Abstract Abstract
In patients with idiopathic pulmonary arterial hypertension (iPAH), iron deficiency is common and has been associated with reduced exercise capacity and worse survival. Previous studies have shown beneficial effects of intravenous iron administration. In this study, we investigated the use of intravenous iron therapy in iron-deficient iPAH patients in terms of safety and effects on exercise capacity, and we studied whether altered exercise capacity resulted from changes in right ventricular (RV) function and skeletal muscle oxygen handling. Fifteen patients with iPAH and iron deficiency were included. Patients underwent a 6-minute walk test, cardiopulmonary exercise tests, cardiac magnetic resonance imaging, and a quadriceps muscle biopsy and completed a quality-of-life questionnaire before and 12 weeks after receiving a high dose of intravenous iron. The primary end point, 6-minute walk distance, was not significantly changed after 12 weeks (409 ± 110 m before vs. 428 ± 94 m after; P = 0.07). Secondary end points showed that intravenous iron administration was well tolerated and increased body iron stores in all patients. In addition, exercise endurance time (P < 0.001) and aerobic capacity (P < 0.001) increased significantly after iron therapy. This coincided with improved oxygen handling in quadriceps muscle cells, although cardiac function at rest and maximal  were unchanged. Furthermore, iron treatment was associated with improved quality of life (P < 0.05). In conclusion, intravenous iron therapy in iron-deficient iPAH patients improves exercise endurance capacity. This could not be explained by improved RV function; however, increased quadriceps muscle oxygen handling may play a role. (Trial registration: ClinicalTrials.gov identifier NCT01288651)
were unchanged. Furthermore, iron treatment was associated with improved quality of life (P < 0.05). In conclusion, intravenous iron therapy in iron-deficient iPAH patients improves exercise endurance capacity. This could not be explained by improved RV function; however, increased quadriceps muscle oxygen handling may play a role. (Trial registration: ClinicalTrials.gov identifier NCT01288651)
Keywords: exercise capacity, iron, skeletal muscle, right ventricle
Iron deficiency is common in patients with idiopathic pulmonary arterial hypertension (iPAH) and is closely associated with poor survival and low exercise capacity.1-3 Compromised oxygen handling—that is, oxygen transport and consumption at a skeletal muscle level—has been suggested to cause the decreased exercise capacity in iron-deficient (ID) iPAH patients.2,4 Furthermore, iron deficiency might lead to deterioration of right ventricular (RV) function.5 We have shown that oral iron supplements are ineffective in restoring body iron stores in iPAH.1,2 Recently, it was demonstrated that intravenous iron treatment in ID iPAH patients was associated with higher serum iron levels, improved 6-minute walk distance (6MWD), and quality of life.6,7 However, the exact mechanisms by which iron improves clinical performance in iPAH patients remain to be elucidated.
Therefore, the aim of the present study was twofold: (1) to investigate whether intravenous iron therapy in ID iPAH was safe and could indeed restore iron levels and improve exercise capacity in another iPAH cohort and (2) to explore whether altered exercise capacity can be explained in part by improved RV function and improved skeletal muscle oxygen handling.
Methods
Patient inclusion
Patients who visited the Pulmonology Department of the Vrije Universiteit University Medical Center between January 2011 and January 2013 were approached to participate. Inclusion criteria were iPAH as defined by the European guidelines,8 receipt of optimal PAH-specific treatment, being clinically stable for at least 3 months, and presence of iron deficiency (serum iron level of <10 μmol/L, transferrin saturation of <15% [women] or <20% [men], and serum ferritin level of <100 μg/L). Patients were excluded if they received iron therapy at admission, participated in another pulmonary hypertension study medication trial, or had a history of anemia, liver function impairment, or any other acute or chronic condition other than iPAH. The study was registered at ClinicalTrials.gov (NCT01288651) and was approved by the Institutional Review Board on Research Involving Human Subjects (Amsterdam, Netherlands). All patients provided written informed consent before inclusion.
Clinical study design
All end points were measured before (baseline) and 12 weeks after intravenous iron therapy. The primary end point was change in 6MWD after iron therapy. Secondary end points were change in blood iron parameters, change in maximal exercise parameters and endurance capacity determined by maximal and submaximal cardiopulmonary exercise tests (CPETs), RV function determined by cardiac magnetic resonance imaging (MRI), pulmonary function determined by means of spirometry and diffusion capacity measurements, quality of life determined by the SF-36 questionnaire, and skeletal muscle oxygen handling at the cellular level determined by quadriceps muscle biopsy. A detailed description of all tests and methods is provided in the appendix. After baseline measurements, an intravenous infusion of ferric carboxymaltose (Ferinject; Vifor Pharma, Glattbrugg, Switzerland) at 1,000 mg was given, in 20 mL of NaCl 0.9% over 2 hours. The study was not placebo controlled, since no permission for a placebo group was given by the medical ethics committee. At the end point of the study, 12 weeks after first admission, all measurements were repeated in the same order.
Statistical analysis
All data from patients before and after iron administration were analyzed using the paired Student t test or repeated-measures analysis of variance with Bonferroni post hoc tests to correct for multiple measurements. All analyses were performed with GraphPad Prism (ver. 5.00; GraphPad Software, San Diego, CA) and SPSS (ver. 20.0; SPSS, Chicago, IL). Log transformation was performed to obtain a normal distribution of the data when necessary. Data are presented as mean ± standard deviation, unless stated otherwise. Differences with a P value of <0.05 were considered statistically significant.
Results
Tolerability and effects on blood iron levels
Eighteen iPAH patients were included in this study, of whom three did not finish the protocol. One patient presented with atrial flutter during second admission, 1 patient withdrew from the study before finishing the protocol, and 1 patient was excluded because erythropoietin was administered to that patient in another hospital during the follow-up period. Baseline characteristics of the 15 patients who were analyzed are shown in Table 1. All patients received the total amount (1,000 mg) of intravenous iron after the baseline measurements were obtained. Two patients had minor complaints of frontal headache during the infusion, which resolved shortly thereafter. No other adverse events were noted. Serum iron parameters were significantly increased 12 weeks after iron administration, without a change in N-terminal prohormone of brain natriuretic peptide levels. Serum hepcidin values were low at baseline and remained low until the end of the study (Table 2).
