Abstract
Wnts are a conserved family of secreted glycoproteins that regulate various developmental processes in metazoans. Three of the five C. elegans Wnts, CWN-1, CWN-2 and EGL-20, and the sole Wnt receptor of the Ror kinase family, CAM-1, are known to regulate the anterior polarization of the mechanosensory neuron ALM. Here we show that CAM-1 and the Frizzled receptor MOM-5 act in parallel pathways to control ALM polarity. We also show that CAM-1 has two functions in this process: an autonomous signaling function that promotes anterior polarization and a nonautonomous Wnt-antagonistic function that inhibits anterior polarization. These antagonistic activities can account for the weak ALM phenotypes displayed by cam-1 mutants. Our observations suggest that CAM-1 could function as a Wnt receptor in many developmental processes, but the analysis of cam-1 mutants may fail to reveal CAM-1’s role as a receptor in these processes because of its Wnt-antagonistic activity. In this model, loss of CAM-1 results in increased levels of Wnts that act through other Wnt receptors, masking CAM-1’s autonomous role as a Wnt receptor.
Keywords: C. elegans, CAM-1, Ror kinase, neuronal polarity
Introduction
Wnts belong to a conserved family of secreted glycoproteins that are important for a wide range of developmental processes that includes cell-fate specification, directed cell motility, organogenesis and stem cell renewal (Komiya and Habas 2008). Wnts can bind to seven-pass transmembrane Frizzled receptors and signal through a canonical pathway that leads to the stabilization of β-catenin or through β-catenin-independent “non-canonical” pathways (Komiya and Habas 2008). Numerous positive and negative regulators of Wnt signaling have been identified, and the observation that deregulated Wnt signaling can lead to cancer (Logan and Nusse 2004) underscores the need for a precise control of molecules that regulate Wnt function.
Rors are a family of conserved receptor tyrosine kinases (RTK) defined by an extracellular immunoglobulin (Ig) domain, cysteine-rich domain (CRD) and kringle domain (Green et al. 2008b). Mutations in Ror genes of humans and mice lead to defects in skeletal and cardiac development (Forrester 2002). Similar to the CRD of Frizzled receptors, the CRDs of vertebrate Rors have been shown to bind to Wnts (Hikasa et al. 2002; Oishi et al. 2003; Kani et al. 2004; Billiard et al. 2005; Mikels and Nusse 2006). Ror2 becomes phosphorylated in response to Wnt5a stimulation, suggesting that it can function as a genuine RTK (Liu et al. 2007; Liu et al. 2008).
However, a recent study using highly purified Ror2 shows that the protein lacks kinase activity in vitro (Bainbridge et al. 2014). Ror2 is best characterized as a positive regulator of a non-canonical Wnt signaling pathway, functioning in mouse embryonic fibroblast (MEF) migration (Nishita et al. 2006), in mouse hair cell orientation (Yamamoto et al. 2008) and in Xenopus convergent extension (Hikasa et al. 2002; Schambony and Wedlich 2007). Ror2 can also function in canonical Wnt signaling, with earlier reports showing that Ror2 can attenuate the expression of a canonical Wnt signaling reporter (Billiard et al. 2005; Mikels and Nusse 2006) and more recent reports arguing for a stimulatory function (Li et al. 2008; Winkel et al. 2008).
The C. elegans Ror ortholog is CAM-1, originally identified in a genetic screen for mutants with defective CAN neuron migrations (Forrester and Garriga 1997; Forrester et al. 1999). cam-1 was shown to function autonomously in CAN migration (Forrester et al. 1999), in positioning of an axon-rich structure called the nerve ring (Kennerdell et al. 2009), in neurite elimination (Hayashi et al. 2009), in neurite outgrowth (Koga et al. 1999; Song et al. 2010) and in synaptic plasticity (Jensen et al. 2012). CAM-1 also has nonautonomous functions. In the migrations of the HSN motor neurons, egl-20/Wnt and cam-1 mutants exhibit reciprocal phenotypes, and expression of the CAM-1 CRD mimics the egl-20 mutant phenotype, consistent with CAM-1 antagonizing EGL-20 through its ability to bind Wnts (Kim and Forrester 2003; Forrester et al. 2004). In vulval development, loss of canonical Wnt signaling leads to a phenotype that is similar to the phenotype caused by expression of the extracellular domain of CAM-1 in non-vulval tissues (Green et al. 2007). This observation suggests that CAM-1 can inhibit Wnt signaling non-autonomously by restricting the amount of Wnts that reach the vulval tissue. However, the site of CAM-1 function in this process remained unclear until a recent study showed that the CANs, a pair of bipolar neurons that extend axons along the entire anterior-posterior axis and express CAM-1, could sequester excess Wnts to ensure proper vulval patterning (Modzelewska et al. 2013). In C. elegans, Wnts are also critical regulators of neuronal polarity along the anterior-posterior axis (Hilliard and Bargmann 2006; Prasad and Clark 2006; Fleming et al. 2010). The signaling from the Wnt LIN-44 to the Frizzled receptor LIN-17 is important for the polarization of PLM mechanosensory neurons. The polarization of the ALM mechanosensory neurons is more complex, requiring the three Wnts CWN-1, CWN-2 and EGL-20. The Ror kinase CAM-1 regulates ALM polarity (Babu et al. 2011), but it is unclear whether other Wnt receptors are involved in this process. Here we show that CAM-1 and at least one Frizzled receptor, MOM-5, act in parallel to control ALM polarity. We also show that CAM-1 has dual and antagonistic functions in this process: an autonomous signaling function that promotes anterior polarization and an antagonistic nonautonomous function that inhibits anterior polarization. These antagonistic activities result in weak ALM phenotypes in the absence of CAM-1. We propose that CAM-1 plays autonomous roles in many processes that require Wnt signaling, but that these roles are masked by increased Wnt levels that result from CAM-1 loss in tissues involved in regulating the distribution of Wnts.
Materials and methods
Strains and genetics
Strains were maintained at 20° C as described (Brenner 1974). The following alleles are used in this study:
LG I: lin-17(n671) (Sawa et al. 1996), mig-1(e1787) (Pan et al. 2006), mom-5(ne12) (Rocheleau et al. 1997), mom-5(gm409) (this study)
LG II: cwn-1(ok546) (Zinovyeva and Forrester 2005), mig-5(rh147) (Walston et al. 2006), dsh-1(ok1445) (Klassen and Shen 2007), mig-14(ga62) (Eisenmann and Kim 2000), cam-1 alleles include gm105, gm122 (Forrester et al. 1999), ks52 (Koga et al. 1999), cw82 (this study), xd13 (Song et al. 2010), sa692 (Ailion and Thomas 2003), ak37 (Francis et al. 2005)
LG III: ncl-1(e1865) (Hedgecock and Herman 1995)
LG IV: eri-1(mg366) (Kennedy et al. 2004), cwn-2(ok895) (Zinovyeva and Forrester 2005), egl-20(n585) (Maloof et al. 1999)
LG V: cfz-2(ok1201) ((Zinovyeva and Forrester 2005)
LG X: lin-18(e620) (Inoue et al. 2004), vang-1(tm1422) (Hoffmann et al. 2010)
Strains used for this study are:
SK4005: zdIs5I; NG4427: cwn-1(ok546)II; egl-20(n585) IV, NG5739: zdIs5I; cam-1(gm122)II, NG5962: mom-5(ne12)/hT2 zdIs5 I; +/hT2 III, NG6032: mom-5(ne12)/hT2 zdIs5 I; cam-1(gm122) II; +/hT2 III, NG4696: mig-1(e1787) zdIs5 I, NG4633: lin-17(n671) zdIs5 I, NG4888: zdIs5 I; cfz-2(ok1201) IV, NG5428: mig-1(e1787) lin-17(n671) zdIs5 I, NG4744: mig-1(e1787) mom-5(ne12) zdIs5/hT2 zdIs5 I; +/hT2 III, NG4902: lin-17(n671) mom-5(ne12) zdIs5/hT2 zdIs5 I; +/hT2 III, NG6126: mig-1(e1787) lin-17(n671) mom-5(ne12) zdIs5/hT2 zdIs5 I; +/hT2 III, NG5877: mig-1(e1787) lin-17(n671) zdIs5 I, cfz-2(ok1201) IV, NG6960: mig-1(e1787) lin-17(n671) mom-5(ne12) zdIs5/hT2 zdIs5 I; +/hT2 III; cfz-2(ok1201) IV, NG6099: mig-1(e1787) lin-17(n671) zdIs5 I; cam-1(gm122) II, NG4629: zdIs5I; cwn-1(ok546) II, NG5514: dpy-5(e61) mom-5(ne12) zdIs5/hT2 zdIs5 I; cwn-1(ok546) II; +/hT2 III, NG6932: mom-5(gm409) zdIs5 I, NG6936: mom-5(gm409) zdIs5 I, cwn-1(ok546) II, NG6974: mom-5(gm409) zdIs5 I, cwn-1(ok546) II, gmEx583[Pmec-3::mom-5::gfp; pRF4[rol-6(su1006)] NG5546: zdIs5 I; cwn-1(ok546) mig-5(rh147) II, NG5593: zdIs5 I; cwn-1(ok546) dsh-1(ok1445) II, NG4630: zdIs5 I; egl-20(n585) IV, NG5745: zdIs5I, cam-1(gm122) II; egl-20(n585) IV, NG5854: zdIs5I; cam-1(gm122) II; cwn-2(ok895) IV, NG5521: dpy-20(e61) mom-5(ne12)/hT2 zdIs5 I; egl-20(n585) IV, NG6270: zdIs5 I; cam-1(gm122) II; xdEx636, NG6532: zdIs5 I; cam-1(gm122) II; nuIs465, NG6138: zdIs5 I; cwn-1(ok546) cam-1(gm122) II; xdEx636, NG6204: gmEx632[Punc-86::cam-1(ΔIntra)::yfp; Pmyo-2::mcherry], NG6304: zdIs5 I; mig-14(ga62) II, xdEx636, NG6309: zdIs5 I; cwn-1(ok546) II; cwn-2(ok895) IV; xdEx636, NG6771:zdIs5 I; cam-1(gm122) II; ncl-1(e1865) III; gmEx188, NG5729: zdIs5 I; cam-1(ks52) II, NG5753: zdIs5 I; cam-1(sa692) II, NG5754: zdIs5 I; cam-1(ak37) II, NG6607: zdIs5 I; cwn-1(ok546) cam-1(cw82) II, NG5731: zdIs5 I; cwn-1(ok546) cam-1(ks52) II, NG5755: zdIs5 I; cwn-1(ok546) cam-1(sa692) II, NG6125: zdIs5 I; cwn-1(ok546) cam-1(gm122) II, NG5874: zdIs5 I; cwn-1(ok546) cam-1(ak37) II, NG5762: zdIs5 I; gmEx191, NG5747: zdIs5 I; cwn-1(ok546) II; gmEx191, NG5761: zdIs5 I; cwEx34, NG5795: zdIs5 I; cwn-1(ok546) II; cwEx34, NG5851: zdIs5 I; cwn-1(ok546) II; cwEx152,NG4603: zdIs5I; cwn-1(ok546)II; nuIs465.
Transgenic animals
The following previously published transgenic strains were used in this study: zdIs5[Pmec-4::gfp, lin-15(+)] (Clark and Chiu 2003); gmEx191[Pcam-1::cam-1::gfp; pRF4[rol-6(su1006)]] and gmEx188[Pcam-1::cam-1; ncl-1(+); pRF4[rol-6(su1006)]] (Forrester et al. 1999); xdEx636[Punc-86::cam-1(b)::yfp; Podr-1::dsRed] (Song et al. 2010); cwEx34[Pcam-1::cam-1(ΔCRD)::gfp; pRF4[rol-6(su1006)]] and cwEx152[Pcam-1::cam-1(ΔIgKriIntra)::gfp; pRF4[rol-6(su1006)]] (Kim and Forrester 2003); nuIs465[Pmyo-3::cam-1::gfp] (Babu et al. 2011)
Isolation of a new mom-5 allele
The mom-5 allele gm409 was isolated in a forward genetic screen for mutations that generated ALM polarity defects in a cwn-1 background. Mutagenesis was carried out as described (Brenner 1974). Mutagenized zdIs5; cwn-1 P0 hermaphrodites were transferred to fresh plates every 4 to 5 hours. After two days, F2 animals were screened for presence of ALM polarity defects. gm409 is a missense mutation that changes a conserved isoleucine in the CRD domain of MOM-5 to phenylalanine (I105F).
Molecular biology and germline transformation
Pmec-3::mom-5(cDNA)::gfp was generated by PCR amplifying mom-5 cDNA from zuIs145 worms (Park et al. 2004) using the following primers: CCCGGGGTAATAATGGGCGCGCCA and ACCGGTGGCCTCATATTAACCTGATCAACATGAGC. The mom-5 cDNA was inserted into a Pmec-3::gfp backbone using XmaI and AgeI restriction enzymes. gmEx583, gmEx584, gmEx585 and gmEx586 were generated by injecting Pmec-3::mom-5(cDNA)::gfp into wild-type hermaphrodites at 75 ng/μl with 25ng/μl pRF4[rol-6(su1006). Punc-86::cam-1(ΔIntra)::yfp was generated by modifying Punc-86::cam-1(b)::yfp (Song et al. 2010) with PCR-based mutagenesis (QuikchangeII XL, Agilent Technologies). The following primers are used: GTCGAGCAAAGAAGAAGTACCCAGCTTTCTTG and its reverse complement. gmEx632, gmEx633, gmEx634, gmEx635 and gmEx636 were generated by injecting Punc-86::cam-1(ΔIntra)::yfp into wild-type hermaphrodites at 10 ng/μl with 3 ng/μl Pmyo-2::mcherry (pCFJ90). gmEx740, gmEx741 and gmEx742 were generated by injecting PCAN::cam-1(ΔIntra)::yfp (Modzelewska et al. 2013) into wild-type hermaphrodites at 100 ng/ul with 3 ng/μl Pmyo-2::mcherry (pCFJ90).
Germline transformation was performed by direct injection of various plasmid DNAs into the gonads of adult wild-type animals as described (Mello et al. 1991).
RNA interference
RNAi was performed using the bacterial feeding method as described (Timmons and Fire 1998; Kamath et al. 2001). In all experiments, worms were grown on plates supplemented with 25 mM Carbenicillin and 1 mM IPTG. The RNAi cultures were prepared by inoculating bacterial strains in LB with 25 mM Carbenicillin for 15 hours at 37° C, followed by addition of 6 mM IPTG and incubation for another hour at 37° C. Bacterial strains used to inactivate genes by feeding were obtained from the library designed by the Ahringer lab (Fraser et al. 2000).
Scoring of the ALM polarity phenotype
Neuronal polarity of ALM was scored using the integrated array zdIs5 [Pmec-4::gfp], which expresses GFP in the ALM, PLM, AVM and PVM mechanosensory neurons. For ALM, the bipolar phenotype was defined as a normal anterior process and a posterior process that is longer than five ALM cell diameters in length.
CAM-1 antibody
A 73 amino acid portion of CAM-1 C-terminal to the kinase domain (from Arg 856 to Asp 928 as translated from isoform a) was cloned into a GST-tagged expression vector pGEX, expressed in bacteria and purified using Glutathione-agarose-coupled resin (Sigma). Polyclonal antiserum was produced by injection of the recombinant protein into rabbit (Cocalico). Anti-CAM-1 antibody was purified by first passing the serum over an Affi-Gel GST column to remove the anti-GST antibodies, and then the flow-through was affinity purified using the CAM-1 C-terminal fragment coupled to Affi-Gel resin (BioRad).
Immunoblot analysis
Worm lysates were run on a 10% SDS-PAGE polyacrylamide gel. The secondary antibody used to detect anti-CAM-1 antibody was horseradish peroxidase affinipure goat anti-rabbit IgG (HRP) (Jackson Immunoresearch).
GST pull-down
Purified GST protein was immobilized on PVDF membrane then probed with 5ml of the CAM-1 antibody solution (1:1000 dilution). After overnight incubation, the CAM-1 antibody solution was removed and subsequently used to probe other membranes.
Three plasmids were used: pGEX-1, H1 (contains the cam-1 fragment encoding the C-terminal 73 a.a. used as the epitope for the anti-CAM-1 antibody), and P1 (contains this cam-1 fragment shortened by 24 bp and corresponds to the sequences present in the cam-1(ks52) mutant). Bacterial cultures of each were induced with 0.1 mM IPTG for 3 hours; samples were removed before and after induction. Each pelleted sample was resuspended in Sample Buffer (50 μl per 0.5 OD600) and boiled 5 minutes prior to use.
Samples were electrophoresed on 12% polyacrylamide gels at 150V for 2–2.5 hours and then transferred to PVDF membranes at 40V for 2 hours. After blocking with 5% nonfat dry milk, membranes were probed with either GST antibody (1:4000) or the CAM-1 antibody (1:1000; prepared as described above).
