Abstract
Innate lymphoid cells (ILCs) are part of a heterogeneous family of innate immune cells with newly identified roles in mediating immunity, tissue homeostasis and pathologic inflammation. Here, we review recent studies delineating the roles of ILCs in the pathogenesis of multiple inflammatory skin disorders and their unique effector functions. Finally, we address how these studies have informed our understanding of the regulation of ILCs and the therapeutic potential of targeting these cells in the context of skin inflammation.
Introduction
Classically, it is believed that adaptive helper T (TH) cells underlie the pathogenesis of multiple inflammatory skin disorders such as atopic dermatitis (AD) (Bieber, 2008) and psoriasis (Nestle et al., 2009). Unique cytokine profiles distinguish TH cells into different subsets and define their effector functions. For example, T helper type 2 (TH2) cells produce hallmark type 2 cytokines IL-4, IL-5 and IL-13 are believed to underlie the pathogenesis of AD (Brandt and Sivaprasad, 2011). In contrast, T helper type 17 (TH17) cells that produce IL-17 and IL-22 contribute to the development and progression of psoriasis (Lowes et al., 2014). Traditionally, immunosuppressive therapeutics for the treatment of AD (e.g., calcineurin inhibitors) and psoriasis (e.g., methotrexate) are thought to broadly inhibit the function of these TH cell subsets. However, recent studies suggest that previously unrecognized innate immune cells may regulate TH cells in the skin and have direct contributions to the development of pathologic skin inflammation. Specifically, innate lymphoid cells (ILCs) have emerged as key players in the pathogenesis of multiple inflammatory skin disorders. This review will focus on the recent advances in our understanding of how ILCs drive skin inflammation and how they may differentially respond to tissue-specific cytokines and factors. We highlight recent studies that reveal how ILCs are regulated and discuss the therapeutic potential of targeting ILCs in the context of skin inflammation.
Definition of Innate Lymphoid Cell Subsets
ILCs are part of a family of innate immune cells that are derived from a common lymphoid progenitor and their development is partially or completely dependent on the common γ-chain (γc or CD132), IL-7, Notch and the transcription factor Inhibitor of DNA binding 2 (Id2) (Mjosberg and Eidsmo, 2014). More recent studies indicate that multiple ILC lineages are also dependent on the transcriptional repressor PLZF, while others (e.g., natural killer cells and lymphoid tissue inducers) are not (Constantinides et al., 2014). Further, all ILC subsets have also been shown to arise from a common lineage-negative (Lin−) Id2+ CD127+ CD25− α4β7+ c-Kit+ precursor (Constantinides et al., 2014; Klose et al., 2014). ILCs are currently categorized into three distinct populations on the basis of their developmental requirements, transcription factor expression profile and/or expression of effector cytokines (Figure 1): group 1 ILCs (ILC1s) are T-bet-dependent IFN-γ- and TNF-α-producing ILCs; RORα-dependent, TCF-1-dependent and GATA3-expressing group 2 ILCs (ILC2s) produce IL-4, IL-5 and IL-13; and RORγt-dependent group 3 ILCs (ILC3s) produce IL-17A and/or IL-22 (Sonnenberg et al., 2013). These ILC groups are analogous to the previously described IFN-γ and TNF-α-producing TH1, IL-4-, IL-5- and IL-13-producing TH2 and IL-17- and IL-22-producing TH17 CD4+ TH cell subsets. However, although ILCs appear to have parallel functions to adaptive CD4+ T cells, they are unique in that they respond to innate signals in the absence of antigen-specificity and have distinct phenotypic and functional profiles.
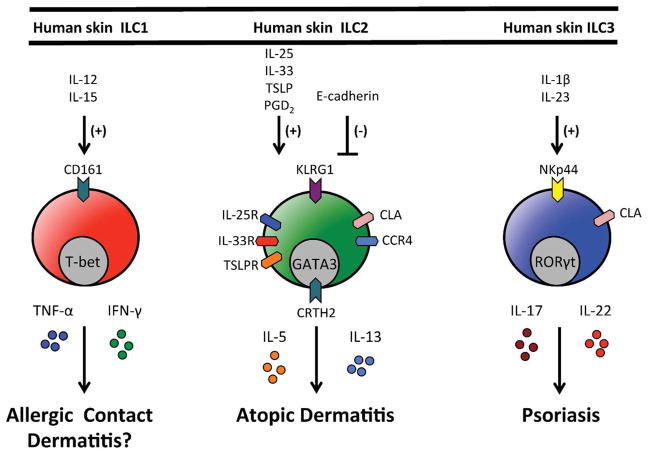
Figure 1. Regulation and function of human skin ILC responses.
