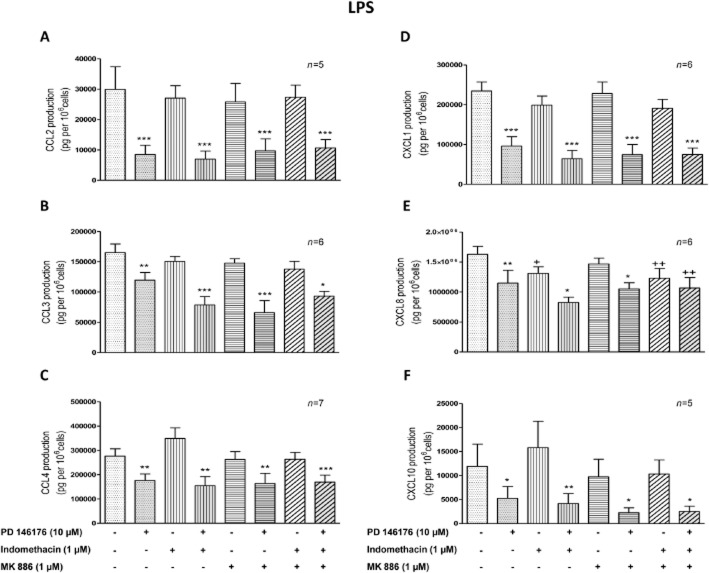
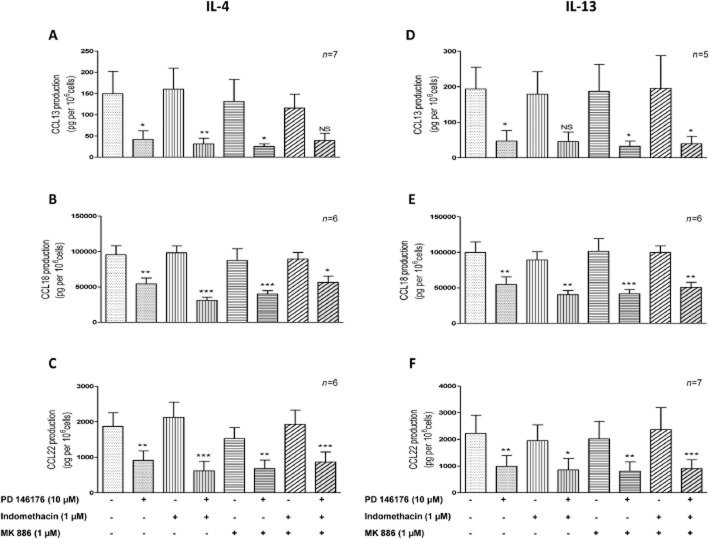
The ALOX5, ALOX5AP, PTGS1 and PTGS2 genes are expressed and modulated by LPS and Th2 cytokines in human LMs. The corresponding enzymes share a substrate (arachidonic acid) with the 15-LOXs. We hypothesized that inhibition of 15-LOXs might deviate the arachidonic acid towards other eicosanoid pathways that might be involved in the effect of 15-LOX inhibitors on cytokine production. To ascertain the role of the 15-LOX pathways, LMs were pretreated with an inhibitor of 5-LOX-activating protein (MK886) and a non-selective COX inhibitor (indomethacin) before the addition of PD146176 or ML351. Neither indomethacin nor MK886 (whether present alone or together) was associated with differences in the production of cytokines (other than CXCL8) and TNF-α (Figure 2). The production of CXCL8 was found to be lower after incubation with indomethacin alone and with indomethacin + MK886 (Figure 2E). However, TNF-α production was higher with indomethacin alone and with indomethacin + MK886 (Table 2014). Inhibition of the production of both the LPS- and the IL-4/IL-13-induced chemokines by PD146176 was maintained in the presence of indomethacin and MK886 (whether present alone or together) (Figure 2A–F and Figure 3A–F).
Figure 2.
