Abstract Abstract
Pulmonary arterial hypertension (PAH) is a noninfectious complication of human immunodeficiency virus (HIV) infection that has gained in importance since the advent of antiretroviral therapy. HIV-associated PAH (HIV-PAH) has a higher prevalence than idiopathic PAH (IPAH), although the vascular pathology seen in HIV-PAH is virtually identical to that seen in IPAH. Initiating therapy for PAH at an early stage is associated with a better prognosis; however, because of the nonspecific symptoms associated with PAH, the diagnosis is often delayed. In addition, because of the low prevalence of HIV-PAH, routine screening for this condition has never been recommended. We hypothesize that the failure to create screening guidelines for HIV-PAH has resulted in underdiagnosis of the condition. This, in turn, results in individuals with HIV-PAH remaining undetected, allowing the disease to progress to more advanced stages or even remain unrecognized until death. If this hypothesis is correct, it may provide a strong argument for HIV-PAH screening guidelines, because HIV-PAH portends a poor prognosis and creates a significant economic burden if left untreated. To address this issue, we conducted a retrospective review of the National Hospital Discharge Survey data and the multiple-cause mortality data to determine the prevalence of HIV-PAH at hospital discharge and death. Using these large data sets, we observed that the prevalence of HIV-PAH among HIV-infected individuals at hospital discharge and death was significantly lower than the reported prevalence in the literature. In addition, we found that PAH was designated as the most common cause of mortality in patients with HIV-PAH.
Keywords: HIV, pulmonary, hypertension, prevalence, discharge, death
Over a million individuals in the United States and approximately 35 million individuals worldwide live with human immunodeficiency virus (HIV) infection or AIDS.1 Deaths from noninfectious complications of HIV infection, such as pulmonary arterial hypertension (PAH), have increased since the advent of antiretroviral therapy.2 The prevalence of PAH among the HIV-infected population (0.5%) is significantly higher than the prevalence of idiopathic PAH or PAH in the general population, estimated to be 0.0015% and 0.003%–0.005%, respectively.3-5 Although controversial, the best evidence suggests that the prevalence of confirmed HIV-associated PAH (HIV-PAH) has remained unchanged even after the advent of antiretroviral therapy.3 There have been recent reports of higher prevalences of pulmonary hypertension (PH) among individuals with HIV infection than previously observed.6,7 However, the majority of these studies determined prevalence rates on the basis of echocardiographic findings without confirmation of PH or PAH by right heart catheterization (RHC), and only approximately 28% of HIV-infected patients with suspected PH by echocardiography are confirmed as having PAH by RHC.3 Recently, Hsue and colleagues reported that 35.2% of their HIV cohort were noted to have echocardiographic findings suggestive of PH.8 Although the study by Hsue et al.8 suggests that preclinical HIV-PAH may be more common than recognized, additional evaluation by RHC is necessary to confirm the presence of PAH because of the known limitations of echocardiography.
Survival rates are poorer for patients with HIV-PAH than for patients with HIV infection in the absence of PAH.9 In 2003, Nunes et al.10 reported that 3-year survival for individuals with HIV-PAH diagnosed at New York Heart Association (NYHA) functional classes I and II (84%) is greater than 3-year survival for patients who received a diagnosis of NYHA functional classes III and IV (28%). More recently, Degano et al.11 reported that the overall survival rate among patients with HIV-PAH was 72% at 3 years, but survival rate varied on the basis of NYHA class, with a 3-year survival rate of just greater than 90% for persons with NYHA class II and just less than 30% for persons with NYHA class IV. This highlights the importance of making a diagnosis and initiating therapy early. Furthermore, in the earlier stages of PAH, the vascular changes that occur may be more likely to be reversible and associated with an imbalance between vasodilators and vasoconstrictors, as opposed to later stages, in which irreversible changes in the pulmonary vasculature may have occurred.12
Despite an established link between HIV infection and PAH, very little is known about the mechanism by which HIV infection contributes to the development of PAH. There is no correlation between CD4 cell count or HIV load and the development of HIV-PAH. However, a correlation has been suggested between HIV-PAH and the duration of HIV infection. HIV-PAH is typically seen 6 or more years after individuals have been infected with HIV.4,10 Given this observation, the incidence and prevalence of HIV-PAH are anticipated to increase as persons live longer with HIV infection. Studies involving nonhuman primates have implicated the HIV protein Nef in the pathogenesis of HIV-PAH.13,14 However, the specifics of the mechanism have not yet been determined. Moreover, similar studies are needed to define the role of Nef in the pathogenesis and development of HIV-PAH in humans.