Human skin ILC1s express CD161 and would be predicted to be activated by IL-12 and IL-15 and produce the effector cytokines TNF-α and IFN-γ. Human skin ILC2s express KLRG1, CLA, CCR4, IL-25R (IL-17Rb), IL-33R (ST2), CRTH2 and TSLPR. In response to IL-25, IL-33 and/or TSLP they produce effector cytokines IL-5 and IL-13 and have been implicated in atopic dermatitis. Human skin ILC2s also migrate in response to PGD2. Human skin ILC3s express NKp44 and CLA, produce IL-17 and IL-22 in response to IL-1β and IL-23 and have been implicated in psoriasis.
Although similar to TH cell subsets in their cytokine profile, ILCs are distinguished from the common cell lineages in that they do not express cell lineage (Lin) markers associated with T cells, B cells, DCs, macrophages and granulocytes but express CD25 (IL-2Ra), CD90 (Thy1 Ag) and CD127 (IL-7Ra) (Spits and Cupedo, 2012). Further, ILC2s can be readily identified by their expression of the receptor for IL-33 (IL-33R or T1/ST2 in mice or ST2 in humans). Different subsets of ILCs promote either tissue homeostasis or detrimental inflammatory processes at multiple epithelial barrier surfaces. Further, these cells have been implicated in a variety of different disease states including allergy, autoimmunity, cancer, infection and obesity (Hams et al., 2013; Kim et al., 2013b; Molofsky et al., 2013; Nussbaum et al., 2013; Sonnenberg and Artis, 2012). The roles of ILCs in various disease states and at other barrier surfaces have been covered extensively elsewhere (Fuchs and Colonna, 2013; Hepworth et al., 2013; Locksley and Fahy, 2014; Monticelli et al., 2012; Sonnenberg and Artis, 2012). However, the diverse functional properties of ILCs in the skin remain less well defined. Therefore in this review we will focus primarily on the emerging role of ILCs in skin health and disease. First, we will introduce ILC2s given that multiple groups have defined their contribution to AD. Second, we will highlight recent studies identifying skin ILC3s and their role in psoriasis. Finally, we will briefly outline the function of ILC1s and their potential role in skin inflammation.
ILC2s and Atopic Dermatitis
ILC2s were originally identified in the gut and associated lymphoid tissues and found to contribute to immunity to helminth parasites in the absence of the adaptive immune system (Fallon et al., 2006; Fort et al., 2001; Moro et al., 2010; Neill et al., 2010; Price et al., 2010; Schmitz et al., 2005). In addition to being distinguished by their expression of the effector type 2 cytokines IL-5 and IL-13, ILC2s also express IL-33R (Kim et al., 2013b). Subsequent studies showed that ILC2s play key roles in airway hyper-reactivity (Chang et al., 2011) and epithelial tissue repair in the lung (Monticelli et al., 2011) in mice and chronic rhinosinusitis in humans (Mjosberg et al., 2012; Mjosberg et al., 2011). These early studies demonstrated that ILC2s were predominantly regulated by the epithelial cell-derived cytokines IL-25 and IL-33 (Monticelli et al., 2012).