The roles of indomethacin and MK886 in the suppression of LPS-induced chemokine release from human LMs by PD146176. Human LMs were exposed to medium alone (the baseline condition) or medium supplemented with indomethacin (1 μM), MK886 (1 μM) or vehicles for 30 min before being pre-incubated for 1 h with PD146176 (10 μM) or vehicle. The cells were then stimulated with LPS (10 ng·mL−1). Levels of chemokines (A) CCL2, (B) CCL3, (C) CCL4, (D) CXCL1, (E) CXCL8 and (F) CXCL10 in the culture supernatant were determined after 24 h of incubation. Data are reported as the mean ± SEM of five to seven independent experiments. *Indicates a significant difference relative to the corresponding control (*P < 0.05; **P < 0.01; ***P < 0.001). +Indicates a significant difference relative to LPS (+P < 0.05, ++P < 0.01).
Figure 3.
The roles of indomethacin and MK886 in the suppression of IL-4- or IL-13-induced chemokine release from human LMs by PD146176. Human LMs were exposed to medium alone (the baseline condition) or medium supplemented with indomethacin (1 μM), MK886 (1 μM) or vehicles for 30 min before being pre-incubated for 1 h with PD146176 (10 μM) or vehicle. The cells were then stimulated with IL-4 (10 ng·mL−1) or IL-13 (50 ng·mL−1). Levels of chemokine CCL13; CLL18 and CCL22 either induced by IL-4 (A, B, C) or by IL-13 (D, E, F) in the culture supernatant were determined after 24 h of incubation. Data are reported as the mean ± SEM of five to seven independent experiments. *Indicates a significant difference relative to the corresponding control (*P < 0.05; **P < 0.01; ***P < 0.001).
PD146176 still inhibited CXCL8 production when the LMs were pretreated with indomethacin (Figure 2E) or MK886 alone. However, the inhibitory effect of PD146176 on CXCL8 production was not observed when LMs were incubated with indomethacin and MK886 together (Figure 2E). Inhibition of COXs by indomethacin revealed the inhibitory effect of PD146176 on LPS-induced TNF-α production (Table 2014).
Similar results were obtained with ML351. Inhibition of the production of chemokines induced by LPS and IL-4 by ML351 was maintained in the presence of indomethacin and MK886: the relative reductions in the LPS-induced production of CCL2 and CCL3 were respectively 47 and 37% (P < 0.05 for both), and the relative reductions of the IL-4-induced production of CCL13 and CCL22 were respectively 55 and 57% (P < 0.05 for both).
Discussion and conclusions
Our results demonstrate that ALOX15 and ALOX15B transcripts are expressed in human LMs and are up-regulated by IL-4/IL-13 and by LPS respectively. The inhibition of 15-LOXs by PD146176 and ML351 was associated with reductions in both the LPS-induced release of CCL2, CCL3, CCL4, CXCL1, CXCL8 and CXCL10, and the IL-4/IL-13-induced release of CCL13, CCL18 and CCL22.
In human blood monocytes, IL-4 and IL-13 strongly up-regulate the expression of ALOX15 (Chaitidis et al., 2005). During the in vitro differentiation of human monocytes to macrophages, markedly greater ALOX15B expression has been reported, whereas ALOX15 and ALOX12 expression remained low (Wuest et al., 2012). In the present study, ALOX15B was found to be strongly expressed in unstimulated human LMs. The stimulation of human MDMs with Th2 cytokines was associated with greater expression of ALOX15B, whereas increased ALOX15 expression was observed with IL-4 and (to a much lesser extent) IL-13 (Wuest et al., 2012). In contrast, IL-4 and IL-13 were only associated with greater ALOX15 expression in human LMs. Incubation with LPS is associated with greater expression of ALOX15B in both human MDMs (Wuest et al., 2012) and human LMs (the present study). Our data suggest that the LPS-induced activation of human LMs led specifically to the expression of ALOX15B, whereas the Th2 cytokine-induced activation led to the expression of ALOX15. In Western blot experiments, a marked increase in protein expression of 15-LOX-1 and 15-LOX-2 was seen after 48 h of stimulation by Th2 cytokines and LPS but not after 24 h of stimulation (Supporting Information Fig. S2).
