Abstract Abstract
Sustained-release oral treprostinil, an oral prostacyclin, led to significant improvement in 6-minute walk distance (6MWD) versus placebo in treatment-naive patients with pulmonary arterial hypertension (PAH) but failed to lead to significant improvement in two 16-week trials in patients receiving background PAH therapies (FREEDOM studies). Long-term studies are lacking. Our objective was to evaluate 6MWD, functional class, hemodynamics, and other long-term outcomes during oral treprostinil administration in PAH. Patients receiving oral treprostinil through the FREEDOM studies at our institution were included and were followed for up to 7 years. The primary end point was change in pulmonary vascular resistance (PVR) at first follow-up catheterization. Other end points included 6MWD, functional class, and other hemodynamic results. Thirty-seven patients received oral treprostinil for a median of 948 days, with 81%, 61%, and 47% continuing therapy at 1, 2, and 3 years, respectively. Mean treprostinil dose at 3, 12, and 24 months was 4.3 ± 2.3, 8.6 ± 3.2, and 11.7 ± 5.8 mg/24 h, respectively. Compared with pretreatment values, there was no significant change in 6MWD at 3 or 12 months, no improvement in functional class at 12 months, and no significant change in hemodynamics at the first follow-up catheterization (N = 34). Oral treprostinil dose was inversely associated with change in PVR (r = −0.42, P < 0.05), and change in PVR was numerically better among patients in the highest dosing quartile. No significant improvement in 6MWD, functional class, or hemodynamics versus pretreatment values was seen with long-term oral treprostinil therapy, potentially because of inability to achieve a clinically effective dose.
Keywords: hemodynamics, pulmonary arterial hypertension, survival, treatment, prostacyclin
Systemic prostacyclins are considered the gold-standard treatment for pulmonary arterial hypertension (PAH). Intravenous epoprostenol (prostacyclin; Flolan/Veletri) and subcutaneous treprostinil (Remoduin) have both been shown to lead to significant improvement in 6-minute walk distance (6MWD), dyspnea scores, quality of life, and hemodynamics compared with controls in 12-week clinical trials in PAH,1-3 and epoprostenol led to improvement in survival compared with controls in one 12-week study.2 In longer-term observational studies, both therapies have been associated with improvement in hemodynamics; moreover, observed survival with epoprostenol and treprostinil therapy is greater than that expected from historical data.4-7
In contrast, studies of oral prostacyclins have had mixed results. Oral beraprost led to improvement in 6MWD versus placebo in treatment-naive PAH patients at 12 weeks but did not lead to significant improvement in 6MWD or clinical worsening in a longer 12-month study.8,9 More recently, oral treprostinil led to improvement in 6MWD in treatment-naive PAH patients (FREEDOM M study: 26-m improvement vs. placebo, P < 0.05)10 but failed to lead to significant improvement in two studies enrolling patients receiving background PAH therapies (FREEDOM C11 and C2,12 11- and 10-m improvement vs. placebo, P = 0.07 and 0.09, respectively). There was also no improvement across all three studies in secondary end points of World Health Organization functional class and clinical worsening. Although the cause for this lack of improvement is unclear, potential contributors, as suggested by the authors, include the short study duration and the relatively low doses of oral treprostinil achieved during the study. We therefore explored whether longer-term therapy with oral treprostinil is associated with improvement in hemodynamics, functional class, and 6MWD, focusing on these end points because of their prognostic value and because hemodynamics have not previously been reported for oral treprostinil.
Methods
This was an open-label study of patients participating in the FREEDOM studies at University of Texas (UT) Southwestern Medical Center, Dallas. The 12–16-week FREEDOM clinical trials (FREEDOM M, C, and C2) were international, double-blind, randomized, placebo-controlled studies of oral treprostinil in PAH. Articles detailing study methods and results have been published.10-12 Patients entered the FREEDOM M or C trials at our center between 2007 and 2010, and completing patients were then eligible to enter the open-label FREEDOM extension study. All patients in the extension study received active therapy, and patients were followed at our center for up to 7 years. Patients underwent routine study follow-up plus assessment of functional class and 6MWD every 3–6 months and right heart catheterization approximately annually, or earlier when clinically required. Baseline 6MWD and functional class were assessed just before the first active therapy with oral treprostinil, while “baseline” catheterizations were performed before the placebo-controlled portion of the study. Informed consent was obtained from all participants, and the studies were conducted in accordance with the amended Declaration of Helsinki and were approved by UT Southwestern’s Institutional Review Board (FREEDOM extension: 082007–076; clinicaltrials.gov identifier NCT01027949). The sample size was predetermined and was based on the number of patients enrolled in the FREEDOM extension study at our center. Sample size calculations around the primary end point (change in pulmonary vascular resistance [PVR]) found that a sample of ≥32 patients would have 80% power to detect a change in PVR ≥20% of baseline, with α = 0.05 the standard of significance.