Fluorescence microscopy
For fluorescence microscopy, L4 to young adult hermaphrodite animals were anesthetized with 1% sodium azide, mounted on an agar pad, and observed with a Zeiss Axioskop2 microscope.
Results
Wnts regulate the initial polarity of the ALM neurons
The C. elegans ALMs are a pair of bilaterally symmetric neurons that are located in the middle of the animal and that sense light touch to the anterior body (Chalfie and Sulston 1981). During embryogenesis, the cell body of each ALM migrates a short distance toward the posterior (Sulston et al. 1983). The cells then extend a single anterior process toward the head. In wild-type animals, the ALM cell body and anterior process can be observed using a Pmec-4::gfp trangene (Figure 1A, B). While cwn-1 is the only Wnt single mutant that has a weak bipolar phenotype, the polarity defect of the ALM is enhanced in cwn-1; cwn-2 and cwn-1; egl-20 double mutants (Hilliard and Bargmann 2006; Prasad and Clark 2006), and in cwn-1; egl-20 cwn-2 triple mutants (Fleming et al. 2010). In Wnt double and triple mutants, the ALMs can be bipolar or their polarity can be reversed, extending a single process toward the tail (Figure 1C, D, E, and F).
Figure 1.
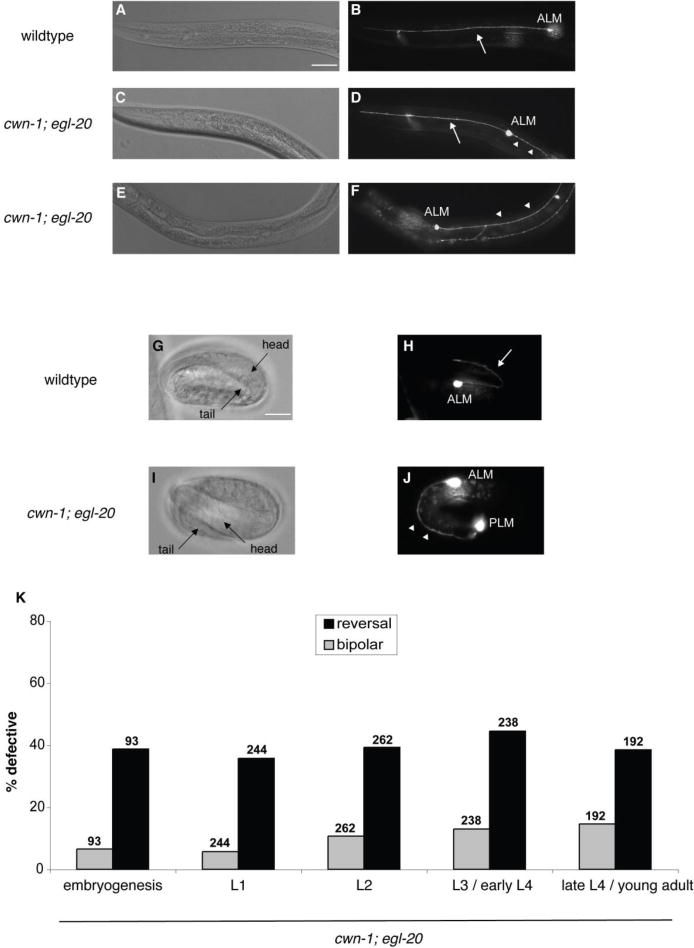
Wnts orient the initial polarity of ALM. (A, C, E) Nomarski and (B, D, F) fluorescence photomicrographs showing the anterior of fourth larval stage zdIs5 [Pmec-4::gfp] animals. (A, B) A wild-type larva. (C, D, E, F) cwn-1; egl-20 mutant larvae. The ALM cell bodies are labeled. Arrows indicate anterior processes, and arrowheads indicate posterior processes. Anterior is to the left, dorsal is up. Scale bar represents 30 μm. (B) A wild-type ALM extends a single, anterior process. (D) A cwn-1; egl-20 ALM is bipolar, extending both a normal anterior process and an ectopic posterior process. (F) A cwn-1; egl-20 ALM extends a single posterior process, indicating a reversal of polarity. (G–J) Photomicrographs of zdIs5 embryos showing ALM neuronal morphology. G and I are Nomarski images of H and J, respectively. (G, H) A wild-type embryo. (I, J) A cwn-1; egl-20 mutant embryo. Scale bar represents 10 μm. (H) A wild-type 3-fold embryo extends a normal anterior process. (J) A cwn-1; egl-20 3-fold embryo extends a single posterior process toward the tail, indicating a reversal of polarity. (K) Graph shows the percentage of ALM neurons with defective polarity for cwn-1; egl-20 animals at different developmental stages. Gray bars indicate the bipolar phenotype and black bars indicate reversed polarity. The number of ALMs scored is indicated above the bars for each genotype. L1, L2, L3 and L4 are the first, second, third and fourth larval stages, respectively. cwn-1; egl-20 animals at different stages displayed comparable ALM reversal defects. We found significant differences between the number of bipolar ALMs in L1 and L3 / early L4 animals and between L1 and late L4 / young adult animals (p < 0.001; Fisher’s exact test). We believe this difference is due to a technicality in how the numbers of bipolar neurons are scored (see Results).
The ALM polarity defects of the Wnt mutants could result from an early defect in the initial orientation of ALM polarity, or they could result from a failure to maintain ALM polarity. If the Wnts regulate polarity establishment, we would detect the ALM defects early, and the defects would not change as the animals developed. If the Wnts regulate polarity maintenance, the ALMs would appear normal early, and the frequency of the defects would increase as the animals developed. We took two approaches to distinguish between these two models. First, we asked when the ALMs begin to extend their processes in wild-type animals and whether these early events were altered in the cwn-1; egl-20 double mutant. Using the Pmec-4::gfp transgene, we could find ALMs with short neurites that extended forward toward the head at the 3-fold stage (Figure 1G, H). Scoring ALMs at the 3-fold stage in the Wnt double mutants, we found ALMs with neurites that extended both forward and backward (data not shown) and ALMs with single neurites that extended backwards toward the tail (Figure 1I, J). Second, we scored the ALM phenotypes in the cwn-1; egl-20 mutants at each larval stage and found that the penetrance of reversed ALMs did not substantially change with time (Figure 1K). The bipolar phenotype in embryogenesis and the first larval stage appeared lower compared to later stages. We suspect that this difference does not reflect a real increase in the penetrance of abnormal neurons but rather how we score the bipolar phenotype. We defined the bipolar phenotype as a normal anterior process and a posterior process that is longer than five ALM cell diameters in length. In early stages the ALM cell bodies often appeared disproportionately large relative to the animal when compared to later stages, thereby masking a potential bipolar phenotype. These observations support the hypothesis that the Wnts orient the initial polarity of the ALMs. This is similar to the role of the Wnt LIN-44 in establishing the polarity of the PLM, a mechanosensory neuron that plays a similar role to ALM, but with a dendritic field that senses light touch to the posterior body (Hilliard and Bargmann 2006).
CAM-1/Ror, MOM-5/Frizzled and all three Dishevelled are required for ALM neuronal polarity
We previously showed that the Ror kinase CAM-1 regulates ALM polarity (Babu et al. 2011), but it is unclear whether other Wnt receptors are involved in this process. We examined animals bearing single mutations in the four C. elegans Frizzled genes mig-1, cfz-2, lin-17, and mom-5 (Figure 2A). We also examined animals with a mutation in lin-18/Ryk, which encodes the single C. elegans ortholog of Ryk, a receptor tyrosine kinase-like molecule with an extracellular Wnt-binding WIF (Wnt-inhibitory factor) domain (Inoue et al. 2004). We found that, with the exception of cam-1, which showed a modest bipolar phenotype, none of the other single mutants had ALM polarity defects (Figure 2A and data not shown). Dishevelleds are cytoplasmic proteins that transduce Frizzled signaling to downstream effectors. Mutations in the three Dishevelled genes, mig-5, dsh-1 or dsh-2, also failed to produce an ALM polarity defect (data not shown; n=100, 100 and 100, respectively).
Figure 2.
