Abstract
Parental history of chronic pain has been associated with self-reported pain in child offspring. This suggests that there may be neurobiological mechanisms associated with pain heritability. Because emotional circuitry is an important component of pain processing and may also influence cognition, we used functional magnetic resonance imaging to examine affective processing and cognitive control using an Emotional Go/NoGo Task in youth with (FH+Pain, N=8) and without (FH−Pain, N=8) a parental history of chronic pain (mean age = 14.17±.34). FH+Pain youth had widespread reductions in brain activity within limbic and visual processing regions during processing of positively valenced emotional stimuli, as well as reduced fronto-parietal response while processing negatively valenced emotional stimuli compared with their peers. Additionally, during inhibition within a positive emotional context, FH+Pain youth had reduced cognitive control and salience-related brain activity. On the other hand, default mode-related brain response was elevated during inhibitory control within a negative emotional context in these adolescents compared with their peers (p/α < 0.05). The current findings indicate differences in both emotional processing and cognitive control brain response in FH+Pain compared with FH−Pain youth, suggesting that both affective and executive functioning pathways may be important markers related to the intergenerational transmission of pain.
Keywords: chronic pain, family history, youth, emotion, cognitive control
1. Introduction
Chronic pain lasting >3 months is present in about 10-30 percent of children and adults 21, 24, 52, 59, 60. In childhood, it can decrease quality of life and lead to lifelong pain and disability 18, 49. Chronic pain is quite heritable 1, 26, 31, 35, 44, 52, 58, and youth with parental chronic pain experience more pain compared to youth without parental history of chronic pain 38, 46, 50. Headache occurs with greater prevalence in children with a family history of chronic headache 1, 35, 52, while twin and family studies report greater concordance of pain in monozygotic twins 31, 58, and heritability estimates of 30-45% in extended families 26. Chronic pain heritability is associated with shared genetic factors 58, but youth with a family history of chronic pain may be at risk for pain due to the interaction of genetic and environmental factors 26. However, efforts towards understanding the mechanisms underlying familial pain vulnerability are in their infancy (for review, see Denk et al., 2014 14). Identifying neurobiological markers would allow us to better understand neural pathways of familial chronic pain risk without confounds of current pain symptoms.
Neuroimaging studies in chronic pain suggest that a number of regions show atypical activity during cognitive and affective tasks 3, 15, 22, 48, 62, implicating both executive functioning and emotional regions. Adults with fibromyalgia show reduced activity in regions implicated in response inhibition (i.e. inferior frontal gyrus) during a go/no-go task, despite comparable task performance to controls 22. Limbic regions involved in affective processing display heightened response to pain-related stimuli and spontaneous pain 3, 15, 62, some of which correlate with depressive symptoms 48. Functional connectivity studies suggest network organization is altered in chronic pain, particularly in networks involved in self-referential processing (default mode network; DMN) 34, 39, 41, and salience attribution (salience network) 28, 29, 39, 41. Atypical functional connectivity in chronic pain is also seen in a pain processing network 6. While very few studies have investigated chronic pain and brain activity in pediatric populations, likely due to the challenges of neuroimaging youth 47, one study in complex regional pain syndrome (CRPS) showed widespread cortical deactivation in the presence vs. absence of pain, interpreted as loss of inhibitory control in the painful state 36. Resting state studies in pediatric CRPS found significant hyperconnectivity of the amygdala to cortical and subcortical areas that reduced following treatment 51, while reduced synchrony of the habenula with cortical and subcortical regions has also been reported 17.
Despite growing literature on chronic pain neurobiology, to our knowledge, no studies have examined brain functioning in youth with a family history of chronic pain. This investigation is critically important to identifying neurobiological markers of pain risk. This study examines brain activity during emotional processing, and the impact of affective context on cognitive control brain response in youth with a biological mother with a chronic pain condition (FH+Pain). Since both affective and cognitive control risk pathways may increase vulnerability towards developing chronic pain, we chose to implement an Emotional Go/NoGo Task 11, 25 using functional magnetic resonance imaging (fMRI), to compare brain activity between FH+Pain youth and their low-risk peers (FH−Pain). This task allowed the examination of brain activity to emotional stimuli and tested the influence of emotional context on top-down inhibitory control brain response in at-risk youth. Based on findings in adults with chronic pain, we hypothesized that relative to their low-risk peers, FH+Pain youth would have increased activity in the amygdala, in response to negatively valenced emotional stimuli, and reduced brain activity during emotional contexts in executive functioning networks, including fronto-parietal areas.