When ILC2s were first identified in both murine (Kim et al., 2013a; Roediger et al., 2013) and human skin, they were also found to be highly enriched in lesional skin of patients with AD (Kim et al., 2013a). Adaptive CD4+ TH2 cells that produce the effector type 2 cytokines IL-4, IL-5 and IL-13 are classically considered the main contributors to the pathogenesis of AD. It is believed that type 2 inflammation underlies acute to subacute AD which manifests histologically as a combination of irregular acanthosis (epidermal thickening), spongiosis (interepidermal edema) and superficial perivascular inflammation in the dermis composed predominantly of lymphocytes and histiocytes with occasional neutrophils and eosinophils. Strikingly, ILC2s that produce IL-5 and IL-13 were found to be both necessary and sufficient for the development of AD-like disease in a mouse model (Kim et al., 2013a; Roediger et al., 2013). Further, in contrast to the previously ascribed roles of IL-25 and/or IL-33 in the gut and lung, proinflammatory skin ILC2s were found to be dependent on another epithelial cell-derived cytokine called thymic stromal lymphopoietin (TSLP) (Kim et al., 2013a). Beyond TSLP, a more recent study showed that transgenic overexpression of IL-33 under a keratin 14 promoter is sufficient to induce AD-like disease in association with the accumulation of ILC2s (Imai et al., 2013). In addition to IL-33, Salimi et al. found that skin ILC2 responses demonstrate dependence on IL-25, IL-33 and and/or TSLP depending on the mouse strain employed (Salimi et al., 2013). Taken together, these studies identified a previously unrecognized role for skin ILC2s in the development of AD-like disease and their diverse mechanisms of regulation.
Similar to murine ILC2s, human skin ILC2s express IL-33R (Kim et al., 2013a) as well as chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) (Salimi et al., 2013; Xue et al., 2014). Xue et al. isolated CRTH2+ human skin ILC2s and showed that they migrate in response to the CRTH2 ligand prostaglandin D2 (PGD2) (Xue et al., 2014). Additionally, they express skin-homing receptors such as cutaneous lymphocyte antigen (CLA), CCR4 and CCR10, and are activated by IL-33 to produce the type 2 cytokines IL-5 and IL-13 (Salimi et al., 2013; Teunissen et al., 2014). In terms of regulation, Salimi et al. found that IL-25 or TSLP alone did not directly activate healthy human skin ILC2s but significantly potentiated the effects of IL-33 (Salimi et al., 2013). In contrast, Teunissen et al. found that TSLP alone was sufficient to activate expanded skin ILC2s and this effect was further enhanced by the addition of IL-25. (Teunissen et al., 2014). Therefore, the key epithelial cell-derived cytokines that regulate human skin ILC2s remain to be fully clarified in future studies. Notably, lesional AD skin from patients demonstrated increased expression of IL-17Rb (IL-25R), IL-33R and TSLP receptor (TSLPR) on ILC2s (Figure 1) (Salimi et al., 2013). These findings provoke the question as to whether activated human skin ILC2s in lesional AD skin respond differently to IL-25, IL-33 and/or TSLP than ILC2s derived from healthy control skin. Addressing this question may help to distinguish the relative importance of these cytokines in the context of established disease and the therapeutic potential of targeting them.
In addition to epithelial cell-derived cytokines, ILC2s are also activated by IL-2 and express the receptor for IL-7, indicating that they also respond to other stromal and hematopoietic cell-derived cytokines (Roediger et al., 2013). More recently, ILC2s were found to express IL-4Rα (Bando et al., 2013) and respond to basophil-derived IL-4 by increasing the production of the chemokine CCL11 as well as effector cytokines IL-5, IL-9 and IL-13 (Motomura et al., 2014). Along these lines, we recently identified that basophil-derived IL-4 critically regulates ILC2 proliferation in the context of AD-like inflammation (unpublished data). Taken together, these studies suggest that a variety of cytokines, chemokines and/or lipid mediators can differentially regulate the activation, migration, proliferation and effector function of ILC2s.
Beyond cytokine-mediated regulation, E-cadherin has been found to directly inhibit the effector cytokine production of human skin ILC2s, possibly via binding cell surface KLRG1 (Salimi et al., 2013). Given that E-caderin is known to be downregulated in lesional AD skin (Trautmann et al., 2001), this may be a key upstream mechanism by which loss of epithelial adhesion molecules in the context of skin barrier dysfunction leads to dysregulated ILC2 responses in AD (Figure 2). Indeed, these studies found that shRNA-mediated knockdown of filaggrin, a key skin barrier protein that is diminished in AD, results in loss of E-cadherin expression (Salimi et al., 2013). Collectively, these studies implicate ILC2s as a direct link between skin barrier defects and early immune dysregulation in the skin.
