Abstract
Background
In US cervical screening, immediate colposcopy is recommended for women with HPV-positive ASC-US (equivocal) cytology. We evaluated whether partial typing by Onclarity™ (BD) might identify HPV-positive women with low enough CIN3+ risk to permit 1-year follow-up instead.
Methods
The NCI-Kaiser Permanente Northern California Persistence and Progression Cohort includes a subset of 13,890 women aged 21+ with HC2 (Qiagen)-positive ASC-US at enrollment; current median follow-up is 3.0 years. Using stratified random sampling, we typed 2,079 archived enrollment specimens including 329 women subsequently diagnosed with CIN3+, 563 with CIN2, and 1,187 with <CIN2. Adjusting for sampling, we computed 3-year cumulative CIN3+ risks for each Onclarity typing channel, using Kaplan-Meier methods.
Results
The 3-year CIN3+ risk for all HC2-positive women with ASC-US was 5.2%; this establishes the “benchmark” risk for colposcopic referral. Hierarchically, 3-year cumulative risks for each typing channel were 16.0% for HPV16, 7.4% for HPV18, 7.0% for HPV31, 7.1% for grouped HPV33/58, 4.4% for HPV52, 3.9% for HPV45, 2.7% for HPV51, 1.6% for HPV39/68/35, and 1.3% for HPV59/56/66.
Discussion
ASC-US linked to HPV16, HPV18, HPV31, or HPV33/58 warrants immediate colposcopy. Optimal management of women with HPV52 or HPV45 is uncertain. Risk of women with only HPV51, HPV39/68/35, or HPV59/56/66 might be low enough to recommend 1-year retesting permitting viral clearance. This strategy would defer colposcopy for 40% of women with HPV-positive ASC-US, half of whom would be cotest-negative at 1-year return. Approximately 10% of those with CIN3 diagnosable at enrollment would be delayed 1 year instead. Cost-effectiveness analyses are needed.
Keywords: ASC-US, HPV testing, cervical screening, triage
INTRODUCTION
Cervical cancer arises via well-established steps including cervical infection with one of approximately a dozen high-risk human papillomavirus (HPV) types, viral persistence (rather than usual clearance), progression of a clone of persistently infected epithelial cells to a precancer, and invasion [1]. Prophylactic vaccination of adolescents against HPV infection will provide the ultimate prevention of cervical cancer but will take decades to achieve this goal. In the meantime in the US, prevention of cancer by screening programs relies on excision or ablation of the cancer-prone cervical transformation zone when precancers (CIN3) are found. Depending on age and parity, women with more equivocal precancers (CIN2) may be treated as well [2].
To define which women have treatable precancer, screening in the US relies on a combination of cytology (conventional or liquid-based Pap tests) and HPV molecular tests [3]. Women judged to be at sufficient risk are sent for colposcopic biopsy. However, there is currently no consensus on the most effective combination of the two methods. In some screening programs, HPV testing is limited to clarification (triage) of equivocal cytologic results called atypical squamous cells of undetermined significance (ASC-US) [2]. At the other extreme, an HPV assay has recently been approved by the US FDA for primary standalone screening; in this approach, the use of cytology may be limited to triage of HPV-positive women lacking the 2 most important carcinogenic types, HPV16 and HPV18 (detection of either generally leads to immediate colposcopy) [4].
Although primary HPV testing might eventually be employed broadly, cytology still remains part of most cervical screening in the US, either alone or in the context of HPV/cytology cotesting. But ASC-US (equivocal) is by far the most common non-normal cytology result, which is more common than all other non-normal cytology results combined [5]. An ASC-US result means that there are subtle microscopic abnormalities of the cervical squamous epithelial cells that might or might not represent the effects of HPV infection. ASC-US is not a biological entity; rather it is an expression of interpretive uncertainty [6]. Accordingly, the great majority of ASC-US results are now managed according to results of concurrent or reflex HPV molecular testing.
The use of HPV-positive results to triage ASC-US (colposcopy if positive, normal if not) is complicated by the great differences in cancer risk between different high-risk HPV types that occur commonly in HPV-positive ASC-US. HPV16 is uniquely carcinogenic, causing 60% of cancers and 50% of precancers [7]. At the other extreme, a type like HPV56 rarely causes cancer (and so it is included as “high-risk”), but only in a very small percentage of cases [7, 8].