Currently, routine PAH screening is not recommended for individuals with HIV infection because of the reportedly low prevalence. The executive summary for the Fifth World Pulmonary Hypertension Symposium states that PAH screening “could be considered” for persons with HIV infection.15 However, no strong screening recommendations were made for HIV-PAH. This strategy raises questions as to how to determine the true burden of HIV-PAH if there is no systematic screening for the condition. An additional concern is the fact that, although the prevalence of HIV-PAH is low, the condition is often diagnosed at a later stage, when prognosis is poorer, because of the nonspecific symptoms associated with HIV-PAH. It may be necessary to implement better screening guidelines to increase the likelihood of diagnosing HIV-PAH at an early stage. This, in turn, could potentially improve the prognosis and decrease spending for individuals with HIV-PAH.
Current literature does not define the prevalence of HIV-PAH among discharged patients with HIV/AIDS or among decedents with HIV infection. Likewise, the prevalence of HIV-PAH in specific subgroups (such as African Americans [AAs]) is not well defined. AAs may be at increased risk for HIV-PAH, because AAs are among those populations with the fastest growing prevalence of HIV infection and do demonstrate endothelial dysfunction related to a potentially genetic difference in response to nitric oxide.16,17 In addition, AAs are disproportionately affected by HIV infection, accounting for 44% of both new and existing cases of HIV infection in the United States in 2010, even though they only accounted for 12% of the US population at that time.18
Our group is currently engaged in a study involving targeted HIV-PAH screening in a predominantly AA community to determine whether this will improve the likelihood of an earlier diagnosis as well as better define the prevalence of HIV-PAH among AAs. Before initiating targeted screening, we reviewed International Classification of Diseases, Ninth Revision, (ICD-9) code data for this population (82% AAs) to determine the baseline HIV-PAH prevalence. The baseline HIV-PAH prevalence for our study group (data not shown) was significantly lower (0.02%) than HIV-PAH prevalence previously reported in the literature (0.5%; P < 0.001). The observation that the HIV-PAH prevalence was lower for this study site than previously reported led us to question whether HIV-PAH was underreported or underdiagnosed on a larger scale in the United States. If so, this would be important, because the underdiagnosis or underreporting of HIV-PAH may result in delayed treatment, which worsens prognosis of affected persons. If we were able to demonstrate that HIV-PAH is underdiagnosed or underreported, a stronger case could be made to advocate for PAH screening guidelines and targeted screening among persons with HIV infection.
A secondary question raised by our observation pertained to the impact of data source on differences in observed prevalence. To further illustrate the potential impact of data source on observed prevalence, consider the fact that bias attributable to previously reported low prevalence of HIV-PAH may bias physicians to not consider PAH in patients with HIV infection, even when dyspnea is present. As a result, the HIV-PAH prevalences determined on the basis of community data may be lower than the prevalences noted in studies where HIV-PAH prevalence was obtained by screening cohorts. Similarly, HIV-PAH prevalence among a discharged patient population may be different than the prevalence of HIV-PAH noted at death, because unrecognized disease would remain untreated and could progress to the point at which it is unmasked at the time of death. If this premise is accurate, then prevalence at death will likely be higher than prevalence observed at hospital discharge. The significance of this question relates to the fact that, if lower prevalence is observed because of missed diagnosis, this may lend support to advocating for targeted screening, because failure to screen could increase the risk that, in some individuals, HIV-PAH would remain undiagnosed and thus untreated.
We therefore determined the prevalence of HIV-PAH among patients discharged from the hospital and HIV-PAH reported at death. Our main hypothesis was that HIV-PAH prevalence is lower among patients discharged from the hospital and at death in the United States than HIV-PAH prevalences reported in the literature. Our primary aim was to determine the prevalence of HIV-PAH among patients discharged from the hospital and at death in the United States compared with previously reported HIV-PAH prevalences. Our secondary aim was to compare the prevalence of HIV-PAH among patients discharged from the hospital with the prevalence of HIV-PAH reported at death.
