Abstract Abstract
We propose an exploratory clinical study, the first of its kind to our knowledge, to determine the safety and potential clinical benefit of the combination of the HIV protease inhibitors (HIV-PIs) saquinavir and ritonavir (SQV+RIT) in patients with idiopathic pulmonary arterial hypertension (IPAH). This study is based on evidence that (1) HIV-PIs can improve pulmonary hemodynamics in experimental models; (2) both Toll-like receptor 4 and high-mobility group box 1 (HMGB1) participate in the pathogenesis of experimental pulmonary hypertension; and (3) a high-throughput screen for inhibitors of HMGB1-induced macrophage activation yielded HIV-PIs as potent inhibitors of HMGB1-induced cytokine production. In this proposed open-label, pre-post study, micro, low, and standard doses of SQV+RIT will be given to IPAH patients for 14 days. Patients will receive follow-up for the next 14 days. The primary outcome to be evaluated is change in HMGB1 level from baseline at 14 days. The secondary outcome is changes in tumor necrosis factor α, interleukin 1β, interleukin 6, C-reactive protein, pulmonary arterial pressure based on echocardiography parameters and New York Heart Association/World Health Organization functional class, and Brog dyspnea scale index from baseline at 14 days. Other secondary measurements will include N-terminal pro-brain natriuretic peptide, atrial natriuretic peptide, and 6-minute walk distance. We propose that SQV+RIT treatment will improve inflammatory disorders and pulmonary hemodynamics in IPAH patients. If the data support a potentially useful therapeutic effect and suggest that SQV+RIT is safe in IPAH patients, the study will warrant further investigation. (ClinicalTrials.gov identifier: NCT02023450.)
Keywords: pulmonary hypertension, HIV protease inhibitors, inflammation, clinical trial
Pulmonary arterial hypertension (PAH) is a relatively rare and devastating illness characterized by high mortality.1,2 The 1993 American College of Chest Physicians consensus statement reported that the incidence of PAH ranges from 1 to 2 cases per million people.3 Median age at diagnosis was 36 years, and median survival for all patients in the National Heart, Lung, and Blood Institute Patient Registry for the Characterization of Primary Pulmonary Hypertension was 2.8 years; 1-, 3-, and 5-year survival was 68%, 48%, and 34%, respectively.1 With the advent of new drug therapy, PAH 1-, 3-, 5-, and 7-year survival improved to 91%, 74%, 65%, and 59%, respectively, for patients with idiopathic/familial PAH, in the REVEAL registry study reported in 2012.4 Despite these improvements in outcome, mortality remains unacceptably high. The lack of a routine screening test for PAH and the fact that early symptoms are nonspecific mean that patients typically present with advanced disease. Previous studies5,6 showed that mutations in the bone morphogenetic protein receptor type 2 (BMPR2) gene are linked to susceptibility to both the familial and sporadic forms of PAH. However, because the penetrance of disease for known BMPR2 mutations ranges from 15% to 80%,7 additional “hits” must be required for disease initiation. The exact nature of these factors remains unclear, but inflammation and immunity have been widely implicated.
Inflammation is increasingly recognized as a feature of PAH, as suggested by infiltration of inflammatory cells, including macrophages, T and B lymphocytes, and dendritic cells within pulmonary perivascular spaces and the plexiform lesions associated with PAH.8,9 In addition, increased cytokine and growth factor expression is observed in the remodeled pulmonary vessels, while elevated circulating levels of certain chemokines, cytokines, and autoantibodies are also associated with pulmonary hypertension.10-17 These observations have led to the proposal that a cascade of pathological vascular events, combined with persistent local inflammation, leads to vasoconstriction and vascular remodeling. Preclinical trials targeting specific inflammatory pathways have shown promising results in animal models.18-20
Toll-like receptors (TLRs) play a key role in innate immune responses by initiating specific antimicrobial response pathways after the recognition of signature molecular motifs in molecules of invading pathogens. Some TLRs (e.g., TLR4) can also be activated by endogenous molecules released by stressed or damaged tissue. This initiates signaling cascades that result in the upregulation of inflammatory mediators.21 Experimental evidence indicates that both TLR422,23 and one of its ligands, high-mobility group box 1 (HMGB1), participate in the pathogenesis of experimental pulmonary hypertension.16 It has been recently shown that either neutralizing HMGB1 or deleting TLR4 confers protection in experimental PAH models.24 Our preliminary data also revealed elevated HMGB1 blood levels in idiopathic PAH (IPAH) patients (Fig. 1). Histological assay has also demonstrated increased HMGB1 expression in the pulmonary arteries of IPAH patients.24 Recently, two groups confirmed that HMGB1 was a promoting factor in experimental pulmonary hypertension and that inhibiting HMGB1 or blocking the activity of HMGB1 attenuates pulmonary hypertension progression.25,26
Figure 1.