Table 1.
Baseline patient characteristics
| Value | |
|---|---|
| General characteristics | |
| Sex, no. | |
| Male | 1 |
| Female | 14 |
| Age, years | 57 ± 13 |
| NYHA functional class, no. | |
| II | 9 |
| III | 6 |
| Body mass index | 30.1 ± 5.7 |
| Pulmonary hypertension treatment | |
| Single ERA, no. | 4 |
| Single prostanoids, no. | 4 |
| Combination ERA/PDE5 inhibitor, no. | 2 |
| Combination ERA/prostanoid, no. | 1 |
| Triple therapy ERA/PDE5/prostanoid, no. | 4 |
| Treatment duration, months | 70 ± 42 |
| Hemodynamic parameters at diagnosis | |
| Mean pulmonary arterial pressure, mmHg | 46 ± 15 |
| Mean right atrial pressure, mmHg | 7 ± 4 |
| Pulmonary vascular resistance, dyn-s/cm5 | 672 ± 394 |
| Cardiac output, L/min | 5.7 ± 2.7 |
| Cardiac index, L/min/m2 | 2.9 ± 1.3 |
| Arterial saturation, % | 94 ± 4 |
| Mixed venous saturation, % | 69 ± 10 |
Except where otherwise noted, data are mean ± standard deviation. Hemodynamic parameters were obtained from right heart catheterization performed during the pulmonary hypertension diagnosis period. NYHA: New York Heart Association; ERA: endothelin receptor antagonist; PDE5: phosphodiesterase 5.
Table 2.
Laboratory parameters in idiopathic pulmonary arterial hypertension patients at baseline and end point
| Before iron | After iron | P | |
|---|---|---|---|
| C-reactive protein, mg/L | 7 ± 8 | 5 ± 4 | NS |
| Hemoglobin, g/dL | 13.4 ± 1.7 | 15.1 ± 1.5 | NS |
| Hematocrit, L/L | 0.40 ± 0.05 | 0.45 ± 0.06 | NS |
| Erythrocytes, ×1012/L | 4.8 ± 0.6 | 5.2 ± 0.7 | NS |
| Mean corpuscular volume, fL | 82 ± 4 | 88 ± 3 | NS |
| Creatinine, μmol/L | 74.3 ± 15.7 | 71.7 ± 15.3 | NS |
| NT-proBNP,a ng/L | 1,339 ± 2,545 | 1,753 ± 4,559 | NS |
| Serum iron, μmol/L | 9.6 ± 4.8 | 16.1 ± 6.1 | <0.05 |
| Total iron binding capacity, μmol/L | 73 ± 10 | 61 ± 9 | <0.001 |
| Transferrin saturation, % | 13.6 ± 6.7 | 27.3 ± 13.4 | <0.001 |
| Serum ferritin,a μg/L | 44 ± 79 | 199 ± 225 | <0.05 |
| Soluble transferrin receptor,a nmol/L | 36 ± 15 | 25 ± 7 | NS |
| Interleukin 6, pg/mL | 3.2 ± 0.5 | 3.2 ± 0.3 | NS |
| Hepcidin,a ng/mL | 4.5 ± 4.5 | 6.6 ± 4.4 | NS |
Data are mean ± standard deviation and were analyzed using repeated-measures analysis of variance with Bonferroni post hoc tests to correct for multiple measurements. NS: not significant; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
Data were log transformed to obtain a normal distribution.
Iron improved submaximal exercise capacity
Intravenous iron therapy did not significantly change 6MWD after 12 weeks (409 ± 110 m before vs. 428 ± 94 m after; P = 0.07; Fig. 1A). Maximal workload (55 ± 23 W before vs. 59 ± 27 W after; not significant [NS]) and peak oxygen uptake (0.97 ± 0.22 L/min before vs. 0.97 ± 0.26 L/min after; NS), determined by maximal CPET, were also unchanged by iron therapy (Table S1). However, the time to reach anaerobic threshold was significantly increased after iron therapy (175 ± 33 seconds before vs. 238 ± 43 seconds after; P < 0.001; Fig. 1B; Table S1). Exercise endurance capacity was markedly improved, as iPAH patients were able to exercise 51% longer after iron therapy compared with baseline (269 ± 89 seconds before vs. 405 ± 210 seconds after; P < 0.001; Fig. 1C; Table S2). Spirometry parameters were unchanged after iron therapy (Table S3).
Figure 1.
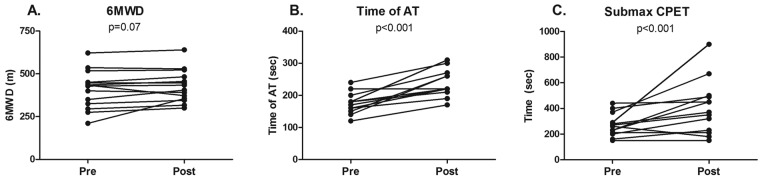
Exercise capacity in iron-deficient idiopathic pulmonary arterial hypertension patients before and after iron administration. Six-minute walk distance (6MWD) was unchanged after iron therapy (A). Time to anaerobic threshold (AT) during maximal cardiopulmonary exercise testing (CPET) was significantly postponed after iron therapy (B). At a submaximal level (75% of maximum achieved workload during CPET), patients were able to bike 51% longer after iron therapy (C).
