Abstract
Incomplete clearance of apoptotic cells and reactive oxygen species (ROS) release are known to trigger inflammasome activation causing severe inflammation in acute lung injury and various metabolic and autoimmune diseases. Moreover, it has been reported that apoptotic cell clearance and ROS-mediated apoptosis critically depend on mitochondrial uncoupling protein-2 (UCP2). However, the relationship between UCP2 and inflammasome activation has not been studied. This report investigates the role of UCP2 in the expression and activation of NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome in human macrophages. We found that UCP2 overexpression significantly enhanced the expression levels of NLRP3. The NLRP3 expression levels were significantly suppressed in THP1 cells treated with genipin, a UCP2 inhibitor, compared to controls. In addition, genipin altered adenosine triphosphate (ATP)- and hydrogen peroxide (H2O2)-mediated interleukin-1 beta (IL-1β) secretion and significantly suppressed caspase-1 activity in inflammasome-activated human macrophages. Taken together, our results suggest that genipin modulates NLRP3 inflammasome activation and ATP- or H2O2-mediated IL-1β release.
Keywords: IL-1β, ASC, caspase-1, THP1, nigericin
Introduction
Inflammasomes are multimeric protein complexes that recognize a diverse set of inflammation inducing stimuli such as pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs); they are involved in caspase-1-mediated processing and secretion of pro-inflammatory cytokines like interleukin-1β (IL-1β), IL-18, and IL-33 [1]. The nucleotide-binding domain and leucine-rich repeat (NLR) proteins act as a central shaft, and recruit the adaptor component of the inflammasome apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 to process IL-1β [2]. Among the NLRs, the NLR family pyrin domain protein 3 (NLRP3) known as cryopyrin/NALP3 is well documented to be involved in sensing a large variety of inflammasome stimuli such as reactive oxygen species (ROS) stress response, extracellular ATP, cholesterol, and crystals such as monosodium urate [3–6]. The inflammasome is stimulated by a two-step process; the first step is achieved by the activation of receptors known as the pathogen response receptors (PRRs). The PRRs in turn activate stimuli such as LPS (lipopolysaccharide) or NF-κB and prime the formation of the NLRP3 complex [7] [8]. In the second step, various stimuli such as ROS or intracellular stimuli such as sterile inflammation activate the NLRP3 complex [9]. In activated human monocytic THP1 cells, the cells have the unique property of displaying inflammasome activation without the priming steps. In addition, it has been shown that similar outcomes are observed in human primary PBMC as both human PBMC and THP1 cells are capable of inducing NLRP3-mediated IL-1β release [10]. The induction of NLRP3 is important for inflammasome activation. The NLRP3 inflammasome is activated by ATP through the P2X7 receptor and is in response to low intracellular potassium levels [11]. Previous reports have also demonstrated the involvement of ROS in the activation of inflammasome [12].
The inflammasome is part of the innate immune system and acts through caspase-1 activation and production of cytokine IL-1β against microorganisms [13]. IL-1β is known to be an important mediator of inflammatory response and can cause a variety of auto-inflammatory diseases including cryopyrin-associated periodic syndrome (CAPS) and Muckle–Wells syndrome if genetic mutations are present in NLRP3 [14,15]. The inflammasome also plays a role in cellular functions such as apoptosis and pyroptosis [16].
Uncoupling protein-2 (UCP2) is a proton carrier protein found in the inner membrane of mitochondria; it is known to help regulate the activation of ROS and partakes in cell signaling mechanisms [17–19]. Evidence suggests that UCP2 also controls macrophage activation by moderating the production of mitochondrial ROS and MAPK signaling [20]. In addition, UCP2 in glial cells have been shown to control neuroinflammation and ER stress. UCP2 has also been reported for its role in regulating apoptosis in different cell systems [21,22]. In addition, the overexpression of UCP2 in the liver causes acute liver injury [23]. Genipin, which was initially isolated from the Chinese medicinal plant Gardenia jasminoides, is well-known to be a potent inhibitor of UCP2 [24,25]. However, the mechanism by which UCP2 regulates NLRP3 inflammasome activation is still not understood. Since we consider that UCP2 regulates NLRP3, the inhibition of UCP2 with genipin should consequently inhibit NLRP3. Therefore, we investigated the role of UCP2 and its inhibitor, genipin, in the regulation of inflammasome expression and activation in human macrophages.