Treatment strategy
Oral treprostinil was initiated at 0.5 or 0.25 mg twice daily (bid) and up-titrated at the discretion of the treating clinician. Up-titration was recommended unless the patient had dose-limiting side effects or had achieved therapeutic goals (functional class II, 6MWD > 450 m, and improved hemodynamics). Add-on oral therapy (phosphodiesterase type 5 inhibitor [PDE5i] and/or endothelin receptor antagonist [ERA]) was allowed. During the first 12 months of therapy, add-on PDE5i or ERA therapy was prescribed only for clinical worsening, defined as either right ventricular failure or persistent functional class III symptoms with a >15% decline in 6MWD. After 12 months, all patients with persistent functional class III symptoms or a 6MWD of <400 m were prescribed at least one approved oral PAH therapy and were considered for a second approved oral therapy at the treating physician’s discretion. Transition to a parenteral prostacyclin therapy was recommended throughout the trial for class IV symptoms, for hemodynamic instability, and for patients with evidence of severe right heart failure (right atrial pressure of ≥15 mmHg or a cardiac index of ≤2.0 L/min/m2). After 12 months of oral treprostinil, parenteral therapy was also recommended for patients with persistent functional class III symptoms, hemodynamics worse than baseline values, and at least one other poor prognostic marker (6MWD < 400 m, abnormal right atrial pressure or cardiac index on catheterization, elevated N-terminal brain natriuretic protein, or worsening findings on echocardiogram). The treatment-escalation recommendations above are consistent with the PAH guidelines at the time of the study.13,14
Statistical analysis
The primary end point was change in PVR, chosen because change in PVR with therapy is a prognostic marker in PAH and because it is also thought to be a predictor of efficacy for novel therapies. “Posttreatment” catheterizations performed before 3 months of oral treprostinil therapy were excluded (N = 1) because the time frame may be too short to see improvement. Secondary end points included changes in other catheterization measures at first follow-up catheterization, change in 6MWD at 3 months (90–120 days) and 12 ± 1 months, and change in functional class at 12 ± 1 months. An exploratory analysis focused on dosing was completed and included (1) absolute value of the change in hemodynamics and 6MWD after stratification by oral treprostinil dose (quartiles for hemodynamics and median for 6MWD), (2) percent change in PVR by dosing quartile and by subgroup (idiopathic PAH, connective tissue disease, baseline PVR, therapy during trial, background PAH therapy), and (3) a regression analysis looking at oral treprostinil dose as a predictor of change in PVR at first follow-up alone and after adjustment for PAH subtype, age, sex, background therapy (yes/no), and baseline PVR and 6MWD. Results are presented as means ± standard deviation or as median and interquartile range. Medians were used for 6MWD because of a nonnormal distribution with the Wilcoxon rank-sum test. Hemodynamic results were tested with a paired Student t test. Kaplan-Meier curves were created for continued oral treprostinil therapy and survival; data were censored on January 15, 2014. Results are “as observed” except for functional class, where the last observed functional class was carried forward except for deceased patients, who were classified as functional class IV. A P value of <0.05 was considered statistically significant.
Results
Demographics
Thirty-eight patients were enrolled in the 12–16-week randomized controlled FREEDOM studies (FREEDOM M or C) at our institution, and 37 patients entered the FREEDOM extension study (Fig. 1). One patient randomized to placebo exited early because of clinical worsening and was excluded from this analysis. Mean age at study entry was 48 ± 14 years, and time since diagnosis was 2.2 ± 1.8 years (Table 1). Five patients were treatment naive at enrollment, and 32 were receiving background oral PAH therapy, including 10 patients receiving combination oral therapy. Add-on therapy with a PDE5i and/or an ERA was permitted, and 16 patients (43%) began one or both therapies during the study. Add-on therapy was initiated before the first follow-up catheterization in 3 patients and during later follow-up in 13 patients.
Figure 1.
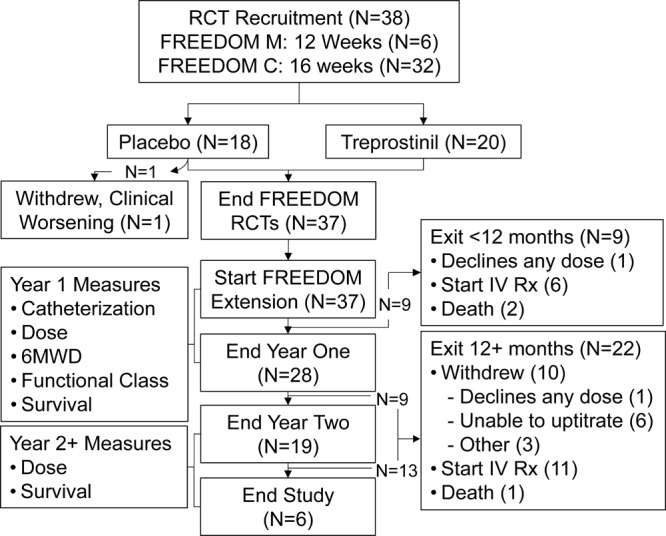
Patient disposition. Treatment-naive patients entered the FREEDOM M study, and patients receiving background pulmonary arterial hypertension therapies entered the FREEDOM C study. After completion, patients entered an extension study, where all patients received open-label oral treprostinil. “Other” reasons for withdrawal were thrombocytopenia (N = 1), worsening hypoxia (N = 1), and incarceration with inability to obtain consent from prison officials to continue experimental therapy (N = 1). IV Rx: intravenous therapy; RCT: randomized clinical trial; 6MWD: 6-minute walk distance.