Iron administration improved quality of life
Quality of life was improved after iron treatment, as indicated by a higher total score in the SF-36 questionnaire (47% ± 19% before vs. 56% ± 19% after; P < 0.05). Subdivision of the different components of the questionnaire showed that mental health was significantly better after iron treatment (49% ± 10% before vs. 60% ± 17% after; P < 0.01), whereas physical health was unaltered (39% ± 20% before vs. 46% ± 20% after; P = 0.09).
Improved exercise capacity could not be explained by improved RV function
Three patients could not undergo cardiac MRI because of obesity or claustrophobia; therefore, cardiac MRI was performed in a subset of 12 patients. Cardiac function at rest, represented by cardiac index (2.8 ± 0.9 L/min/m2 before vs. 2.5 ± 0.8 L/min/m2 after; NS) and ventricular ejection fraction (left ventricle [LV]: 62% ± 12% before vs. 59% ± 14% after; RV: 40% ± 21% before vs. 39% ± 21% after; both NS), was unchanged by iron therapy. In addition, LV and RV free wall mass index remained stable (LV: 59 ± 15 g/m2 before vs. 62 ± 17 g/m2 after; RV: 51 ± 29 g/m2 before vs. 56 ± 31 g/m2 after; both NS; Table S4).
Skeletal muscle oxygen transport was enhanced after intravenous iron therapy
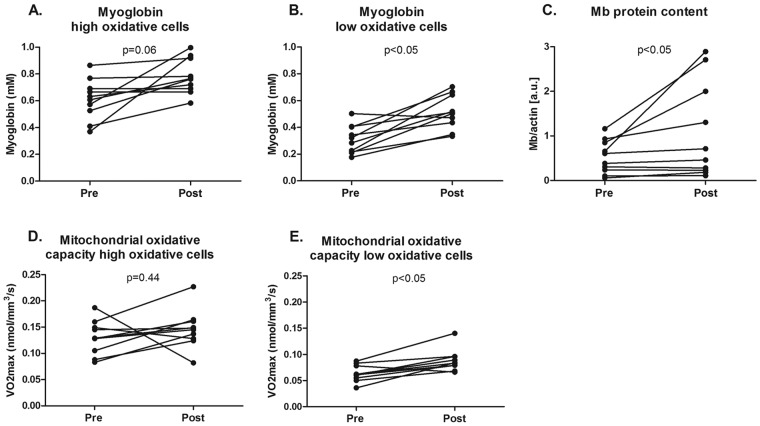
Twelve patients consented to a quadriceps muscle biopsy. In 2 patients, the biopsy procedure did not result in sufficiently high-quality material. Biopsy analysis was therefore performed in a subset of 10 patients. Myoglobin concentration (0.34 ± 0.17 mM before vs. 0.44 ± 0.11 mM after; P < 0.05) and mitochondrial oxidative capacity (0.06 ± 0.01 nmol/mm3/s before vs. 0.09 ± 0.02 nmol/mm3/s after; P < 0.05) were both significantly increased in low oxidative cells after iron therapy, as was total myoglobin protein content (0.5 ± 0.4 AU before vs. 1.1 ± 0.6 AU after; P < 0.05; Figs. 2, 3). Furthermore, the number of capillaries per myocyte in the quadriceps muscle (1.0 ± 0.4 cap/myocyte before vs. 1.2 ± 0.2 cap/myocyte after; P = 0.37) was similar after iron therapy (Figs. 2, 3).
Figure 2.
Oxygen handling in quadriceps muscle fibers after iron therapy in iron-deficient idiopathic pulmonary arterial hypertension patients. Myoglobin concentration was unaltered in high oxidative cells after iron therapy (A); however, a significant increase was observed in low oxidative cells (B) and total myoglobin protein content (C). Mitochondrial oxidative capacity, expressed as maximal oxygen consumption (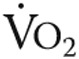 ) in high oxidative cells, was unchanged by iron therapy (D). In low oxidative cells,
) in high oxidative cells, was unchanged by iron therapy (D). In low oxidative cells, 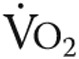 was higher after iron therapy (E).
was higher after iron therapy (E).
Figure 3.
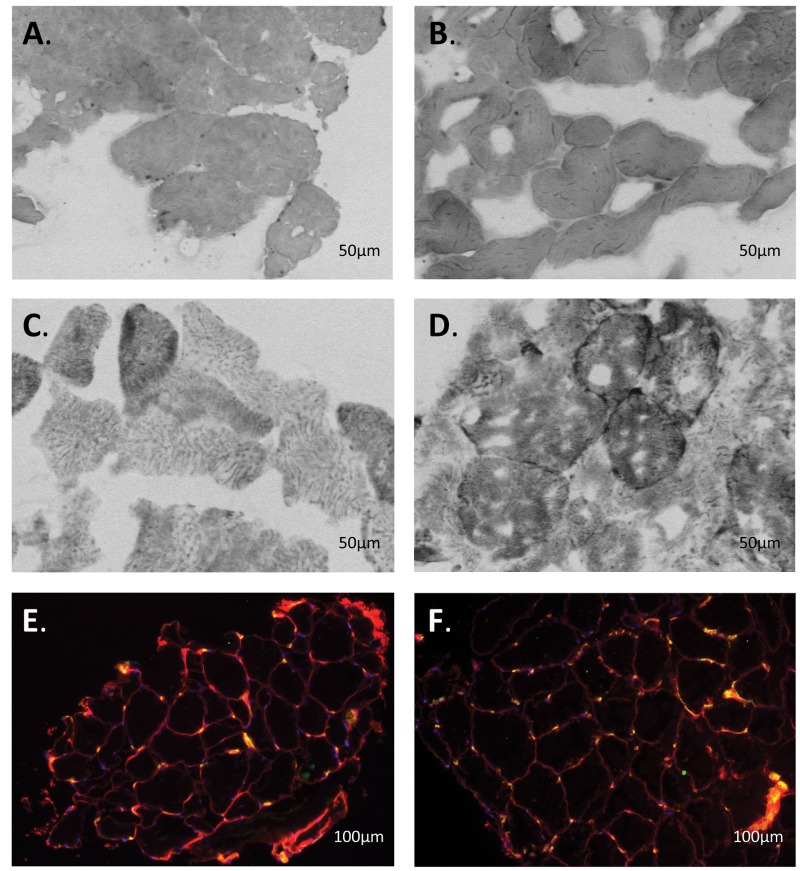
Quadriceps biopsy samples from idiopathic pulmonary arterial hypertension (iPAH) patients. Shown are typical examples of quadriceps biopsy samples from iPAH patients before (A, C, E) and after (B, D, F) intravenous iron therapy. A, B, Myoglobin staining. C, D, Succinate dehydrogenase activity staining. Type I and II cells are distinguished by color, as type I cells have more myoglobin and succinate dehydrogenase activity (darker cells) than type II cells. E, F, CD31 staining. Every yellow dot represents a capillary.