Materials and methods
Cells and reagents
THP1 cells derived from human blood with acute monocytic leukemia were obtained from American Type Culture Collection (ATCC, Manassas, VA) and were maintained in RPMI-1640 medium as per vendor instructions. PMA (Sigma-Aldrich, St. Louis, MO), nigericin (Sigma-Aldrich, St. Louis, MO), and ATP (Enzo Life Sciences, Farmingdale, NY) were purchased from its respective vendors. Anti-mouse (Cell Signaling Technology, Danvers, MA) and anti-rabbit (Cell Signaling Technology, Danvers, MA) secondary antibodies were used to detect protein expression in Western blots. Primary antibodies specific to NLRP3, ASC (Enzo Life Sciences, Farmingdale, NY), caspase-1 (Santa Cruz, Dallas, TX), IL-1β (Cell signaling Technology, Danvers, MA), and UCP2 (R&D systems, Minneapolis, MN) were purchased from its respective companies.
Treatment with inflammasome stimulators and Genipin
Inflammasome was stimulated in THP1 cells as previously mentioned [26]. In brief, THP1 cells (monocytes) were differentiated by adding 0.5 μg/ml of PMA for 3 hours, and medium was replaced with complete RPMI overnight. After 24 hours, cells were briefly washed with PBS and 10 μg/ml of nigericin; 5 mM H2O2 or 5 mM ATP was added to THP1 cells pretreated with or without genipin (50 μM, 100 μM, 250 μM). The activation of the inflammasome was confirmed by measuring IL-1β secretion in supernatants and comparing the expression of mature IL-1β or caspase-1 expression by Western blot analysis.
Transfection of THP1 cells
THP1 cells were seeded at 0.5 million cells per well in a six-well plate and differentiated with 0.5 μg/ml of PMA for 3 hours. To remove the residual effect of PMA, the medium was replaced with complete RPMI-1640 overnight. After 24 hours, the medium was replaced by serum-free RPMI-1640 and cells were transfected with 2.5 μg/well of control vector or UCP2-GFP (OriGene, Rockville, MD) plasmids using Lipofectamine® LTX and PLUS Reagent (Invitrogen, CA, USA) as per the manufacturer instructions. After 4 hours post-transfection, the medium was replaced with complete RPMI-1640. After 24 hours, the cells were washed with PBS and medium was replaced by serum-free RPMI-1640 for 2 hours and the cells were treated with different inflammasome activators or UCP2 inhibitor genipin at different concentrations. Transfection efficiency was analyzed by fluorescence.
Cell Viability Assays
MTT assay was performed using a commercial kit (R&D Systems, Minneapolis, MN) as per the manufacturer instructions. Trypan blue exclusion assay was performed as described previously [27].
Western Blot
Cells were lysed with RIPA buffer and protein quantification was performed using the BCA assay kit. Uniform quantity of protein was loaded on to SDS-PAGE, and protein was transferred to PVDF membrane as previously mentioned [28]. The supernatant was acetone-precipitated, resuspended in RIPA buffer, and used to detect IL-β and caspase-1. The membrane was blocked for 1 hour in 5% nonfat dry milk and PBS containing 0.05% TWEEN-20 (PBS/T). Primary and secondary antibodies were diluted to 1000-fold in blocking buffer, and washing steps were followed as previously described [28]. A Pierce ECL Western blotting substrate kit (Thermo Scientific, Rockford, IL) was used to detect the protein and exposed in a ChemiDoc XRS (Biorad, Hercules, CA).
ELISA
Levels of IL-1β in supernatants were estimated using a commercial ELISA kit (eBioscience, San Diego, CA) as per the manufacturer instructions.
Statistics
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software Inc, San Diego, CA). Statistical difference for data sets was determined using one- and two-way ANOVA. P < 0.05 was considered significant.
Results
Overexpression of UCP2 increases NLRP3 expression in human THP1 cells
Increased inflammasome activation and NLRP3 expression have been reported in several autoinflammatory disorders [11,29,30]. To investigate the role of UCP2 in inflammasome activation, we initially evaluated if overexpression of UCP2 alters NLRP3 levels in differentiated THP1 cells (Fig. 1A). The overexpression of UCP2 caused a significant increase in NLRP3 compared to vector control-transfected THP1 cells or untransfected THP1 cells (Fig. 1B). These results suggest that UCP2 is associated with an increased expression of NLRP3 in human macrophages (Fig. 1C).
Figure 1. Increased NLRP3 expression levels in UCP2-transfected human macrophages.