2. Materials and Methods
2.1 Participant Recruitment and Exclusionary Criteria
Adolescents, ages 11 to 16 years old, were recruited through a study on family history and chronic pain. This age range was selected for the pilot study because the incidence of chronic pain increases during the adolescent years (especially in females 52), thereby allowing us to focus on a population at particularly high risk for developing chronic pain. However, the restricted age range, which excluded younger or older adolescents, was chosen to limit developmental confounds in the study, as age and risk for pain may interact across development and differentially affect brain activity in actively developing limbic and cognitive control circuitry 23. Parents with a history of chronic pain were recruited through pain and fibromyalgia specialty clinics. Healthy parents with no history of chronic pain were recruited through the community and through the university’s research recruitment website. Informed consent and assent were obtained from all participants and their parents. Exclusionary criteria included left handedness 40, serious medical problems, current use of psychotropic medications, mental retardation or severe learning disabilities, prenatal exposure to drugs or alcohol, acute head trauma, and MRI contraindications. Of the eight FH−Pain participants, three reported use of non-psychotropic medications. Two of the youth reported use of allergy medications and medication to treat asthma, while one youth reported use of Synthroid to treat a thyroid condition. Additionally, one youth from the FH+Pain group reported using melatonin. One youth did not complete tests of intellectual functioning or a questionnaire on mood symptoms (see section 2.2). However, due to the preliminary nature of this study, and the small sample size, this participant was included in all analyses. Finally, as the purpose of the study was to investigate the effects of family history of chronic pain on emotional processing and cognitive control without the interference of self-reported pain, youth who reported experiencing pain frequency >1 time per week on a pain questionnaire were excluded 12, 52. All study procedures were approved by the Oregon Health & Science University (OHSU) Institutional Review Board.
2.2 Participant Characteristics
Youth with a 1st degree biological mother who had an active chronic pain condition were classified as family history positive for pain (FH+Pain). These mothers were currently receiving specialty medical care for chronic pain. Only youth with biological mothers with chronic pain were included in the study. Youth with no history of chronic pain in a 1st degree biological mother, biological father, or other cohabiting parent (e.g., step-parent) were classified as family history negative for pain (FH−Pain). All family history information was collected via the biological mother’s report during the initial study screening. Based on this classification of family history of chronic pain, 8 FH+Pain (2 females) and 8 FH−Pain (5 females) completed study visits. Parents of all participants were administered the Hollingshead Index of Social Position 27 to compare socioeconomic status of FH+Pain and FH−Pain youth. Fifteen of the sixteen participants were administered the 2-subtest (Vocabulary and Matrix Reasoning) Wechsler Abbreviated Scale of Intelligence (WASI) to estimate intelligence 61. Youth completed self-reported pubertal measurements using the Pubertal Development Scale 43, and these scores were converted to Crockett Stages, ranging 1-5, with higher scores indicating more advanced maturity 5. Additionally, all but one participant completed the Children’s Depression Inventory (CDI 33), which was used to compare depression levels between FH+Pain and FH−Pain youth. Youth and parent versions of the Early Adolescent Temperament Questionnaire-Revised (EATQ-R; 16) were administered to both the adolescent and the participating parent, and the Fear Scaled sub-score was compared between groups to assess sub-clinical levels of anxiety symptoms as reported by either the youth or parent. A modified version of the Sleep Habits Survey 63 was administered to youth to examine whether group differences in sleepiness, sleep/wake problems, or preference for sleep time were present between FH+Pain and FH−Pain youth. Due to the fact that chronic pain and mood/anxiety disorders are highly co-morbid 2, 32, 37, all biological parents of participants were administered a modified version of the DSM-IV family history assessment module to assess family history of major depressive disorder (MDD) and generalized anxiety disorder (GAD) 45. The prevalence of maternal MDD and GAD were compared between FH+Pain and FH−Pain youth. Additionally, a score was calculated for a family history of MDD and GAD, in which presence of MDD and GAD in 1st and/or 2nd degree relatives (biological parents, grandparents, and aunts/uncles) resulted in a Family History Density (FHD) score for MDD and GAD, a measure previously used to assess density of familial alcoholism 53. FHD scores of 0 indicate no family history of the psychiatric disorder in 1st or 2nd degree relatives. Higher scores represent greater density of familial MDD or GAD. A score of 0.5 was assigned to youth who had a 1st degree biological parent with a history of the psychiatric condition, 0.25 was assigned to grandparents with the disorder, while 0.25 divided by total number of aunts/uncles on the maternal or paternal side of the family with the disorder was assigned for aunts/uncles who met criteria for MDD or GAD. Using the FHD score, FH+Pain and FH−Pain youth were also compared on degree of familial MDD and GAD.