The different effects of IL-4 and IL-13 on the expression of ALOX5, ALOX5AP, PTGS2 and other genes of the eicosanoid pathways (Supporting Information) are probably explained by the fact that IL-4 only binds to type I receptors, whereas IL-13 binds to both type I and type II receptors. Both types of receptor are expressed by macrophages, although the respective downstream signalling components differ. When compared with IL-13, IL-4 induces stronger expression of markers of alternative polarization of macrophages and may also yield a somewhat different biological response profile (Bhattacharjee et al., 2013; Van Dyken and Locksley, 2013).
The slight increase in 15-HETE levels in the supernatant after exposure to LPS and the absence of an IL-4/IL-13-induced increase in the production of 15-HETE is probably explained by the incorporation of 15-HETE into the cell’s phospholipids – thus limiting its release into the supernatant (Norris et al., 2014). Profita et al. (1999) demonstrated that IL-4 not only enhances 15-LOX activity but also increases the incorporation of 15-HETE into phospholipids in pulmonary epithelial cells. This IL-4-induced increase in the incorporation of 15-HETE into phospholipids further limits the release of 15-HETE into the supernatant, and so may explain (at least in part) the absence of an increase in 15-HETE levels in the supernatant after exposure to IL-4 in the present study. The incorporation of 15-HETE into phospholipids might regulate or alter the intracellular signal transduction by reducing the amount and/or production of second messengers. Accordingly, the 15-HETE incorporated into cellular phospholipids has been shown to modulate the receptor-mediated activation of human neutrophils (Takata et al., 1994). Although further investigation of these intracellular mechanisms was beyond the scope of the present study, the mechanisms might explain why inhibition of 15-LOX can reduce cytokine release in the absence of a change in 15-HETE release into the supernatant.
The overexpression of 15-LOX-2 in macrophages derived from human monocyte THP-1 cells was seen to be associated with greater CXCL10 and CCL2 production (Danielsson et al., 2008). Conversely, knock-down of ALOX15B expression in human MDMs was associated with lower spontaneous production of CXCL8 (Magnusson et al., 2012) and lower hypoxia-induced production of CXCL10, whereas levels of CCL2, CCL5 and CXCL8 did not vary (Danielsson et al., 2008). In the present study in human LMs, inhibition of the two 15-LOX isoenzymes by PD146176 and inhibition of 15-LOX-1 by ML351 were associated with a markedly lower production of LPS-induced chemokines (including CCL2, CCL5, CXCL8 and CXCL10) and Th2 cytokine-induced chemokines – suggesting the involvement of the 15-LOX pathways. Involvement of the 12-LOX-pathway can be ruled out, as human macrophages do not express 12-LOX (as seen in the present study and Wuest et al., 2012).
Arachidonic acid is a common substrate for LOXs and COXs. Hence, inhibition of 15-LOXs by PD146176 or ML351 can cause the shunting of arachidonic acid metabolism to the 5-LOX and COX-1/2 (PTGS1/2) pathways, with a potential increase in the levels of associated eicosanoids (e.g. PGs E2 and I2, and cysteinyl leukotrienes). In turn, these eicosanoids can modulate chemokine production in monocytes and macrophages (Hashimoto et al., 2009; Ichiyama et al., 2009; Kuo et al., 2011; Buenestado et al., 2012; Tsai et al., 2014). Furthermore, 15-HETE can be also produced via COX-dependent pathways (Levy et al., 1993). As (i) human LMs expressed the 5-LOX and COX-1/2 (PTGS1/2) pathways, (ii) LPS was primarily associated with greater PTGS2 expression, and (iii) IL-13 was associated with lower expression of PTGS2, ALOX5 and ALOX5AP, we decided to ascertain the role of the 15-LOX pathways in the LPS- and IL-4/IL-13-induced production of chemokines. To this end, we assessed the effect of 15-LOX inhibition by PD146176 or ML351 after blockade of the 5-LOX and COX-1/2 (PTGS1/2) pathways. Our results confirmed that 15-LOXs have a regulatory role in the production of chemokines induced by LPS and IL-4/IL-13.