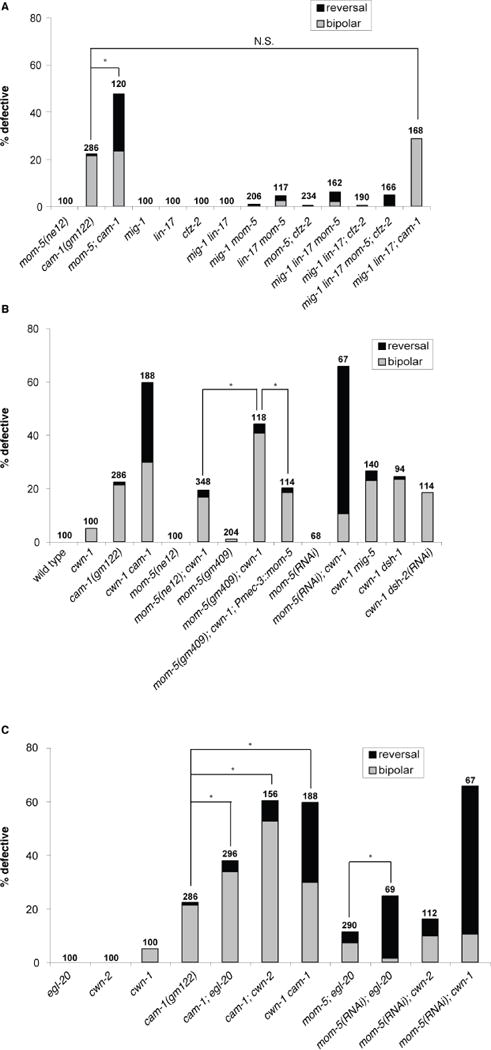
CAM-1 and MOM-5 act in parallel pathways in ALM neuronal polarity. (A) A mutation in mom-5 significantly enhanced the polarity defect in cam-1. Mutations in mig-1 and lin-17 weakly enhanced the polarity defect in mom-5. (B) Mutations in cam-1/Ror, mom-5/Frizzled, and the dishevelled genes mig-5 and dsh-1 generated defects in ALM neuronal polarity in a cwn-1 sensitized background. A mom-5 cDNA expressed from the mec-3 promoter partially rescued the polarity defect of a mom-5; cwn-1 double mutant. (C) cam-1 and mom-5 genetically interacted with Wnts. Data in A–C are presented as in Fig. 1K. N.S., not significant; *p < 0.001 (Fisher’s exact test).
We reasoned that a lack of a phenotype for the Frizzled and Dishevelleds mutants might be due to overlapping functions. Since cwn-1 is the only Wnt mutant that has a weak bipolar phenotype, we reasoned that it might provide a sensitized background to identify additional components that regulate ALM polarity. We performed RNAi against Wnt receptors and Dishevelleds using a strain containing a cwn-1 mutation and a mutation in eri-1, which sensitizes animals to the effects of RNAi (Kennedy et al. 2004). RNAi against cam-1, mom-5 and all three dishevelled mRNAs showed a significant enhancement of the ALM polarity defects compared to control RNAi (Figure 2B and data not shown).
To confirm the RNAi results, we constructed double mutants with putative null alleles. The cwn-1 mutation in combination with mutations in cam-1, dsh-1, or mig-5 generated ALM polarity defects comparable to the RNAi phenotypes (Figure 2B), indicating the RNAi was effective in eliminating the function of these genes. On the other hand, mom-5(ne12); cwn-1 double mutants had a much weaker phenotype than mom-5(RNAi); cwn-1 animals (Figure 2B). The mom-5 mutation ne12 leads to a maternal-effect embryonic lethality; therefore, we analyzed homozygous mom-5 mutants derived from heterozygous mothers. The weak phenotype of the double mutant could result from a maternal contribution of wild-type mom-5. Consistent with this hypothesis, the partial loss of both maternal and zygotic mom-5 function by the weak gm409 mutation was more effective in enhancing the cwn-1 mutant phenotype than the complete loss of only zygotic function by the null mutation ne12 (Figure 2B). A transgene containing a mom-5 cDNA driven from the mec-3 promoter that expresses MOM-5 in mechanosensory neurons partially rescued the ALM defects of the cwn-1; mom-5(gm409) double mutant, indicating that MOM-5 acts in the neurons (Figure 2B). The lack of a significant ALM phenotype in cwn-1 dsh-2 mutants could also result from a maternal contribution of dsh-2 (data not shown; n=100).
CAM-1 and MOM-5 act in parallel pathways in ALM polarity
Compared with either single mutant, we observed a significant increase in the ALM polarity defect of mom-5; cam-1 double mutants (Figure 2A), suggesting that the two receptors act in parallel pathways. However, we did not detect an enhancement of the polarity phenotype in other cam-1; frizzled double mutants: lin-17; cam-1, mig-1; cam-1 and cam-1; cfz-2 (data not shown; n=190, 268 and 190, respectively). A lin-18/Ryk mutation also failed to enhance the polarity defect of the cam-1 mutant (data not shown; n=160). Considering the findings that the Frizzleds MIG-1, CFZ-2 and LIN-17 may act as CAM-1 co-receptors (Kennerdell et al. 2009; Song et al. 2010; Jensen et al. 2012), we asked whether these Frizzleds could be CAM-1 co-receptors functioning in parallel to MOM-5 to regulate ALM polarity. Although certain Frizzled doubles mutants (mig-1 lin-17 and mig-1; cfz-2) did not display ALM polarity defects, mig-1 mom-5 and lin-17 mom-5 mutants displayed a weak synthetic polarity phenotype (Figure 2A and data not shown). These observations suggest that MIG-1 and LIN-17 might also act as co-receptors to CAM-1 in ALM polarity. Consistent with this hypothesis, the combined loss of mig-1 and lin-17 failed to enhance the ALM polarity phenotype of a cam-1 mutant (Figure 2A). However, the ALM phenotype in the triple Frizzled mutant mig-1 lin-17 mom-5 was much weaker than in the mom-5; cam-1 double mutant (Figure 2A). In addition, the ALM phenotype in the triple Frizzled mutant mig-1 lin-17; cfz-2 was not comparable to cam-1, and the ALM phenotype of the quadruple Frizzled mutant mig-1 lin-17 mom-5; cfz-2 was much weaker compared to that of mom-5; cam-1 (Figure 2A). These results indicate either that there are additional CAM-1 co-receptors or that CAM-1 can also act alone.
CAM-1 and MOM-5 do not appear to mediate the effects of specific Wnts
To determine whether CAM-1 and MOM-5 mediate the effects of specific Wnts (CWN-1, CWN-2 or EGL-20) in ALM polarity, we analyzed the polarity phenotype when a Wnt was removed from a receptor-mutant background. The prediction is that if a receptor specifically responds to a particular Wnt ligand, then removal of the Wnt from the receptor-mutant background should not enhance the polarity defect. On the other hand, if the Wnt and the receptor act in parallel pathways, the double mutant lacking both Wnt and receptor function may display an enhanced polarity phenotype. Double mutant analysis with cam-1 and the Wnts showed that while mutations in all three Wnt genes enhanced the polarity defect of a cam-1 mutant, the cwn-1 mutation enhanced the most, while the egl-20 mutation enhanced the least (Figure 2C). Similarly, cwn-1 enhanced mom-5 more than egl-20 or cwn-2 did (Figure 2C). These observations suggest that CAM-1 and MOM-5 do not mediate the effects of specific Wnts.
CAM-1 possesses autonomous and nonautonomous functions that act antagonistically
To test whether CAM functions in the ALM, we expressed a cam-1 cDNA under the control of the unc-86 promoter, which is expressed in many neurons including the ALMs, but not in non-neuronal cells (Baumeister et al. 1996). This transgene partially rescued the ALM defects in both cam-1 single and cwn-1 cam-1 double mutants (Figure 3A), suggesting that CAM-1 can act in the ALM to regulate its polarity. The Punc-86::cam-1 transgene was also able to partially rescue the polarity defects of a mig-14 mutant and of a cwn-1; cwn-2 double Wnt mutant (Figure 3B). MIG-14 is the homolog of Wntless, a chaperone necessary for Wnt secretion (Banziger et al. 2006; Bartscherer et al. 2006; Myers and Greenwald 2007); the ga62 allele reduces but does not eliminate mig-14 function (Eisenmann and Kim 2000). The ability to rescue these mutants with this transgene suggests that excess CAM-1 in the ALM can enhance residual Wnt function in the mig-14 hypomorph.
Figure 3.