Methods
We conducted a retrospective review of the National Hospital Discharge Survey (NHDS) data and the multiple-cause mortality data sets from the National Vital Statistics Systems (NVSS) data compiled by the National Center for Health Statistics. Patients with HIV infection who were ≥15 years of age were included in the study.
NHDS is a national probability survey of patients discharged from non-Federal acute stay hospitals in the United States from 1965 to 2010. Mortality data from the NVSS serves as a source of demographic, geographic, and cause-of-death information covering a long time period in the United States. The NHDS and NVSS data sets were selected to assess HIV-PAH prevalence at hospital discharge and death because the data sources were considered to be credible and consisted of large samples of data collected over long time periods. In addition, these data sets have been used for similar studies in the past.
Analysis of the NHDS data was performed for the time period 2001–2010. Cases of HIV infection or AIDS were identified by inclusion of ICD-9 codes 042, 043, 044, 079.53, and V08 in any of the discharge diagnoses. PAH (primary PH) was identified using ICD-9 code 416.0. The NHDS data were also used to determine the prevalence of nonprimary pulmonary hypertension (ICD-9 codes 416.8 and 416.9) among individuals with HIV infection. In addition, we identified the number of patients without HIV infection who received a diagnosis of PAH. The prevalence of PAH among patients with HIV infection was compared with the prevalence among patients without HIV infection. Prevalence was stratified by age, sex, and race. We also documented the discharge status for patients with HIV-PAH, patients with HIV infection without PAH, and patients with PAH without HIV infection to make comparisons between these groups.
Mortality data sets from the NVSS were accessed via a publicly available website (http://wonder.cdc.gov) for the time period 1999–2010. Patients with HIV infection or AIDS were identified using International Classification of Diseases, 10th edition, (ICD-10) revision codes B20–B24, whereas patients with PAH were identified using ICD-10 revision code I27.0.
The prevalences of HIV-PAH at hospital discharge and death, obtained from our review of the NHDS and NVSS data sets, respectively, were compared with previously observed prevalences for HIV-PAH.3 Racial comparisons between the NHDS and NVSS data sets were only performed for AA and white patients, because the other races were not defined uniformly for the two data sets. In addition, AA and white patients made up the majority of the study group. Comparisons between the NHDS and NVSS prevalences were also made with respect to age groups and sex. In-hospital mortality was calculated for the NHDS group using data available for individuals whose discharge status was listed as death.
IBM SPSS, version 20, software was used to perform the data analysis. Prevalences obtained from descriptive statics were compared for statistical significance using χ2 tests.
Results
The mean age of patients from the NHDS (hospital discharge) data set was just over 42 years old, and AAs made up the majority of the study group (Table 1). Race was not reported for 29.1% of patients with HIV-PAH in the NHDS data set. Mean age could not be obtained for the NVSS data set, but it was observed that the group consisted of a slightly higher percentage of white patients than AA patients.
Table 1.
Demographic characteristics of patients with human immunodeficiency virus infection–associated pulmonary arterial hypertension
| Characteristic | NHDS data (n = 797) |
NVSS data (n = 254) |
Sitbon et al.3 (n = 35) |
|---|---|---|---|
| Age, years, mean (SD) | 42.5 (7.8) | …a | 41.81 (8.04) |
| Male sex | 59.7 | 60.2 | 71 |
| Race: | |||
| White | 5.8 | 57.1 | 80 |
| African American | 54.2 | 42.5 | 17 |
| Other | 10.9 | 0.4 | 3 |
| Not reported | 29.1 | …b | …b |
Data are percentage of patients, unless otherwise indicated. NHDS: National Hospital Discharge Survey; NVSS: National Vital Statistics Systems; SD: standard deviation.
Mean age cannot be calculated using Centers for Disease Control and Prevention Wide-ranging Online Data for Epidemiologic Research website.
Race was reported for all cases.