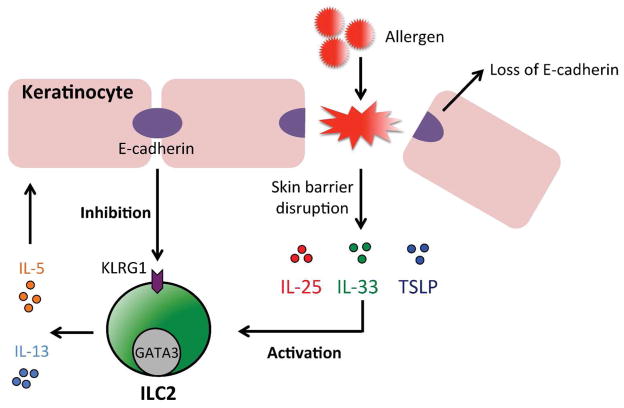
Figure 2. ILC2s and skin barrier dysfunction.
Human skin ILC2s have been implicated in the pathogenesis of atopic dermatitis and are activated by the epithelial cell-derived cytokines IL-25, IL-33 and/or TSLP to produce IL-5 and IL-13. In contrast, the keratinocyte cell adhesion molecule E-cadherin has been shown to inhibit the activation of skin ILC2s, possibly via KLRG1. Atopic dermatitis-associated inflammation and loss of skin barrier protein filaggrin expression is associated with the loss of E-cadherin expression in keratinocytes.
Finally, although skin ILC2s could promote AD-like disease in the absence of adaptive immunity, they were also sufficient to drive adaptive TH2 cell responses in vivo (Kim et al., 2013a). Recent studies show that this can occur indirectly through the effect of ILC2-derived IL-13 on DCs (Halim et al., 2014) and/or direct stimulation of T cells via MHC II (Mirchandani et al., 2014). Further, ILC2s were visualized in the skin time employing intravital multiphoton microscopy and found to selectively interact with mast cells in vivo (Roediger et al., 2013). Employing in vitro assays to monitor mast cell function, it has been proposed that ILC2s suppress mast cell function via the production of IL-13 in the steady state. In contrast, IL-2-mediated activation of ILC2s was sufficient to induce ILC2-dependent AD-like disease, enhanced production of IL-5 and the recruitment of eosinophils (Roediger et al., 2013). Collectively, these studies demonstrated novel regulatory and proinflammatory functions of ILC2s in the skin.
ILC3s and Psoriasis
CD4+ TH17 cells that produce the effector cytokines IL-17 and IL-22 are classically believed to underlie the pathogenesis of psoriasis in humans (Nestle et al., 2009). This TH17 cell-associated cytokine repertoire results in histologic features of regular acanthosis (uniform elongation of the epidermal rete ridges), parakeratosis (retention of nuclei in the stratum corneum) and a neutrophilic inflammatory infiltrate (Zheng et al., 2007). However, Pantelyushin et al. employed a murine model of psoriasis-like disease and found that TH17 cells were not the dominant source of these cytokines but rather RORγt+ γδ T cells and ILC3s (Pantelyushin et al., 2012). In support of this, they demonstrated that lymphocyte-deficient mice develop psoriasiform inflammation in a similar manner to wild-type mice that harbor TH17 cells. Taken together, these findings provoked the hypothesis that IL-17- and IL-22-producing ILC3s may directly contribute to the pathogenesis of psoriasis-like disease. Indeed, loss-of-function studies employing ILC-deficient Rag2−/− γc−/− mice demonstrated a reduction in disease severity (Pantelyushin et al., 2012). Collectively, these studies suggested that ILC3s may actually play a direct role in psoriasis in humans.
