Abstract
Selection theories of drug use propose that individuals choose or self-select into peer groups based on perceived similarities with other group members in regard to their beliefs, attitudes, and histories of drug use. The purpose of the present study was to determine whether a shared history of drug exposure would influence choice of a social partner. Adolescent male rats were treated with either cocaine (3.0 mg/kg, ip) or saline and their preference for a cocaine-treated rat or a saline-treated rat was measured in a partner preference test. Next, a series of conditioning trials were conducted in which rats were paired with the cocaine-treated and saline-treated partner on alternating days for 10 days. Finally, a second partner preference test was conducted in which preference for the cocaine-treated and saline-treated partner was reassessed. Relative to baseline, rats increased the amount of time they spent with their similarly treated partner, and this effect was driven by cocaine-treated rats increasing the amount of time spent in proximity to their cocaine-treated partner after conditioning. These findings support a selection model of drug use by showing that a shared history of drug exposure is sufficient to establish a social preference for one individual over another.
Keywords: cocaine, partner preference, rat, selection, social, social choice
Introduction
Selection models of drug use propose that individuals self-select into social groups because of perceived similarities with other members of the group. According to these models, individuals choose their peers based on similar beliefs, attitudes, and histories of drug use (Kandel, 1986; Andrews and Hops, 2010; Pandina et al., 2010). Very few experimental studies have examined the role of selection in drug use, possibly because of a lack of experimental models that allow a subject to choose or select another subject based on a shared drug use history.
We recently reported that a rat self-administering cocaine will choose to respond on a lever in close proximity to another rat self-administering cocaine, rather than on a lever in close proximity to a rat that is not self-administering cocaine (Smith and Pitts, 2014). This finding suggests that rats “prefer” to be in close proximity to another rat with a shared behavioral history during periods of drug self-administration; however, several limitations of that study were noted: (1) all testing took place in the absence of human observers so a preference for a response lever was used as a proxy to infer social preference; (2) it was not determined what aspect of the shared behavioral history (e.g., drug exposure, response-contingent reinforcement) was responsible for the preference; and (3) it was not clear whether the preference was due to an attraction to one partner or aversion to the other partner.
The purpose of this study was to determine if a shared history of cocaine exposure is sufficient to establish a preference for one individual over another as revealed by an increase in time spent with that partner.
Methods
Sujects
Male, Long-Evans rats were obtained at 42 days of age and housed in triads (three rats to a cage) for one week until the beginning of partner preference testing. At that time, each subject was moved to single housing and assigned to one of three conditions: (1) choice rat, (2) cocaine-treated partner, or (3) saline-treated partner. Choice rats (total n = 32) were further subdivided at that time into cocaine-treated (n = 16) or saline-treated (n = 16) subjects.
Apparatus
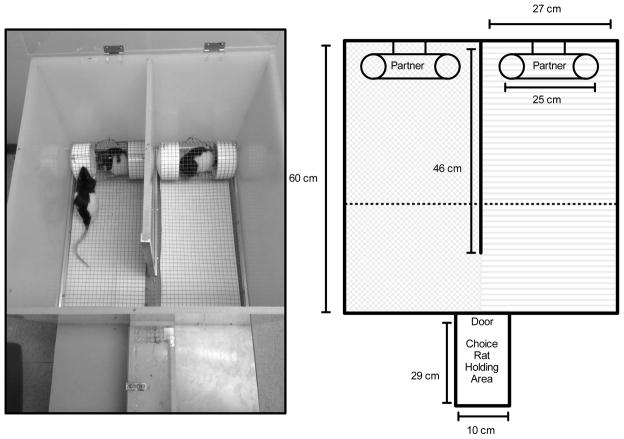
Partner preference testing was conducted in a custom-built chamber (Fig. 1). The chamber consisted of a small holding area for choice rats and an open-field partner preference area, partially bisected by a solid partition. Social partners were confined on opposite sides of the chamber in small wire-screen cages. All test sessions were video recorded.
Figure 1.