Seven hundred ninety-seven of 1,944,365 discharged patients were identified as having HIV-PAH, leading to an estimated prevalence of 0.04%. In comparison, 254 of 165,714 decedents with HIV infection or AIDS were identified as having HIV-PAH, resulting in a prevalence of 0.15%. The prevalences of HIV-PAH at hospital discharge and death were significantly lower (P < 0.001) than the HIV-PAH prevalence of 0.5% reported previously in the literature (Fig. 1). The lower prevalences of HIV-PAH observed in the NHDS (hospital discharge) and NVSS (mortality) data sets were independent of race (Table 2).
Figure 1.
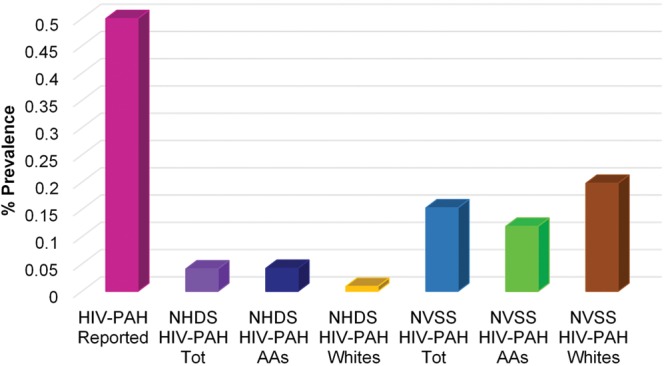
Comparison of human immunodeficiency virus infection associated with pulmonary arterial hypertension (HIV-PAH) prevalence at hospital discharge and death versus reported HIV-PAH prevalence. AAs: African American patients; NHDS: National Hospital Discharge Survey; NVSS: National Vital Statistics Systems; Tot: total; Whites: white patients.
Table 2.
Prevalence of human immunodeficiency virus (HIV) infection–associated pulmonary arterial hypertension (PAH) by race
| Race | NHDS | NVSS | Sitbon et al.3 | P value (NHDS vs. Sitbon et al.3) |
|---|---|---|---|---|
| White | 0.009 (46/534,728) | 0.198 (145/7,319) | 0.488 (28/5,736) | <0.001 |
| African American | 0.042 (432/1,021,622) | 0.119 (108/90,452) | 0.327 (6/1,835) | <0.001 |
| Overalla | 0.041 (797/1,944,365) | 0.153 (254/165,714) | 0.458 (35/7,648) | <0.001 |
Data are prevalence (no. of patients with PAH/no. of patients with HIV infection). Prevalence was compared between white and African American patients only. NHDS: National Hospital Discharge Survey; NVSS: National Vital Statistics Systems.
Overall includes patients of races other than white and African American; therefore, total number of cases exceeds the sum of white and African American patients.
Prevalences for HIV-infected patients discharged from the hospital were highest among those 25–44 years old, followed by those 45–64 years old. As a group, women had higher HIV-PAH prevalences than men for both the NHDS (hospital discharge) and NVSS (mortality) data sets (Fig. 2). On the basis of the NHDS (hospital discharge) data, AA patients had higher prevalences of PAH than white patients irrespective of HIV status (Fig. 3). Review of the NVSS (mortality) data, however, showed a higher prevalence of HIV-PAH among white patients (Fig. 4). The prevalence of nonprimary pulmonary hypertension (ICD-9 codes 416.8 and 416.9) was 0.57% for both AA and white HIV-infected patients at hospital discharge.
Figure 2.
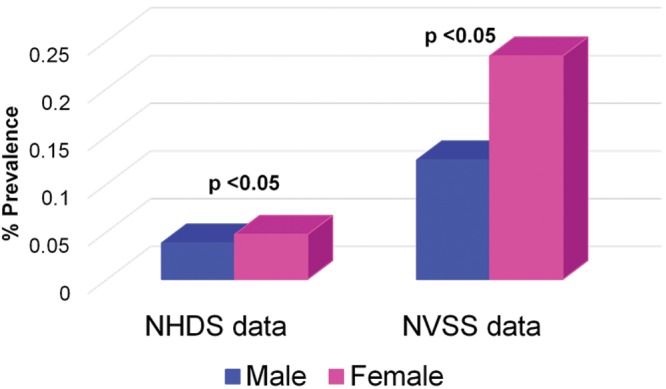
Prevalence of human immunodeficiency virus infection associated with pulmonary arterial hypertension (HIV-PAH) by sex. NHDS: National Hospital Discharge Survey; NVSS: National Vital Statistics Systems.