Clinical use of HPV typing might exploit the heterogeneous gradient of risk by type, and possibly create a more finely tuned management approach to ASC-US if such precision is desired and resources permit. At present, HPV typing is recommended by guidelines committees only to help guide the management of HPV-positive women with negative cytology [2]. In general, such women are retested in 1 year, but finding HPV16 or HPV18 elevates risk estimates, and mandates colposcopy instead. We examined a complementary possibility, i.e., whether HPV partial typing using the BD Onclarity™ assay might uncover a sizable subset of women with HPV-positive ASC-US having low enough precancer/cancer risk to permit follow-up in one year with the expectation of viral clearance, thus avoiding colposcopy.
MATERIALS AND METHODS
The PaP Cohort
As described previously in detail, Kaiser Permanente Northern California (KPNC) initiated cervical screening with cytology-HPV cotesting in 2003 at 3-year intervals for women 30 and older [9]. HPV triage of ASC-US was already routinely performed at all ages 21 and older. The Persistence and Progression (PaP) cohort study was created as a very large “convenience sample” by storing residual cervical specimens from approximately 55,000 mainly HPV-positive women co-tested or triaged at KPNC from 2007–2010 (with <1% enrolled in late 2006 or January 2011) [10]. Approximately 8% of women opted out of specimen use.
Clinical HPV testing was performed using Hybrid Capture 2 (HC2, Qiagen, Germantown, MD). Cytology tests varied by time and KPNC laboratory: during the study period, conventional Pap smears were replaced by liquid-based cytology (SurePath, BD Diagnostics, Sparks, MD). HC2 results were known at the time of cytology review, which was also informed by FocalPoint (BD, Diagnostics) automated pre-screening for slightly more than half of readings. Residual exfoliated cervical specimen in specimen transport medium (STM, Qiagen) left after HC2 testing was stored for study use. The specimen was neutralized and stored at −70–80 degrees C, and transported on dry ice until HPV typing was performed [11]. The clinical cut-off for the STM sample type was deduced using a pre-established cut-off for 0.5 mL of PreservCyt liquid-based cytology (LBC) media. Briefly, equal numbers of C-33 A cells harboring eight different HPV E6/E7 target sequences were spiked into both collection vials (1 mL STM; 20 mL ThinPrep) and the Ct response with increasing amounts of STM specimen was compared to 0.5 mL of ThinPrep media. 25 ml of STM specimen was found to give a similar Ct response to 0.5 mL of LBC medium and had a similar clinical performance to Hybrid Capture 2.[12] An equivalent aliquot of neutralized specimen was used in this study.
Study Population
During the years 2007–2010 at KPNC, 809,315 women aged 21 or older had at least one cotest (concurrent cytology and HC2, including for this analysis those performed reflexively for management of cytologic results in women aged 21–29). The cytologic result of the first cotest in the 4-year enrollment period was ASC-US for 39,305, of which 17,190 (43.7%) were HC2 positive. The percentage of HC2 positivity decreased substantially with age (from >60% at ages 21–24 to ~30% at ages 60+). As shown in Figure 1, we successfully collected residual HC2 specimens from 13,890 women with enrollment HC2-positive ASC-US, or about 81% of the 17,190 eligible specimens. The missed fraction was random with regard to risk of disease, e.g., we did not collect specimens from women attending Friday afternoon clinics because prompt processing was not possible. Thus, the PaP collection is highly likely to represent the full KPNC population.
Figure 1.
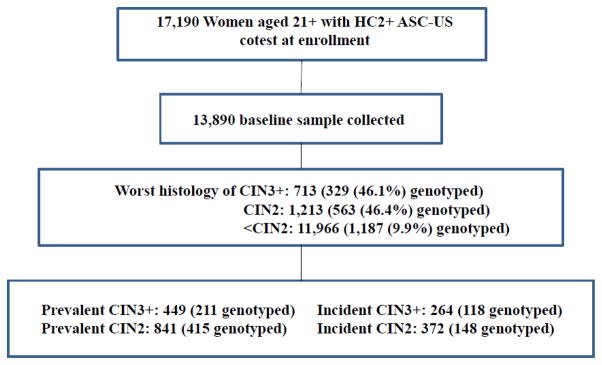
For Onclarity testing, we drew a random sample of half of women whose worst histologic diagnosis during follow-up to date was CIN3+, half of those diagnosed with CIN2, and approximately10% of those never (to date) diagnosed with CIN2+ (≤CIN1). A subsequent data update of the cohort after the initial draw in mid-2014 revealed several additional, interval incident cases of CIN2 or CIN3+; they were switched from the control group to the applicable incident case category for the data analyses.