Partner preference apparatus. The left panel is a photograph of a typical partner preference session. The choice rat was allowed to move freely between the cocaine-treated and saline-treated partner during a 10-minute test session. When on a given side, each choice rat had full visual, auditory, and olfactory contact with the partner, as well as limited tactile contact. The right panel is a schematic of the chamber used to collect partner preference data. The chamber was custom designed (60 × 54 cm) and was functionally divided into two sides by a solid partition that extends 46 cm into the center of the chamber. In the first analysis, the compartment was divided into two equal halves and time spent on each side was determined (note shaded regions in schematic indicating two sides). In the second analysis, the compartment was divided into three equal areas (two areas in close proximity to each social partner and a third “neutral” area on the opposite side of the chamber) and the time spent in each area was determined (note dotted line in schematic delineating three equal areas).
Partner Preference Procedure
Partner preference conditioning and testing took place during three phases: (1) habituation and baseline testing, (2) conditioning, and (3) post-conditioning testing. During habituation, rats were placed in the partner preference chamber for 10 minutes in isolation. On the next day, a second habituation session occurred in which all three rats were placed in the chamber together for 10 minutes under drug-free conditions, with the choice rat allowed to move freely and the partners confined in wire-screen cages. On the following day, a baseline partner preference test was conducted in which each choice rat and both social partners received an i.p. injection of their assigned drug, either 3.0 mg/kg cocaine or saline. After 5 minutes, the choice rat was placed in the holding area, and the two social partners were confined in their wire-screen cages on opposite sides of the chamber. The door to the holding area was then opened and the choice rat was free to move about the chamber for 10 minutes.
Conditioning took place in standard polycarbonate cages. During conditioning sessions, the choice rat and one social partner received their assigned injection (cocaine or saline), and both rats were placed together in the conditioning cage for 30 minutes. Rats were unconfined during these sessions and allowed to interact freely. On the following day, the same procedure was repeated with the choice rat and the other social partner. A total of 10 conditioning sessions were conducted, resulting in 5 conditioning sessions with the cocaine-treated partner and 5 conditioning sessions with the saline-treated partner. The order of conditioning trials was counterbalanced such that approximately half of subjects received their first conditioning session with their similarly treated partner and half with their other partner. Conditioning sessions were not recorded.
Partner preference testing commenced one day following the final conditioning session. During the partner preference test, all procedures were identical to those used during the baseline test.
Data Analysis
Two analyses were conducted. For the first analysis, time spent with a partner was defined simply as being on the same side of the chamber as the partner by dividing the chamber into two equal halves. Because the chamber was sufficiently large that a choice rat could be on the same side of the chamber as a social partner but could avoid social interaction with that partner, the second analysis divided the chamber into three equal areas that (1) defined social preference as being in close physical proximity to a social partner and (2) included a neutral area at the back of the chamber to determine if the rats were avoiding social interaction (see Fig. 1). The outcome measure for both analyses was the change in the percentage of time spent with the similarly treated partner before conditioning versus after conditioning. Positive scores indicated an increase in time spent with the similarly treated partner. Means and 95% confidence intervals were determined for all rats, as well as for the cocaine-treated and saline-treated subgroups. Social preferences were considered statistically significant when the 95% confidence intervals did not overlap zero, the theoretical point of indifference.
Results
In the first analysis (two equal areas), rats did not exhibit a preference for either side of the compartment during the baseline test (data not shown). Following conditioning, rats significantly increased the percentage of time spent in the side of the compartment with their similarly treated partner (Fig. 2A), increasing the time spent with that partner by 29.7 seconds. This effect was driven by cocaine-treated choice rats spending more time with their cocaine-treated partner following conditioning. In this subgroup, the time spent with the cocaine-treated partner increased by 48.9 seconds.
Figure 2.