Table 1.
Demographics: FREEDOM extension patients
| Characteristic | Result |
|---|---|
| Age, years | 48 ± 14 |
| Female, N (%) | 29 (76) |
| Weight, kg | |
| Female | 76 ± 24 |
| Male | 85 ± 21 |
| Etiology, N | |
| Idiopathic PAH | 26 |
| CTD-PAH | 11 |
| Background therapy (at randomization), N | None: 5, PDE5i/ERA: 22, both: 10 |
| Hemodynamics | |
| RAP, mmHg | 9 ± 6 |
| PAP, mmHg | 53 ± 15 |
| PCWP, mmHg | 9 ± 4 |
| CI, L/min/m2 | 2.5 ± 0.7 |
| SVo2, % | 61 ± 9 |
| PVR, Wood units | 11 ± 6 |
| Baseline 6MWD, median (IQR), m | 366 (314–413) |
Data are reported as mean ± standard deviation unless otherwise specified. CI: cardiac index; CTD: connective-tissue disease; ERA: endothelin 1 receptor antagonist; IQR: interquartile range; PAH: pulmonary arterial hypertension; PAP: pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; PDE5i: phosphodiesterase type 5 inhibitor; PVR: pulmonary vascular resistance; RAP: right atrial pressure; SD: standard deviation; SVo2: mixed venous oxygen saturation; 6MWD: 6-minute walk distance.
Oral treprostinil dosing
Oral treprostinil was initiated at doses of 0.5 or 0.25 mg bid, with the lower dose added per protocol later in the study for the purpose of improving tolerability. Overall survival was 92%, 89%, and 86% at 1, 2, and 3 years, respectively (N = 37; Fig. 2). Of the surviving patients, 81% continued to receive oral treprostinil at 1 year, but only 61% and 47% were still receiving this therapy at 2 and 3 years, respectively. The maximum tolerated oral treprostinil dose during the first year of therapy ranged from 1 to 20 mg/24 h (Fig. 3), and the highest tolerated dose at any time point was 32 mg/24 h. Side effects were similar to those for other systemic prostacyclins and included headache (86%), nausea (68%), diarrhea (59%), jaw pain (51%), and flushing (49%). Side effects limited up-titration during the first year of therapy in all but 1 patient. Overall, 8 patients discontinued oral treprostinil because of either side effects alone (N = 2) or inability to tolerate up-titration despite ≥12 months of therapy (N = 6, all at ≤7 mg/day; Fig. 1). Dosing was bid until 2013, when a protocol amendment allowed three-times-daily (tid) dosing, if preferred. All remaining patients (N = 6) chose to transition to tid dosing and reported improved side effects, but patients were unable to increase their total daily dose (median daily dose after 6 months decreased from 18 to 17.5 mg/24 h).
Figure 2.
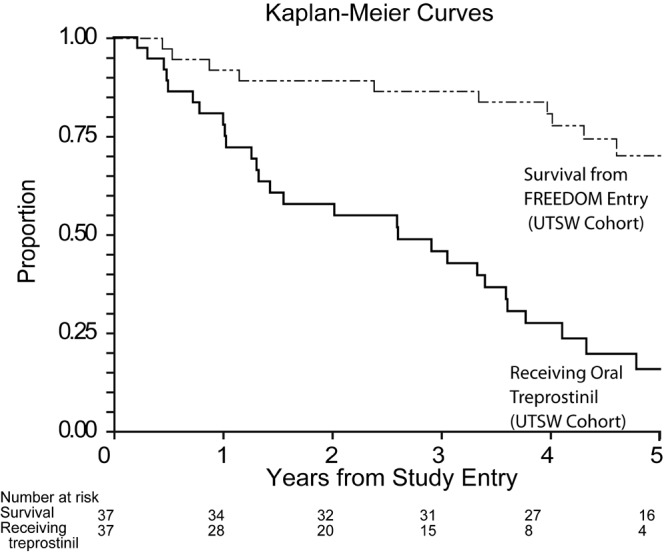
Survival and proportion receiving oral treprostinil over a 5-year period. UTSW: University of Texas Southwestern Medical Center.
Figure 3.