To permit a methodologic ancillary analysis, we also selected a random sample of 200 specimens from women with HC2-negative ASC-US at enrollment. The objective was to estimate crudely the impact had we used Onclarity rather than HC2 on HPV prevalence and colposcopy referrals.
Follow-up of the PaP Cohort
Information on subsequent histologic diagnoses of women in the PaP cohort was obtained. The median length of follow-up of the women sampled for this analysis who were not diagnosed with CIN2+ was 3.6 years (IQR of 1.2 to 5.0 years, maximum 7.7 years). Median time until the screening visit leading to diagnosis of CIN2 was 0.0 years (prevalent diagnosis, IQR of 0.0 to 0.5 years, maximum 6.0 years); median time until the screening visit leading to diagnosis of CIN3+ was 0.0 years (IQR of 0.0 to 1.1 years, maximum 6.3 years). Thus, the majority of cases of precancer/cancer diagnosed in the PaP Cohort to date among women with an HPV-positive ASC-US result were prevalent cases diagnosed based on enrollment screening. The average age at enrollment of women in the three diagnostic groups was very similar, with means of 35–36 years of age and the majority of women in all three groups between 25 and 40.
BD Onclarity HPV Typing
The BD Onclarity HPV Assay is a multi-plex Real-Time PCR assay that targets HPV type-specific E6 or E7 sequences [13]. The assay detects 14 HPV types, providing extended genotyping information. The assay was designed to distinguish the most carcinogenic HPV types from those less likely to cause CIN3+. Output results include individual genotype results for HPV 16, 18, 31, 45, 51 and 52, with HPV 33_58 as a paired channel result (which cumulatively account for approximately 91% of cervical cancers) [14]; the remaining 6 HPV types are combined in two channels of three (HPV 56, HPV59, and HPV66 and HPV35, HPV39, and HPV68). DNA extraction, PCR amplification and detection are performed on the integrated BD Viper LT System. The clinical performance of the assay has been described previously and it fulfills guidelines for clinically validated assays [15, 16]; an FDA trial for U.S. licensure is currently underway.
Statistical Methods
First, we compared Onclarity and HC2 results (positive/negative). We noted the Onclarity typing results of test-positive specimens in the random sample of 200 HC2-negative ASC-US specimens.
As the main analysis, we estimated the 3-year CIN3+ risks for each typing channel among the HC2-positive ASC-US women, including Onclarity-negative results as one possible result. We calculated the risk estimates by reconstituting the sampling fractions and using Kaplan-Meier methods that accounted for loss-to-follow-up. The risk estimations were repeated for CIN2+ for completeness. The calculations were performed using the kapmeier procedure in SAS callable SUDAAN version 11.
As an important feature of the risk estimation, it was performed in a hierarchical manner, to take into account the relatively common (27.4%) occurrence of multiple infections among Onclarity-positive women. The typing channel with the highest risk was estimated (HPV16 turned out as expected to be the highest-risk result). We then calculated the risk of the remaining channels, to find the second highest-risk channel for specimens that lacked HPV16; we made this exclusion in order to avoid confusion due to multiple infections containing HPV16. We proceeded channel-by-channel in a hierarchical manner, excluding higher-risk type channels, until we arrived at the lowest-risk one.
In ancillary analyses, we estimated outcomes among women whose HPV infections fell only into the 3 lowest-risk typing channels. Because their 3-year risk was significantly lower than the recommended threshold for colposcopy referral in the current management guidelines ([2], see Results and Discussion), we estimated what might have happened if these women had not been referred immediately to colposcopy.
In ancillary, supplemental analyses, we also recalculated the main findings in two more restricted cohorts: 1) women aged 25 and older enrolled at the time of a screening visit (those following a negative cotest without known history of precancer), to inform the possible use of HPV typing in the context of primary HPV screening [4], and 2) women aged 30 and older coming for a screening HPV-cytology cotest visit (again excluding those being managed for prior abnormalities) in line with current cotesting guidelines [2]. Finally, we estimated crudely what would have resulted if Onclarity had been used in place of HC2 as the screening test.
RESULTS
Among the 196 of 200 HC2-negative ASC-US specimens whose specimens were available for retesting by Onclarity, 15 (7.7%) tested positive: 3 HPV16, 2 HPV45, 3 HPV52, 1 HPV51, and 7 HPV59/56/66 (1 sample was positive for both HPV16 and HPV52). This sample of 196 HC2-negative specimens yielded 2 incident cases of CIN2, of which 1 was HPV59/56/66 positive by Onclarity at enrollment, and 1 incident case of CIN3 (HPV16 positive at enrollment).