Figure 3.
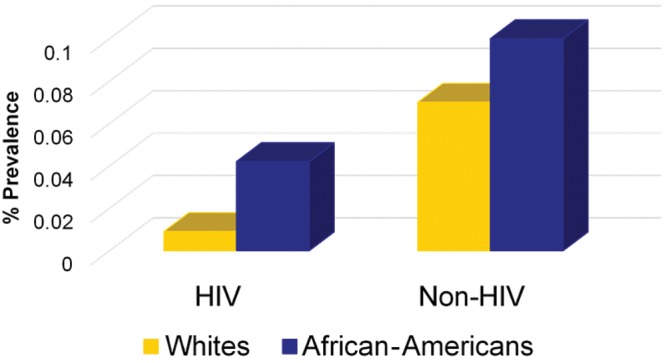
Prevalence of pulmonary arterial hypertension (PAH) among white and African American patients at hospital discharge, comparing patients with human immunodeficiency virus (HIV) infection versus patients without HIV infection (Non-HIV).
Figure 4.
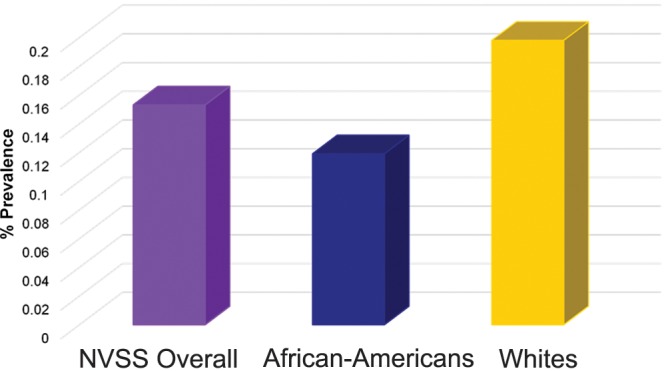
Prevalence of human immunodeficiency virus infection associated with pulmonary arterial hypertension at death for National Vital Statistics Systems (NVSS) data overall and by white or African American race.
The majority of patients discharged from the hospital, irrespective of diagnosis, were classified as routine discharges to home. The most common discharge status for patients with HIV-PAH after discharge to home (84.7%) was either leaving against medical advice (7.5%) or death (6%). In contrast, both HIV-infected patients without PAH and non–HIV-infected patients with PAH had “transfer to a long-term care facility” documented as the second most common discharge category (Fig. 5). The differences between the percentage of patients within each discharge status for patients with HIV-PAH versus either HIV-infected patients without PAH or non–HIV-infected patients with PAH was statistically significant for all categories of discharge status reviewed (P < 0.05).
Figure 5.
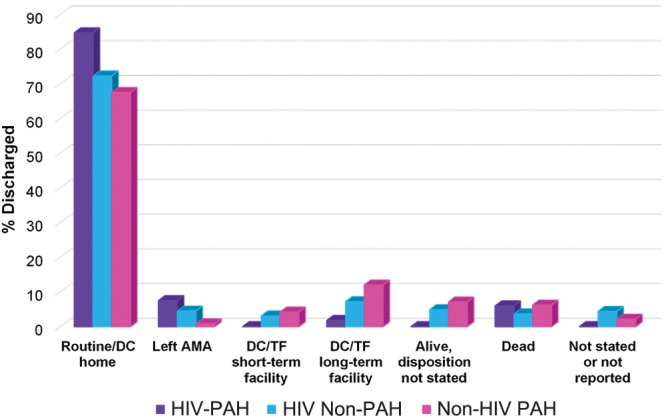
Discharge status based on presence or absence of human immunodeficiency virus (HIV) or pulmonary arterial hypertension (PAH). Differences in the discharge status between patients with HIV-associated PAH (HIV-PAH) and HIV-infected patients without PAH (HIV Non-PAH) was statistically significant for all discharge categories with P value < 0.05. Differences in the discharge status between HIV-PAH and Non-HIV PAH were also statistically significant for all discharge categories with P value < 0.05. DC/TF long-term facility: discharged/transferred to long-term care institution; DC/TF short-term facility: discharged/transferred to short-term care facility; Left AMA: left against medical advice; Routine/DC home: routine/discharged home.