In support of the murine studies by Pantelyushin et al., a more recent study identified the presence of IL-17- and IL-22-producing NKp44+ ILC3s in the blood and skin of psoriasis patients (Villanova et al., 2014). Further investigation revealed that these NKp44+ ILC3s are highly enriched in both lesional and non-lesional skin of psoriasis patients in comparison to healthy control skin. These findings were confirmed by two separate studies demonstrating increased frequencies of RORγt+ CD56+ ILC3s that express NKG2D in both lesional and non-lesional skin of psoriasis patients (Dyring-Andersen et al., 2014) and NKp44+ ILC3s selectively enriched in lesional psoriasis skin (Teunissen et al., 2014). In support of a potential role for ILC3s in the pathogenesis of psoriasis, a case study in a patient on anti-tumor necrosis factor therapy (adalimumab) demonstrated correlation between therapeutic response and a reduction in ILC3s (Villanova et al., 2014). Further, Teunissen et al. found that NKp44− ILC3s convert to NKp44+ ILC3s following stimulation with IL-1β and IL-23 (Figure 1). Given that disruption of the p40 subunit of IL-23 is an effective therapy (ustekinumab) for psoriasis, these findings implicate ILC3s as a potential mechanism by which IL-23 may mediate disease. Similar to skin ILC2s, ILC3s were also found to express the skin-homing marker CLA (Teunissen et al., 2014; Villanova et al., 2014). Strikingly CLA expression was much higher on ILC3s in comparison to conventional CD4+ or CD8+ T cells (Villanova et al., 2014). Collectively, homologous to ILC2s in AD, these studies highlight a potential role for skin ILC3s in the pathogenesis of psoriasis.
Prior studies on ILC3s have demonstrated diverse functions in the intestine ranging from maintenance of epithelial barrier function to inflammation and protective immunity to extracellular bacteria (Hepworth and Sonnenberg, 2014). Furthermore, ILC3s have been found to directly regulate the function of CD4+ T cells to promote tissue homeostasis in the intestine (Hepworth et al., 2013). Although ILC3s have been implicated as proinflammatory cells in psoriasis (Pantelyushin et al., 2012), whether they interact with adaptive CD4+ T cells or help to maintain tissue homeostasis in the skin remains to be determined.
ILC1s and Skin Inflammation
ILC1s are not as well-defined as the ILC2 and ILC3 subsets. In addition to conventional NK (cNK) cells, multiple ILC1-like cells have been reported. Cytotoxic intestinal intraepithelial NKp44+ CD103+ (human) and CD160+ NKp46+ NK1.1+ (murine) ILC1-like cells resemble NK cells but are IL-15-independent and lack CD127 expression (Fuchs et al., 2013). More recently, non-cytotoxic T-bet+ Eomes− CD127+ non-NK cell ILC1s, which arise from a distinct precursor that does not give rise to cNK cells, but are dependent on IL-15 for their maintenance and survival have been reported (Klose et al., 2014). Based on their distinctly non-NK cell lineage, these cells now appear to be bona fide ILC1s. Further, T-bet-expressing “ex-RORγt” ILC3s arise from RORγt+ precursors and can be instructed by IL-12 and/or commensal microbiota for their differentiation into IFN-γ-expressing NK receptor-expressing ILC1-like cells (Bernink et al., 2013; Klose et al., 2013; Rankin et al., 2013; Vonarbourg et al., 2010). Despite these differences, ILC1-like cells and ILC1s are similar in that all express T-bet and IFN-γ (Bernink et al., 2013; Fuchs et al., 2013; Klose et al., 2014; Klose et al., 2013) (Figure 1). They have also been shown to produce TNF-α and mediate immunity to intracellular infections with Toxoplasma gondii and Salmonella (Klose et al., 2014; Klose et al., 2013). In addition, human ILC1s are associated with Crohn’s disease (Bernink et al., 2013; Fuchs et al., 2013) and their murine counterpart has been shown to contribute to the development of experimental colitis (Fuchs et al., 2013). Although CRTH2− c-Kit− NKp44− CD161+ ILC1s have been identified in human skin as a rare population (Teunissen et al., 2014; Villanova et al., 2014), their function in skin disease remains unknown and is an exciting area of future inquiry. Recent studies have demonstrated that allergic contact dermatitis (ACD) can exhibit mixed TH cell responses (Dhingra et al., 2014) in addition to classical TH1 cytokine responses (Gorbachev et al., 2001; Ishizaki et al., 2007; Kehren et al., 1999; Saint-Mezard et al., 2005). Similarly, based on the recent studies with ILC2s and ILC3s, it is possible that skin ILC1s may also contribute to the development of ACD.