The remaining analyses focused on the HC2-positive specimens. Table 1 shows the HPV typing results by case group; the percentages sum to >100% because specimens could test positive concurrently for multiple infections. Among the <CIN2 specimens, HPV16 was the most common single type identified, although two grouped channels yielded higher percentages of positivity. The relative prevalence of HPV16 increased with severity of outcome, and more than half of enrollment specimens from women diagnosed with CIN3+ were positive for HPV16.
Table 1.
Onclarity Typing Results of HC2+ ASC-US, by Worst Subsequent Histologic Result
| <CIN2 (n = 1187) | CIN2 (n = 563) | CIN3+ (n = 329) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HPV type channel | HPV Channel + | % | HPV Channel + | % | HPV Channel + | % |
| HPV16 | 190 | 16.0% | 185 | 32.9% | 179 | 54.4% |
| HPV18 | 71 | 6.0% | 37 | 6.6% | 24 | 7.3% |
| HPV31 | 130 | 11.0% | 81 | 14.4% | 42 | 12.8% |
| HPV45 | 56 | 4.7% | 22 | 3.9% | 15 | 4.6% |
| HPV51 | 131 | 11.0% | 44 | 7.8% | 20 | 6.1% |
| HPV52 | 148 | 12.5% | 104 | 18.5% | 47 | 14.3% |
| HPV33 or HPV58 | 105 | 8.9% | 68 | 12.1% | 32 | 9.7% |
| HPV39 or HPV68 or HPV35 | 264 | 22.2% | 116 | 20.6% | 45 | 13.7% |
| HPV59 or HPV56 or HPV66 | 314 | 26.5% | 87 | 15.5% | 26 | 7.9% |
| Any channel positive | 1047 | 88.2% | 537 | 95.4% | 319 | 97.0% |
Approximately 12% of HC2-positive ASC-US specimens tested negative by Onclarity. Of note, 26/563 (4.6%) HC2-positive CIN2 cases and 10/329 (3.0%) HC2-positive CIN3+ cases tested negative with Onclarity. There were few (n=8) invasive cancers; of which 5 were HPV16 positive, 2 were HPV18 positive and 1 was Onclarity negative (but HPV18 positive in earlier testing in another laboratory). Five of the 8 cases were localized adenocarcinomas (ages 32–59 at time of testing that preceded histologic diagnosis by ≤ 16 months), while the 3 SCC included 1 localized case (age 27; 21 months to diagnosis), 1 with regional lymph node extension (age 34; 41 months to diagnosis), and 1 with distant spread (age 54, the Onclarity-negative case diagnosed 62 months after the HPV testing). All 7 cases of AIS tested Onclarity positive, with HPV16 and/or HPV18.accounting for all but 1).
The 3-year CIN3+ risk estimates by type result are detailed in Table 2. The analysis is hierarchical for both the type-positive and type-negative results (e.g., HPV16-positive specimens are excluded when HPV18 positivity/negativity is evaluated). There was a greatly elevated 16.0% 3-year risk of CIN3+ associated with HPV16 positivity, which was more than 10 times greater than the 1.3% risk associated with the lowest-risk channel (HPV59/HPV56/HPV66). Of note, the risk when HPV16 was absent was low (3.7%), more than 12% lower in absolute risk than when HPV16 was present. HPV16 (present/absent) was clearly the most powerful risk stratifying type.
Table 2.