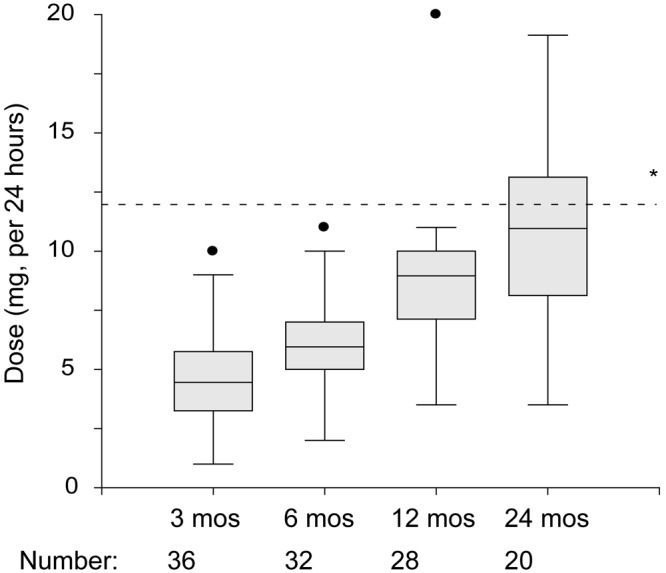
Daily oral treprostinil dose, in milligrams. Median doses were 4.38, 6.0, 9.0, and 11.0 mg/24 h at 3, 6, 12, and 24 months, respectively; mean doses were 4.3 ± 2.3, 6.0 ± 2.1, 8.6 ± 3.2, and 11.7 ± 5.8 mg/24 h, respectively. Circles indicate outliers. *Dashed line: 12 mg, thought to be equivalent to approximately 20 ng/kg/min subcutaneous or intravenous treprostinil.15
Functional class and 6MWD
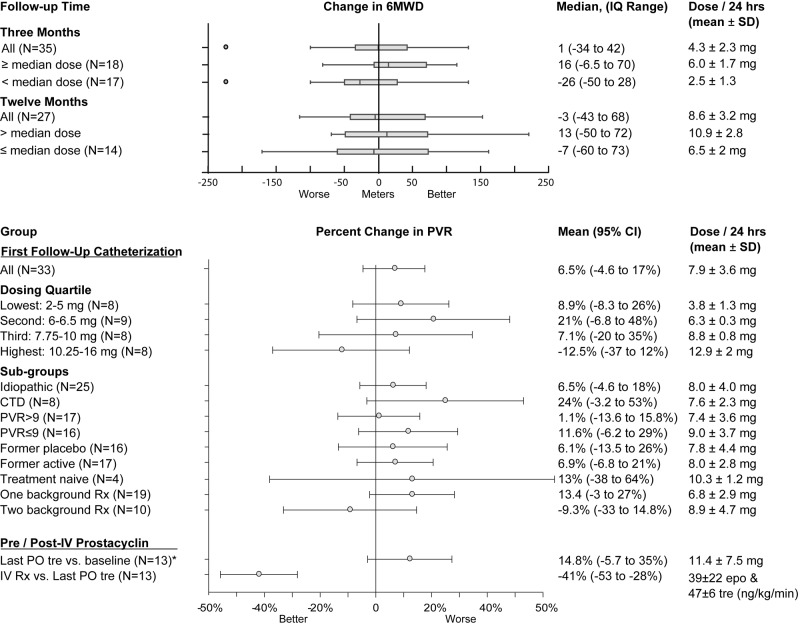
Functional class at the start of active therapy was class II (N = 2, 5%), class III (N = 34, 92%), or class IV (N = 1, 3%). There was no statistically significant change in functional class after 12 months of therapy: 6 patients improved, 2 worsened, 3 were deceased, and there was no change in 26 patients (P not significant). There was also no significant change in 6MWD from baseline values at 3 or 12 months, with median changes of 1 and −3 m, respectively (P not significant). Patients receiving greater than the median dose of oral treprostinil at each time point did have numerically greater increases in 6MWD (Fig. 4).
Figure 4.
Change in 6MWD and change in PVR with oral treprostinil therapy. No significant improvement in PVR was seen in the group as a whole or in any tested subgroup. Circles in the top plot indicate outliers. CTD: connective-tissue disease; epo: epoprostenol; IQ: interquartile; IV Rx: intravenous therapy; PVR: pulmonary vascular resistance; SD: standard deviation; tre: treprostinil; 6MWD: 6-minute walk distance. *Last PO tre: last catheterization while still receiving oral treprostinil.
Hemodynamics
Baseline hemodynamics were consistent with severe PAH (Table 1). After therapy with oral treprostinil, there was no significant improvement in hemodynamics over baseline values. Among patients with catheterization both before and after oral treprostinil therapy, PVR was 10.74 Wood units at baseline and 10.75 Wood units at first follow-up (overall change in PVR: 0.01 ± 3.6 Wood units; Table 2). Results were similar across subgroups of etiology, severity, receipt of placebo or active therapy in the FREEDOM studies, and whether treatment naive (FREEDOM M participants) or receiving background therapy (FREEDOM C participants), but percent change in PVR was numerically better for patients in the highest dosing quartile (Table 2; Fig. 4). Specifically, patients receiving at least 10.125 mg/24 h oral treprostinil had a 12.4% (95% confidence interval [CI]: −37% to 12%) decline in PVR versus baseline (N = 8), while all other dosing quartiles showed increases in PVR. Oral treprostinil dose was also a modest predictor of change in PVR in a linear regression analysis (r = −0.42, P < 0.05; Fig. 5), and this association remained significant after adjustment for age, sex, etiology, background therapy, baseline PVR, and baseline 6MWD.