The 15-LOX pathway is a major metabolic pathway for arachidonic acid in human lung homogenates and airway epithelial cells. In human bronchial epithelial cells, both 15-LOX isoforms are present and are expressed even more strongly in patients with severe asthma (Brown et al., 2001; Zhao et al., 2002; Gras et al., 2012; Jakiela et al., 2013; Mabalirajan et al., 2013). Exposure of human bronchial epithelial cells to IL-4 or IL-13 is associated with greater expression of ALOX15 but not ALOX15B (Brown et al., 2001; Jakiela et al., 2013), as observed in human LMs in the present study. In work on bronchial epithelial cells, the induction of ALOX15 expression by Th2 cytokines was associated with increased expression of two chemokines (CCL24 and CCL26). In the A549 epithelial alveolar cell line, the expression of chemokines (CCL3, CCL4, CCL5, CCL20, CXCL3, CXCL10, CXCL11 and CX3CL1) was up-regulated by 15-LOX-1 overexpression and down-regulated by inhibition of 15-LOX-1 activity (Liu et al., 2009). Taken as a whole, these data show that 15-LOXs are involved in the regulation of chemokine expression by both human LMs (in the present study) and airway epithelial cells.
Conversely, inhibition of 15-LOXs did not alter LPS-induced TNF-α production in human LMs, as previously shown in thioglycolate-elicited peritoneal murine macrophages (Middleton et al., 2006). We and others have reported that LPS-induced release of TNF-α is potently inhibited by PGE2 and PGI2 (Raychaudhuri et al., 2002; Aronoff et al., 2007; Ratcliffe et al., 2007; Buenestado et al., 2012), and we have previously shown that substantial amounts of PGE2 and 6-ketoPGF1α accumulate in the cell culture supernatant of human LMs exposed to LPS (Buenestado et al., 2012). Inhibition of prostanoid synthesis with indomethacin not only increases the LPS-induced production of TNF-α (in the present study, and as also shown with alveolar macrophages; Hempel et al., 1996) by eliminating the predominant downregulatory role of prostaglandins, but also reveals the inhibitory effect of PD146176 and the role of the 15-LOXs.
Furthermore, our results indicate that inhibition of the 5-LOX pathway had no effect on LPS- and Th2 cytokine-induced chemokine production by human LMs; this fits with similar results in human alveolar macrophages for CXCL8 (Parkar et al., 1990) and in human peritoneal macrophages for the pro-inflammatory cytokines IL-1β and IL-6 (Pruimboom et al., 1994).
In conclusion, the in vitro activation of human LMs (by exposure to either LPS or IL-4/IL-13) is associated with an increase in the expression of ALOX15B and ALOX15 respectively. Specific inhibition of the 15-LOXs was associated with a reduction in the production of chemokines induced by LPS and Th2 cytokines. However, it is known that 15-LOXs also contribute to the resolution of inflammation via the formation of specialized proresolving mediators (e.g. resolvins, protectins, maresins and new ketophospholipids) (Hammond et al., 2012; Levy and Serhan, 2014). Our present results in LMs suggest that the 15-LOX pathways have several roles in the pathogenesis of inflammatory lung disorders and may thus constitute a potential drug target.
Acknowledgments
The authors thank David Fraser (Biotech Communication SARL, Damery, France) for copy-editing services.
Glossary
- FCS
fetal calf serum
- 15-HETE
15-hydroxyeicosatetraenoic acid
- 13-HODE
13-hydroxy octadecadienoic acid
- HPRT
hypoxanthine phosphoribosyltransferase
- LM
lung macrophage
- LOX
lipoxygenase
- MDM
monocyte-derived macrophage
Author contributions
C. A., H. S. and M. B. prepared the lung macrophages and carried out immunoassays, the 15-lipoxygenase activity assay and RT-qPCRs. E. N. provided the human tissues and was also involved in preparation of the lung macrophages. C. A. analysed the results, prepared the figures, performed the statistical analysis and drafted the manuscript. P. D. and S. G. D. conceived the study, participated in its design, managed the work in the laboratory, analysed the results and drafted the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Supporting Information
Figure S1 Time course of ALOX15 and ALOX15B mRNA expression in polarized human lung macrophages.