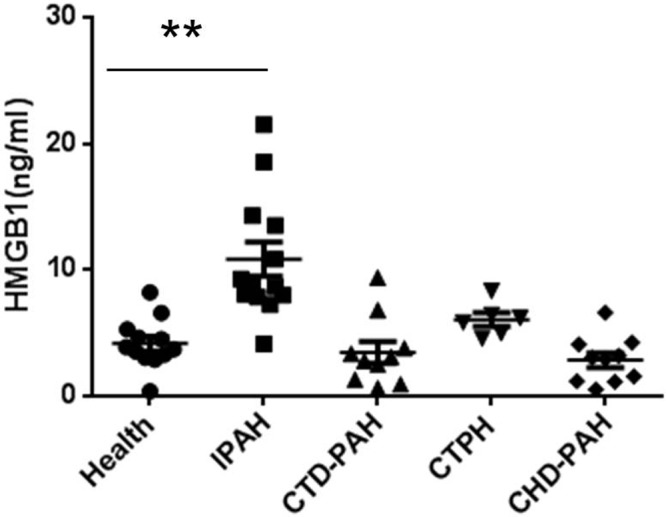
Serum high-mobility group box 1 (HMGB1) levels in patients with different types of pulmonary arterial hypertension (PAH). CTD-PAH: connective tissue disease–associated PAH; CTPH: chronic thromboembolic pulmonary hypertension; CHD-PAH: congenital heart disease–associated PAH; IPAH: idiopathic PAH. **P < 0.01.
HMGB1 is passively released during cell injury and necrosis or actively secreted during cell activation and stress. Thus, HMGB1 has emerged as an important damage-associated molecular pattern.27 HMGB1 expresses inflammatory cytokine activity by binding to the TLR4/MD2 receptor complex on macrophages and stimulating release of tumor necrosis factor α (TNF-α) and other cytokines.28 TNF-α is a key cytokine that is largely produced by activated macrophages but, importantly, is also released by vascular smooth muscle cells29 and endothelial cells.30 In clinical studies of PAH patients, serum levels of TNF-α and other proinflammatory cytokines, including interleukin 1β (IL-1β), interleukin 2 (IL-2), interleukin 6 (IL-6), interleukin 8 (IL-8), and interleukin 12p70 (IL-12p70), are elevated compared to those of healthy controls. Elevated levels of these cytokines are associated with lower survival rates.17 Inflammatory processes appear to play an important role in the vascular remodeling of PAH, and the activated HMGB1/TLR4 signaling pathway might be an important target for PAH therapy.
Whether blocking TLR4 signaling could reduce the inflammatory response (including HMGB1) during PAH and improve the clinical course of PAH patients is unclear. A high-throughput screen for inhibitors of HMGB1-induced macrophage TNF production yielded first-generation HIV protease inhibitors (HIV-PIs) as potent inhibitors of HMGB1-induced cytokine production.31 The most potent inhibitor of macrophage activation via TLR4 identified in the screen is saquinavir (SQV; T. R. Billiar, unpublished data). SQV has been shown to inhibit activity of several mammalian proteases, including the matrix metalloproteinase 2, 20S, and 26S proteasomes. This activity has been associated with a suppression of PI3K/Akt signaling, nuclear factor κB activation, and TNF levels.32-37 First-generation HIV-PIs have been used for prolonged periods without severe side effects in HIV seropositive subjects of any age. Recently, a 6-month course of SQV was shown to improve steroid-resistant nephrotic syndrome in humans.35 Interestingly, Gary-Bobo et al.34 recently showed that HIV-PIs can improve pulmonary hemodynamics in experimental models of PAH.
On the basis of these studies in humans and experimental systems, we propose a trial to determine whether HIV-PIs will alter the inflammation biomarkers and pathobiology of IPAH. Patients will receive SQV plus ritonavir (RIT; SQV+RIT) treatment. RIT is also a first-generation HIV-PI but is used primarily to increase the bioavailability of SQV.38,39 A 28-day, open-label, single-blind, pre-post study will investigate whether SQV+RIT will reduce biomarkers of inflammation and improve pulmonary hemodynamics in IPAH patients. If results of this study yield positive results, we would propose further investigation of this drug combination to treat refractory IPAH.
IPAH patients whose condition has been stable with their current therapy for the preceding 3 months will be enrolled into the study, following informed consent. Patients will be divided into 3 cohorts. Groups will receive SQV+RIT in either micro (SQV 0.3 mg/kg, RIT 0.03 mg/kg), low (SQV 3 mg/kg, RIT 0.3 mg/kg), or standard (SQV 15 mg/kg, RIT 1.5 mg/kg) doses twice daily. Trials will continue only when the dose is well tolerated.
Methods
Ethics statement
This study is an open-label, single-blind, and pre-post pilot trial with 3 cohorts to evaluate the safety and efficacy of different doses of SQV+RIT in IPAH patients. The study will take place at Xiangya Hospital, Central South University, Changsha, China. After 14 days of SQV+RIT treatment, all patients will be followed for an additional 14 days to measure biomarkers, pulmonary artery pressures, and other indices. The protocol (885.6KB, pdf) and the informed-consent document (207.7KB, pdf) for this trial are available as supporting information. The Institutional Review Board (IRB) of the Third Xiangya Hospital, Central South University (no. 13022), and the IRB of Xiangya Hospital, Central South University (no. 201401013), have approved the study as an off-label use of an approved drug. IRB membership included experts on medicine and law and community representatives. This trial has been registered in the ClinicalTrials.gov protocol registration system (identifier no. NCT02023450).
Study subjects
Twenty patients with IPAH will be recruited in the study. Diagnosis of PAH via right heart catheterization will be required, with evidence of a resting mean pulmonary artery pressure (mPAP) of ≥25 mmHg, a pulmonary capillary wedge pressure of ≤15 mmHg, and normal or reduced cardiac output. The inclusion and exclusion criteria are listed in Table 1.