Table 2.
Change in hemodynamics versus baseline catheterization
| All (N = 34) | Q1 (2–5 mg) | Q2 (6–6.5 mg) | Q3 (7.75–10 mg) | Q4 (10.25–16 mg) | |
|---|---|---|---|---|---|
| RAP, mmHg | −0.2 ± 7 | −3 ± 8 | 2 ± 8 | −1 ± 4 | 0 ± 6 |
| PAP, mmHg | 1 ± 8 | 2 ± 7 | 5 ± 5a | 0 ± 7 | −4 ± 12 |
| PCWP, mmHg | 0.9 ± 5 | 0 ± 6 | 2 ± 4 | −1 ± 3 | 3 ± 5 |
| CI, L/min/m2 (N = 33)a | 0.1 ± 0.7 | 0 ± 0.4 | −0.2 ± 0.5 | 0.3 ± 0.9 | 0.2 ± 0.7 |
| SVo2, % | −2 ± 8 | −3 ± 8 | −5 ± 10 | −3 ± 6 | 3 ± 6 |
| PVR, Wood units (N = 33)a | 0.01 ± 3.6 | 0.7 ± 2.4 | 1.2 ± 3 | −0.1 ± 2.2 | −2.3 ± 5.2 |
| Daily treprostinil dose, mg | 7.8 ± 3.6 | 3.8 ± 1.3 | 6.3 ± 0.3 | 8.8 ± 0.8 | 12.9 ± 2 |
| Median daily treprostinil dose, mg | 6.5 | 3.5 | 6.25 | 8.75 | 13.125 |
Data are reported as mean ± standard deviation unless otherwise specified. First follow-up catheterization was performed after 11 ± 7 months of therapy. Three patients (9%) received add-on PDE5i or ERA therapy before follow-up. Missing data are due to clinical worsening and catheterization before 3 months of therapy (N = 1), medication discontinuation without catheterization (N = 1), or insurance issues (N = 1). No comparisons were statistically significant. CI: cardiac index; ERA: endothelin 1 receptor antagonist; PAP: pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; PDE5i: phosphodiesterase type 5 inhibitor; PVR: pulmonary vascular resistance; Q: dosing quartile; RAP: right atrial pressure; SVo2: mixed venous oxygen saturation.
N = 33 because baseline CI data were missing for 1 patient.
Figure 5.
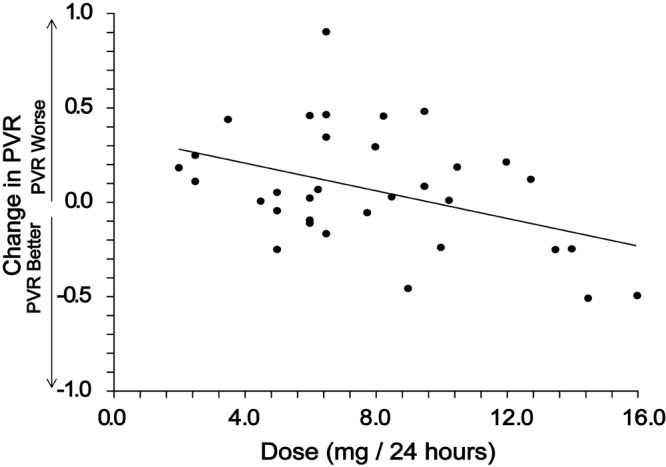
Percent change in pulmonary vascular resistance (PVR). There was no overall improvement in PVR (mean change in PVR: 6.5%), but oral treprostinil dose at first catheterization did correlate inversely with change in PVR (r = −0.42, P < 0.05).
Discontinuations and transitions to other therapies
Eleven patients exited the study because of prostacyclin side effects and inability to up-titrate (N = 8) or other reasons (N = 3; Fig. 1). All patients were down-titrated over a period of several months so as to avoid any rebound related to prostacyclin withdrawal. Patients also received one or more additional PAH therapies during this time, including approved oral therapies (N = 9) and/or inhaled prostacyclin analogs (N = 4). Long-term outcomes for these patients are beyond the scope of this article, but no patients died during the transition or during the first year after discontinuation of oral treprostinil therapy.
Seventeen patients (46%) required transition to intravenous prostacyclin therapy (epoprostenol or treprostinil). As shown in Table 3, these patients had a number of concerning hemodynamic findings, both at baseline and after oral treprostinil therapy. Dosing and up-titration of the intravenous therapies were individualized and were based on PAH severity, prior oral treprostinil dose and tolerability, and intravenous therapy tolerability. We utilized minimum intravenous-therapy dosing targets of 20 ng/kg/min for epoprostenol and 40 ng/kg/min for treprostinil, consistent with our center’s usual 3–4-month goals. Sixteen of 17 patients reached their individual dosing targets uneventfully, achieving mean doses at 4 months of 33 ± 9 ng/kg/min for epoprostenol (N = 13) and 43 ± 6 ng/kg/min for treprostinil (N = 3). One patient reported continued severe prostacyclin side effects with intravenous therapy and chose to discontinue prostacyclin therapy altogether.