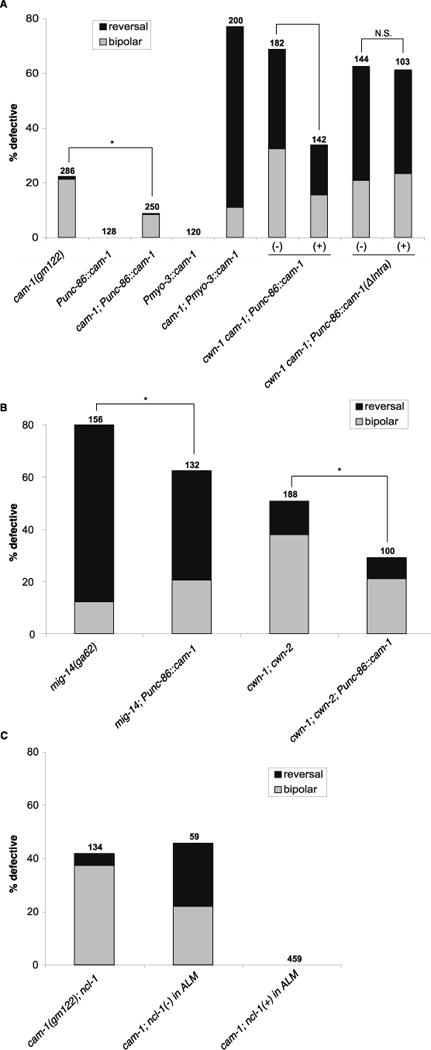
CAM-1 functions in the ALM to promote its polarity. (A) A CAM-1 transgene expressed from the unc-86 promoter rescued the polarity defect in cam-1 and cwn-1 cam-1. The unc-86 promoter is expressed in a number of neuronal lineages, including the lineage that generates the ALM (Baumeister et al. 1996). A CAM-1 transgene expressed from the muscle-specific myo-3 promoter enhanced the polarity defect in cam-1. A CAM-1 transgene lacking its intracellular domain (ΔIntra) failed to rescue the polarity defect in cwn-1 cam-1. cam-1(gm122) was used in these experiments. (B) CAM-1 expressed from the unc-86 promoter suppressed the polarity defects in mig-14 and cwn-1; cwn-2. (C) Loss of CAM-1 in the ALM (identified by the cell-autonomous marker ncl-1) resulted in polarity defects. Data in A–C are presented as in Fig. 1K. N.S., not significant; *p < 0.05 (Fisher’s exact test).
To further test the autonomous role for CAM-1 in ALM polarity, we generated animals that were mosaic for cam-1 function. We started with a strain that was mutant for cam-1 and ncl-1. The ncl-1 mutation results in enlarged nucleoli (the Ncl phenotype) and can be used as a cell autonomous marker in mosaic analysis (Hedgecock and Herman 1995). The strain also bore an extrachromosomal transgenic array that contained the wild-type cam-1 and ncl-1 genes as well as a dominant allele of the rol-6 gene that causes animals to roll. This is the transgene that was used to determine the site of cam-1 function in CAN migration (Forrester et al. 1999). We scored the ALM polarity and Ncl phenotypes of rolling animals. Rolling animals carry the extrachromosomal array, but the mitotic instability of the array leads to mosaicism: only a subset of cells contain the array, and the remaining cells are mutant for cam-1 and ncl-1 and have enlarged nucleoli. Approximately 45% of the Ncl ALMs displayed a polarity defect, but none of the 459 ALMs with wild-type nucleoli displayed a polarity defect (Figure 3C). We attempted to determine where in the lineage the losses occurred, but most of the cells that were related lineally to ALM were of hypodermal origin, and we were unable to unambiguously determine whether they exhibited the Ncl phenotype. Nonetheless, these observations strongly support the hypothesis that cam-1 acts in the ALM to promote normal polarity.
One interesting finding from this experiment is that the mosaic animals with Ncl ALMs have a higher frequency of reversed ALMs than cam-1; ncl-1 mutants that do not carry the transgenic array (Figure 3C), which we interpret as an enhancement of the ALM defect. One explanation for this effect is that CAM-1 has antagonistic activities in ALM polarity: an autonomous function as a Wnt receptor that promotes normal polarity, and a nonautonomous function that antagonizes Wnt function. In this model, Wnt signaling is altered in two ways in the mosaic animals where ALM had lost the transgene,. First, CAM-1 no longer acts autonomously as a receptor because it is absent from ALMs. Second, the presence of CAM-1 in other cells reduces the amount of Wnt ligand that reaches the ALM. In combination, these two factors lead to more frequent ALM polarity defects. In this model, Wnt signaling is also impaired in nontransgenic mutant animals because CAM-1 is absent from ALMs, but its absence in other cells leads to an increase in the amount of Wnt ligand that reaches the ALM. Increased Wnt levels compensate for the lack of CAM-1 in the ALM, resulting in weaker polarity defects. To test this hypothesis further, we expressed CAM-1 from the myo-3 promoter in muscle cells (Figure 3A). This expression resulted in a strong ALM polarity defect in a cam-1 mutant background, consistent with our hypothesis that CAM-1 has antagonistic functions.
CAM-1 has both kinase-dependent and kinase-independent functions in ALM polarity
We next addressed whether the intracellular domain of CAM-1 is required for ALM polarity. Through analysis of mutants or transgenes that lack the intracellular domain, several studies have shown that this domain is at least partially, if not completely, dispensable for CAM-1 function (Kim and Forrester 2003; Francis et al. 2005; Kennerdell et al. 2009; Song et al. 2010) We first examined the ALM phenotypes of seven cam-1 alleles, both alone and in combination with cwn-1 (Figure 4A, B). Based on the cam-1 lesions and ALM phenotypes, we placed these mutants into three classes (Figure 6). Class 1 mutant ALMs displayed a bipolar phenotype (Figure 4B). The gm122 allele is a nonsense mutation that truncates CAM-1 in the CRD (Forrester et al. 1999), sa692 is a missense mutation in the CRD (Ailion and Thomas 2003), and ak37 is a large deletion removing sequences from the kringle domain to the C-terminus (Francis et al. 2005). These alleles behave like strong loss-of-function, if not null, mutations in other developmental processes (Forrester et al. 1999; Kim and Forrester 2003; Kennerdell et al. 2009; Song et al. 2010).
Figure 4.
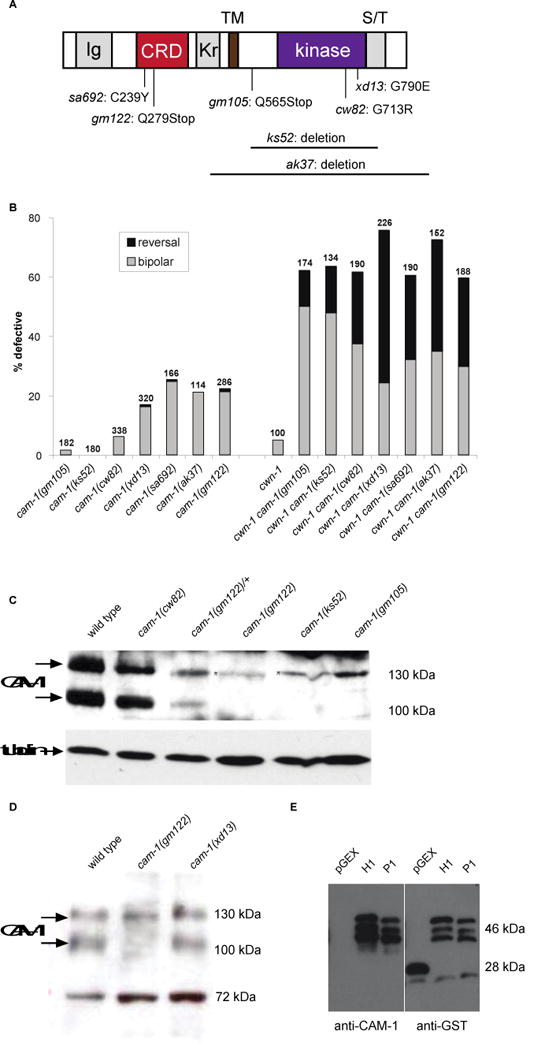
CAM-1 intracellular domain is important for ALM polarity. (A) Molecular lesions of cam-1 alleles. See text for details. (B) Putative null alleles of cam-1 (gm122, sa692 and ak37) and kinase-defective alleles (cw82 and xd13) generated a polarity phenotype, but putative hypomorphic alleles (gm105 and ks52) did not. A mutation in cwn-1 enhanced polarity defects in all cam-1 alleles. Data in B are presented as in Fig. 1K. (C, D) Immunoblots of extracts from different cam-1 mutant backgrounds using an anti-CAM-1 antibody raised against its C-terminus. Tubulin and a non-specific 72 kDa band were used as loading controls for (C) and (D), respectively. There are two bands that are CAM-1 specific. The lower molecular weight band is the predicted size for CAM-1. The higher molecular weight band appears to run at the same position as a nonspecific band recognized by the anti-CAM-1 antibody since it is present in the nonsense mutants gm122 and gm105. (E) The anti-CAM-1 antibody recognizes the truncated CAM-1 C-terminal fragments. H1 enocodes the full length fragment of CAM-1 used to produce the antibodies (73 a.a.) and P1 encodes the fragment from which the 8 amino acids missing in ks52 have been deleted (65 a.a.).