Hierarchical Results of Onclarity Testing of HC2-Positive ASC-US, with 3-Year Risks of CIN3+
| Positive | Negative | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HPV Typing Result (Hierarchical) | N women | 3-year risk of CIN3 + if channel is positive | Lower 95 % CI | Upper 95 % CI | N women | 3-year risk of CIN3 + if channel is negative | Lower 95 % CI | Upper 95 % CI | N women |
| HPV16 | 554 | 16.0% | 14.1% | 18.0% | 1525 | 3.7% | 3.3% | 4.1% | 2079 |
| HPV18 | 106 | 7.4% | 5.1% | 10.7% | 1419 | 3.4% | 3.1% | 3.8% | 1525 |
| HPV31 | 204 | 7.0% | 5.3% | 9.4% | 1215 | 2.9% | 2.5% | 3.3% | 1419 |
| HPV33 or HPV58 | 160 | 7.1% | 5.1% | 9.6% | 1055 | 2.4% | 2.0% | 2.8% | 1215 |
| HPV52 | 226 | 4.3% | 3.2% | 5.9% | 829 | 1.9% | 1.5% | 2.3% | 1055 |
| HPV45 | 55 | 3.9% | 1.9% | 7.7% | 774 | 1.7% | 1.4% | 2.2% | 829 |
| HPV51 | 114 | 2.7% | 1.7% | 4.5% | 660 | 1.6% | 1.2% | 2.1% | 774 |
| HPV39 or HPV68 or HPV35 | 270 | 1.6% | 1.0% | 2.3% | 390 | 1.6% | 1.1% | 2.3% | 660 |
| HPV59 or HPV56 or HPV66 | 214 | 1.3% | 0.7% | 2.3% | 176 | 2.0% | 1.2% | 3.3% | 390 |
Overall 3-year
CIN3+ risk among
HC2+ ASC-US 5.2%
In this hierarchical analysis, HPV18 (in the absence of HPV16), HPV31 (in the absence of HPV16 or HPV18), and HPV33/HPV58 (in the absence of HPV16, HPV18, or HPV31) all yielded CIN3+ risks larger than the 5.2% risk associated with HC2 positivity overall. However, the lower 95% confidence bounds were very close to the 5.2% HC2-positive ASC-US overall risk, which was taken as the threshold for colposcopy referral. HPV52 and HPV45 were associated with non-significantly lower risks than the threshold. The remaining channels were associated with significantly lower risks. Of note, Onclarity-negative (but HC2-positive) specimens yielded a 2.0% 3-year risk of CIN3+, non-significantly higher than the risk associated with HPV39/68/35 or HPV59/56/66 positive results.
In Table 3, the comparable 3-year risk estimates are given for CIN2, representing equivocal precancer, to demonstrate that choice of disease endpoint did not, in this instance, change the conclusions. HPV16 remained the type conferring very high risk of CIN2, and the rough ordering of risk was similar to that for CIN3+.
Table 3.
Hierarchical Results of Onclarity Testing of HC2-Positive ASC-US, with 3-Year Risks of CIN2+
| Positive | Negative | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HPV Typing Result (Hierarchical) | N women | 3-year risk of CIN2 + if channel is positive | Lower 95 % CI | Upper 95 % CI | N women | 3-year risk of CIN2 + if channel is negative | Lower 95 % CI | Upper 95 % CI | N women |
| HPV16 | 554 | 30.6 % | 27.7 % | 33.8 % | 1525 | 10.9 % | 10.4 % | 11.4 % | 2079 |
| HPV18 | 106 | 19.1 % | 14.6 % | 24.7 % | 1419 | 10.4 % | 9.8 % | 10.9 % | 1525 |
| HPV33 or HPV58 | 173 | 18.4 % | 14.9 % | 22.5 % | 1246 | 9.5% | 8.9 % | 10.1 % | 1419 |
| HPV31 | 191 | 16.4 % | 13.4 % | 19.9 % | 1055 | 8.4% | 7.8 % | 9.1 % | 1246 |
| HPV52 | 226 | 14.8 % | 12.3 % | 17.7 % | 829 | 7.0% | 6.3 % | 7.7 % | 1055 |
| HPV45 | 55 | 10.5 % | 6.8 % | 16.0 % | 774 | 6.7% | 6.1 % | 7.5 % | 829 |
| HPV39 or HPV68 or HPV35 | 285 | 8.6% | 7.2 % | 10.3 % | 489 | 5.7% | 4.9 % | 6.7 % | 774 |
| HPV51 | 99 | 8.2% | 5.9 % | 11.3 % | 390 | 5.2% | 4.3 % | 6.2 % | 489 |
| HPV59 or HPV56 or HPV66 | 214 | 4.8% | 3.7 % | 6.3 % | 176 | 5.6% | 4.3 % | 7.4 % | 390 |
Overall 3-year
CIN2+ risk among 14.1
HC2+ ASC-US %
The 3 channels that, in the absence of higher risk types, yielded significantly lowered risks compared with the benchmark threshold for colposcopy referral comprised an estimated 34.7% of all the HC2-positive ASC-US specimens, or 38.9% of HC2 positive specimens if Onclarity-negative specimens were excluded. Of the 449 CIN3+ cases diagnosed at enrollment, 9.9% tested positive only for a type or types in the 3 lowest-risk channels.
The remaining analyses explored to the extent possible the “downstream effects” of using HPV typing in ASC-US triage. Specifically, we wished to estimate what might have happened to women testing positive only for types in the 3 lowest-risk channels if they (hypothetically) had not been referred immediately to colposcopy. This represents in part a “counterfactual” analysis, in that most women were in fact referred immediately to colposcopy based on HC2 positivity. For this projection, we tabulated the outcomes associated with the specimens testing positive only for the 3 lowest-risk channels. In order to estimate risks and counts for all HC2-positive ASC-US in the PaP Cohort, taking into account the fact that we only partially tested the total, we re-expanded the sampling fractions of each histologic outcome category.