2.3 Emotional Go/NoGo Task
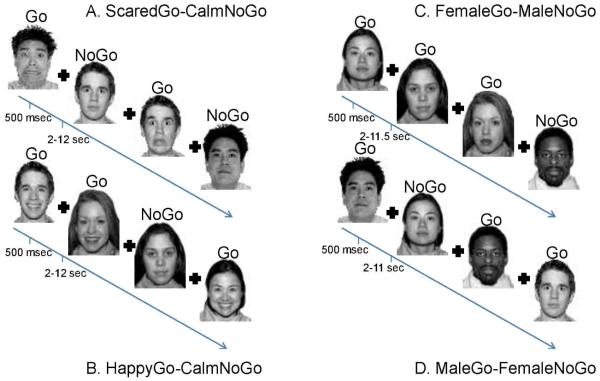
Participants completed a modified version (Fig 1; 11) of the previously published Emotional Go/NoGo Task 25 in E-Prime Version 1.2 (Figure 1). Youth completed four runs in the scanner with scared, happy, or calm faces (go trials) and calm non-target faces (nogo trails). Two runs with scared or happy go stimuli consisted of 60 go and 26 no go faces. Two other runs only included calm faces: female go/male nogo, and male go/female nogo. Each of these runs had 30 go and 13 no go trials concatenated, which resulted in 60 calm go trials and 26 calm no go trials. In all trials, stimuli were presented for 500 milliseconds. Interstimulus intervals, during which a fixation cross appeared, were jittered between 2,000-12,000 ms, as determined optimal by Freesurfer’s 19 OptSeq (http://surfer.nmr.mgh.harvard.edu/optseq/), an fMRI experiment timing and optimization tool. Only calm non-target faces were used for nogo trials because this study aimed to explore emotional processing and cognitive control during emotional and non-emotional contexts (go trials). Since neutral faces have been shown to elicit different responding in children vs. adults 56, calm faces were selected as non-emotional non-target trials from the NimStim dataset 57.
Figure 1. Emotional Go-NoGo Task.
Participants completed the Emotional Go-NoGo fMRI task in the scanner. There were four runs of the task: two emotional runs (A and B) and two control runs (C and D). Emotional runs always followed controls runs, but the order in which happy and scared runs appeared was counterbalanced across participants. The task instructions were to respond as quickly and as accurately to the target (go) face that was specified for a particular run and to not respond when a non-target (nogo) face appeared. Each face was presented for 500 milliseconds with a 2-12 second jitter used as the intertrial interval for the emotional runs of the task, and 2-11.5 second jitter used for the control runs of the task. A fixation cross appeared during the jitter period.
2.4 Behavioral Data Analyses
Demographic variables were compared between groups using independent-samples t-tests, Mann-Whitney U tests, and chi-square tests. All participants (8 FH+Pain and 8 FH−Pain) completed four runs of the Emotional Go/NoGo Task and met the required performance criteria (≥13 correct rejections on nogo trials within the emotional and non-emotional runs of the task). A multivariate analysis of variance (MANOVA) was used to analyze the Emotional Go/NoGo Task behavioral data. The MANOVA included Hits, Correct Rejections, Reaction Time, and D-Prime as the within subjects measures, and family history status (FH+Pain or FH−Pain) as the between-group variable. A second MANOVA was run to analyze post-scan self-reported ratings of valence and arousal for the faces presented during the fMRI task, with valance and arousal ratings as the within-subjects measures and family history status (FH+Pain or FH−Pain) as the between-subjects variable. All statistical analyses were completed in IBM SPSS Statistics version 20.0 8.