In-hospital mortality (calculated on the basis of individuals whose discharge status was listed as death) was higher among HIV-infected patients with PAH than among HIV-infected patients without PAH (Fig. 6). Sixty-four percent of decedents with HIV-PAH had PAH listed as the primary cause of death.
Figure 6.
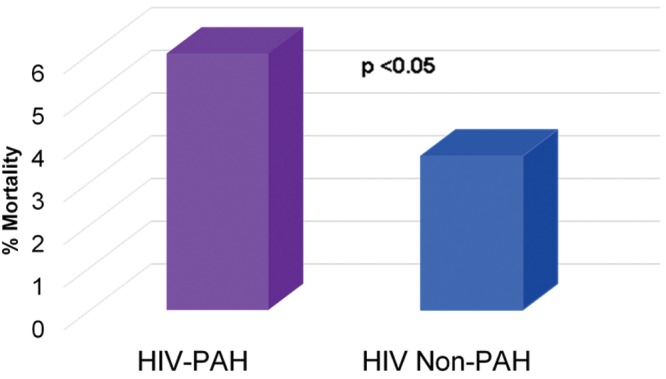
National Hospital Discharge Survey in-hospital mortality rate. HIV-PAH: patients with human immunodeficiency virus infection–associated pulmonary arterial hypertension; HIV Non-PAH: patients with human immunodeficiency virus infection without pulmonary arterial hypertension.
Discussion
A recent study observed that hospitalization rates for persons living with HIV infection in 2009 were higher than previously estimated, and AA patients with HIV infection had a 42% higher hospitalization rate for any illness than did white patients with HIV infection.19 The prevalence of HIV-PAH was highest among the 25–44-year-old age group for the NHDS/hospital discharge patients. This age group also represented individuals with the highest prevalence of HIV infection. This is consistent with findings of the study on HIV-PAH prevalence by Sitbon et al.,3 which reported a mean age for their HIV-PAH cohort of 41.5 years. Similarly, Mehta et al.20 reported that, in their study cohort, the average age at presentation for HIV-PAH was 33 years. Although a larger percentage of patients with HIV-PAH were male than female, the HIV-PAH prevalence was significantly higher among female patients than male patients (P < 0.05) in both the NVSS and NHDS data sets. This is in contrast to previous reports that men had a higher risk for HIV-PAH.21 No clear racial disparity was noted with regard to a predisposition to HIV-PAH, because HIV-PAH prevalence was higher for AAs in the NHDS group, whereas it was higher for white patients in the NVSS group. No earlier studies have specifically investigated the possibility of a race-associated predilection for HIV-PAH. The prevalence of nonprimary pulmonary hypertension (0.57%) among HIV-infected patients at hospital discharge was the same for AA and white patients and was noted to be closer to the prevalence reported for HIV-PAH.
The HIV-PAH prevalences among HIV-infected individuals at hospital discharge (NHDS data) and at death (NVSS data) were significantly lower than previously published HIV-PAH prevalence (0.5%). The lower prevalences observed for HIV-PAH among patients at hospital discharge and at death may be due to underreporting and/or underdiagnosis of the condition. The underreporting of HIV-PAH among discharged patients may be attributable to the fact that a large number of HIV comorbidities were ranked higher than PAH on the list of diagnoses reported for individuals with HIV infection. This raises concerns as to whether HIV-PAH was, in many instances, unrecognized and untreated.
The prevalence of HIV-PAH varied widely between the NHDS data set and the NVSS data set (0.04% vs. 0.15%). The higher HIV-PAH prevalences observed at death than at hospital discharge would be expected if a disease unrecognized at discharge remained untreated, allowing it to progress to the point where it was more likely to be identified at time of death.