Discussion
In the present study, we investigated the effects of iron therapy in ID iPAH patients. We showed that intravenous iron therapy in ID iPAH patients increased exercise endurance capacity. This could not be explained by better RV function; however, skeletal muscle biopsies of these patients revealed improvements in oxygen handling capacity after iron treatment.
Intravenous iron safely increased body iron stores and quality of life
Patients received a single fixed high dose of intravenous iron. An alternative approach would have been to supply multiple smaller doses using the Ganzoni formula to calculate the total amount of iron needed to restore body iron.5,9-11 One reason for using the present approach was that ferric carboxymaltose (Ferinject) is safe to administer in high iron dosages (up to 1,000 mg at one time) and allowed a single visit for every patient.12 Second, use of the Ganzoni formula may not be reliable in iPAH patients with hypoxemia-driven erythropoiesis and consequent hemoglobin (Hb) levels within the normal range despite iron deficiency.11,13 Third, previous studies with iron therapy showed that the mean dose of iron needed was around 1,000 mg or even higher.10 One of the concerns was that patients might increase their Hb levels above reference values, which could be detrimental since this increases blood viscosity. However, although we observed a slight increase in Hb after iron treatment, it was not above the upper limit of normal. In addition, in contrast to oral iron treatment, which does not produce a response in 44% of ID iPAH patients, ferric carboxymaltose led to higher iron stores in all patients, as represented by increased ferritin levels.2
Furthermore, intravenous iron therapy was accompanied by improved quality of life, as shown by a mean increase of 9 points on a 100-point scale. Better quality of life after iron therapy has been previously described in ID patients with left heart failure as well as those with pulmonary hypertension.5,6 Whether the observed improvement is a result of better exercise capacity or an independent result of iron therapy, however, is difficult to determine.
Iron and exercise capacity
The achieved distance in the 6-minute walk test (6MWT) was unchanged by iron therapy. In addition, maximal CPET results were also unaltered. However, in the submaximal CPET, a significant improvement in exercise endurance time and improved aerobic capacity was demonstrated. It seems contradictory that the 6MWT, which is mostly regarded to be a submaximal exercise test, was unchanged by iron therapy. This might be explained in part by the very different type of exercise (i.e., biking vs. walking). In addition, the 6MWT has been associated with higher aerobic capacity and less metabolic stress than the CPET, which might indicate a different effect of iron therapy in both exercise tests.14 Also, any potential beneficial effect on the 6MWT is difficult to extrapolate to resting pulmonary hemodynamics.15 To observe changes in hemodynamics after iron therapy in future studies, right heart catheterization should be performed.
Iron deficiency by itself (without pulmonary hypertension) has been associated with diminished exercise capacity and fatigue in otherwise healthy subjects,16-18 although improvements in exercise capacity were observed only when anemia was also present.4,18-21 In the present study, increased Hb or higher maximal 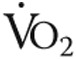 did not explain the improved exercise endurance. Although Hb levels were slightly higher after iron therapy (nonsignificant), there was no significant correlation with exercise endurance time (data not shown), which corresponds with previous findings in iPAH patients where the absence or presence of anemia did not influence 6MWD.2,5 In addition, maximal
did not explain the improved exercise endurance. Although Hb levels were slightly higher after iron therapy (nonsignificant), there was no significant correlation with exercise endurance time (data not shown), which corresponds with previous findings in iPAH patients where the absence or presence of anemia did not influence 6MWD.2,5 In addition, maximal 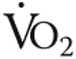 was unaltered after iron therapy and could thus not explain the better exercise endurance. Other mechanisms that may play a role in better exercise endurance are improved cardiac function, increased skeletal muscle oxygenation, and reduced pulmonary vascular resistance.14
was unaltered after iron therapy and could thus not explain the better exercise endurance. Other mechanisms that may play a role in better exercise endurance are improved cardiac function, increased skeletal muscle oxygenation, and reduced pulmonary vascular resistance.14
Intravenous iron did not alter cardiac function but enhanced skeletal muscle oxygen handling
Iron therapy did not improve RV function in iPAH patients. However, it is difficult to relate cardiac function at rest with exercise parameters. In addition, restoring iron levels at the cellular level might have beneficial effects in the long term by improving cellular oxygen handling in the RV, similar to the alterations in skeletal muscles after intravenous iron treatment.
Alterations in skeletal muscle oxygen handling may have been responsible for the improved exercise endurance capacity with iron treatment. A relationship between skeletal muscle ID and reduced exercise capacity has been suggested previously in ID rats and humans (without PH).17,19,22,23 We showed an increase in myoglobin and mitochondrial oxidative capacity in low oxidative cells after iron therapy, suggesting a higher capacity for intracellular oxygen transport.