Conclusions
The mammalian immune system has evolved a sophisticated array of adaptive immune responses to ward off invading microbial pathogens at the skin barrier. Specifically, adaptive immune cells such as T cells can recognize and combat infections in a specialized manner by recognizing specific antigens encountered over time. Additionally, recent studies have also demonstrated that adaptive T cells also limit the translocation of skin commensal bacteria into the lymphatic system (Shen et al., 2014). However, it is widely appreciated that inappropriate immune responses in the skin against environmental allergens or self-antigens can result in the development of a variety of inflammatory skin disorders. Therefore, interventions for diseases such as AD and psoriasis have classically attempted to target adaptive T cells.
The identification of different ILC populations that are resident at the skin barrier indicates that these cells may have evolved to respond immediately to pathogenic signals and to aid the adaptive immune system in mounting a robust immune response. The studies outlined in this review highlight that dysregulation of ILCs can result in the development of multiple inflammatory skin diseases. Although commensal microbiota in the gut are known to interact with ILC1-like cells (Klose et al., 2013; Vonarbourg et al., 2010) and ILC3s (Hepworth et al., 2013) (Qiu et al., 2013; Sonnenberg et al., 2012), their interactions with ILC2s and their role in skin barrier homeostasis is less well defined. It would be expected that innate signals from skin commensal microbiota are required to maintain normal frequencies and functions of skin ILCs and this remains an exciting area of future investigation. More broadly, how ILCs are regulated in the skin, the array of effector mechanisms they employ and their potential role in maintaining skin barrier homeostasis remains unclear. Inflammatory skin diseases are the most common conditions affecting the skin and it is possible that ILCs will play critical roles in a variety of disease states including, but not limited to, ACD, bullous pemphigoid, arthropod bites and Sweet’s syndrome. Based on the histopathology of these disease states it can be hypothesized which ILC subsets (e.g., ILC2s for eosinophilic or ILC3s for neutrophilic inflammation) may play a role in their pathogenesis. To improve our understanding of the function of skin ILCs, further investigation is necessary to (i) understand the contributions of ILC populations to different disease states, (ii) examine how ILCs regulate skin homeostasis and (iii) identify novel ILC-specific markers for therapeutic intervention. Studying the mechanisms that maintain skin immune homeostasis in health and disease may identify new therapeutic targets for the treatment of numerous chronic inflammatory skin diseases.
Abbreviations
- ILCs
Innate lymphoid cells
- ILC1s
group 1 ILCs
- ILC2s
group 2 ILCs
- ILC3s
group 3 ILCs
- TH2
T helper type 2
- TH17
T helper type 17
- TSLP
thymic stromal lymphopoietin
- TSLPR
TSLP receptor
- CRTH2
chemoattractant receptor-homologous molecule expressed on TH2 cells
- PGD2
prostaglandin D2
References
- Bando JK, Nussbaum JC, Liang HE, et al. Type 2 innate lymphoid cells constitutively express arginase-I in the naive and inflamed lung. J Leukoc Biol. 2013;94:877–84. doi: 10.1189/jlb.0213084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernink JH, Peters CP, Munneke M, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221–9. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. The New England journal of medicine. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. Journal of clinical & cellular immunology. 2011:2. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–8. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, et al. A committed precursor to innate lymphoid cells. Nature. 2014 doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Dyring-Andersen B, Geisler C, Agerbeck C, et al. Increased number and frequency of group 3 innate lymphoid cells in nonlesional psoriatic skin. The British journal of dermatology. 2014;170:609–16. doi: 10.1111/bjd.12658. [DOI] [PubMed] [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of experimental medicine. 2006;203:1105–16. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–95. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Colonna M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr Opin Gastroenterol. 2013;29:581–7. doi: 10.1097/MOG.0b013e328365d339. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Vermi W, Lee JS, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–81. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbachev AV, Heeger PS, Fairchild RL. CD4+ and CD8+ T cell priming for contact hypersensitivity occurs independently of CD40-CD154 interactions. J Immunol. 2001;166:2323–32. doi: 10.4049/jimmunol.166.4.2323. [DOI] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–35. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hams E, Locksley RM, McKenzie AN, et al. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. Journal of immunology. 2013;191:5349–53. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Monticelli LA, Fung TC, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–7. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepworth MR, Sonnenberg GF. Regulation of the adaptive immune system by innate lymphoid cells. Curr Opin Immunol. 2014;27:75–82. doi: 10.1016/j.coi.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Yasuda K, Sakaguchi Y, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13921–6. doi: 10.1073/pnas.1307321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Yamada A, Yoh K, et al. Th1 and type 1 cytotoxic T cells dominate responses in T-bet overexpression transgenic mice that develop contact dermatitis. J Immunol. 2007;178:605–12. doi: 10.4049/jimmunol.178.1.605. [DOI] [PubMed] [Google Scholar]