For each discharge status, the percentage of patients with HIV-PAH compared with either HIV-infected patients without PAH or HIV-uninfected patients with PAH was significantly different across all discharge status categories. Regardless of diagnosis, the most common discharge status observed was a routine discharge to home. Interestingly, the HIV-PAH group had the highest percentage of patients returning home after hospital discharge. Both HIV-infected patients without PAH and HIV-uninfected patients with PAH were noted to have “transfer to a long-term care facility” as the second most common discharge status, whereas patients with HIV-PAH had “left against medical advice” as their second most common discharge status, followed closely by “death.” Patients with HIV-PAH were the only group to have no discharges to a short-term care facility. Irrespective of HIV status, a higher percentage of individuals with PAH had “death” as their discharge status compared with the HIV-infected patients without PAH.
The in-hospital mortality was higher for patients with HIV-PAH than for HIV-infected patients without PAH according to the NHDS data analysis. This is consistent with previous observations that HIV-PAH portends a poorer prognosis than does HIV infection without PAH. Review of NVSS data revealed that PAH was the leading cause of death (64.2%) among HIV-infected decedents with PAH, similar to findings from a case series in which PAH was the direct cause of death in 72% of their 82 patients with HIV-PAH.10 The fact that the in-hospital mortality rate is higher among patients with HIV-PAH than among HIV-infected patients without PAH, coupled with the observation that PAH is the leading cause of death for persons with HIV-PAH, underscores the need to diagnose and treat this condition. The lower HIV-PAH prevalences observed on the basis of the community data that we reviewed suggest that perhaps the lack of clear screening guidelines for PAH among persons infected with HIV has resulted in HIV-PAH going largely undetected.
The data source used in our study was credible and consisted of a large sample with data collected over a long period. However, our review has the limitations of any epidemiology study that employs ICD coding to identify cases.22 Possible limitations include physician error in selection of the ICD code, transcribers entering incorrect codes, or inaccuracies resulting from missing data. Also our study populations (discharged and deceased patients) differ from populations previously studied to define HIV-PAH prevalence; these earlier studies determined HIV-PAH prevalence using outpatient HIV infection cohorts. In addition, the HIV-PAH prevalence calculated using the mortality data from the NVSS may have led to an observed prevalence higher than the actual HIV-PAH prevalence, because other comorbidities may have contributed to PAH. This, however, would not affect our final conclusion, because accounting for other comorbidities would mean the actual HIV-PAH prevalence for HIV-infected individuals at the time of death would be even lower (compared with reported rates) than that observed in our analysis. Similarly, in the case of the NHDS data, persons admitted to the hospital more than once would be represented more than once, and thus the observed prevalence would be greater than the true HIV-PAH prevalence for the discharged patients. However, controlling for repeat admissions would result in “true” prevalences that were even lower than those observed in our analysis. Thus, our conclusion would remain the same.
Our data analysis revealed that the prevalence of HIV-PAH among the discharged patient population was lower than that observed at death. To explain this discrepancy, we suggest that cases unrecognized at hospital discharge would remain untreated and be allowed to progress to the point at which they would be more likely to be identified at the time of death. This argument is speculative and could only be conclusively confirmed if autopsy information were available for all deceased individuals. Because this is not the case, we are not, unfortunately, able to determine more definitively whether PAH was, in fact, more prevalent among the HIV-infected decedents than among the HIV-infected discharged population.
We were unable to clearly identify any racial disparities related to the prevalence of HIV-PAH on the basis of our review of the discharge and mortality data sets. However, of note, race was not reported in 29.1% of HIV-PAH cases in the NHDS data set.
We have commenced a study to determine the impact of targeted screening on the earlier detection of HIV-PAH. The study site has a high prevalence of HIV infection and the fastest-growing HIV-infected population in the metro Atlanta area.
Prevalences of HIV-PAH at hospital discharge and death were lower than reported HIV-PAH prevalence, irrespective of age, sex, or race. This raises questions about whether the condition is likely to be treated if it is not reported. Although HIV-PAH prevalence is low, lack of screening guidelines may put individuals with HIV-PAH at risk for poorer outcomes due to a late or missed diagnosis.
Conclusions
Prevalences of HIV-PAH among HIV-infected individuals at hospital discharge and death were significantly lower than previously reported HIV-PAH prevalences. The prevalence of HIV-PAH was lower among patients discharged from the hospital than at death. PAH was the leading cause of mortality for HIV-infected decedents with PAH.
Acknowledgments
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Minority Health and Health Disparities, National Center for Advancing Translational Sciences, or National Institutes of Health.