2.5 Image Acquisition
Magnetic resonance imaging took place on a 3.0 T Siemens Magnetom Tim Trio (Siemens Medical Solutions, Erlangen, Germany) scanner at OHSU’s Advanced Imaging Research Center. Each participant was oriented to scanning procedures prior to the imaging session by a trained scan technician and research assistant who discussed the scanner environment, emphasized the importance of remaining still during scanning, and had participants perform a practice session of the fMRI task. In the scanner, participants laid in the supine position and were given earplugs and headphones to minimize scanner noise, but were able to communicate with the scan technician in between scan sessions. Foam pillows were placed around the participants’ heads to minimize head motion. Task responses were made on a 4-button optical button box, and participants viewed the fMRI task through a mirror mounted on a 12-channel head coil that reflected the projector screen at the back of the bore. Following a localizer scan to estimate the participant’s position in the bore, a T1-weighted magnetization prepared rapid acquisition gradient echo (MPRAGE) anatomical scan was acquired (Time to Repetition (TR) = 2300 ms, Time to Echo (TE) = 3.58 ms, flip angle = 10°, resolution = 1 × 1 × 1.1 mm, inversion time (TI) = 900 ms, Field of View (FOV) = 240 × 256 mm, 160 slices, time of acquisition: 9:14). Next, T2*-weighted echo planar imaging was used to image the blood oxygen level-dependent (BOLD) response across four runs of the Emotional Go/NoGo Task (TR = 2000 ms, TE = 30 ms, flip angle = 90°, resolution = 3.75 × 3.75 × 3.8 mm, FOV = 240 mm2, 33 slices; HappyGo/CalmNoGo and ScaredGo/CalmNoGo: ~8:00 minutes; MaleGo/FemaleNoGo and FemaleGo/MaleNoGo: ~4:00 minutes).
2.6 Image Preprocessing
Standard image preprocessing procedures were performed using Analysis of Functional NeuroImages 9. These steps included slice timing correction, identification of artifact, skull-stripping of subject-specific brain anatomy, and co-registration of functional to anatomical images. To account for head motion, a least squares algorithm was used to identify the volume number of the time series to which all other volumes in each of the four runs of the task were least adjusted in the rotational or translational directions 10. TRs in which any of the six rotational or translational directions exceeded 2.5 mm or degrees were censored prior to further analyses. Furthermore, mean root mean square (RMS) across the four runs of the task was calculated for the 6 motion parameters, and was used to compare group differences in head motion. All functional data were blurred with a 6 mm full width half maximum Gaussian kernel to increase signal-to-noise ratio. Non-brain areas were masked out, followed by signal normalization, and concatenation of the two control runs of the task. Individual subject data were then modeled with a gammavariate function, while modeling delays, to estimate the hemodynamic response function (HRF; 7). Stimulus onset times were convolved with the HRF for regressors of interest, including Hits (HappyGo, ScaredGo, CalmGo) and Correct Rejections (CalmNoGo in the emotional (HappyGo and ScaredGo) and non-emotional contexts (CalmGo)) from the four runs of the task. Regressors of non-interest included False Alarms, Misses, and motion parameters. Contrasts of interest for emotional processing included: HappyGo vs. CalmGo, and ScaredGo vs. CalmGo. Contrasts of interest for inhibitory control within emotional contexts included: HappyGo(CalmNoGo) vs. CalmGo(CalmNoGo), and ScaredGo(CalmNoGo) vs. CalmGo(CalmNoGo) 11. Individual subject data were transformed to 3 mm3 voxels in standardized Talairach space 55.
2.7 fMRI Group-level Analyses
For each contrast of interest, functional data were voxel thresholded (p < 0.05) in each group of participants and added together to comprise a task-related activity map. AFNI’s 3dttest++ was used to examine group differences in brain activity within this task-related activity map for each contrast, thereby only examining group differences in brain activity in task-relevant brain regions. Monte Carlo simulation estimated the minimum cluster size needed to correct for multiple comparisons with a voxel and cluster threshold (p/α < 0.05) 20 in each of the four contrasts of interest. Due to differences in task-related activity maps based on each contrast of interest, the minimum cluster size needed for multiple comparison correction differed slightly between analyses: HappyGo vs. CalmGo (25 voxels), ScaredGo vs. CalmGo (33 voxels), HappyGo(CalmNoGo) vs. CalmGo(CalmNoGo) (48 voxels), ScaredGo(CalmNoGo) vs. CalmGo(CalmNoGo) (18 voxels).
3. Results
3.1 Demographic Characteristics
FH+Pain and FH−Pain youth did not significantly differ on any of the demographic characteristics listed in Table 1, including age, sex ratio, ethnicity, IQ, SES, pubertal maturity, subclinical depressive or anxiety symptoms, sleep quality or preference, or in-scanner head motion. FH+Pain youth and their peers had similar FHD of MDD (t14 = 1.53, p = 0.15), and GAD (U14 = 24.0, Z = −0.97, p = 0.44). However, not surprisingly, six of the eight FH+Pain youth had biological mothers who met criteria for MDD, while only two of the eight biological mothers met this criterion in the FH−Pain group (Χ21 = 4.00, p = 0.05). When examining biological mothers alone, one FH+Pain youth had a mother who met criteria for GAD, while no FH−Pain youth had mothers who met criteria for GAD (Χ21 = 1.07, p = 0.30).