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria |
| Age 18–60 years |
| Idiopathic PAH |
| Personally signed and dated informed-consent document indicating that the subject (or a legally acceptable representative) has been informed of all pertinent aspects of the study |
| Subject willing and able to comply with scheduled visits, treatment plans, laboratory tests, and other study procedures |
| Diagnosis of PH confirmed by cardiac catheterization: mPAP ≥ 25 mmHg (at rest), pulmonary capillary wedge pressure ≤ 15 mmHg, and normal or reduced cardiac output |
| Stable PAH therapy for at least 3 months |
| Exclusion criteria |
| Baseline systemic hypotension, defined as MAP < 50 mmHg |
| Requires intravenous inotropes within 30 days before study participation |
| Has uncontrolled systemic hypertension, as evidenced by sitting systolic BP > 160 mmHg or sitting diastolic BP > 100 mmHg at screening |
| History of portal hypertension or chronic liver disease, including cirrhosis, chronic alcoholism, hepatitis B, and/or hepatitis C (with evidence of recent infection and/or active virus replication), defined as moderate-to-severe hepatic impairment (Child-Pugh class B or C) |
| Chronic renal insufficiency, as defined by serum creatinine > 2.5 mg/dL at screening, or requires dialysis support |
| Hemoglobin concentration < 9 g/dL at screening |
| History of atrial septostomy |
| Repaired or unrepaired congenital heart disease |
| Pericardial constriction |
| Restrictive or congestive cardiomyopathy |
| LVEF <40%, as shown by MUGA, angiography, or echocardiography |
| Symptomatic coronary disease with demonstrable ischemia |
| Other severe acute or chronic medical or laboratory abnormality that may increase the risk associated with study participation or investigational product administration or may interfere with the interpretation of study results and, in the judgment of the investigator, would make the subject inappropriate for this study |
| Psychiatric, addictive, or other disorder that compromises the ability to give informed consent for participating in this study; this includes recent history of alcohol or illicit drug abuse 30 days before study screening (day 1) and for the duration of the study |
| Poorly controlled asthma, defined by active wheezing and/or cough with FEV1 < 70% predicted, responsive to inhaled BD (>15% increase in FEV1 with BD) |
| Clinically significant intercurrent illness (including lower respiratory tract infection) or clinically significant surgery within 4 weeks before the administration of study drug |
| History of hypersensitivity or idiosyncratic reaction to drugs from multiple drug classes |
| Receipt of an investigational product or device or participation in a drug research study within a period of 15 days (or 5 half-lives of the drug, whichever is longer) before the first dose of study drug |
| Blood loss or blood donation > 550 mL within 90 days or plasma donation > 500 mL within 14 days before administration of study drug |
| QTc interval > 450 ms |
| Diabetes mellitus, as defined by symptoms of hyperglycemia and serum fasting plasma glucose level ≥ 7.0 mmol/L or casual plasma glucose ≥ 11.1 mmol/L at screen |
| Hyperlipidemia, defined as TC ≥ 6.22 mmol/L, LDL-C ≥ 4.14 mmol/L, or TG ≥ 2.26 mmol/L |
| History of Crohn’s disease, ulcerative colitis, inflammatory bowel disease, etc. |
| Unwilling to take contraceptive measures during the study |
| Use of certain other medications requires evaluation for possible exclusion based on the potential for adverse drug interactions |
BD: bronchodilator; BP: blood pressure; FEV1: forced expiratory volume after 1 s; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; MAP: mean arterial pressure; mPAP: mean pulmonary artery pressure; MUGA: multiple-gated acquisition scan; PH: pulmonary hypertension; PAH: pulmonary arterial hypertension; TC: total cholesterol; TG: triglycerides.
Study design rationale
The three main considerations in the study design are (1) study entry criteria, (2) SQV+RIT dosing and frequency of administration, and (3) timing of end points to capture onset and duration of effect.
1. Recently, increased circulating levels of monocyte chemoattractant protein 1, TNF-α, and other proinflammatory cytokines, including IL-1β, IL-2, IL-6, IL-8, and IL-12p70, have been observed in patients with IPAH, compared to healthy controls.17 Preliminary data from studies conducted in China and the United States by the authors also revealed elevated HMGB1 blood levels in IPAH patients. To include patients with a range of inflammatory biomarker levels, patients will be enrolled by considering clinical criteria and not baseline biomarker levels.
2. Patients will be divided into 3 cohort studies in sequence. These include groups to be treated with micro (1/50 of the standard dose), low (1/5 of the standard dose), and standard doses of SQV (15 mg/kg) and RIT (1.5 mg/kg) twice daily. The two main considerations of the design are as follows. (a) The primary purpose is to determine the potential targeting effect of SQV+RIT on the levels of HMGB1 and other inflammatory biomarkers in IPAH patients. The 2 low-dose groups will provide insight on the minimal dose needed to observe any effect. The protocol may be revised on the basis of results from micro and low-dose groups. (b) The safety of SQV+RIT is well established in Western countries.38 However, SQV+RIT has not been extensively studied in the Chinese population. Our dosing strategy is also intended to establish the safety of SQV+RIT. Only if the lower doses are well tolerated will the standard HIV dose be given.
3. Results from animal experiments indicate that treatment for 7 days with HIV-PI (amprenavir, RIT, or nelfinavir) improves pulmonary hemodynamics and prevents muscularization of pulmonary vessels.24 Patients will undergo 14 days of treatment and another 14 days of observation. The expected outcome in IPAH patients is a reduction in the level of HMGB1 and other inflammatory factors. Pulmonary artery pressure and symptom improvement will also be assessed. HMGB1 and other inflammatory factors will be measured at day 1 and day 2 to establish the baseline levels and then again on days 14 and 28. Pulmonary artery pressure changes will be tested with echocardiography. The flow diagram of the study is shown in Figure 2.