Table 3.
Hemodynamic results among patients transitioning to intravenous therapy
| Death before IV Rx cath (N = 4) | Survived to IV Rx cath (N = 13) | ||||||
|---|---|---|---|---|---|---|---|
| Oral treprostinil | IV Rx | Oral treprostinil | IV Rx | ||||
| Baseline | First f/u: 12 ± 9 mo |
None | Baseline | First f/u: 9 ± 7 mo |
Last f/u: 18 ± 17 mo |
First f/u: 11 ± 4 mo |
|
| Dose, mean ± SD, mg/24 h | … | 7.6 ± 3 | … | … | 7.8 ± 4 | 10.6 ± 7 | Combinationa |
| RAP, mmHg | 10 | 21 | … | 12 | 8 | 9 | 6b |
| PAP, mmHg | 49 | 55 | … | 52 | 55 | 60 | 47c |
| PCWP, mmHg | 9 | 10 | … | 9 | 9 | 9 | 10 |
| CI, L/min/m2 | 2.5 | 2.0 | … | 2.4 | 2.6 | 2.5 | 3.3bc |
| SVo2, % | 55 | 48 | … | 61 | 56 | 55 | 66c |
| PVR, Wood units | 10.3 | 13.3 | … | 10.5 | 10.8 | 11.3 | 6.63bc |
Data are reported as means unless otherwise specified. Seventeen patients transitioned to intravenous therapy (IV Rx). Four died before undergoing a follow-up catheterization (left). Catheterization results for the remaining 13 patients showed significant hemodynamic improvement versus baseline and versus results of the last catheterization with oral treprostinil therapy (right). CI: cardiac index; f/u: follow-up; IV Rx cath: catheterization while receiving intravenous therapy; PAP: pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure; SVo2: mixed venous oxygen saturation.
Dose at catheterization: treprostinil: 47 ± 6 ng/kg/min; epoprostenol: 39 ± 22 ng/kg/min.
P < 0.05 versus baseline.
P < 0.05 versus last follow-up with oral therapy.
Survival from the start of intravenous therapy was 71%, 64%, and 56% at 1, 2, and 3 years, respectively, including 5 deaths during the first 6 months of therapy. Thirteen patients (76%) underwent catheterization at 11 ± 4 months of intravenous therapy. Catheterization results showed significant improvement versus both baseline (pre–oral treprostinil) and catheterization while receiving oral therapy (Table 3). Of note, because 4 (24%) of the 17 transition patients died before follow-up catheterization, these results could overestimate the hemodynamic benefits of intravenous therapy.
Discussion
In this study, we evaluated long-term outcomes in 37 patients enrolled in the FREEDOM extension study at our institution. We found no significant improvement in functional class, 6MWD, or hemodynamics versus baseline during long-term follow-up. These results suggest that oral treprostinil may not lead to significant long-term improvement in these end points at the doses most commonly achieved in the FREEDOM clinical trials and extension study. This contrasts with the significant benefits shown with intravenous and subcutaneous prostacyclins in clinical trials and open-label studies.1-3
Systemic treprostinil therapy: optimal dosing
Our findings suggest that insufficient prostacyclin dosing was most likely the major contributor to our overall negative results. This is supported by our findings of a moderate inverse association between oral treprostinil dose and change in PVR, by the 12% decline in PVR in the highest dosing quartile (P not significant), and by the large decline in PVR (41%) among patients whose therapy transitioned from oral treprostinil to intravenous epoprostenol or treprostinil. Concerns about dosing have previously been raised with oral treprostinil. The authors of the primary FREEDOM C and C2 papers speculated that the failure to achieve the primary end point (6MWD) could have related to the relatively low doses achieved, the short study durations, and premature discontinuations due to tolerability, particularly before the introduction of lower tablet strengths later in the FREEDOM series.
Although optimal dosing for systemic prostacyclins remains unknown, studies of intravenous and subcutaneous prostacyclins have suggested a significant dose response for both 6MWD16 and hemodynamics.16-18 Survival also appears to be associated with subcutaneous treprostinil doses, with greater survival seen for every 10 ng/kg/min-higher dose achieved at 12 weeks (hazard ratio: 0.66 [95% CI: 0.48–0.9]) and for those achieving a dose ≥40 ng/kg/min (hazard ratio: 0.29 [95% CI: 0.20–0.44]) at any time point.5,19
Partially because of these results, our center utilizes a minimum intravenous and subcutaneous treprostinil target dose of 40 ng/kg/min, typically achieved during the first 3–4 months of therapy.20,21 For epoprostenol, we target 20 ng/kg/min, chosen on the basis of treprostinil-to-epoprostenol conversion studies, other epoprostenol studies, and our own clinical experience.6,18,22 Notably, dosing equivalencies between epoprostenol and treprostinil have not been established; studies looking at eporprostenol-to-treprostinil transitions typically utilize lower initial doses of treprostinil (∼1.25 times the epoprostenol dose) followed by gradual up-titration, with maintenance doses at 12 weeks of about 2 times the prior epoprostenol dose. Our dosing targets are also slightly lower than prostacyclin maintenance doses reported at other pulmonary hypertension centers in the United States,23 but these are only our minimum targets, and many patients undergo subsequent additional up-titration based on both clinical and hemodynamic response.