First, we estimated the number of CIN2+ lesions diagnosable at enrollment that would have been delayed if colposcopy had not been performed for the lowest-risk HPV types. Of the 4823/13890 women projected to have enrollment HPV positivity restricted to the lowest-risk HPV types, we had no follow-up data (biopsy or retesting) on 4.8%. Including in the denominator solely the 95.2% of women who had either a biopsy or subsequent retesting visit that permitted risk estimation, 84.0% had an enrollment biopsy result. There were no cancers but an estimated 46 (1.0%) women had CIN3/AIS diagnosed at enrollment and 205 (4.5%) had CIN2 diagnosed at enrollment. Thus, we estimate that approximately 5% of women who would have been told to return in 1 year could have had CIN2+ diagnosed at enrollment, mainly CIN2, if referred immediately to colposcopy.
We next estimated what percentage of women not referred immediately to colposcopy might have avoided it altogether. Of the estimated 4341 women with the lowest-risk HPV types and <CIN2 or no biopsy at enrollment, 66.0% had a subsequent cotest; the median time to repeat testing was 1.2 years (IQR 0.9–1.4 years). We did not have Onclarity testing on these repeat specimens, and can report only that 55.3% had negative cotests suggesting viral clearance (which might have meant avoidance of colposcopy for these women); 38.6% were again HC2 positive, and an additional 6.1% were HPV-negative but had ASC-US or worse cytologic results. (Of note, of those women with colposcopy following non-normal repeat testing, there were 1.6% cases of CIN2 and 0.5% cases of CIN3. Thus, the yield of CIN2+ among women with persistent abnormalities was low, only about 2%.)
In additional ancillary analyses, we re-estimated the performance of Onclarity testing of HC2-positive ASC-US for women aged 25+ or 30+, restricted to screening visits, i.e., those following a previous negative cotest without known history of precancer. The objective was to make the data concordant with two applications of HPV testing: 1) primary standalone HPV testing starting at age 25 (based on 1,344 selected for Onclarity testing) with ASC-US cytology leading to immediate colposcopy and 2) cotesting beginning at age 30 (based on 945 selected for testing), in which ASC-US cytology is the threshold for immediate colposcopy when HPV is positive. The results, shown in Supplemental Tables 1 and 2, demonstrated that the results, despite reduced precision due to smaller numbers, were unchanged compared with all HC2-positive ASC-US; the same 3 channels would have lowest risk of CIN3+.
Finally, we crudely estimated the impact on the results if Onclarity had been used from the beginning as the screening and ASC-US triage test instead of HC2. To obtain these estimates, we expanded the sampling fractions of HC2-positive and for HC2-negative ASC-US; the tested sample of HC2-negative ASC-US was small, introducing unreliability into the expanded estimates. The results indicate that if Onclarity had been used for screening instead of HC2, the % of ASC-US found to be HPV-positive would be virtually unchanged (~44%). Of the HPV-positives, the % in the 3 lowest risk Onclarity channels would be similar (40.3% using Onclarity for screening compared with 38.9% using HC2).
DISCUSSION
In this study, typing of HPV-positive ASC-US revealed a type-dependent 10-fold range in 3-year risks of CIN3+, ranging from high risk (16.0%) that should mandate immediate colposcopy to low risk (2.0%) that might be better managed with repeat testing at 1 year. Current US guidelines for the clinical management of cervical screening abnormalities are based on risks of CIN3+ of the various screening combinations [2, 5]. The underlying principle is “equal management of equal risk”. Currently, all women with HPV-positive ASC-US are recommended for immediate colposcopy. If more precise, risk-based management is desired, and if our results are confirmed, it is possible that HPV-positive women found to have only the lowest risk HPV types in the high-risk pool (approximately 40% of women with HPV-positive ASC-US) might be followed instead for viral clearance. Among women found to have only the lowest risk types detected by Onclarity, the 3-year risk of CIN3+ was consistent with those leading in current management guidelines to 1-year retesting. For example, their risk was similar to the risk of CIN3+ following ASC-US cytology unqualified by HPV testing in the larger KPNC cohort [5]. This strategy would delay colposcopy for 1 year in about 10% of women with CIN3+ diagnosable at baseline; from another perspective, that same number of deferred diagnosable CIN3 cases represents 1% of women testing positive only for the low-risk channels. The risk of a 1-year delay is unknown. On the other hand, we estimate that about half of the 40%, or about 20% of women with HPV+ ASC-US (i.e., >100,000 women per year in the US) retested at 1 year would have normal repeat cotests, and possibly avoid colposcopy altogether.