Figure 2.
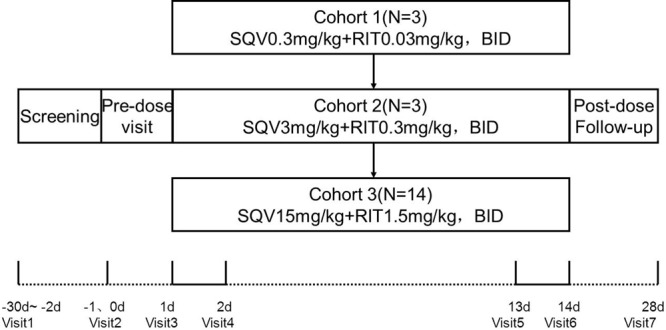
Schematic of the pilot study. The dotted line indicates outpatient assessment, and the solid line indicates inpatient assessment. BID: twice daily; RIT: ritonavir; SQV: saquinavir.
Testing will be considered complete for the purpose of this study once subjects reach the end of day 28. The end of the study is defined as the last visit of the last subject undergoing the trial.
Recruitment
Patients will be identified and screened on the basis of clinical data collected during routine outpatient appointments at Xiangya Hospital. Potential subjects will be identified by the physician investigator, who is also the treating physician. The physician investigator, who already has knowledge of and access to the patients’ information, will review records to identify potential research subjects for the study. After identifying potentially eligible subjects, the physician investigator will approach them to discuss the research opportunity.
To minimize the possibility that patients will feel obligated to participate, investigators will reiterate to them that their participation is voluntary, that they do not have to participate, and that the decision not to participate will not affect their care now or in the future. The physician investigator will also allow patients to make further inquiries if they are interested.
Twenty patients who are eligible to participate in the study will be required to read and sign the informed consent. The first 3 eligible patients will be entered into the micro-dose group, and next 3 patients will be entered into the low-dose group. After a 1-month washout period, those 6 patients will be offered the opportunity to be entered into the standard-dose group.
Investigational drugs
Patients who are eligible to participate in the study will receive SQV+RIT treatment. SQV was the first HIV-PI approved by the US Food and Drug Administration (FDA) and has been shown to be safe even when chronically administered to humans. SQV is typically administered with RIT, which increases the bioavailability of SQV.38,39 The FDA-approved dosage of SQV+RIT for HIV infection is SQV 1,000 mg twice daily (5 × 200-mg capsules or 2 × 500-mg tablets), in combination with RIT 100 mg twice daily. RIT is to be taken at the same time as SQV and within 2 hours after a meal. An abundance of clinical studies have demonstrated that this regimen is well tolerated and safe in HIV-infected subjects.38-42 A pilot study was conducted in steroid-dependent and steroid-resistant nephrotic syndrome patients treated with SQV (30 mg/kg/day) for at least 6 months; clinical benefit was observed, and no adverse drug reaction was reported, apart from transitory mild diarrhea.35 To ensure the safety of SQV+RIT in IPAH patients, besides contraindicated drugs, we will define potential interaction medications and formulate management of each drug (Table 2). All SQV and RIT capsules are labeled, packaged, and distributed by Roche Pharmaceuticals and directly transported and repacked at Fangsheng Pharmaceuticals in Changsha, China.
Table 2.
Potential drug interactions and management in PAH patients
| Concomitant drugs | Potential clinical effects | Management |
|---|---|---|
| PDE5 inhibitors | Increased potential for sildenafil-associated AEs (which include visual disturbances, hypotension, prolonged erection, and syncope); a safe and effective dose has not been established when used with SQV+RIT | Use sildenafil with caution at reduced doses of 25 mg every 48 h, with increased monitoring of AEs when administered concomitantly with SQV+RIT |
| HMG-CoA reductase inhibitors | Potential for myopathy, including rhabdomyolysis | Titrate atorvastatin dose carefully and use the lowest dose necessary; do not exceed atorvastatin 20 mg/day |
| Endothelin receptor antagonists: bosentan | Increases in serum bosentan concentration when bosentan was coadministered with SQV+RIT; SQV’s effect on bosentan is not well established | Discontinue use of bosentan at least 36 h before initiation of SQV+RIT; at least 10 days after the initiation of SQV+RIT, resume bosentan at 62.5 mg once daily or every other day, depending on individual tolerability |
| Anticoagulant: warfarin | Increased warfarin effects (e.g., increased INR and risk of bleeding) | Concentrations of warfarin may be affected; it is recommended that INR be monitored |
| Calcium channel blockers (CCBs) | Impact on the PR interval of coadministration of SQV+RIT with other drugs that prolong the PR interval | Use with caution; titrate CCB dose and monitor closely; electrocardiographic monitoring is recommended when a CCB is used with SQV+RIT |
AEs: adverse events; HMG-CoA: 3-hydroxy-3-methyl-glutaryl coenzyme A; INR: international normalized ratio; PDE5: phosphodiesterase 5; RIT: ritonavir; SQV: saquinavir.
Outcome measures and data collection
All visits and assessments described in this section are summarized in Table 3.
Table 3.