Iron and the lungs
Besides negatively influencing oxygen transport, it has been suggested that ID also has direct effects on the lungs. Under hypoxic conditions and at high altitude, ID resulted in an increase in pulmonary arterial pressure in otherwise healthy subjects.24,25 This has been mainly attributed to stabilization and transcription of hypoxia-inducible factors linked to contraction, proliferation, and migration of pulmonary artery smooth muscle cells.25-27 Another proposed concept is that (ID-induced) anemia may limit the conversion of nitrite to nitric oxide, thereby inhibiting the antiproliferative and vasodilatation effects of nitric oxide on the pulmonary vasculature.28-30 Because the present study did not include right heart catheterization, we can only hypothesize about the alterations in the pulmonary circulation in ID iPAH patients. However, Howard et al.31 are currently performing a similar clinical study in the United Kingdom in which right heart catheterization is performed, and this study may provide more mechanistic insights into pulmonary vascular changes.
Limitations
The two main limitations of the present study were that we did not include a placebo group and right heart catheterization was not performed. However, a larger placebo-controlled trial that includes this measurement is currently pending in the United Kingdom.31
Conclusions
Intravenous iron therapy in ID iPAH patients increased iron stores and was well tolerated without significant adverse events. 6MWD was unaltered by iron therapy, but endurance capacity improved significantly after iron therapy. This could not be explained by altered RV function at rest; however, increased skeletal muscle oxygen handling at the cellular level might be a cause.
Acknowledgments
We thank Frank Oosterveer, Iris van der Mark, Martha Wagenaar, Colette ter Heerdt, Pia Trip, Sylvia Bogaards, John Wharton, Patrick Jak, Jerica Sinkeldam, Rita Visser, Tim Marcus, and Lara Konijnenberg for their help in patient inclusion and clinical patient care.
Appendix. Additional descriptions of methods and tables
Exercise tests
To measure the effects of intravenous iron therapy on the primary end point, 6MWD, a 6MWT was performed as described elsewhere.32 Briefly, patients walked as far as possible for 6 minutes without running or jogging along a long, flat, straight, indoor corridor with a hard surface. Secondary exercise end points of a maximal and a submaximal constant load exercise test were performed according to European Respiratory Society guidelines.33 Briefly, the protocol of the maximal CPET consisted of 3 minutes of seated rest, 3 minutes of unloaded cycling, and subsequent minute-by-minute increases in work load until exhaustion followed by a 3-minute recovery period. The maximal CPET was performed after a resting period of at least 3 hours after performing the 6MWD. Minute ventilation (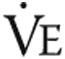 ), tidal volume (
), tidal volume (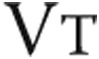 ), breathing frequency, oxygen consumption (
), breathing frequency, oxygen consumption (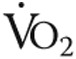 ), and carbon dioxide output (
), and carbon dioxide output (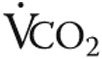 ) were measured. Ventilatory equivalents of O2 and CO2 and respiratory quotients were calculated, and the anaerobic threshold was determined using the
) were measured. Ventilatory equivalents of O2 and CO2 and respiratory quotients were calculated, and the anaerobic threshold was determined using the 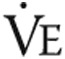 /
/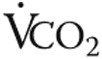 slope method. On the second day, exercise endurance was determined during a protocol of 3 minutes of seated rest and 3 minutes of unloaded cycling followed by a constant load of 75% of the previous estimated maximal workload determined during the maximal CPET. Patients cycled for as long as possible. The test was stopped when patients indicated exhaustion, when they were no longer able to pedal at a rate of 60 per minute, or when the test exceeded 15 minutes. The same parameters were measured as during the maximal CPET, as well as total exercise time. Pulmonary function measurements consisted of spirometry and single-breath diffusion capacity.34,35
slope method. On the second day, exercise endurance was determined during a protocol of 3 minutes of seated rest and 3 minutes of unloaded cycling followed by a constant load of 75% of the previous estimated maximal workload determined during the maximal CPET. Patients cycled for as long as possible. The test was stopped when patients indicated exhaustion, when they were no longer able to pedal at a rate of 60 per minute, or when the test exceeded 15 minutes. The same parameters were measured as during the maximal CPET, as well as total exercise time. Pulmonary function measurements consisted of spirometry and single-breath diffusion capacity.34,35
Cardiac MRI
To measure the effects on cardiac function, a cardiac MRI was performed at baseline and end point. A 1.5-T Avanto scanner (Siemens Medical Solutions, Erlangen, Germany) was used. A stack of short-axis images was obtained covering the ventricles from base to apex. All technical details were similar to those described elsewhere.36 During postprocessing, the cardiovascular MRIs were assessed using the MASS software package (Medis, Medical Imaging Systems, Leiden, Netherlands). On end-diastolic and end-systolic images, endocardial and epicardial contours of the ventricles were obtained by manual tracing. Papillary muscles and trabeculae were excluded from the blood volume and included in ventricular masses. Masses and volumes were indexed to body surface area.
Blood iron parameters
Whether intravenous iron therapy could increase body iron stores was assessed by measuring Hb and mean corpuscular volume (MCV) using spectrophotometry (Cell-Dyn Sapphire; Abbott, Hoofddorp, Netherlands) and hematocrit (Ht) as calculated from the product of MCV and erythrocyte number.2 Serum iron and total iron-binding capacity (TIBC) were determined using photometry (Modular P800 system; Roche, Almere, Netherlands), and transferrin saturation was calculated as serum iron divided by TIBC. Sandwich immunoassays with electrochemical luminescence technology were used to measure serum ferritin levels (Modular E170 system; Roche). Circulating soluble transferrin receptor and interleukin 6 were measured using an enzyme-linked immunoadsorbent assay (R&D Systems Europe, Abingdon, United Kingdom) in EDTA samples. Hepcidin concentrations were determined using a competitive radioimmunoassay.37
Quadriceps muscle biopsy
To measure the effects of iron therapy on cellular oxygen handling in skeletal muscle, two biopsy samples from the vastus lateralis part of the quadriceps muscle were obtained under local anaesthesia with 2% lidocaine solution using the microbiopsy technique with a 16-G spring-loaded biopsy needle (QC-16-15.0-10T; Cook Medical, Limerick, Ireland), as described elsewhere.38-40 Biopsy samples were directly evaluated under a light microscope, snap-frozen in liquid nitrogen, and stored at −80°C for histochemical analysis (as described below).