HPV16 was associated with a uniquely strong elevation in risk of CIN3+. However, the finding of HPV16 with ASC-US does not change clinical management compared to HPV-positive ASC-US overall, whose risk is quite similar to LSIL and which already mandates referral to colposcopy.
In the case of HPV-positive ASC-US, it is the absence of HPV16 that lowered risk to a level that suggested immediate colposcopy might not be needed. This conclusion contrasts with a previous analysis from the ALTS group, which showed similar risk stratification from HPV16 typing, but was interpreted as not warranting a change in management [17]. The combined data now look more compelling. When HPV18, HPV31, and HPV33/58 were also absent, the remaining HPV-positive ASC-US cases were still numerous but even less risky as a group than HC2-positive ASC-US overall. HPV52 and HPV45 were intermediate in risk in this study, and their proper management is uncertain. These types might be more important in other regions; HPV45 in particular is an important cause of invasive cervical cancer worldwide [7]. Therefore, it might be just the lowest risk HPV types in the high-risk pool, when found alone with ASC-US, which could justify follow-up instead of colposcopy.
When we re-estimated the risks for CIN2, the conclusions were similar. In general, it is preferable to base screening decisions on CIN3, a more certain surrogate of invasive cancer risk. Surrogate endpoints are required in decision-making because we cannot practically or ethically follow women to cervical cancer mortality or even incidence. CIN2 can be caused by HPV types, such as HPV53 or HPV66, that very rarely cause invasive cancer [7]. In this case, the results were consistent for CIN2 and CIN3+.
The conclusions were similar for women aged 25 and older (matching primary HPV testing with reflex cytology), or 30 and older (matching cotesting).
As a limitation of the study, we do not know what HPV types if any were present in the HC2-positive, Onclarity-negative specimens. The 3-year risk of CIN3+ in this group was low (2.0%); it is possible, supported by some scant published evidence [13], that many of these specimens contained HPV types known to cross-react with HC2 probes (e.g., HPV53, HPV73, HPV82), whose cumulative risk for CIN2 and CIN3 is elevated, but whose risk for invasive cancer is lower when grouped than the overall risk of the 12 established carcinogenic types assayed by Onclarity. Of note, HPV66 is targeted by Onclarity but is now defined like HPV53 as “possibly carcinogenic”; it is rarely found in invasive cancers alone [8]. These subtleties at the low end of carcinogenicity of the high-risk HPV types, compared to HC2 positive but untyped specimens, probably explain the odd-looking reversal in the risks of positivity versus negativity of the lowest-risk Onclarity channels among HC2-positive women [18].
Another limitation of this study is that the definition of HPV positivity was based on another test, HC2. The clinical sensitivity and specificity of the 2 assays is roughly similar, but Onclarity is slightly more analytically sensitive for the established carcinogenic types, and has not been reported to cross-react with genetically related types classified as “possibly carcinogenic” [18]. Thus, although we cannot estimate precisely what the HPV positivity rates would have been if the cohort had been screened with Onclarity instead, it appears that the positivity rates would remain about the same. Of the women with HC2-positive ASC-US, 10.7% tested Onclarity-negative and would have been recommended for 3-year follow-up (because the cancer risk from HPV-negative ASC-US is similar to that of negative cytology). On the other hand, 7.7% of 196 HC2-negative ASC-US specimens tested Onclarity-positive. Because the majority of ASC-US specimens were HPV-negative, the two kinds of discrepant results virtually balance. The analysis also showed, albeit with limited precision that the results, including the percentages in the 3 lowest-risk typing channels, would have been very similar had Onclarity been used instead of HC2 for screening.
Discovery of persistent HPV infection as the necessary cause of cervical cancer is a major achievement; but translation into cancer prevention is far from complete. Two desirable objectives are in apparent conflict, precision in clinical management versus simplicity in public health activities. It is now possible to estimate risk of CIN3+ with unprecedented accuracy and precision. But at the same time, it is debatable whether most clinicians or patients can make good use of knowing the complexities underlying those estimates.