Figure 6.
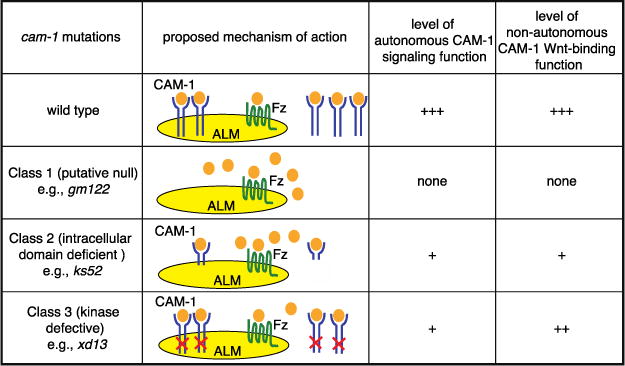
A model for how CAM-1 possesses autonomous and nonautonomous functions that act antagonistically to regulate ALM polarity. See text for details.
Class 2 mutants had little to no ALM polarity defects. The gm105 allele is a nonsense mutation that is predicted to result in a receptor that lacks most of the intracellular domain (Forrester et al. 1999), and the ks52 allele is an in-frame deletion that removes the kinase domain (Koga et al. 1999). While these mutations had little effect on ALM polarity in a wild-type background, they interacted synergistically with cwn-1 (Figure 4B). These alleles behaved like hypomorphic mutations in other developmental processes (Forrester et al. 1999; Kim and Forrester 2003; Kennerdell et al. 2009; Song et al. 2010).
Class 3 mutants are particularly interesting. The cw82 and xd13 mutants had bipolar ALM phenotypes that were less penetrant than Class 1 mutants (Figure 4B). The cw82 (this study) and xd13 alleles (Song et al. 2010) are missense mutations in the kinase domain, changing conserved glycines to charged amino acids. A recent study shows that CAM-1, unlike vertebrate Rors, possesses kinase activity in vitro (Bainbridge et al. 2014), suggesting that the cw82 and xd13 alleles disrupt kinase activity. The finding that these kinase missense mutations have stronger effects on ALM polarity than the Class 2 mutations that are predicted to eliminate large parts of the intracellular domain is paradoxical. Based on our model that CAM-1 has two antagonistic functions, we speculate that the different effects of the two types of mutations might result from differences in the stability of the mutant proteins. If kinase function is required for the autonomous role but not the nonautonomous role of CAM-1, then the Class 3 mutations might significantly reduce CAM-1’s signaling function but not its role in antagonizing Wnts, leading to ALM defects. The mutations that truncate the protein or remove large parts of the intracellular domain would also eliminate CAM-1’s signaling function, but might also destabilize the protein, leading to increased Wnt levels that compensate for the loss or reduction of CAM-1 signaling.
To test this protein-stability hypothesis, we measured the levels of CAM-1 in the different cam-1 mutants (Figure 4C, D). Consistent with the hypothesis, the Class 2 mutant ks52 had much lower levels of CAM-1 than the Class 3 mutants (cw82 and xd13). These observations support the hypothesis that the kinase missense mutations affect specifically the autonomous role of CAM-1. One potential caveat with this interpretation is that 8 of 73 amino acids in the protein fragment used to make the antibodies are missing from ks52. However, the CAM-1 antibody was still able to recognize this truncated protein fragment on immunoblots of proteins from bacterial cultures, suggesting that the epitope recognized by our antibodies is present in the cam-1(ks52) mutant (Figure 4E).
We further tested the idea that the kinase domain is important for CAM-1 signaling by using the unc-86 promoter to express in a cwn-1 cam-1 mutant background a cam-1 cDNA lacking the sequence that encodes the intracellular domain of CAM-1 (Punc-86::cam-1ΔIntra). Unlike the transgene expressing the full-length cam-1 cDNA, which partially rescued the ALM phenotype of the double mutant, the Punc-86::cam-1ΔIntra transgene failed to rescue the ALM polarity defects (Figure 3A). We were able to observe punctate YFP expression in the ALM from the ΔIntra transgene that is fluorescently tagged at the C-terminus (data not shown), so it is unlikely that the lack of rescue of this transgene was caused by the instability of truncating CAM-1. This experiment further supports the hypothesis that kinase function is important for CAM-1 function in the ALM.
CAM-1 CRD is both necessary and sufficient to antagonize Wnts that regulate ALM polarity
Our model predicts that CAM-1 has a nonautonomous Wnt antagonistic function. The expression of full-length CAM-1 from its endogenous promoter or a muscle-specific myo-3 promoter induced ALM polarity defects in a cwn-1 sensitized background (Figure 5), suggesting that excess CAM-1 antagonizes Wnts (i.e. CWN-2 or EGL-20) nonautonomously to cause polarity phenotypes. In addition, expression in a cwn-1 background of either a membrane-tethered CAM-1 CRD or full-length CAM-1 led to polarity phenotypes that were indistinguishable from one another. On the other hand, a CAM-1 transgene that lacks the CRD failed to induce polarity defects (Figure 5). These findings suggest that the CAM-1 CRD is both necessary and sufficient for Wnt antagonism.
Figure 5.
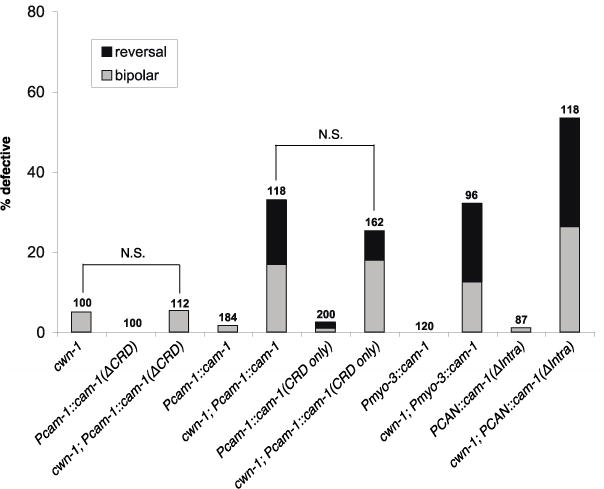
CAM-1 CRD is both necessary and sufficient to antagonize Wnt function in ALM polarity. Expression in a cwn-1 background of either a membrane-tethered CAM-1 CRD or full-length CAM-1 led to polarity phenotypes that were not significantly different from one another. A CAM-1 transgene that lacks the CRD failed to induce polarity defects above background. A CAM-1 transgene expressed from the muscle-specific myo-3 promoter or a CAN neuron-specific promoter induced polarity defects in a cwn-1 background. Data are presented as in Fig. 1K. N.S., not significant.
A recent study showed that CAM-1 expressed from the axons of the CAN neurons can function nonautonomously to sequester Wnts, which is important for proper vulval patterning (Modzelewska et al. 2013). However, CANs extend their axons in late embryogenesis (Wu et al. 2011), after the ALMs have already polarized. To test whether the CAN cell bodies have an effect on ALM polarity, we used a CAN-specific promoter to express a cam-1 cDNA that lacks the sequence for its intracellular domain (PCAN::cam-1 ΔIntra::gfp) (Modzelewska et al. 2013). We found that this transgene induced a polarity defect in a cwn-1 background (Figure 5), suggesting that CAM-1 expressed on the CAN cell body can modify Wnt distribution to regulate ALM polarity.
Discussion
The outcome of Wnt signaling is intricately regulated by an ensemble of positive and negative factors acting both within and outside of the Wnt responding cell. Here we propose a model where the C. elegans Ror kinase CAM-1 has dual and antagonistic functions in ALM neuronal polarity, acting as a receptor to promote Wnt signaling within the ALM while antagonizing Wnt signaling outside of ALM. We speculate that CAM-1 antagonizes Wnt function by sequestering Wnts and altering their distribution. This model makes three testable predictions that were supported by our observations. First, excess CAM-1 expression in tissues that are involved in the distribution of Wnts should lead to polarity phenotypes. We found that CAM-1 expressed in muscle cells and in the two CAN neurons induced ALM polarity defects in a sensitized background, and cam-1 loss enhanced these defects (Figures 3A and 5). This enhancement presumably results from the loss of CAM-1’s autonomous signaling function. The CAM-1 CRD is both necessary and sufficient for this antagonistic activity (Figure 5). Second, the presence of CAM-1 in cells other than the ALM should enhance the polarity phenotype caused by the absence of CAM-1 in the ALM, a prediction that is supported by our mosaic analysis (Figure 3C). Third, expression of CAM-1 in neurons should provide signaling function and suppress the ALM polarity defect of cam-1 mutants. Our transgene experiments and mosaic analysis corroborate these predictions (Figures 3A and 3C).