Cellular analysis of iPAH quadriceps muscle biopsy samples
Cryosections of 5 μm thick were cut from the biopsy samples. Functional myoglobin concentration was calculated from myoglobin peroxidase activity, and succinate dehydrogenase (SDH) activity was measured as described elsewhere.41-43 To measure capillarization, the number of capillaries per myocyte was counted.38 Immunofluorescence image acquisition was performed on a Marianas digital imaging microscopy workstation, and semiautomatic analysis was done with SlideBook 5.1 imaging analysis software (Intelligent Imaging Innovations, Denver, CO).
Myoglobin protein content in quadriceps muscle was determined by gel electrophoresis (4%–15% acrylamide gels) and Western blotting protein analysis in homogenized tissue. Primary monoclonal mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and secondary goat anti–mouse antibody (Dako, Glostrup, Denmark) were used to demonstrate myoglobin. All signals were normalized to actin on the Ponceau staining. Quantification was performed using AIDA software (ver. 4.21.033; Raytest, Straubenhardt, Germany).38
Table S1.
Maximal cardiopulmonary exercise test parameters at baseline and end point in idiopathic pulmonary arterial hypertension patients
| Before iron | After iron | P | |
|---|---|---|---|
| Work, W | 55 ± 23 | 59 ± 27 | NS |
| % predicted | 55 ± 24 | 61 ± 26 | NS |
Maximal 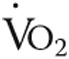 , L/min , L/min |
0.97 ± 0.22 | 0.97 ± 0.26 | NS |
| % predicted | 75 ± 32 | 80 ± 32 | NS |
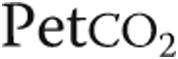 , kPa , kPa |
3.3 ± 0.7 | 3.4 ± 0.8 | NS |
| Heart rate, bpm | 125 ± 22 | 127 ± 26 | NS |
| % predicted | 78 ± 11 | 80 ± 13 | NS |
| O2 pulse, mL/beat | 7.6 ± 0.9 | 7.7 ± 1.2 | NS |
| % predicted | 96 ± 43 | 102 ± 43 | NS |
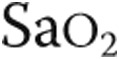 pulse, % pulse, % |
88 ± 9 | 87 ± 8 | NS |
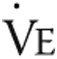 , L/min , L/min |
48.7 ± 13.1 | 49.6 ± 16.7 | NS |
| % predicted | 57 ± 9 | 59 ± 12 | NS |
| RR, breaths/min | 36 ± 7 | 37 ± 8 | NS |
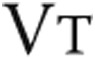 , L , L |
1.39 ± 0.34 | 1.36 ± 0.37 | NS |
| % predicted | 99 ± 21 | 102 ± 20 | NS |
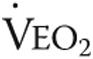 |
51 ± 12 | 52 ± 16 | NS |
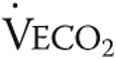 |
46 ± 11 | 46 ± 12 | NS |
| Time to reach AT, seconds | 175 ± 33 | 238 ± 43 | <0.001 |
Data are mean ± standard deviation. Data are from 14 patients because 1 patient was not able to bike and were analyzed using repeated-measures analysis of variance with Bonferroni post hoc tests to correct for multiple measurements. NS: not significant; 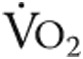 : oxygen uptake;
: oxygen uptake; 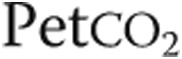 : partial pressure of end-tidal carbon dioxide;
: partial pressure of end-tidal carbon dioxide; 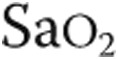 : oxygen saturation of arterial blood;
: oxygen saturation of arterial blood; 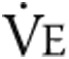 : minute ventilation; RR: respiration rate;
: minute ventilation; RR: respiration rate; 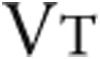 : tidal volume;
: tidal volume; 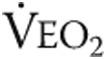 : ventilatory equivalent for oxygen;
: ventilatory equivalent for oxygen; 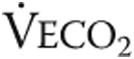 : ventilatory equivalent for carbon dioxide; AT: anaerobic threshold.
: ventilatory equivalent for carbon dioxide; AT: anaerobic threshold.
Table S2.
Submaximal cardiopulmonary exercise test (CPET) parameters at baseline and end point in idiopathic pulmonary arterial hypertension patients
| Before iron | After iron | P | |
|---|---|---|---|
| Time, seconds | 269 ± 89 | 405 ± 210 | <0.001 |
Maximal 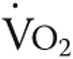 , L/min , L/min |
0.94 ± 0.19 | 0.94 ± 0.24 | NS |
| % predicted | 76 ± 34 | 77 ± 38 | NS |
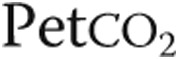 , kPa , kPa |
3.2 ± 0.6 | 3.3 ± 0.7 | NS |
| Heart rate, bpm | 120 ± 21 | 120 ± 22 | NS |
| % predicted | 76 ± 11 | 76 ± 13 | NS |
| O2 pulse, mL/beat | 7.9 ± 1.4 | 7.8 ± 1.6 | NS |
| % predicted | 101 ± 44 | 101 ± 46 | NS |
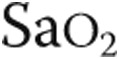 pulse, % pulse, % |
86 ± 9 | 87 ± 8 | NS |
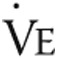 , L/min , L/min |
49.4 ± 12.6 | 46.2 ± 14.2 | NS |
| % predicted | 56 ± 15 | 56 ± 12 | NS |
| RR, breaths/min | 39 ± 10 | 35 ± 8 | NS |
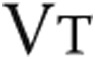 , L , L |
1.37 ± 0.33 | 1.35 ± 0.32 | NS |
| % predicted | 109 ± 29 | 109 ± 23 | NS |
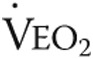 |
54 ± 14 | 51 ± 17 | NS |
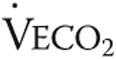 |
49 ± 10 | 47 ± 13 | NS |
Data are mean ± standard deviation. Data are from 14 patients because 1 patient was not able to bike and were analyzed using repeated-measures analysis of variance with Bonferroni post hoc tests to correct for multiple measurements. Workload was 75% of maximal achieved load during maximal CPET. NS: not significant; 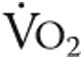 : oxygen uptake;
: oxygen uptake; 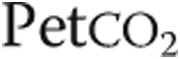 : partial pressure of end-tidal carbon dioxide;
: partial pressure of end-tidal carbon dioxide; 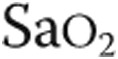 : oxygen saturation of arterial blood;
: oxygen saturation of arterial blood; 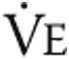 : minute ventilation; RR: respiration rate;
: minute ventilation; RR: respiration rate; 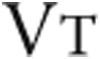 : tidal volume;
: tidal volume; 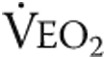 : ventilatory equivalent for oxygen;
: ventilatory equivalent for oxygen; 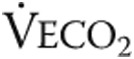 : ventilatory equivalent for carbon dioxide.