The underlying answer, we believe, is to return to the principal function of cancer screening, which is to condense risks for presentation and use, even when finely estimated by whatever methods, into “risk bands”, each linked to a distinct course of action. Regardless of test methods and exact risk estimates, there are really only 5 clinical responses to cervical screening and triage: 1) treatment in case of extremely high risk (e.g., persistent HPV16 and HSIL regardless of biopsy result), 2) colposcopic biopsy of women at less extreme but substantial risk, 3) intensified retesting (e.g., at 6 months - 2 years) primarily of women with HPV infections but no precancer, 4) routine screening (at 3–5 years) of women without infection, and 5) exiting from screening for women at virtually no risk (post benign hysterectomy or after sustained normal screening at older ages). It will be important for diagnostic companies to cooperate in producing the comparative data needed to translate between their results. An emphasis on minor product distinctions rather than broad similarities leading to consistent risk-based actions would ultimately be counter-productive in the rational introduction of HPV diagnostics into cervical screening.
It is not feasible to conduct randomized clinical trials to answer the many questions faced in the design of cervical screening programs that combine varieties of cytology, HPV tests, and novel biomarkers. To create optimal cervical screening guidelines, an activity at the intersection of public health and clinical medicine, demands that we rely on large-scale realistic longitudinal data and focused, broadly accepted health decision simulation modeling analyses. The risks presented in the present analysis merit consideration by such decision analysts, in the formulation of future management guidelines.
Supplementary Material
Highlights.
HPV genotyping might be useful as part of ASC-US triage.
The absence of the highest-risk genotypes reduces risk sufficiently to consider one-year retesting rather than immediate colposcopy.
A fifth of colposcopy referrals might be avoided with little delay in diagnosis of CIN3 or worse lesions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 2.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829–46. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]
- 3.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012 doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 4.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of Primary High-Risk Human Papillomavirus Testing for Cervical Cancer Screening: Interim Clinical Guidance. Obstet Gynecol. 2015 doi: 10.1097/AOG.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 5.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. Journal of lower genital tract disease. 2013;17:S28–35. doi: 10.1097/LGT.0b013e318285423c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayar R, Solomon D. Second edition of ‘The Bethesda System for reporting cervical cytology’ - atlas, website, and Bethesda interobserver reproducibility project. CytoJournal. 2004;1:4. doi: 10.1186/1742-6413-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjose S, Franceschi S, et al. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int J Cancer. 2012 doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 8.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 9.Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595–600. doi: 10.1097/AOG.0b013e3181996ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiffman M, Burk RD, Boyle S, Raine-Bennett T, Katki HA, Gage JC, et al. A Study of Genotyping for the Management of Human Papillomavirus-Positive, Cytology-Negative Cervical Screening Results. J Clin Microbiol. 2014 doi: 10.1128/JCM.02116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaMere BJ, Kornegay J, Fetterman B, Sadorra M, Shieh J, Castle PE. Human papillomavirus genotyping after denaturation of specimens for Hybrid Capture 2 testing: feasibility study for the HPV persistence and progression cohort. J Virol Methods. 2007;146:80–5. doi: 10.1016/j.jviromet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle PE, Gutierrez EC, Leitch SV, Maus CE, McMillian RA, Nussbaumer WA, et al. Evaluation of a new DNA test for detection of carcinogenic human papillomavirus. J Clin Microbiol. 2011;49:3029–32. doi: 10.1128/JCM.00422-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright TC, Jr, Stoler MH, Agreda PM, Beitman GH, Gutierrez EC, Harris JM, et al. Clinical performance of the BD Onclarity HPV assay using an adjudicated cohort of BD SurePath liquid-based cytology specimens. Am J Clin Pathol. 2014;142:43–50. doi: 10.1309/AJCP53KMHNRDICBL. [DOI] [PubMed] [Google Scholar]
- 14.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 15.Cuzick J, Cadman L, Mesher D, Austin J, Ashdown-Barr L, Ho L, et al. Comparing the performance of six human papillomavirus tests in a screening population. Br J Cancer. 2013;108:908–13. doi: 10.1038/bjc.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbyn M, Snijders PJ, Meijer CJ, Berkhof H, Cuschieri K, Kocjan BJ, et al. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2015 doi: 10.1016/j.cmi.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 17.Gage JC, Schiffman M, Solomon D, Wheeler CM, Gravitt PE, Castle PE, et al. Risk of precancer determined by HPV genotype combinations in women with minor cytologic abnormalities. Cancer Epidemiol Biomarkers Prev. 2013;22:1095–101. doi: 10.1158/1055-9965.EPI-12-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agreda PM, Beitman GH, Gutierrez EC, Harris JM, Koch KR, LaViers WD, et al. Analytical and Clinical Performance of the BD Onclarity(tm) HPV Assay on the BD Viper(tm) LT System. J Clin Virol. Submitted. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