Figure S2 Western blot analysis of 15-LOX-1 and 15-LOX-2 protein expression in human lung macrophages stimulated by LPS, IL-4 or IL-13 for 24 and 48 h.
Figure S3 Effect of PD146176 on ALOX15 and ALOX15B expression in human lung macrophages.
Figure S4 Effect of PD146176 on mRNA expression of genes involved in the eicosanoid pathways in human lung macrophages.
Table S1 Effect of PD146176 (10 μM) on lung macrophage viability in an LDH assay.
Table S2 Effect of LPS on production of the panel of IL-4-/IL-13-induced chemokines.
Table S3 Effect of IL-4 and IL-13 on production of the panel of LPS-induced chemokines.
Table S4 Effect of LPS and Th2 cytokines on mRNA expression of M1- and M2a-related markers of polarization in human lung macrophages.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: catalytic receptors. Br J Pharmacol. 2013a;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, et al. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol. 2007;178:1628–1634. doi: 10.4049/jimmunol.178.3.1628. [DOI] [PubMed] [Google Scholar]
- Back M, Powell WS, Dahlen SE, Drazen JM, Evans JF, Serhan CN, et al. International Union of Basic and Clinical Pharmacology. Update on leukotriene, lipoxin and oxoeicosanoid receptors: IUPHAR Review 7. Br J Pharmacol. 2014;171:3551–3574. doi: 10.1111/bph.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Shukla M, Yakubenko VP, Mulya A, Kundu S, Cathcart MK. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradding P, Feather IH, Wilson S, Holgate ST, Howarth PH. Cytokine immunoreactivity in seasonal rhinitis: regulation by a topical corticosteroid. Am J Respir Crit Care Med. 1995;151:1900–1906. doi: 10.1164/ajrccm.151.6.7767538. [DOI] [PubMed] [Google Scholar]
- Brown CD, Kilty I, Yeadon M, Jenkinson S. Regulation of 15-lipoxygenase isozymes and mucin secretion by cytokines in cultured normal human bronchial epithelial cells. Inflamm Res. 2001;50:321–326. doi: 10.1007/PL00000251. [DOI] [PubMed] [Google Scholar]
- Buenestado A, Grassin Delyle S, Arnould I, Besnard F, Naline E, Blouquit-Laye S, et al. The role of adenosine receptors in regulating production of tumour necrosis factor-alpha and chemokines by human lung macrophages. Br J Pharmacol. 2010;159:1304–1311. doi: 10.1111/j.1476-5381.2009.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenestado A, Grassin-Delyle S, Guitard F, Naline E, Faisy C, Israel-Biet D, et al. Roflumilast inhibits the release of chemokines and TNF-alpha from human lung macrophages stimulated with lipopolysaccharide. Br J Pharmacol. 2012;165:1877–1890. doi: 10.1111/j.1476-5381.2011.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitidis P, O’Donnell V, Kuban RJ, Bermudez-Fajardo A, Ungethuem U, Kuhn H. Gene expression alterations of human peripheral blood monocytes induced by medium-term treatment with the TH2-cytokines interleukin-4 and -13. Cytokine. 2005;30:366–377. doi: 10.1016/j.cyto.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, et al. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002;32:1558–1565. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- Danielsson KN, Rydberg EK, Ingelsten M, Akyurek LM, Jirholt P, Ullstrom C, et al. 15-Lipoxygenase-2 expression in human macrophages induces chemokine secretion and T cell migration. Atherosclerosis. 2008;199:34–40. doi: 10.1016/j.atherosclerosis.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2011;50:115–131. doi: 10.1016/j.plipres.