: ventilatory equivalent for carbon dioxide.
Table S3.
Spirometry and diffusion parameters of idiopathic pulmonary arterial hypertension patients at baseline and end point
| Before iron | After iron | P | |
|---|---|---|---|
| FEV1, L | 2.1 ± 0.4 | 2.0 ± 0.4 | NS |
| % predicted | 85 ± 21 | 83 ± 18 | NS |
| FEV1/VC, % | 68 ± 9 | 63 ± 19 | NS |
| % predicted | 86 ± 112 | 88 ± 15 | NS |
| VC, L | 3.1 ± 0.6 | 3.1 ± 0.7 | NS |
| % predicted | 104 ± 20 | 105 ± 21 | NS |
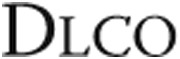 , mmol/min/kPa , mmol/min/kPa |
4.8 ± 1.8 | 4.6 ± 1.9 | NS |
| % predicted | 59 ± 18 | 58 ± 20 | NS |
Data are mean ± standard deviation and were analyzed using repeated-measures analysis of variance with Bonferroni post hoc tests to correct for multiple measurements. NS: not significant; FEV1: forced expiratory volume in 1 second; FEV1/VC: Tiffeneau index, or ratio of FEV1 to VC; VC: vital capacity; 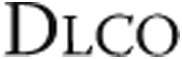 : diffusion capacity.
: diffusion capacity.
Table S4.
Cardiac function in idiopathic pulmonary arterial hypertension patients determined by cardiac magnetic resonance imaging
| Before iron | After iron | P | |
|---|---|---|---|
| LV ejection fraction, % | 62 ± 12 | 59 ± 4 | NS |
| RV ejection fraction, % | 40 ± 21 | 39 ± 21 | NS |
| LV EDV index, mL/m2 | 58 ± 21 | 53 ± 17 | NS |
| RV EDV index, mL/m2 | 104 ± 61 | 98 ± 56 | NS |
| SV index, mL/m2 | 35 ± 15 | 32 ± 12 | NS |
| Heart rate, bpm | 85 ± 18 | 81 ± 15 | NS |
| Cardiac index, L/min/m2 | 2.8 ± 0.9 | 2.5 ± 0.8 | NS |
| LV free wall mass index, g/m2 | 59 ± 15 | 62 ± 17 | NS |
| RV free wall mass index, g/m2 | 51 ± 29 | 56 ± 31 | NS |
Data are mean ± standard deviation and were analyzed using repeated-measures analysis of variance with Bonferroni post hoc tests to correct for multiple measurements. All parameters are corrected for body surface area of the patients. LV: left ventricular; NS: not significant; RV: right ventricular; SV: stroke volume; EDV: end-diastolic volume.
References Cited Only in the Appendix
- 32.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed]
- 33.ERS Task Force on Standardization of Clinical Exercise Testing. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. Eur Respir J 1997;10:2662–2689. [DOI] [PubMed]
- 34.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed]
- 35.Cotes JE, Chinn DJ, Quanjer PH, et al. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:41–52. [PubMed]
- 36.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011;58:2511–2519. [DOI] [PubMed]
- 37.Busbridge M, Griffiths C, Ashby D, et al. Development of a novel immunoassay for the iron regulatory peptide hepcidin. Br J Biomed Sci 2009;66:150–157. [DOI] [PubMed]
- 38.de Man FS, Handoko ML, Groepenhoff H, et al. Effects of exercise training in patients with idiopathic pulmonary arterial hypertension. Eur Respir J 2009;34:669–675. [DOI] [PubMed]
- 39.Bekedam MA, van Beek-Harmsen BJ, Boonstra A, et al. Maximum rate of oxygen consumption related to succinate dehydrogenase activity in skeletal muscle fibres of chronic heart failure patients and controls. Clin Physiol Funct Imaging 2003;23:337–343. [DOI] [PubMed]
- 40.Hayot M, Michaud A, Koechlin C, et al. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J 2005;25:431–440. [DOI] [PubMed]
- 41.Lee-de Groot MB, Tombe AL, van der Laarse WJ. Calibrated histochemistry of myoglobin concentration in cardiomyocytes. J Histochem Cytochem 1998;46:1077–1084. [DOI] [PubMed]
- 42.van Beek-Harmsen BJ, Bekedam MA, Feenstra HM, et al. Determination of myoglobin concentration and oxidative capacity in cryostat sections of human and rat skeletal muscle fibres and rat cardiomyocytes. Histochem Cell Biol 2004;121:335–342. [DOI] [PubMed]
- 43.des Tombe AL, van Beek-Harmsen BJ, Lee-de Groot MB, et al. Calibrated histochemistry applied to oxygen supply and demand in hypertrophied rat myocardium. Microsc Res Tech 2002;58:412–420. [DOI] [PubMed]
Source of Support: Ferinject was kindly provided by Vifor Pharma. The rat study was funded by Nutricia Advanced Medical Nutrition. GR and AV-N were funded by the Netherlands Organization for Scientific Research (NWO; VIDI grant 917.96.306). MRW and LSH were funded by the British Heart Foundation (RG/10/16/28575) and the National Institute for Health Research.