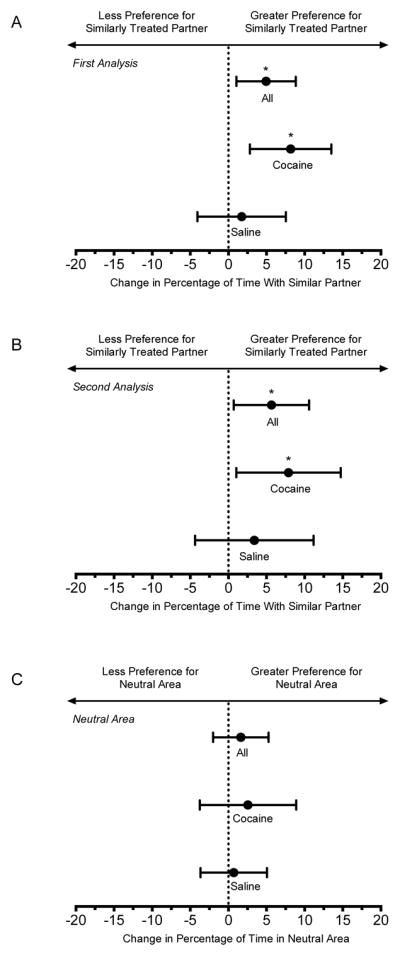
Change in partner preference measures. The hoirzontal axis depicts the change in partner preference (A and B) or time spent in the neutral area (C) after conditioning relative to baseline. All change scores were calculated as post-conditioning minus preconditioning. Percentage of time with similarly treated partner was calculated as time with similarly treated partner divided by the total time with partners (×100). Positive values indicated an increase in time spent with the similarly treated partner. Percentage of time in the neutral area (for the second analysis) was calculated as time in neutral compartment divided by total time. Positive values indicated an increase in time spent in the neutral area after conditioning. Means and 95% confidence intervals were determined for all choice rats (n = 32), and separately for the cocaine-treated (n = 16) and saline-treated (n = 16) subgroups. Asterisks (*) indicate statistical significance.
In the second analysis (three equal areas), rats did not exhibit a preference for any of the three areas of the compartment during the baseline test (data not shown). Following conditioning, rats significantly increased the percentage of time spent in the area of the compartment in close proximity to their similarly treated partner (Fig. 2B), increasing the time spent with that partner by 12.9 seconds and decreasing the time spent with the opposite partner by 31.6 seconds. Again, this effect was driven by cocaine-treated choice rats spending more time with their cocaine-treated partner following conditioning. In this subgroup, the time spent with the cocaine-treated partner increased by 17.8 seconds and the time spent with the saline-treated partner decreased by 42.1 seconds. The percentage of time in the neutral area did not change significantly after conditioning (Fig. 2C).
Discussion
The principal finding of this study is that rats increased the amount of time they spent in proximity to a social partner with a shared history of drug exposure. The time spent in a neutral area did not change following conditioning, suggesting that this effect was not due to rats simply avoiding social interaction. These findings support our previous study reporting that rats choose to self-administer cocaine in close proximity to another rat that is also self-administering cocaine (Smith and Pitts, 2014), and are consistent with studies reporting that the rewarding effects of drugs and social contact are enhanced when combined with one another (Thiel et al., 2008; 2009; Watanabe 2011; 2013).
The change in social preference was driven primarily by cocaine-treated rats increasing the amount of time spent in proximity to other cocaine-treated rats. We do not know why saline-treated rats did not develop a preference for other saline-treated rats, but it is possible that additional conditioning sessions would have produced a significant preference in this group. Future studies will need to determine the minimum amount of conditioning required to establish a preference in different populations.
One limitation of this study is that only a single dose of cocaine was examined. The 3.0 mg/kg dose was selected because it produces distinct discriminative stimulus effects (Costanza et al., 2001) and a significant place preference in place conditioning studies (Cordery et al., 2014). We expect that the effects of cocaine on social choice vary across dose in a nonlinear manner. Higher doses of cocaine often produce aversive (Kosten et al., 1994) and anxiogenic (Schank et al., 2008) effects that would likely interfere with the establishment of a social preference. Furthermore, higher doses of cocaine produce motor stereotypies (Tang et al., 2008) that would interfere with play and other species-typical social behaviors.