CAM-1 possesses autonomous and nonautonomous functions that act antagonistically
CAM-1 has been shown to act as a signaling receptor and as a Wnt antagonist in various developmental contexts (Green et al. 2008b). The site of non-autonomous CAM-1 function has remained elusive until a recent study showed that the CANs, which extend a pair of axons that span the entire anterior-posterior axis and express CAM-1, could sequester Wnts to ensure proper vulval patterning during larval development (Modzelewska et al. 2013). As CANs only fully extend their axons following hatching, it is surprising that CAM-1 expressed in these neurons can disrupt proper polarization of the ALM, which takes place during embryogenesis. This result suggests that CAM-1 expressed from the CAN cell body can modify Wnt distribution to regulate ALM polarity and is consistent with a finding by Modzelewska et al. that a similar transgene has an effect on the embryonic migration of another neuron, the HSN (Modzelewska et al. 2013).
Here we propose that Wnt sequestration by the CANs, muscle cells and possibly other cells provide a non-autonomous function for CAM-1. However, it has recently been shown that muscle-expressed CAM-1 has a signaling function in synaptic plasticity (Jensen et al. 2012). It remains to be determined whether CAM-1 non-autonomous function in ALM polarity is to sequester Wnts or to play a more complex signaling role.
CAM-1 also has been shown to have both autonomous and non-autonomous functions during the development of the vulva, albeit in different contexts. CAM-1 acts non-autonomously to sequester Wnts to prevent over-induction of vulval precursor cells (VPCs) (Green et al., 2007) while also acting autonomously in the orientation of VPCs by responding to the Wnt EGL-20 (Green et al., 2008a). This is slightly different from what we are proposing in this study, in which both functions of CAM-1 affect the same process (i.e. ALM polarization).
The dual roles of CAM-1 are also reminiscent of the roles Frizzled receptors play in Wnt-regulated wing patterning in Drosophila. Double fz; fz2 mutants fail to activate the expression of Wnt target genes in the presumptive wing cells, indicating that the Frizzled receptors mediate Wnt signaling (Chen and Struhl 1999). In addition, these Frizzled mutants have elevated extracellular Wnt levels. It is thought that the receptors can down-regulate Wnt signaling through internalization of the Wnts (Baeg et al. 2004; Han et al. 2005). In C. elegans, the Wnt receptors CAM-1, LIN-17 and LIN-18 have also been proposed to sequester Wnts non-autonomously (Green et al. 2007). Receptors involved in other pathways such as the Hedgehog, receptor tyrosine kinase and Netrin signaling pathways have also been shown to be involved in regulating both signaling and ligand distribution (Casanova and Struhl 1993; Chen and Struhl 1996; Hiramoto et al. 2000).
CAM-1 has both kinase-dependent and kinase-independent functions in ALM polarity
Our experiments support the idea that the kinase domain of CAM-1 is important for its function in ALM polarity. Several previous studies have also addressed the function of this the CAM-1 intracellular region (Kim and Forrester 2003; Francis et al. 2005; Kennerdell et al. 2009; Song et al. 2010). In many cases, the investigators found the intracellular domain to be at least partially, if not completely, dispensable for CAM-1 function. This may be due to the ability of CAM-1 to form co-receptor complexes. Genetic experiments suggest that CAM-1 can act as a co-receptor with the Frizzled receptors MIG-1, CFZ-2 and LIN-17 (Kennerdell et al. 2009; Song et al. 2010; Jensen et al. 2012) and with VANG-1/Van Gogh (Green et al. 2008a; Hayashi et al. 2009). The intracellular domain of CAM-1 is required when CAM-1 acts with VANG-1 (Green et al. 2008a; Hayashi et al. 2009), but is partially or completely dispensable when CAM-1 acts with Frizzled receptors (Francis et al. 2005; Kennerdell et al. 2009; Song et al. 2010; Jensen et al. 2012). Frizzled and Ror kinases can physically interact via their CRD domains (Oishi et al. 2003). The discrepancy in the requirement of the Ror intracellular domain could be further explained by a recent observation that a mutation in C-terminus of Vangl2 abolishes its interaction with Ror2 (Gao et al. 2011). In light of these observations, our finding that the CAM-1 kinase domain appears to play a role in signaling suggested that the C. elegans Van Gogh homolog VANG-1 might function as a CAM-1 co-receptor in ALM polarity, but neither vang-1 single nor cwn-1; vang-1 double mutants displayed ALM polarity defects (data not shown; n= 76 and 102, respectively). We did find that single mutations in mig-1 and lin-17 generated a weak synthetic polarity phenotype in a mom-5 background, suggesting that the Frizzleds that these genes encode are potential CAM-1 co-receptors functioning in parallel to MOM-5 in ALM polarity.
How may CAM-1 transduce the Wnt signal to downstream components to polarize the ALM? CAM-1 has been proposed to signal to downstream Wnt component Dishevelled DSH-1 to regulate the level of acetylcholine receptors and synaptic plasticity at the neuromuscular junction (Jensen et al. 2012). Similarly, CAM-1 and DSH-1 were shown to act genetically in the same pathway in the outgrowth of motor neurons, and yeast-2-hybrid experiments indicate that the intracellular domain of CAM-1 can physically interact with the PDZ and DEP domains of DSH-1, domains that are traditionally associated with non-canonical Wnt signaling (Song et al. 2010). Consistent with this idea, mutations in β-catenins (bar-1, wrm-1, and sys-1) did not have an effect on motor neuron outgrowth (Song et al. 2010). Our genetic analysis also suggests Wnts signal through a β-catenin-independent pathway to regulate ALM polarity. RNAi inactivation of canonical pathway components such as bar-1/ β-catenin, pry-1/Axin, pop-1/TCF did not generate a significant phenotype in a cwn-1; eri-1 background (unpublished observations).
The finding that CAM-1 can simultaneously function as a positive and negative regulator of Wnt signaling to polarize the ALM raises the intriguing prospect that CAM-1 functions as a Wnt receptor in many processes, but that mutant analysis of cam-1 mutants fails to reveal a role because of CAM-1’s antagonistic activities. For instance, CAM-1 is thought to act cell non-autonomously in HSN and PVM migrations (Kim and Forrester 2003; Forrester et al. 2004), but whether CAM-1 also functions in these neurons to promote their migrations has not been addressed. It also raises the possibility that Wnt-binding receptors that are broadly expressed may have a previously unrecognized function in antagonizing Wnt signaling. For example, the Frizzled receptors LIN-17 and MIG-1 antagonize each other in HSN migration with MIG-1 acting autonomously to promote this migration (Pan et al. 2006). One possible mechanism for LIN-17’s role in this process is to sequester Wnts that act through MIG-1. It is tempting to speculate that a fine balance of autonomous and non-autonomous activities of CAM-1, and possibly other Wnt receptors, determines the outcome of Wnt signaling in different developmental contexts that require their function.
Highlights.
The CAM-1 and MOM-5 Wnt receptors orient the polarity of the ALM neuron.
CAM-1 functions autonomously in the ALM neuron to orient its polarity.
CAM-1 also functions non-autonomously to antagonize Wnt signaling.
Acknowledgments
We thank Mei Ding, Joshua Kaplan and Nadeem Moghal for providing nematode strains and plasmids. We thank Anthony Lilienthal for the isolation of the mom-5(gm409) allele. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by the National Institutes of Health grant NS32057 to G.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This antagonistic function can mask CAM-1’s autonomous role as a Wnt receptor.