Conflict of Interest: None declared.
Supplements
AppendixPulmCirc-005-466.s001.pdf (428.4KB, pdf)
References
- 1.Rhodes CJ, Howard LS, Busbridge M, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol 2011;58:300–309. [DOI] [PubMed]
- 2.Ruiter G, Lankhorst S, Boonstra A, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J 2011;37:1386–1391. [DOI] [PubMed]
- 3.Soon E, Treacy CM, Toshner MR, et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax 2011;66:326–332. [DOI] [PubMed]
- 4.Brownlie T, Utermohlen V, Hinton PS, et al. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr 2004;79:437–443. [DOI] [PubMed]
- 5.Anker SD, Comin CJ, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361:2436–2448. [DOI] [PubMed]
- 6.Viethen T, Gerhardt F, Dumitrescu D, et al. Ferric carboxymaltose improves exercise capacity and quality of life in patients with pulmonary arterial hypertension and iron deficiency: a pilot study. Int J Cardiol 2014;175:233–239. [DOI] [PubMed]
- 7.van Empel VP, Lee J, Williams TJ, et al. Iron deficiency in patients with idiopathic pulmonary arterial hypertension. Heart Lung Circ 2014;23:287–292. [DOI] [PubMed]
- 8.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219–1263. [DOI] [PubMed]
- 9.Bolger AP, Bartlett FR, Penston HS, et al. Intravenous iron alone for the treatment of anemia in patients with chronic heart failure. J Am Coll Cardiol 2006;48:1225–1227. [DOI] [PubMed]
- 10.Okonko DO, Grzeslo A, Witkowski T, et al. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC-HF: a randomized, controlled, observer-blinded trial. J Am Coll Cardiol 2008;51:103–112. [DOI] [PubMed]
- 11.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities [in German]. Schweiz Med Wochenschr 1970;100:301–303. [PubMed]
- 12.Moore RA, Gaskell H, Rose P, et al. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord 2011;11:14. [DOI] [PMC free article] [PubMed]
- 13.Rhodes CJ, Wharton J, Howard L, et al. Iron deficiency in pulmonary arterial hypertension: a potential therapeutic target. Eur Respir J 2011;38:1453–1460. [DOI] [PubMed]
- 14.Deboeck G, Niset G, Vachiéry JL, et al. Physiological response to the six-minute walk test in pulmonary arterial hypertension. Eur Respir J 2005;26:667–672. [DOI] [PubMed]
- 15.Provencher S, Hervé P, Sitbon O, et al. Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. Eur Respir J 2008;32:393–398. [DOI] [PubMed]
- 16.Davies KJ, Maguire JJ, Brooks GA, et al. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol 1982;242:E418–E427. [DOI] [PubMed]
- 17.Brutsaert TD, Hernandez-Cordero S, Rivera J, et al. Iron supplementation improves progressive fatigue resistance during dynamic knee extensor exercise in iron-depleted, nonanemic women. Am J Clin Nutr 2003;77:441–448. [DOI] [PubMed]
- 18.Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr 2001;131:676S–688S. [DOI] [PubMed]
- 19.Hinton PS, Giordano C, Brownlie T, et al. Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J Appl Physiol 2000;88:1103–1111. [DOI] [PubMed]
- 20.Celsing F, Blomstrand E, Werner B, et al. Effects of iron deficiency on endurance and muscle enzyme activity in man. Med Sci Sports Exerc 1986;18:156–161. [PubMed]
- 21.Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail 2012;14:423–429. [DOI] [PubMed]
- 22.Finch CA, Miller LR, Inamdar AR, et al. Iron deficiency in the rat: physiological and biochemical studies of muscle dysfunction. J Clin Invest 1976;58:447–453. [DOI] [PMC free article] [PubMed]
- 23.Willis WT, Brooks GA, Henderson SA, et al. Effects of iron deficiency and training on mitochondrial enzymes in skeletal muscle. J Appl Physiol 1987;62:2442–2446. [DOI] [PubMed]
- 24.Smith TG, Balanos GM, Croft QP, et al. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol 2008;586:5999–6005. [DOI] [PMC free article] [PubMed]
- 25.Smith TG, Talbot NP, Privat C, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA 2009;302:1444–1450. [DOI] [PubMed]
- 26.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ 2008;15:621–627. [DOI] [PubMed]
- 27.Shimoda LA. 55th Bowditch Lecture: effects of chronic hypoxia on the pulmonary circulation: role of HIF-1. J Appl Physiol 2012;113:1343–1352. [DOI] [PMC free article] [PubMed]
- 28.Kim-Shapiro DB, Gladwin MT, Patel RP, et al. The reaction between nitrite and hemoglobin: the role of nitrite in hemoglobin-mediated hypoxic vasodilation. J Inorg Biochem 2005;99:237–246. [DOI] [PubMed]
- 29.Crawford JH, Isbell TS, Huang Z, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 2006;107:566–574. [DOI] [PMC free article] [PubMed]
- 30.Krasuski RA, Hart SA, Smith B, et al. Association of anemia and long-term survival in patients with pulmonary hypertension. Int J Cardiol 2011;150:291–295. [DOI] [PubMed]
- 31.Howard LS, Watson GM, Wharton J, et al. Supplementation of iron in pulmonary hypertension: rationale and design of a phase II clinical trial in idiopathic pulmonary arterial hypertension. Pulm Circ 2013;3:100–107. [DOI] [PMC free article] [PubMed]