Source of Support: MH-F receives salary support from the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454, and her research is supported in part by grant number 8G12MD007602 from the National Institute of Minority Health and Health Disparities. As a KL2 Scholar, she receives funding via the KL2TR000455, and she was also enrolled in the Master of Science in Clinical Research program at Morehouse School of Medicine, which is supported by the Clinical Research Education and Career Development grant 8R25MD007589-10.
Conflict of Interest: None declared.
References
- 1.Centers for Disease Control and Prevention HIV Surveillance—United States, 1981–2008. Morb Mortal Wkly Rep MMWR 2011 60(21);689–693. [PubMed]
- 2.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr 2006;41(2):194–200. [DOI] [PubMed]
- 3.Sitbon O, Lascoux-Combe C, Delfraissy J-F, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008;177(1):108–113. [DOI] [PubMed]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–1030. [DOI] [PubMed]
- 5.Peacock A. Treatment of pulmonary hypertension. BMJ 2003;326:835–836. [DOI] [PMC free article] [PubMed]
- 6.Quezada M, Martin-Carbonero L, Soriano V, et al. Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS 2012;26:1387–1392. [DOI] [PubMed]
- 7.Isasti G, Moreno T, Pérez I, et al. High prevalence of pulmonary arterial hypertension in a cohort of asymptomatic HIV infected patients. AIDS Res Hum Retroviruses 2013;29(2):231–234. [DOI] [PubMed]
- 8.Hsue P, Deeks S, Faraf H, et al. Role of HIV and human herpesvirus-8 in pulmonary arterial hypertension AIDS 2008; 22(7):825–833. [DOI] [PMC free article] [PubMed]
- 9.Opravil M, Pechère M, Speich R, et al. HIV-associated pulmonary hypertension: a case control study. Am J Resp Crit Care Med 1997;155(3):990–995. [DOI] [PubMed]
- 10.Nunes H, Humbert M, Sitbon O, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2003;167:1433–1439. [DOI] [PubMed]
- 11.Degano B, Guillame M, Savale L, et al. HIV-associated pulmonary arterial hypertension survival and prognostic factors in the modern therapeutic era. AIDS 2010;24:67–75. [DOI] [PubMed]
- 12.Gaine S. Pulmonary hypertension. JAMA 2000;284(24):3160–3168. [DOI] [PubMed]
- 13.George MP, Champion HC, Simon M, et al. Physiologic changes in a non-human primate model of HIV-associated pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2013;48(3):374–381. [DOI] [PMC free article] [PubMed]
- 14.Marecki J, Cool C, Parr J, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef–infected macaques. Am J Respir Crit Care Med 2006;174:437–445. [DOI] [PMC free article] [PubMed]
- 15.Galiè N, Simmoneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol 2013;62(25 suppl):D1–D3. [DOI] [PubMed]
- 16.Lapu-Bula R, Quarshie A, Lyn D, Oduwole A, et al. The 894T allele of endothelial nitric oxide synthase gene is related to left ventricular mass in African Americans with high-normal blood pressure. J Natl Med Assoc 2005;97:197–205. [PMC free article] [PubMed]
- 17.Li R, Lyn D, Lapu-Bula, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004;17:560–567. [DOI] [PubMed]
- 18.Centers for Disease Control and Prevention. Estimated HIV incidence in the United States, 2007–2010. HIV Surveillance Supp Rep 2012;17(4). http://www.cdc.gov/hiv/pdf/statistics_hssr_vol_17_no_4.pdf. Accessed June 23, 2015.
- 19.Bachhuber MA, Southern WN. Hospitalization rates of people living with HIV in the United States, 2009. Public Health Rep 2014;129(2):178–186. [DOI] [PMC free article] [PubMed]
- 20.Mehta NJ, Khan IA, Mehta RN, et. al. HIV-related pulmonary hypertension: analytic review of 131 cases. Chest 2000;118(4):1133–1141. [DOI] [PubMed]
- 21.Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med 2010;11:620–634. [DOI] [PubMed]
- 22.O’Malley KJ, Cook KF, Price MD, et al. Measuring diagnoses: ICD code accuracy. Health Serv Res 2005;40(5 pt 2):1620–1639. [DOI] [PMC free article] [PubMed]