Oral treprostinil can theoretically be administered in these dosing ranges, but tolerability appears to be a significant issue for most patients. The average oral treprostinil dose at 1 year in our FREEDOM extension patients was 8.6 mg/24 h, similar to the average of 8.4 mg/24 h at 1 year in the FREEDOM extension study overall.24 In addition, fewer than 10% of patients at our center were able to reach 12 mg/24 h within the first year of therapy, and no patients reached 24 mg/24 h within 1 year—with the latter thought to be roughly equivalent to our minimum intravenous treprostinil dosing target of 40 ng/kg/min.15 This occurred despite continuous attempts to up-titrate throughout the first year of therapy in 36 of 37 patients and despite our center’s considerable experience in managing systemic prostacyclins and their side effects.
Limitations
The small sample size (N = 37) and observational design may limit the conclusiveness of our results. The lack of a control group, in particular, makes it difficult to exclude a beneficial effect on hemodynamic stability, because PAH is frequently progressive. However, while our data do not exclude the possibility that oral treprostinil could prevent hemodynamic worsening, most approved PAH therapies lead to significant hemodynamic improvement versus baseline. Decreases in PVR have been in the 20%–42% range with most other approved PAH therapies,2,3,16,18,22,25-28 and our study was well powered to identify improvement of this magnitude.
Other factors could also have contributed to our negative results. Treatment-naive patients may have greater improvement than patients receiving background therapy, and most patients at our center had received background therapy. Our “baseline” catheterizations (but not walk tests and functional class) were also performed before the randomized controlled portions of the FREEDOM studies (i.e., before the 12–16-week placebo periods), meaning that any worsening in hemodynamics before the initiation of oral treprostinil was not captured. On the other hand, our sensitivity analysis did not reveal any significant differences in hemodynamic response in former placebo versus former active-therapy patients or in treatment-naive patients versus patients receiving one or two background therapies (Fig. 4). In addition, most patients who would be considered candidates for oral treprostinil will likely be receiving background therapies.
Finally, the higher (equivalent) prostacyclin dosing achieved in patients after transition to intravenous therapy should also be noted. In our opinion, this is not a true limitation but is instead the main issue: up-titration of oral therapy is more difficult than up-titration of intravenous therapy, even when attempted over a long period of time. The specific cause(s) for the differences in tolerability between oral and intravenous therapy remain unclear but could relate to the peak and trough associated with bid dosing, variability in patients’ adherence to the recommended 500-calorie meal (required to optimize absorption), potential direct gastrointestinal side effects with oral administration, and/or the lack of sufficiently small dose increments. Although it is hoped that oral treprostinil tolerability will improve with the option of tid dosing and smaller pill sizes, this remains unclear and will require further study.
Conclusions
In this report, we present data for 37 patients receiving oral treprostinil in the FREEDOM extension study at our institution. We found no significant improvement in functional class, 6MWD, or hemodynamics after approximately 1 year of therapy. The modest association between higher treprostinil dose and improvement in PVR suggests that oral treprostinil might be effective if sufficiently high doses can be achieved, but our dosing results suggest that this will be difficult to accomplish. Overall, these findings, combined with the negative oral treprostinil clinical trial results in patients receiving background PAH therapy, suggest that parenteral prostanoids remain the treatment of choice for most PAH patients requiring a systemic prostanoid.
Acknowledgments
The data collection, statistical analysis and manuscript preparation were completed solely by the study authors.
Source of Support: The original randomized controlled FREEDOM studies and extension study were sponsored by United Therapeutics, 1040 Spring Street, Silver Spring, Maryland 20910, USA. The sponsor retained the right to review, but not disapprove, publications derived from these data. KMC’s work is supported by National Institutes of Health (NIH) grant 1K23HL105784.
Conflict of Interest: KMC has participated in research sponsored by Actelion, Bayer, Gilead, GlaxoSmithKline, Novartis, United Therapeutics, GeNO, and the NIH and has received honoraria for service on advisory boards for Actelion, Bayer, EKOS, and Gilead. SDB has participated in research sponsored by Actelion, Bayer, Gilead, GlaxoSmithKline, Novartis, United Therapeutics, GeNO, and the NIH and has received honoraria for speaking or for service on advisory boards for Actelion, Bayer, and Gilead. FT has served as a scientific advisor, consultant, and/or investigator in clinical trials for Actelion, Bayer, GeNO, Gilead, Glaxo Smith Kline, Novartis, Pfizer, and United Therapeutics. MK has served on advisory boards and/or speakers’ bureaus for Actelion, Gilead, Bayer, and United Therapeutics. The remaining authors have no disclosures to declare.