Visit and assessment schedule
| Screening | Pretreatment | Treatment | Follow-up | ||||
|---|---|---|---|---|---|---|---|
| Outpatient clinic | Inpatient assessment | Outpatient clinic | |||||
| Days of intervention | |||||||
| Study procedures | Before treatment | Baseline (days −1 and 0) | Day 1 | Day 2 | Day 13 | Day 14 | Day 28 |
| Informed consent | × | ||||||
| Medical history, demographics | × | ||||||
| Inclusion/exclusion criteria | × | ||||||
| Physical exam | × | × | × | × | |||
| 6MWD | × | × | × | ||||
| Vital signs | × | × | × | × | × | × | × |
| Oxygen saturation | × | × | × | × | × | × | × |
| Laboratory tests | × | × | × | × | |||
| NT-proBNP, ANP | × | × | × | ||||
| Inflammatory biomarker | × | × | × | × | × | × | |
| Electrocardiogram | × | × | × | × | × | ||
| Echocardiogram | × | × | × | × | |||
| NYHA/WHO FC assessment | × | × | × | × | × | ||
| Compliance evaluations | × | × | × | × | × | ||
| Adverse-event assessment | × | × | × | × | × | × | |
| Concomitant medications | × | × | × | × | × | × | × |
ANP: atrial natriuretic peptide; NT-proBNP: N-terminal pro–brain natriuretic peptide; NYHA/WHO FC: New York Heart Association/World Health Organization functional class; 6MWD: 6-minute walk distance.
Primary outcome measures. The primary measure of efficacy will be change in HMGB1 level from baseline at 14 days, measured with an enzyme-linked immunosorbent assay (ELISA) kit.
Secondary outcome measures. To determine whether short-term use of SQV+RIT reduces inflammatory biomarkers, the parameters of TNF-α, IL-1β, IL-6, N-terminal pro–brain natriuretic peptide (NT-proBNP), and C-reactive protein (CRP) will be measured in the blood with ELISA kits. Changes in pulmonary artery pressure, total right heart function, New York Heart Association/World Health Organization (NYHA/WHO) functional class, and Brog dyspnea scale index from baseline will also be assessed at 14 days.
A summary of how measurements will be made is as follows:
-
•
Inflammation biomarkers: HMGB1, TNF-α, IL-1β, IL-6, and CRP measurements will be executed with specific ELISA kits.
-
•
Heart failure indicator: NT-ProBNP and atrial natriuretic peptide measurements will be executed with specific ELISA kits.
-
•
Cardiopulmonary hemodynamics: pulmonary arterial pressure and cardiac output measurements will be executed with echocardiography.
-
•
The 6-minute walk distance (6MWD) and Brog dyspnea scale will be measured according to American Thoracic Society guidelines.
-
•
NYHA/WHO functional class will be assessed according to standard guidelines.
Statistical methods
This is an open label, single-blind, pre-post clinical study with 3 cohorts. Both patients and physicians will know the type and dosing of the research drugs. The statistician analyzing the results will be blinded to the study cohort. All causes of missing data will be collected and reviewed to determine the type of missing outcome data. All data will be analyzed at the Center of Clinical Pharmacology at the Third Xiangya Hospital with the single-blind analysis method. A full statistical-analysis plan will be developed for each end point. A T test will be performed to determine difference in mean values for primary and secondary outcome measures at baseline and day 14. Because of the exploratory nature of the study, no multiplicity adjustment will be made to P values for secondary analysis, although results will be interpreted with caution if the primary end point does not achieve significance. Adverse events (AEs) will be recorded in detail. AEs occurring before treatment, during active treatment, and after treatment will be summarized separately. The number and percentage of subject experiencing at least one AE of any type will be recorded. Separate summaries will be provided for all AEs, AEs by maximum severity, drug-related AEs, severe AEs, and AEs leading to withdrawal.
Results
This is an exploratory study, and the sample size was chosen according to the principles of the FDA phase 0 guidelines (<15 patients) for the first and second cohorts and with respect to safety as well. We expect that 6 patients in the first and second cohorts will give us safety information and some insight into the potential changes in inflammatory biomarkers.
In the standard-dose cohort, we hypothesize that treatment with SQV+RIT in IPAH patients will result in a 50% reduction in HMGB1. In our preliminary data, the average levels of HMGB1 were 4.16 ± 1.96 ng/mL in healthy volunteers (n = 12), 10.85 ± 4.88 ng/mL in IPAH patients (n = 13), 3.46 ± 2.73 ng/mL in connective tissue disease–associated PAH patients (n = 10), 6.06 ± 1.33 ng/mL in chronic thromboembolic pulmonary hypertension patients (n = 6), and 2.85 ± 1.84 ng/mL in congenital heart disease–associated PAH patients (n = 10). These levels were significantly raised in the IPAH group compared with healthy control subjects (P < 0.01), but no significant difference was observed in patients with other type of PAHs compared with healthy control subjects. With power set to 0.90 and α to 0.05 and using a 2-sided test and 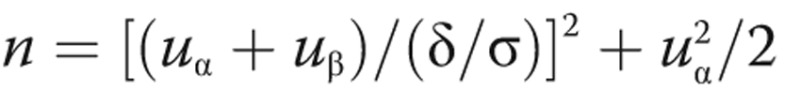 , the number of patients needed to show a treatment effect is 12. An additional 15% increase in sample size was included in the design to address possible incomplete data, measurement variation, and dropout. Therefore, the total number is 14 in the standard-dose group. After a 1-month washout period, patients in cohorts 1 and 2 will be able to join the last cohort under their own will, so the total potential number is 14–20. We will endeavor to minimize loss of patient follow-up and resulting missing outcome data. We expect that a sample size of 14 in the standard-dose group will be sufficient to detect meaningful changes in inflammation biomarkers. We also expect to see trends in cardiopulmonary hemodynamics and symptom improvement in this group.