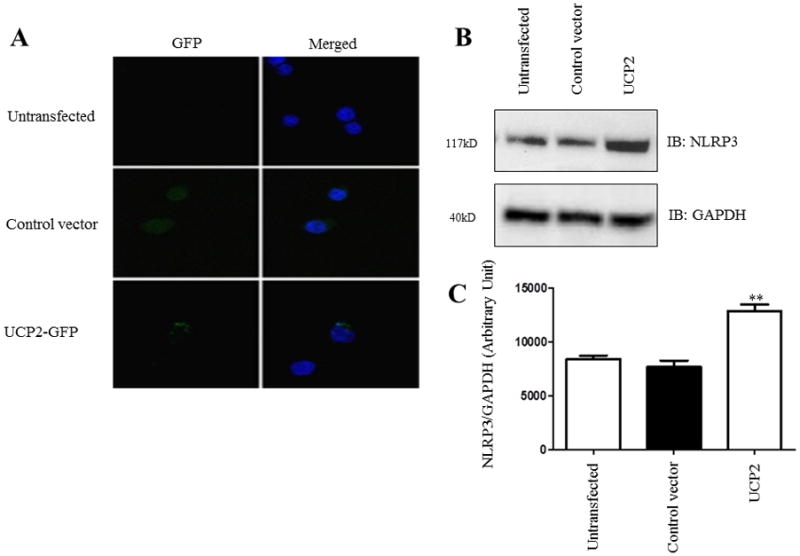
Differentiated THP1 cells were transfected with control vector or UCP2-GFP plasmids. A) Fluorescence imaging of untransfected, control vector-transfected, and UCP2-GFP-transfected human macrophages were produced to confirm transfection efficiency. B) Western blot analysis of NLRP3 in cell lysates was obtained from cells transfected with control vector or UCP2-GFP. Data is representative of at least 3 independent experiments. C) Western blot of NLRP3 was analyzed densitometrically using ImageJ. Data is representative of at least 3 independent experiments. P < 0.05 was considered significant. (** = p < 0.01)
NLRP3 expression is altered by genipin
Genipin is a well-known UCP2 inhibitor and has been recognized to inhibit superoxide-activated UCP2-mediated proton leak in several diseases [24]. Since our results indicated that UCP2 might, in part, regulate the expression of NLRP3 levels in human macrophages, we then checked whether genipin can alter the expression levels of NLRP3 and other inflammasome components such as ASC. Differentiated THP1 cells were treated with different concentrations of genipin (50 μM, 100 μM, and 200 μM) and were incubated for 24 hours. Expression levels of NLRP3 and ASC were assessed by Western blot analysis. Our results suggest a significant drop in the levels of NLRP3 at all the concentrations of genipin when compared to controls, however, the levels of the adaptor protein ASC did not change (Fig. 2A–C). This shows that genipin regulates the expression of the NLRP3 component of the inflammasome. Further, to avoid the possibilities of cytotoxic effects of experimental dosage of genipin, PMA-differentiated THP1 cells were treated with a range of 50 μM, 100 μM, and 250 μM of genipin for 24 hours and the cell viability was compared with untreated control using a proliferation assay. The presence of genipin did not affect the cell viability (Fig. 2D & 2E). Based on the results from cell viability in which we measured concentrations 25–200 μM, we optimized the concentration of genipin at 50 μM [24,31].
Figure 2. Genipin alters NLRP3 and UCP2 expression in differentiated THP1 cells.