References
- 1.Simonneau G, Barst RJ, Galiè N, Naeije R, Rich S, Bourge RC, Keogh A, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165(6):800–804. [DOI] [PubMed]
- 2.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996;334(5):296–301. [DOI] [PubMed]
- 3.Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, Rich S, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: a randomized, controlled trial. Ann Intern Med 2000;132(6):425–434. [DOI] [PubMed]
- 4.Lang I, Gomez-Sanchez M, Kneussl M, Naeije R, Escribano P, Skoro-Sajer N, Vachiéry JL. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest 2006;129(6):1636–1643. [DOI] [PubMed]
- 5.Barst RJ, Galiè N, Naeije R, Simonneau G, Jeffs R, Arneson C, Rubin LJ. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J 2006;28(6):1195–1203. [DOI] [PubMed]
- 6.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002;40(4):780–788. [DOI] [PubMed]
- 7.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106(12):1477–1482. [DOI] [PubMed]
- 8.Barst RJ, McGoon M, McLaughlin V, Tapson V, Oudiz R, Shapiro S, Robbins IM, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003;41(12):2119–2125. [DOI] [PubMed]
- 9.Galiè N, Humbert M, Vachiéry JL, Vizza CD, Kneussl M, Manes A, Sitbon O, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002;39(9):1496–1502. [DOI] [PubMed]
- 10.Jing ZC, Parikh K, Pulido T, Jerjes-Sanchez C, White RJ, Allen R, Torbicki A, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013;127(5):624–633. [DOI] [PubMed]
- 11.Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, Badesch DB, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012;142(6):1383–1390. [DOI] [PubMed]
- 12.Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, Kotlyar E, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest 2013;144(3):952–958. [DOI] [PubMed]
- 13.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest 2007;131(6):1917–1928. [DOI] [PubMed]
- 14.Barst RJ, Gibbs JS, Ghofrani HA, Hoeper MM, McLaughlin VV, Rubin LJ, Sitbon O, Tapson VF, Galiè N. Updated evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 2009;54(1 suppl.):S78–S84. [DOI] [PMC free article] [PubMed]
- 15.White RJ, Torres F, Allen R, Jerjes C, Pulido T, Yehle D, Howell M, Laliberte K, Marier JF, Tapson VF. Pharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol 2013;61(6):474–481. [DOI] [PubMed]
- 16.Tapson VF, Gomberg-Maitland M, McLaughlin VV, Benza RL, Widlitz AC, Krichman A, Barst RJ. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest 2006;129(3):683–688. [DOI] [PubMed]
- 17.McLaughlin VV, Gaine SP, Barst RJ, Oudiz RJ, Bourge RC, Frost A, Robbins IM, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 2003;41(2):293–299. [DOI] [PubMed]
- 18.Homma S. Dose-dependent reduction in pulmonary vascular resistance with epoprostenol in pulmonary arterial hypertension. Circ J 2010;74(10):2062–2063. [DOI] [PubMed]
- 19.Benza RL, Gomberg-Maitland M, Naeije R, Arneson CP, Lang IM. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011;30(9):982–989. [DOI] [PubMed]
- 20.Gomberg-Maitland M, Tapson VF, Benza RL, McLaughlin VV, Krichman A, Widlitz AC, Barst RJ. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med 2005;172(12):1586–1589. [DOI] [PubMed]
- 21.Minai OA, Parambil J, Dweik RA, Davila GH, Peterson L, Rollins KD, Chen H. Impact of switching from epoprostenol to IV treprostinil on treatment satisfaction and quality of life in patients with pulmonary hypertension. Respir Med 2013;107(3):458–465. [DOI] [PubMed]
- 22.Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, Diehl JH, Crow J, Long W. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol): results of a randomized trial. Ann Intern Med 1990;112(7):485–491. [DOI] [PubMed]
- 23.Oudiz RJ, Farber HW. Dosing considerations in the use of intravenous prostanoids in pulmonary arterial hypertension: an experience-based review. Am Heart J 2009;157(4):625–635. [DOI] [PubMed]
- 24.Orenitram (treprostinil) [package insert]. Bethesda, MD: National Library of Medicine. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=8ed2003a-c801-411e-831e-d06079bb0d7c#nlm34089-3.
- 25.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet 2001;358(9288):1119–1123. [DOI] [PubMed]
- 26.Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost AE, Engel PJ, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008;149(8):521–530. [DOI] [PubMed]
- 27.Galiè N, Badesch D, Oudiz R, Simonneau G, McGoon MD, Keogh AM, Frost AE, et al. Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2005;46(3):529–535. [DOI] [PubMed]
- 28.Channick RN, Olschewski H, Seeger W, Staub T, Voswinckel R, Rubin LJ. Safety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertension. J Am Coll Cardiol 2006;48(7):1433–1437. [DOI] [PubMed]