References
- Ailion M, Thomas JH. Isolation and characterization of high-temperature-induced Dauer formation mutants in Caenorhabditis elegans. Genetics. 2003;165:127–144. doi: 10.1093/genetics/165.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu K, Hu Z, Chien SC, Garriga G, Kaplan JM. The immunoglobulin super family protein RIG-3 prevents synaptic potentiation and regulates Wnt signaling. Neuron. 2011;71:103–116. doi: 10.1016/j.neuron.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg GH, Selva EM, Goodman RM, Dasgupta R, Perrimon N. The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors. Dev Biol. 2004;276:89–100. doi: 10.1016/j.ydbio.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Bainbridge TW, DeAlmeida VI, Izrael-Tomasevic A, Chalouni C, Pan B, et al. Evolutionary Divergence in the Catalytic Activity of the CAM-1, ROR1 and ROR2 Kinase Domains. PLoS One. 2014;9:e102695. doi: 10.1371/journal.pone.0102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Liu Y, Ruvkun G. Lineage-specific regulators couple cell lineage asymmetry to the transcription of the Caenorhabditis elegans POU gene unc-86 during neurogenesis. Genes Dev. 1996;10:1395–1410. doi: 10.1101/gad.10.11.1395. [DOI] [PubMed] [Google Scholar]
- Billiard J, Way DS, Seestaller-Wehr LM, Moran RA, Mangine A, et al. The orphan receptor tyrosine kinase Ror2 modulates canonical Wnt signaling in osteoblastic cells. Mol Endocrinol. 2005;19:90–101. doi: 10.1210/me.2004-0153. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J, Struhl G. The torso receptor localizes as well as transduces the spatial signal specifying terminal body pattern in Drosophila. Nature. 1993;362:152–155. doi: 10.1038/362152a0. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- Chen CM, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- Clark SG, Chiu C. C. elegans ZAG-1, a Zn-finger-homeodomain protein, regulates axonal development and neuronal differentiation. Development. 2003;130:3781–3794. doi: 10.1242/dev.00571. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM, Kim SK. Protruding vulva mutants identify novel loci and Wnt signaling factors that function during Caenorhabditis elegans vulva development. Genetics. 2000;156:1097–1116. doi: 10.1093/genetics/156.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T, Chien SC, Vanderzalm PJ, Dell M, Gavin MK, et al. The role of C. elegans Ena/VASP homolog UNC-34 in neuronal polarity and motility. Dev Biol. 2010;344:94–106. doi: 10.1016/j.ydbio.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester WC. The Ror receptor tyrosine kinase family. Cell Mol Life Sci. 2002;59:83–96. doi: 10.1007/s00018-002-8407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester WC, Dell M, Perens E, Garriga G. A C. elegans Ror receptor tyrosine kinase regulates cell motility and asymmetric cell division. Nature. 1999;400:881–885. doi: 10.1038/23722. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Garriga G. Genes necessary for C. elegans cell and growth cone migrations. Development. 1997;124:1831–1843. doi: 10.1242/dev.124.9.1831. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 inhibits EGL-20/Wnt signaling in cell migration. Genetics. 2004;168:1951–1962. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Evans SP, Jensen M, Madsen DM, Mancuso J, et al. The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron. 2005;46:581–594. doi: 10.1016/j.neuron.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, et al. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, et al. Wnt Signaling Gradients Establish Planar Cell Polarity by Inducing Vangl2 Phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. [DOI] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008a;134:646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JL, Kuntz SG, Sternberg PW. Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol. 2008b;18:536–544. doi: 10.1016/j.tcb.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development. 2005;132:667–679. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Hirotsu T, Iwata R, Kage-Nakadai E, Kunitomo H, et al. A trophic role for Wnt-Ror kinase signaling during developmental pruning in Caenorhabditis elegans. Nat Neurosci. 2009;12:981–987. doi: 10.1038/nn.2347. [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Herman RK. The ncl-1 gene and genetic mosaics of Caenorhabditis elegans. Genetics. 1995;141:989–1006. doi: 10.1093/genetics/141.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell. 2006;10:379–390. doi: 10.1016/j.devcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila Netrin receptor Frazzled guides axons by controlling Netrin distribution. Nature. 2000;406:886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Segbert C, Helbig G, Bossinger O. Intestinal tube formation in Caenorhabditis elegans requires vang-1 and egl-15 signaling. Dev Biol. 2010;339:268–279. doi: 10.1016/j.ydbio.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, et al. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Jensen M, Hoerndli FJ, Brockie PJ, Wang R, Johnson E, et al. Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell. 2012;149:173–187. doi: 10.1016/j.cell.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2:RESEARCH0002. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kani S, Oishi I, Yamamoto H, Yoda A, Suzuki H, et al. The receptor tyrosine kinase Ror2 associates with and is activated by casein kinase Iepsilon. J Biol Chem. 2004;279:50102–50109. doi: 10.1074/jbc.M409039200. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Wang D, Ruvkun G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature. 2004;427:645–649. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- Kennerdell JR, Fetter RD, Bargmann CI. Wnt-Ror signaling to SIA and SIB neurons directs anterior axon guidance and nerve ring placement in C. elegans. Development. 2009;136:3801–3810. doi: 10.1242/dev.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Forrester WC. Functional analysis of the domains of the C elegans Ror receptor tyrosine kinase CAM-1. Dev Biol. 2003;264:376–390. doi: 10.1016/j.ydbio.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Koga M, Take-uchi M, Tameishi T, Ohshima Y. Control of DAF-7 TGF-(alpha) expression and neuronal process development by a receptor tyrosine kinase KIN-8 in Caenorhabditis elegans. Development. 1999;126:5387–5398. doi: 10.1242/dev.126.23.5387. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen H, Hu L, Xing Y, Sasaki T, et al. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ross JF, Bodine PV, Billiard J. Homodimerization of Ror2 tyrosine kinase receptor induces 14-3-3(beta) phosphorylation and promotes osteoblast differentiation and bone formation. Mol Endocrinol. 2007;21:3050–3061. doi: 10.1210/me.2007-0323. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rubin B, Bodine PV, Billiard J. Wnt5a induces homodimerization and activation of Ror2 receptor tyrosine kinase. J Cell Biochem. 2008;105:497–502. doi: 10.1002/jcb.21848. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Maloof JN, Whangbo J, Harris JM, Jongeward GD, Kenyon C. A Wnt signaling pathway controls hox gene expression and neuroblast migration in C. elegans. Development. 1999;126:37–49. doi: 10.1242/dev.126.1.37. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. Embo J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewska K, Lauritzen A, Hasenoeder S, Brown L, Georgiou J, et al. Neurons refine the Caenorhabditis elegans body plan by directing axial patterning by Wnts. PLoS Biol. 2013;11:e1001465. doi: 10.1371/journal.pbio.1001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers TR, Greenwald I. Wnt signal from multiple tissues and lin-3/EGF signal from the gonad maintain vulval precursor cell competence in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:20368–20373. doi: 10.1073/pnas.0709989104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–562. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Pan CL, Howell JE, Clark SG, Hilliard M, Cordes S, et al. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell. 2006;10:367–377. doi: 10.1016/j.devcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Park FD, Tenlen JR, Priess JR. C. elegans MOM-5/frizzled functions in MOM-2/Wnt-independent cell polarity and is localized asymmetrically prior to cell division. Curr Biol. 2004;14:2252–2258. doi: 10.1016/j.cub.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, et al. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Sawa H, Lobel L, Horvitz HR. The Caenorhabditis elegans gene lin-17, which is required for certain asymmetric cell divisions, encodes a putative seven-transmembrane protein similar to the Drosophila frizzled protein. Genes Dev. 1996;10:2189–2197. doi: 10.1101/gad.10.17.2189. [DOI] [PubMed] [Google Scholar]
- Schambony A, Wedlich D. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell. 2007;12:779–792. doi: 10.1016/j.devcel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Song S, Zhang B, Sun H, Li X, Xiang Y, et al. A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Walston T, Guo C, Proenca R, Wu M, Herman M, et al. mig-5/Dsh controls cell fate determination and cell migration in C. elegans. Dev Biol. 2006;298:485–497. doi: 10.1016/j.ydbio.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Winkel A, Stricker S, Tylzanowski P, Seiffart V, Mundlos S, et al. Wnt-ligand-dependent interaction of TAK1 (TGF-beta-activated kinase-1) with the receptor tyrosine kinase Ror2 modulates canonical Wnt-signalling. Cell Signal. 2008;20:2134–2144. doi: 10.1016/j.cellsig.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ghitani A, Christensen R, Santella A, Du Z, et al. Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2011;108:17708–17713. doi: 10.1073/pnas.1108494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Zinovyeva AY, Forrester WC. The C. elegans Frizzled CFZ-2 is required for cell migration and interacts with multiple Wnt signaling pathways. Dev Biol. 2005;285:447–461. doi: 10.1016/j.ydbio.2005.07.014. [DOI] [PubMed] [Google Scholar]