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard J, Ford-Hutchinson AW, Chan C, Charleson S, Denis D, Foster A, et al. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 – dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989;67:456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Gras D, Bourdin A, Vachier I, de Senneville L, Bonnans C, Chanez P. An ex vivo model of severe asthma using reconstituted human bronchial epithelium. J Allergy Clin Immunol. 2012;129:1259–1266 e1251. doi: 10.1016/j.jaci.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Gregus AM, Dumlao DS, Wei SC, Norris PC, Catella LC, Meyerstein FG, et al. Systematic analysis of rat 12/15-lipoxygenase enzymes reveals critical role for spinal eLOX3 hepoxilin synthase activity in inflammatory hyperalgesia. FASEB J. 2013;27:1939–1949. doi: 10.1096/fj.12-217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond VJ, Morgan AH, Lauder S, Thomas CP, Brown S, Freeman BA, et al. Novel keto-phospholipids are generated by monocytes and macrophages, detected in cystic fibrosis, and activate peroxisome proliferator-activated receptor-γ. J Biol Chem. 2012;287:41651–41666. doi: 10.1074/jbc.M112.405407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Ichiyama T, Hasegawa M, Hasegawa S, Matsubara T, Furukawa S. Cysteinyl leukotrienes induce monocyte chemoattractant protein-1 in human monocyte/macrophages via mitogen-activated protein kinase and nuclear factor-kappaB pathways. Int Arch Allergy Immunol. 2009;149:275–282. doi: 10.1159/000199724. [DOI] [PubMed] [Google Scholar]
- Hempel SL, Monick MM, Hunninghake GW. Effect of hypoxia on release of IL-1 and TNF by human alveolar macrophages. Am J Respir Cell Mol Biol. 1996;14:170–176. doi: 10.1165/ajrcmb.14.2.8630267. [DOI] [PubMed] [Google Scholar]
- Ichiyama T, Hasegawa M, Hashimoto K, Matsushige T, Hirano R, Furukawa S. Cysteinyl leukotrienes induce macrophage inflammatory protein-1 in human monocytes/macrophages. Int Arch Allergy Immunol. 2009;148:147–153. doi: 10.1159/000155745. [DOI] [PubMed] [Google Scholar]
- Jakiela B, Gielicz A, Plutecka H, Hubalewska M, Mastalerz L, Bochenek G, et al. Eicosanoid biosynthesis during mucociliary and mucous metaplastic differentiation of bronchial epithelial cells. Prostaglandins Other Lipid Mediat. 2013;106:116–123. doi: 10.1016/j.prostaglandins.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Jayawickreme SP, Gray T, Nettesheim P, Eling T. Regulation of 15-lipoxygenase expression and mucus secretion by IL-4 in human bronchial epithelial cells. Am J Physiol. 1999;276(4 Pt 1):L596–L603. doi: 10.1152/ajplung.1999.276.4.L596. [DOI] [PubMed] [Google Scholar]
- Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–356. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Kuo CH, Ko YC, Yang SN, Chu YT, Wang WL, Huang SK, et al. Effects of PGI2 analogues on Th1- and Th2-related chemokines in monocytes via epigenetic regulation. J Mol Med (Berl) 2011;89:29–41. doi: 10.1007/s00109-010-0694-2. [DOI] [PubMed] [Google Scholar]
- Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BD, Romano M, Chapman HA, Reilly JJ, Drazen J, Serhan CN. Human alveolar macrophages have 15-lipoxygenase and generate 15(S)-hydroxy-5,8,11-cis-13-trans-eicosatetraenoic acid and lipoxins. J Clin Invest. 1993;92:1572–1579. doi: 10.1172/JCI116738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xu D, Liu L, Schain F, Brunnstrom A, Bjorkholm M, et al. 15-Lipoxygenase-1 induces expression and release of chemokines in cultured human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L196–L203. doi: 10.1152/ajplung.00036.2008. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lundstrom SL, Yang J, Kallberg HJ, Thunberg S, Gafvelin G, Haeggstrom JZ, et al. Allergic asthmatics show divergent lipid mediator profiles from healthy controls both at baseline and following birch pollen provocation. PLoS ONE. 2012;7:e33780. doi: 10.1371/journal.pone.0033780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabalirajan U, Rehman R, Ahmad T, Kumar S, Singh S, Leishangthem GD, et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci Rep. 2013;3:1349. doi: 10.1038/srep01349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson LU, Lundqvist A, Karlsson MN, Skalen K, Levin M, Wiklund O, et al. Arachidonate 15-lipoxygenase type B knockdown leads to reduced lipid accumulation and inflammation in atherosclerosis. PLoS ONE. 2012;7:e43142. doi: 10.1371/journal.pone.0043142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Menard L, Pilote S, Naccache PH, Laviolette M, Borgeat P. Inhibitory effects of MK-886 on arachidonic acid metabolism in human phagocytes. Br J Pharmacol. 1990;100:15–20. doi: 10.1111/j.1476-5381.1990.tb12044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton MK, Rubinstein T, Pure E. Cellular and molecular mechanisms of the selective regulation of IL-12 production by 12/15-lipoxygenase. J Immunol. 2006;176:265–274. doi: 10.4049/jimmunol.176.1.265. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris PC, Gosselin D, Reichart D, Glass CK, Dennis EA. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:12746–12751. doi: 10.1073/pnas.1404372111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono E, Mita H, Taniguchi M, Higashi N, Hasegawa M, Miyazaki E, et al. Concentration of 14,15-leukotriene C4 (eoxin C4) in bronchoalveolar lavage fluid. Clin Exp Allergy. 2009;39:1348–1352. doi: 10.1111/j.1365-2222.2009.03261.x. [DOI] [PubMed] [Google Scholar]
- Parkar BA, McCormick ME, Foster SJ. Leukotrienes do not regulate interleukin 1 production by activated macrophages. Biochem Biophys Res Commun. 1990;169:422–429. doi: 10.1016/0006-291x(90)90348-q. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechkovsky DV, Prasse A, Kollert F, Engel KM, Dentler J, Luttmann W, et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Profita M, Vignola AM, Sala A, Mirabella A, Siena L, Pace E, et al. Interleukin-4 enhances 15-lipoxygenase activity and incorporation of 15(S)-HETE into cellular phospholipids in cultured pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1999;20:61–68. doi: 10.1165/ajrcmb.20.1.3151. [DOI] [PubMed] [Google Scholar]
- Profita M, Sala A, Riccobono L, Paterno A, Mirabella A, Bonanno A, et al. 15-Lipoxygenase expression and 15(S)-hydroxyeicoisatetraenoic acid release and reincorporation in induced sputum of asthmatic subjects. J Allergy Clin Immunol. 2000;105:711–716. doi: 10.1067/mai.2000.105122. [DOI] [PubMed] [Google Scholar]
- Pruimboom WM, van Dijk JA, Tak CJ, Garrelds I, Bonta IL, Wilson PJ, et al. Interactions between cytokines and eicosanoids: a study using human peritoneal macrophages. Immunol Lett. 1994;41:255–260. doi: 10.1016/0165-2478(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Rai G, Joshi N, Perry S, Yasgar A, Schultz L, Jung JE, et al. 2010. Discovery of ML351, a potent and selective inhibitor of human 15-lipoxygenase-1. Probe Reports from the NIH Molecular Libraries Program, edn. Bethesda (MD). p∧pp.