, the number of patients needed to show a treatment effect is 12. An additional 15% increase in sample size was included in the design to address possible incomplete data, measurement variation, and dropout. Therefore, the total number is 14 in the standard-dose group. After a 1-month washout period, patients in cohorts 1 and 2 will be able to join the last cohort under their own will, so the total potential number is 14–20. We will endeavor to minimize loss of patient follow-up and resulting missing outcome data. We expect that a sample size of 14 in the standard-dose group will be sufficient to detect meaningful changes in inflammation biomarkers. We also expect to see trends in cardiopulmonary hemodynamics and symptom improvement in this group.
Discussion
Inflammation is an increasingly recognized feature of PAH, and this study is the first anti-inflammation therapy trial for IPAH patients, as far as we know. Recent evidence indicates that HIV-PIs can improve pulmonary hemodynamics in experimental models of PAH.34 Experimental evidence also indicates that both TLR4 and HMGB1 participate in the pathogenesis of experimental pulmonary hypertension.16,22-26 We and others have shown elevated circulating HMGB1 levels in IPAH patients.24 A recent high-throughput screen for inhibitors of HMGB1-induced macrophage activation yielded HIV-PIs as potent inhibitors of HMGB1-induced cytokine production.31 On the basis of experimental evidence, we propose a pilot trial to determine whether SQV+RIT treatment will suppress inflammation biomarkers and improve pulmonary hemodynamics in IPAH patients. The proposed pilot study has the potential to lead to new insight into the pathophysiology of PAH.
To accelerate the discovery and development of new molecular entities, the FDA released exploratory investigational new drug (IND) guidance in 2006 to support clinical evaluation before dose escalation, safety, and tolerance studies associated with a traditional IND.43 Three “limited” concepts are described in the guideline: dosing a limited number of subjects with a limited range of doses for a limited period of time. Muller44 summarized the systematic comparison of requirements for exploratory IND studies based on the International Conference on Harmonisation (ICH) M3(R2) guidelines. Starting doses are different in clinical strategy: from less than 1/100 of the dose calculated in preclinical animal toxicology research to 1/50 of the no-observed-adverse-effect level in more sensitive species. The number of participants needed is smaller than that needed for a phase 1 trial, typically less than 15. Generally, the maximum study duration is 7 days, but the guidance allows considerable flexibility in study design. A recent review45 describes how the FDA allowed a pharmaceutical company to conduct a phase 0 trial with a dosing period longer than 7 days but no longer than 14 days.
SQV+RIT has been widely used in HIV patients with good tolerance. In a pilot study conducted in patients with steroid-dependent and steroid-resistant nephrotic syndrome treated with SQV (30 mg/kg/day) for at least 6 months, no adverse drug reaction was reported, apart from transitory mild diarrhea.35 These data suggested that SQV and RIT are safe and well tolerated in the short term. In our study, the starting dose is designed as 1/50 of the standard dose; 10 times the first dose (1/5 of the standard dose) will be given to the second cohort. Those two groups will include 3 patients each, and the treatment period will be 14 days. We hope that these micro- and low-dose groups will provide some guidance for the design of the standard-dose group and will establish whether SQV+RIT is safe for IPAH patients. If the first two cohorts confirm safety, the last cohort (N = 14) will receive the standard dose of SQV+RIT for 14 days to explore the safety and targeted effect of SQV+RIT in IPAH patients.
The primary purpose of this short-term exploratory clinical trial for IPAH patients is to determine whether SQV+RIT reduces HMGB1 and other inflammatory biomarkers in IPAH patients, as seen in experimental models. Furthermore, changes in pulmonary hemodynamics, cardiac function, and exercise ability will be measured. We hope that a significant beneficial change can be achieved with SQV+RIT therapy (especially in the standard-dose group) in this small-sample-size study. This study could provide support subsequent larger phase 2 and 3 studies.
We have chosen to limit the study to patients with IPAH and not those with other subgroups of PAH. This is based, first, on the fact that our preliminary data on elevated HMGB1 levels are only for IPAH patients. In addition, we have excluded other forms of PAH, since many disease-associated factors may influence inflammation in these conditions. For example, systemic inflammation may be a key pathogenic factor in patients with connective-tissue disease. If our study provides evidence that SQV+RIT has efficacy in IPAH patients, studies could be extended to include patients with other etiologies for PAH in the future.
Baseline levels of HMGB1 may be found to vary. If this is the case in our first 6 patients, we will consider using elevated baseline HMGB1 levels as a criterion for inclusion in the third-highest-dose cohort.
In conclusion, this pilot study of SQV+RIT in IPAH patients will address safety and the targeted effects of SQV+RIT on inflammation biomarkers in IPAH patients. We anticipate that the results of the study will provide insights into therapeutic strategies designed to improve aberrant inflammation as drivers of IPAH and potentially other types of pulmonary hypertension.
Source of Support: This study is funded by the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” in China (no. 2012ZX09303014–001, to HY) and National Natural Science Foundation of China funding (no. 81200035, to XL), as well as the Hunan Provincial Innovation Foundation for Postgraduates (no. CX2014B108, to YL) and a Central South University Top Creative PhD scholarship (no. 2014bjjxj061) to YL.