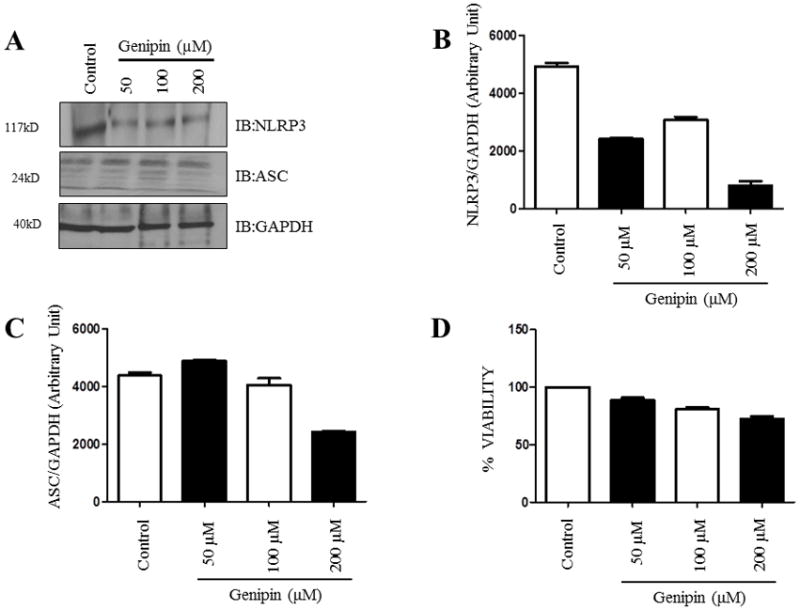
Differentiated THP1 cells were treated with 50 μM, 100 μM, and 200 μM of genipin and incubated for 24 hours. A) Western blot analysis of NLRP3 and ASC from cell lysates was obtained from various treatments with genipin. GAPDH was used as an internal control. B–C) Levels of NLRP3 and ASC were analyzed densitometrically using ImageJ. D–E) Differentiated THP1 cells were treated with various doses of genipin and cell viability was calculated after 24 hours. Data is representative of at least 3 independent experiments. P < 0.05 was considered significant. (** = p < 0.01)
Genipin inhibits ATP-mediated inflammasome activation in human macrophages
Based on the findings reported above, we further checked whether genipin can alter NLRP3 and other inflammasome components in the presence of inflammasome activators such as ATP and nigericin. The NLRP3 inflammasome was stimulated in differentiated THP1 cells by ATP or nigericin in the presence or absence of genipin. Western blot analysis was performed to elucidate the expression levels of NLRP3 and ASC in ATP- or nigericin-treated THP1 cells. No significant differences in NLRP3 or ASC levels were found in cells treated with ATP or nigericin and with/without genipin (Fig. 3A–C). However, there was a significant drop in IL-1β levels in cells pretreated with ATP and post-treated with genipin when compared to cells pretreated with ATP alone. This difference was not found in cells treated with nigericin (Fig. 3D). It is known that macrophage activation releases the proinflammatory cytokines TNF-α (tumor necrosis factor-alpha) and IL-6 (interleukin-6), however these proinflammatory cytokines are not released upon inflammasome activation. Therefore, to check the specificity of genipin suppressed inflammasome activation, we assessed IL-6 and TNF-α in differentiated THP1 cells by ATP or nigericin in the presence or absence of genipin. Our results showed no significant difference in IL-6 and TNF-α secretion levels (Fig. 3E & 3F). These results suggest that genipin suppresses ATP-mediated inflammasome activation and IL-1β secretion without altering the basal levels of the inflammasome components. In addition, our results also suggest that genipin inhibition of ATP-mediated inflammasome activation does not alter proinflammatory cytokines IL-6 and TNF-α.