- Ratcliffe MJ, Walding A, Shelton PA, Flaherty A, Dougall IG. Activation of E-prostanoid4 and E-prostanoid2 receptors inhibits TNF-alpha release from human alveolar macrophages. Eur Respir J. 2007;29:986–994. doi: 10.1183/09031936.00131606. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri B, Malur A, Bonfield TL, Abraham S, Schilz RJ, Farver CF, et al. The prostacyclin analogue treprostinil blocks NFkappaB nuclear translocation in human alveolar macrophages. J Biol Chem. 2002;277:33344–33348. doi: 10.1074/jbc.M203567200. [DOI] [PubMed] [Google Scholar]
- Sachs-Olsen C, Sanak M, Lang AM, Gielicz A, Mowinckel P, Lodrup Carlsen KC, et al. Eoxins: a new inflammatory pathway in childhood asthma. J Allergy Clin Immunol. 2010;126:859–867 e859. doi: 10.1016/j.jaci.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Sendobry SM, Cornicelli JA, Welch K, Bocan T, Tait B, Trivedi BK, et al. Attenuation of diet-induced atherosclerosis in rabbits with a highly selective 15-lipoxygenase inhibitor lacking significant antioxidant properties. Br J Pharmacol. 1997;120:1199–1206. doi: 10.1038/sj.bjp.0701007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples KJ, Hinks TS, Ward JA, Gunn V, Smith C, Djukanovic R. Phenotypic characterization of lung macrophages in asthmatic patients: overexpression of CCL17. J Allergy Clin Immunol. 2012;130:1404–1412 e1407. doi: 10.1016/j.jaci.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata S, Matsubara M, Allen PG, Janmey PA, Serhan CN, Brady HR. Remodeling of neutrophil phospholipids with 15(S)-hydroxyeicosatetraenoic acid inhibits leukotriene B4-induced neutrophil migration across endothelium. J Clin Invest. 1994;93:499–508. doi: 10.1172/JCI116999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson NC, Chaudhuri R, Spears M, Messow CM, Jelinsky S, Miele G, et al. Arachidonic acid metabolites and enzyme transcripts in asthma are altered by cigarette smoking. Allergy. 2014;69:527–536. doi: 10.1111/all.12376. [DOI] [PubMed] [Google Scholar]
- Tsai MK, Hsieh CC, Kuo HF, Yang SN, Kuo CH, Huang MY, et al. Effect of prostaglandin I2 analogs on macrophage inflammatory protein 1alpha in human monocytes via I prostanoid receptor and cyclic adenosine monophosphate. J Investig Med. 2014;62:332–339. doi: 10.2310/JIM.0000000000000042. [DOI] [PubMed] [Google Scholar]
- Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–343. doi: 10.1146/annurev-immunol-032712-095906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest SJ, Crucet M, Gemperle C, Loretz C, Hersberger M. Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis. 2012;225:121–127. doi: 10.1016/j.atherosclerosis.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Zhao J, Maskrey B, Balzar S, Chibana K, Mustovich A, Hu H, et al. Interleukin-13-induced MUC5AC is regulated by 15-lipoxygenase 1 pathway in human bronchial epithelial cells. Am J Respir Crit Care Med. 2009;179:782–790. doi: 10.1164/rccm.200811-1744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Cuff CA, Moss E, Wille U, Cyrus T, Klein EA, et al. Selective interleukin-12 synthesis defect in 12/15-lipoxygenase-deficient macrophages associated with reduced atherosclerosis in a mouse model of familial hypercholesterolemia. J Biol Chem. 2002;277:35350–35356. doi: 10.1074/jbc.M205738200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Time course of ALOX15 and ALOX15B mRNA expression in polarized human lung macrophages.
Figure S2 Western blot analysis of 15-LOX-1 and 15-LOX-2 protein expression in human lung macrophages stimulated by LPS, IL-4 or IL-13 for 24 and 48 h.
Figure S3 Effect of PD146176 on ALOX15 and ALOX15B expression in human lung macrophages.
Figure S4 Effect of PD146176 on mRNA expression of genes involved in the eicosanoid pathways in human lung macrophages.
Table S1 Effect of PD146176 (10 μM) on lung macrophage viability in an LDH assay.
Table S2 Effect of LPS on production of the panel of IL-4-/IL-13-induced chemokines.
Table S3 Effect of IL-4 and IL-13 on production of the panel of LPS-induced chemokines.
Table S4 Effect of LPS and Th2 cytokines on mRNA expression of M1- and M2a-related markers of polarization in human lung macrophages.