- Kehren J, Desvignes C, Krasteva M, et al. Cytotoxicity is mandatory for CD8(+) T cell-mediated contact hypersensitivity. J Exp Med. 1999;189:779–86. doi: 10.1084/jem.189.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013a;5:170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol. 2013b;25:738–44. doi: 10.1016/j.coi.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, Flach M, Mohle L, et al. Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages. Cell. 2014;157:340–56. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- Klose CS, Kiss EA, Schwierzeck V, et al. A T-bet gradient controls the fate and function of CCR6-RORgammat+ innate lymphoid cells. Nature. 2013;494:261–5. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- Locksley RM, Fahy JV. Asthma and the flu: a tricky two-step. Immunol Cell Biol. 2014;92:389–91. doi: 10.1038/icb.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227–55. doi: 10.1146/annurev-immunol-032713-120225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirchandani AS, Besnard AG, Yip E, et al. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. Journal of immunology. 2014;192:2442–8. doi: 10.4049/jimmunol.1300974. [DOI] [PubMed] [Google Scholar]
- Mjosberg J, Bernink J, Golebski K, et al. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–59. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Mjosberg J, Eidsmo L. Update on innate lymphoid cells in atopic and non-atopic inflammation in the airways and skin. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2014 doi: 10.1111/cea.12353. [DOI] [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–49. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–54. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Artis D. Innate lymphoid cells: critical regulators of allergic inflammation and tissue repair in the lung. Curr Opin Immunol. 2012;24:284–9. doi: 10.1016/j.coi.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–4. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Motomura Y, Morita H, Moro K, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–71. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–70. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Kaplan DH, Barker J. Psoriasis. The New England journal of medicine. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–8. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122:2252–6. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–94. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Guo X, Chen ZM, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–99. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin LC, Groom JR, Chopin M, et al. The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat Immunol. 2013;14:389–95. doi: 10.1038/ni.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nature immunology. 2013;14:564–73. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Mezard P, Chavagnac C, Vocanson M, et al. Deficient contact hypersensitivity reaction in CD4−/− mice is because of impaired hapten-specific CD8+ T cell functions. J Invest Dermatol. 2005;124:562–9. doi: 10.1111/j.0022-202X.2005.23567.x. [DOI] [PubMed] [Google Scholar]
- Salimi M, Barlow JL, Saunders SP, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. The Journal of experimental medicine. 2013;210:2939–50. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Shen W, Li W, Hixon JA, et al. Adaptive immunity to murine skin commensals. Proc Natl Acad Sci U S A. 2014;111:E2977–86. doi: 10.1073/pnas.1401820111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37:601–10. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg GF, Mjosberg J, Spits H, et al. SnapShot: innate lymphoid cells. Immunity. 2013;39:622–e1. doi: 10.1016/j.immuni.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Sonnenberg GF, Monticelli LA, Alenghat T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336:1321–5. doi: 10.1126/science.1222551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–75. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- Teunissen MB, Munneke JM, Bernink JH, et al. Composition of Innate Lymphoid Cell Subsets in the Human Skin: Enrichment of NCR ILC3 in Lesional Skin and Blood of Psoriasis Patients. J Invest Dermatol. 2014 doi: 10.1038/jid.2014.146. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Altznauer F, Akdis M, et al. The differential fate of cadherins during T-cell-induced keratinocyte apoptosis leads to spongiosis in eczematous dermatitis. J Invest Dermatol. 2001;117:927–34. doi: 10.1046/j.0022-202x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- Villanova F, Flutter B, Tosi I, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol. 2014;134:984–91. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonarbourg C, Mortha A, Bui VL, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–51. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Salimi M, Panse I, et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol. 2014;133:1184–94. doi: 10.1016/j.jaci.2013.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Danilenko DM, Valdez P, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–51. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]