Figure 3. Genipin suppresses ATP-mediated inflammasome activation in human macrophages.
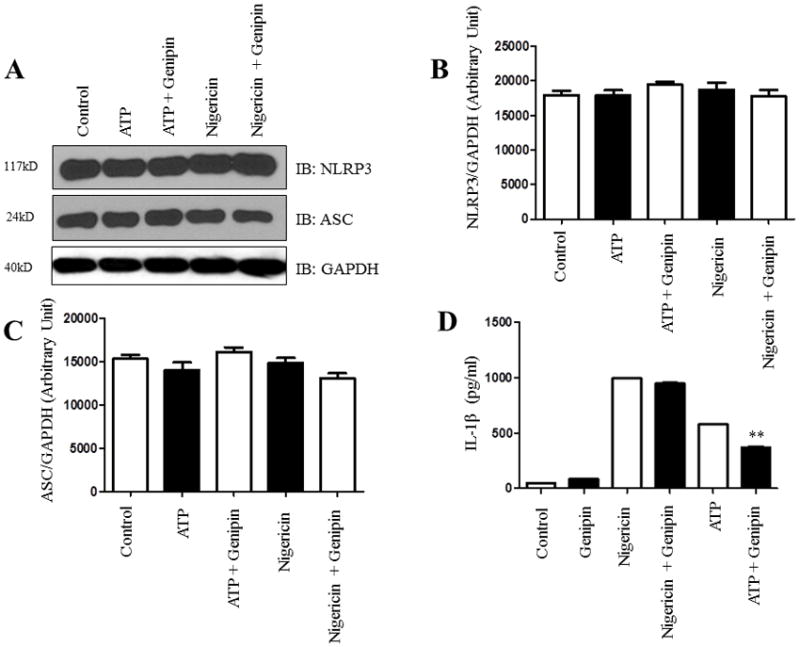
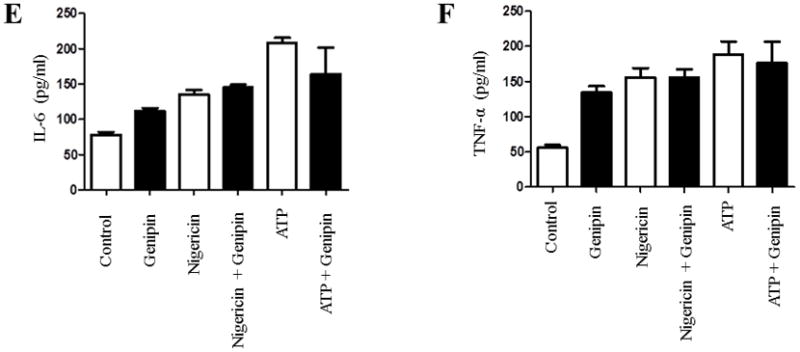
Differentiated THP1 cells were treated with inflammasome activators 5 mM ATP or 10 μg/ml of nigericin for 1 hour with or without 50 μM of genipin. A) The expression levels of NLRP3 and ASC were analyzed in 50 ug of total cell lysate. GAPDH was used as an internal control. B–C) Levels of NLRP3 and ASC were analyzed densitometrically using ImageJ. D–F) The secretions of IL-1β, IL-6, and TNF-α in the supernatants were analyzed by ELISA. Data is representative of at least 3 independent experiments. P < 0.05 was considered significant. (** = p < 0.01)
ROS-mediated inflammasome activation is inhibited by genipin
UCP2 has been implicated in modulating ROS secretion in different pathways such as proliferation, apoptosis, and differentiation [32]. Mitochondrial ROS was previously shown to be involved in signaling the NLRP3 inflammasome and induce the processing and secretion of IL-1β [12,19]. To determine the effects of genipin on ROS-mediated inflammasome activation, we exposed differentiated THP1 cells to H2O2 and then post-treated them with genipin. The supernatants were analyzed for IL-1β secretion by ELISA. Our findings show a significant drop in the levels of IL-1β in cells post-treated with genipin when compared to untreated cells (Fig. 4A). Additionally, H2O2-mediated induction of cleaved IL-1β and caspase-1 in supernatants was significantly inhibited in the presence of genipin (Fig. 4B). These results suggest that genipin regulates H2O2-mediated inflammasome signaling and IL-1β secretion.
Figure 4. Genipin inhibits inflammasome activation stimulated by H2O2.
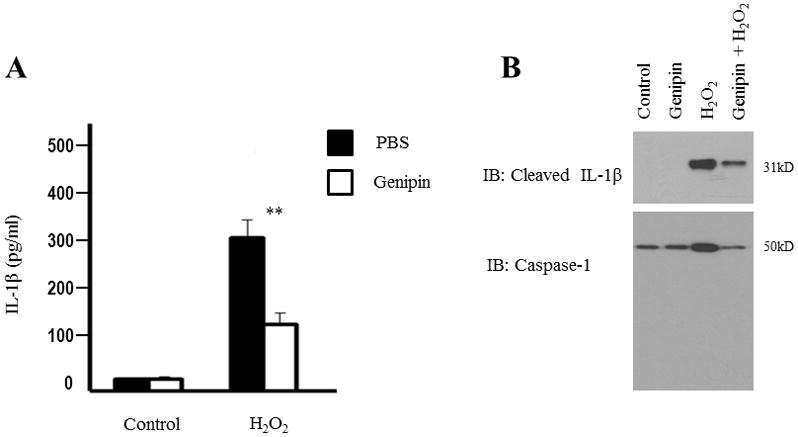
Differentiated THP1 cells were treated with 5 mM H2O2 for 3 hours. A) The secretion of IL-1β in the supernatant was analyzed by ELISA. Data is representative of at least 3 independent experiments. P < 0.05 was considered significant. (** = p < 0.01) B) Active caspase-1 and cleaved IL-1β in the supernatants were analyzed by Western blot. Supernatant from three independent replicate samples was pooled together and is representative of three independent experiments.