Conflict of Interest: None declared.
Supplements
Protocol (885.6KB, pdf)
Informed consent (207.7KB, pdf)
References
- 1.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 2.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiéry JL, Barberà JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34(6):1219–1263. [DOI] [PubMed]
- 3.Rubin J. Primary pulmonary hypertension. Chest 1993;104(1):236–250. [DOI] [PubMed]
- 4.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL registry. Chest 2012;142(2):448–456. [DOI] [PubMed]
- 5.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bonemorphogenetic protein receptor-II gene. Am J Hum Genet 2000;67(3):737–744. [DOI] [PMC free article] [PubMed]
- 6.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, Loyd JE, Nichols WC, Trembath RC; the International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet 2000;26(1):81–84. [DOI] [PubMed]
- 7.Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA III, Loyd JE. Mutations in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med 2001;345(5):319–324. [DOI] [PubMed]
- 8.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 2005;26(6):1110–1118. [DOI] [PubMed]
- 9.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 1998;114(3_suppl.):225S–230S. [DOI] [PubMed]
- 10.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, Duroux P, Galanaud P, Simonneau G, Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995;151(5):1628–1631. [DOI] [PubMed]
- 11.Sakamaki F, Kyotani S, Nagaya N, Sato N, Oya H, Satoh T, Nakanishi N. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000;102(22):2720–2725. [DOI] [PubMed]
- 12.Fartoukh M, Emilie D, Le Gall C, Monti G, Simonneau G, Humbert M. Chemokine macrophage inflammatory protein-1α mRNA expression in lung biopsy specimens of primary pulmonary hypertension. Chest 1998;114(1_suppl.):50S–51S. [DOI] [PubMed]
- 13.Olivetta E, Percario Z, Fiorucci G, Mattia G, Schiavoni I, Dennis C, Jäger J, et al. HIV-1 Nef induces the release of inflammatory factors from human monocyte/macrophages: involvement of Nef endocytotic signals and NF-κB activation. J Immunol 2003;170 (4):1716–1727. [DOI] [PubMed]
- 14.Negi VS, Tripathy NK, Misra R, Nityanand S. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol 1998;25(3):462–466. [PubMed]
- 15.Morse JH, Barst RJ, Fotino M, Zhang Y, Flaster E, Gharavi AE, Fritzler MJ, Dominguez M, Angles-Cano E. Primary pulmonary hypertension, tissue plasminogen activator antibodies, and HLA-DQ7. Am J Respir Crit Care Med 1997;155(1):274–278. [DOI] [PubMed]
- 16.Morse JH, Barst RJ, Fotino M, Zhang Y, Flaster E, Fritzler MJ. Primary pulmonary hypertension: immunogenetic response to high-mobility group (HMG) proteins and histone. Clin Exp Immunol 1996;106(2):89–95. [DOI] [PMC free article] [PubMed]
- 17.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010;122(9):920–927. [DOI] [PubMed]
- 18.Chen L, Nakano K, Kimura S, Matoba T, Iwata E, Miyagawa M, Tsujimoto H, et al. Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension 2011;57(2):343–350. [DOI] [PubMed]
- 19.Wang Q, Zuo XR, Wang YY, Xie WP, Wang H, Zhang M. Monocrotaline-induced pulmonary arterial hypertension is attenuated by TNF-α antagonists via the suppression of TNF-α expression and NF-κB pathway in rats. Vasc Pharmacol 2013;58 (1–2):71–77. [DOI] [PubMed]
- 20.Sewing AC, Kantores C, Ivanovska J, Lee AH, Masood A, Jain A, McNamara PJ, Tanswell AK, Jankov RP. Therapeutic hypercapnia prevents bleomycin-induced pulmonary hypertension in neonatal rats by limiting macrophage-derived tumor necrosis factor-α. Am J Physiol Lung Cell Mol Physiol 2012;303(1):L75–L87. [DOI] [PubMed]
- 21.Paul-Clark MJ, George PM, Gatheral T, Parzych TK, Wright WR, Crawford D, Bailey LK, Reed DM, Mitchell JA. Pharmacology and therapeutic potential of pattern recognition receptors. Pharmacol Ther 2012;135(2):200–215. [DOI] [PubMed]
- 22.Raychaudhuri B, Bonfield TL, Malur A, Hague K, Kavuru MS, Arroliga AC, Thomassen MJ. Circulating monocytes from patients with primary pulmonary hypertension are hyporesponsive. Clin Immunol 2002;104(2):191–198. [DOI] [PubMed]
- 23.George PM, Badiger R, Shao D, Edwards MR, Wort SJ, Paul-Clark MJ, Mitchell JA. Viral Toll Like Receptor activation of pulmonary vascular smooth muscle cells results in endothelin-1 generation; relevance to pathogenesis of pulmonary arterial hypertension. Biochem Biophys Res Commun 2012;426(4):486–491. [DOI] [PubMed]
- 24.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med 2012;18:1509–1518. [DOI] [PMC free article] [PubMed]