One limitation of our model is that only one aspect of the social environment is manipulated (a shared history of drug exposure). Shared attitudes and shared beliefs about drug use also predict drug use among peers (Epstein et al., 2003; Hohman et al., 2014), but these aspects of the social environment are less amenable to manipulation in animal models. Consequently, additional research will be needed to fully characterize the various pharmacological, organismal, and environmental factors that interact to establish and maintain social preference in human substance-using populations.
One of the leading prognosticators of whether an adolescent will use drugs is whether his or her friends use drugs (Walden et al., 2004; Bahr et al., 2005). Selection models of drug use propose that individuals self-select into peer groups that are similar to themselves in terms of attitudes, beliefs, and histories regarding drug use. The present findings support these models by showing that a shared history of drug exposure is sufficient to establish a social preference for one individual over another.
Acknowledgments
This study was supported by NIH grants R01DA031725 and R01DA0274855.
Footnotes
The authors have no financial conflicts of interests to report.
References
- Andrews JA, Hops H. The influence of peers on substance abuse. In: Scheier LM, editor. Handbook of drug use etiology: Theory, methods, and empirical findings. Washington DC: American Psychological Association; 2010. pp. 403–20. [Google Scholar]
- Bahr SJ, Hoffmann JP, Yang X. Parental and peer influences on the risk of adolescent drug use. J Prim Prev. 2005;26:529–51. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP. A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol. 2014;19:575–86. doi: 10.1111/adb.12020. [DOI] [PubMed] [Google Scholar]
- Costanza RM, Barber DJ, Terry P. Antagonism of the discriminative stimulus effects of cocaine at two training doses by dopamine D2-like receptor antagonists. Psychopharmacology. 2001;158:146–53. doi: 10.1007/s002130100872. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Botvin GJ, Spoth R. Predicting smoking among rural adolescents: social and cognitive processes. Nicotine Tob Res. 2003;5:485–91. doi: 10.1080/1462220031000118577. [DOI] [PubMed] [Google Scholar]
- Hohman ZP, Crano WD, Siegel JT, Alvaro EM. Attitude ambivalence, friend norms, and adolescent drug use. Prev Sci. 2014;1:65–74. doi: 10.1007/s11121-013-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB. Processes of peer influences in adolescence. In: Silbereisen RK, Eyferth K, Rudinger G, editors. Development as action in context: Problem behavior and normal youth development. New York: Springer; 1986. pp. 203–28. [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–44. [PubMed] [Google Scholar]
- Pandina RJ, Johnson VL, White HR. Peer influences on substance use during adolescence and emerging adulthood. In: Scheier LM, editor. Handbook of drug use etiology: Theory, methods, and empirical findings. Washington DC: American Psychological Association; 2010. pp. 383–401. [Google Scholar]
- Schank JR, Liles LC, Weinshenker D. Norepinephrine signaling through beta-adrenergic receptors is critical for expression of cocaine-induced anxiety. Biol Psychiatry. 2008;63:1007–12. doi: 10.1016/j.biopsych.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Pitts EG. Social preference and drug self-administration: a preclinical model of social choice within peer groups. Drug Alcohol Depend. 2014;135:140–5. doi: 10.1016/j.drugalcdep.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Mittler T, Duke DC, Zhu Y, Pawlak AP, West MO. Dose- and rate-dependent effects of cocaine on striatal firing related to licking. J Pharmacol Exp Ther. 2008;324:701–13. doi: 10.1124/jpet.107.129734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: a model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–12. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden B, McGue M, Iacono WG, Burt A, Elkins I. Identifying shared environmental contributions to early substance use: The respective roles of peers and parents. J Abnorm Psychol. 2004;113:440–50. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Drug-social interactions in the reinforcing property of methamphetamine in mice. Behav Pharmacol. 2011;22:203–6. doi: 10.1097/FBP.0b013e328345c815. [DOI] [PubMed] [Google Scholar]
- Watanabe S. Social factors in conditioned place preference with morphine in mice. Pharmacol Biochem Behav. 2013;103:440–3. doi: 10.1016/j.pbb.2012.10.001. [DOI] [PubMed] [Google Scholar]