Discussion
Mutations in the NLRP3 inflammasome are known to cause cryopyrin-associated disorders [29,30]. In addition, there have been reports that incomplete phagocytosis leads to inflammasome activation and that UCP2 is involved in phagocytosis [32]. Since IL-1β-mediated disorders and cryopyrinopathies are known to be associated with inflammasomes [33], recent strategies focus to minimize the severity of them by targeting the NLRP3 inflammasome activation.
In our current study, we analyzed the role of UCP2 and its inhibitor, genipin, in regulating inflammasome activation. The results in our report demonstrated that overexpression of UCP2 significantly increases the NLRP3 levels in human macrophages; this revealed that inflammasome regulation can be, in part, due to UCP2 (Fig. 1). We speculated that using genipin, a UCP2 inhibitor, can suppress the inflammasome activation if the mechanism of activation is partly via UCP2. When treated with 50 μM, 100 μM, and 250 μM of UCP2 inhibitor at 24 hours, the human macrophages showed a significant drop in the expression levels of NLRP3 (Fig. 2). Surprisingly, there was no change in levels of the adaptor protein ASC. Since we observed significant alterations in NLRP3 expression when treated with genipin, we next analyzed if genipin can suppress the inflammasome activation in the presence of activators such as ATP, nigericin, or a ROS inducer like H2O2. Our results indicated a significant drop in IL-1β levels in cells post-treated with genipin after ATP-mediated induction (Fig. 3) but not in proinflammatory cytokines IL-6 and TNF-α. Such differences were also not observed in nigericin-mediated human macrophages. This inhibitory effect of IL-1β secretion acts on ATP, but not nigericin, because it inhibits ATP activation on the P2X7 receptor channel. This compelling evidence suggests that UCP2 is involved in regulating mitochondrial ROS-mediated response in several diseases. Next, we analyzed if the UCP2 inhibitor genipin can inhibit H2O2-triggered (ROS-mediated) NLRP3 inflammasome induction and IL-1β release in human THP1 cells. Our results indicated a significant drop in IL-1β levels in THP1 cells post-treated with genipin (Fig. 4). Western blot analysis also revealed a significant drop in the levels of cleaved IL-1β and caspase-1 in genipin-treated human macrophages. As we continue to comprehend the mechanisms of inflammasome activation and the different inhibitors that can suppress inflammation, we believe investigating the role of UCP2 and genipin in inflammasome activation will further help to develop clinically effective therapeutic treatments which can target pathways that cause inflammation. To conclude, we believe that further research on targeting UCP2-mediated inflammasome activation by UCP2 inhibitors like genipin can lead to improvements in treatment for countless patients with inflammatory-related diseases, particularly in NLRP3 inflammasome activation-mediated disorders.
Highlights.
Overexpression of UCP2 increases NLRP3 expression in human THP1 cells
NLRP3 expression is altered by genipin
Genipin inhibits ATP-mediated inflammasome activation in human macrophages
ROS-mediated inflammasome activation is inhibited by genipin
Acknowledgments
NK was funded by the American Heart Association National Scientist Development Grant 09SDG2260957 and National Institutes of Health R01 HL105932 and the Joy McCann Culverhouse Endowment to the Division of Allergy and Immunology.