- 25.Yang PS, Kim DH, Lee Y, Lee SE, Kang W, Chang HJ, Shin JS. Glycyrrhizin, inhibitor of high mobility group box-1, attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling in rats. Respir Res 2014;15:148. doi:10.1186/s12931-014-0148-4. [DOI] [PMC free article] [PubMed]
- 26.Sadamura-Takenaka Y, Ito T, Noma S, Oyama Y, Yamada S, Kawahara K, Inoue H, Maruyama I. HMGB1 promotes the development of pulmonary arterial hypertension in rats. PLoS ONE 2014;9(7):e102482. doi:10.1371/journal.pone.0102482. [DOI] [PMC free article] [PubMed]
- 27.Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao L, Huang J, et al. HMGB1 in health and disease. Mol Asp Med 2014;40:1–116. [DOI] [PMC free article] [PubMed]
- 28.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 2010;107(26):11942–11947. [DOI] [PMC free article] [PubMed]
- 29.Newman WH, Zhang LM, Leeper-Woodford SK, Castresana MR. Human blood vessels release tumor necrosis factor-α from a smooth muscle cell source. Crit Care Med 1996;24(2):294–297. [DOI] [PubMed]
- 30.Ranta V, Orpana A, Carpén O, Turpeinen U, Ylikorkala O, Viinikka L. Human vascular endothelial cells produce tumor necrosis factor-α in response to proinflammatory cytokine stimulation. Crit Care Med 1999;27(10):2184–2187. [DOI] [PubMed]
- 31.Gerö D, Szoleczky P, Módis K, Pribis JP, Al-Abed Y, Yang H, Chevan S, Billiar TR, Tracey KJ, Szabo C. Identification of pharmacological modulators of HMGB1-induced inflammatory response by cell-based screening. PLoS ONE 2013;8(6):e65994. doi:10.1371/journal.pone.0065994. [DOI] [PMC free article] [PubMed]
- 32.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer Res 2005;65(18):8256–8265. [DOI] [PubMed]
- 33.Sgadari C, Monini P, Barillari G, Ensoli B. Use of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growth. Lancet Oncol 2003;4(9):537–547. [DOI] [PubMed]
- 34.Gary-Bobo G, Houssaini A, Amsellem V, Rideau D, Pacaud P, Perrin A, Brégeon J, et al. Effects of HIV protease inhibitors on progression of monocrotaline- and hypoxia-induced pulmonary hypertension in rats. Circulation 2010;122(19):1937–1947. [DOI] [PMC free article] [PubMed]
- 35.Coppo R, Camilla R, Porcellini MG, Peruzzi L, Gianoglio B, Amore A, Daprà V, et al. Saquinavir in steroid-dependent and -resistant nephrotic syndrome: a pilot study. Nephrol Dial Transplant 2012;27(5):1902–1910. [DOI] [PubMed]
- 36.Wolf T, Findhammer S, Nolte B, Helm EB, Brodt HR. Inhibition of TNF-α mediated cell death by HIV-1 specific protease inhibitors. Eur J Med Res 2003;8(1):17–24. [PubMed]
- 37.Equils O, Shapiro A, Madak Z, Liu C, Lu D. Human immunodeficiency virus type 1 protease inhibitors block toll-like receptor 2 (TLR2)- and TLR4-induced NF-κB activation. Antimicrob Agents Chemother 2004;48(10):3905–3911. [DOI] [PMC free article] [PubMed]
- 38.Knechten H, Lutz T, Pulik P, Martin T, Tappe A, Jaeger H. Safety and efficacy in HIV-1-infected patients treated with ritonavir-boosted saquinavir mesylate. Arch Drug Inf 2010;3(1):26–36. [DOI] [PMC free article] [PubMed]
- 39.Stephan C, Jaeger H, Carganico A, Knecht G, Lutz T, Mayr C, Mosthaf FA, et al. Safety and efficacy after switch to a saquinavir-containing antiretroviral regimen in protease inhibitor pretreated HIV-positive patients. Eur J Med Res 2010;15(9):369–376. [DOI] [PMC free article] [PubMed]
- 40.Walmsley S, Avihingsanon A, Slim J, Ward DJ, Ruxrungtham K, Brunetta J, Bredeek UF, et al. Gemini: a noninferiority study of saquinavir/ritonavir versus lopinavir/ritonavir as initial HIV-1 therapy in adults. J Acquir Immune Defic Syndr 2009;50(4):367–374. [DOI] [PubMed]
- 41.Bunupuradah T, van der Lugt J, Kosalaraksa P, Engchanil C, Boonrak P, Puthanakit T, Mengthaisong T, et al. Safety and efficacy of a double-boosted protease inhibitor combination, saquinavir and lopinavir/ritonavir, in pretreated children at 96 weeks. Antivir Ther 2009;14(2):241–248. [PubMed]
- 42.Brunet C, Reliquet V, Jovelin T, Venisse N, Winer N, Bui E, Le Moal G, Perfezou P, de Saint Martin L, Raffi F. Effectiveness and safety of saquinavir/ritonavir in HIV-infected pregnant women: INEMA cohort. Med Mal Infect 2012;42(9):421–428. [DOI] [PubMed]
- 43.Center for Drug Evaluation and Research. Guidance for industry, investigators, and reviewers: exploratory IND studies. US Department of Health and Human Services, Food and Drug Administration. 2006. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm078933.pdf. Accessed February 21, 2008.
- 44.Muller PY. Comparative requirements for exploratory clinical trials—eIND, eCTA and microdosing. Adv Drug Deliv Rev 2011;63(7):511–517. [DOI] [PubMed]
- 45.Robinson WT. Innovative early development regulatory approaches: expIND, expCTA, microdosing. Clin Pharmacol Ther 2008;83(2):358–360. [DOI] [PubMed]