Abbreviations
- PAMPs
pathogen-associated molecular patterns
- DAMPs
danger-associated molecular patterns
- ROS
reactive oxygen species
- UCP2
uncoupling protein 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 2.Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/Caspase-1 axis regulates IL-1beta processing in neutrophils. Eur J Immunol. 2012;42:710–715. doi: 10.1002/eji.201141921. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind F, Bartok E, Rieger A, Franchi L, Nunez G, et al. Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaide O, Hoffman HM. Insight into the inflammasome and caspase-activating mechanisms. Expert Rev Clin Immunol. 2008;4:61–77. doi: 10.1586/1744666X.4.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. J Leukoc Biol. 2007;82:259–264. doi: 10.1189/jlb.1206755. [DOI] [PubMed] [Google Scholar]
- 7.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Land WG. Transfusion-Related Acute Lung Injury: The Work of DAMPs. Transfus Med Hemother. 2013;40:3–13. doi: 10.1159/000345688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy N, Cowley TR, Richardson JC, Virley D, Upton N, et al. The neuroprotective effect of a specific P2X(7) receptor antagonist derives from its ability to inhibit assembly of the NLRP3 inflammasome in glial cells. Brain Pathol. 2012;22:295–306. doi: 10.1111/j.1750-3639.2011.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandyopadhyay S, Lane T, Venugopal R, Parthasarathy PT, Cho Y, et al. MicroRNA-133a-1 regulates inflammasome activation through uncoupling protein-2. Biochem Biophys Res Commun. 2013;439:407–412. doi: 10.1016/j.bbrc.2013.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kone-Paut I, Piram M. Targeting interleukin-1beta in CAPS (cryopyrin-associated periodic) syndromes: what did we learn? Autoimmun Rev. 2012;12:77–80. doi: 10.1016/j.autrev.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, et al. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 16.Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, et al. AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 2013;20:1149–1160. doi: 10.1038/cdd.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pi J, Collins S. Reactive oxygen species and uncoupling protein 2 in pancreatic beta-cell function. Diabetes Obes Metab. 2010;12(Suppl 2):141–148. doi: 10.1111/j.1463-1326.2010.01269.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu M, Sun XL, Qiao C, Liu Y, Ding JH, et al. Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation. Neurobiol Aging. 2014;35:421–430. doi: 10.1016/j.neurobiolaging.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Mailloux RJ, Harper ME. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med. 2011;51:1106–1115. doi: 10.1016/j.freeradbiomed.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Emre Y, Hurtaud C, Karaca M, Nubel T, Zavala F, et al. Role of uncoupling protein UCP2 in cell-mediated immunity: how macrophage-mediated insulitis is accelerated in a model of autoimmune diabetes. Proc Natl Acad Sci U S A. 2007;104:19085–19090. doi: 10.1073/pnas.0709557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen XL, Tang WX, Tang XH, Qin W, Gong M. Downregulation of uncoupling protein-2 by genipin exacerbates diabetes-induced kidney proximal tubular cells apoptosis. Ren Fail. 2014;36:1298–1303. doi: 10.3109/0886022X.2014.930650. [DOI] [PubMed] [Google Scholar]
- 22.Derdak Z, Garcia TA, Baffy G. Detection of uncoupling protein-2 (UCP2) as a mitochondrial modulator of apoptosis. Methods Mol Biol. 2009;559:205–217. doi: 10.1007/978-1-60327-017-5_15. [DOI] [PubMed] [Google Scholar]
- 23.Shang Y, Liu Y, Du L, Wang Y, Cheng X, et al. Targeted expression of uncoupling protein 2 to mouse liver increases the susceptibility to lipopolysaccharide/galactosamine-induced acute liver injury. Hepatology. 2009;50:1204–1216. doi: 10.1002/hep.23121. [DOI] [PubMed] [Google Scholar]
- 24.Zhang CY, Parton LE, Ye CP, Krauss S, Shen R, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Zhao J, Zhang X. Inhibition of uncoupling protein 2 by genipin reduces insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys. 2009;486:88–93. doi: 10.1016/j.abb.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Chuang YT, Lin YC, Lin KH, Chou TF, Kuo WC, et al. Tumor suppressor death-associated protein kinase is required for full IL-1beta production. Blood. 2011;117:960–970. doi: 10.1182/blood-2010-08-303115. [DOI] [PubMed] [Google Scholar]
- 27.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, et al. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol. 2003;29:513–522. doi: 10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- 28.Rajanbabu V, Pan CY, Lee SC, Lin WJ, Lin CC, et al. Tilapia hepcidin 2–3 peptide modulates lipopolysaccharide-induced cytokines and inhibits tumor necrosis factor-alpha through cyclooxygenase-2 and phosphodiesterase 4D. J Biol Chem. 2010;285:30577–30586. doi: 10.1074/jbc.M110.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman HM, Wolfe F, Belomestnov P, Mellis SJ. Cryopyrin-associated periodic syndromes: development of a patient-reported outcomes instrument to assess the pattern and severity of clinical disease activity. Curr Med Res Opin. 2008;24:2531–2543. doi: 10.1185/03007990802297495. [DOI] [PubMed] [Google Scholar]
- 30.Miyamae T. Cryopyrin-associated periodic syndromes: diagnosis and management. Paediatr Drugs. 2012;14:109–117. doi: 10.2165/11595040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Mailloux RJ, Adjeitey CN, Harper ME. Genipin-induced inhibition of uncoupling protein-2 sensitizes drug-resistant cancer cells to cytotoxic agents. PLoS One. 2010;5:e13289. doi: 10.1371/journal.pone.0013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–224. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]