Table 1.
Participant Demographics for Youth with Valid fMRI Data. Means and standard deviations unless otherwise noted.
| FH+Pain | FH−Pain | Statistic | p value | |
|---|---|---|---|---|
| N | 8 | 8 | ||
| Age | 13.61 (1.55) | 14.72 (0.98) | t14 = 1.72 | .11 |
| Gender | 2F/6M | 5F/3M | X21 = 2.29 | .13 |
| Caucasian (%) | 87.5 | 87.5 | ||
| IQab | 116.57 (6.95) | 116.50 (7.46) | t13 = −0.02 | .99 |
| Vocabulary Sub-testb | 58.57 (7.23) | 61.13 (6.15) | t13 = 0.74 | .47 |
| Range | 43-63 | 54-71 | ||
| Matrix Reasoning Sub-testb | 60.43 (5.50) | 57.50 (3.74) | t13 = −1.22 | .24 |
| Range | 54-67 | 53-62 | ||
| SESc | 31.13 (13.86) | 34.0 (14.17) | t14 = 0.41 | .69 |
| PDS Crockett Staged | 3.89 (1.10) | 4.12 (.70) | U14 = 15.0 Z = −1.87 | .08 |
| Head Motion (RMS)e | 0.39 (0.25) | 0.45 (0.56) | U14 = 24.0 Z = −0.84 | .44 |
| CDIfg | 40.71 (5.41) | 42.75 (7.19) | t13 = 0.61 | .55 |
| DSM-IV Psychiatric Diagnoses (N)h | 1 | 0 | ||
| Biological Maternal Major Depressive | 6 | 2 | X21 = 4.00 | .05 |
| Disorder (N)i | ||||
| Family History Density of MDDj | 0.52 (0.38) | 0.27 (0.25) | t14 = −1.53 | .15 |
| Range | 0-1.13 | 0-0.58 | ||
| Biological Maternal Generalized Anxiety | 1 | 0 | X21 = 1.07 | .30 |
| Disorder (N)k | ||||
| Family History Density of GADl | 0.18 (0.26) | 0.09 (0.19) | U14 = 24.0 Z = −0.97 | .44 |
| Range | 0-0.65 | 0-0.50 | ||
| EATQ-Rm Youth Fear Scaled | 2.45 (0.28) | 2.60 (0.60) | t13 = 0.61 | .55 |
| EATQ-R Parent Fear Scaled | 2.07 (0.85) | 1.85 (0.61) | t13 = −0.57 | .58 |
| SHSn Sleepiness Scaleo | 12.0 (2.65) | 13.13 (3.14) | t13 = 0.74 | .47 |
| SHS Sleep/Wake Problems Scalep | 17.0 (7.62) | 14.17 (3.31) | t11 = −0.84 | .42 |
| SHS Morning/Evening Scalelq | 24.86 (3.58) | 28.0 (3.66) | t13 = 1.68 | .12 |
Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)
IQ missing from one FH+Pain participant; the two subtests included Vocabulary and Matrix Reasoning. T-scores on the subtests may range from 20-80, with higher scores reflecting higher IQ. Full-scale IQ scores between 90 and 110 represent average, scores above 110 represent high-average, while scores above 120 represent superior IQ levels
Hollingshead Index of Social Position (Hollingshead, 1957)
Pubertal Developmental Scale Crockett Stage; scores range 1-5, with higher scores reflecting greater maturity (Petersen et al., 1988)
Root Mean Square
Children’s Depression Inventory (Kovacs, 1985)
CDI missing for one FH+Pain participant
One youth diagnoses with Generalized Anxiety Disorder
Modified DSM-IV Family History Assessment Module (Rice et al., 1995)
Family History Density of Major Depressive Disorder (MDD); the presence of MDD in biological parents, grandparents and aunts/uncles was calculated and resulted in a family history density score, with higher scores reflecting greater prevalence of familial MDD
Modified DSM-IV Family History Assessment Module (Rice et al., 1995)
Family History Density of Generalized Anxiety Disorder (GAD); the presence of MDD in biological parents, grandparents and aunts/uncles was calculated and resulted in a family history density score, with higher scores reflecting greater prevalence of familial GAD
Early Adolescent Temperament Questionnaire-Revised (Ellis & Rothbart, 2001); missing for FH+Pain participant
Modified version of the Sleep Habits Survey (Wolfson & Carskadon, 1998)
Lower score reflects less sleepiness; missing for one FH+Pain participant
Lower score reflects less sleep/wake problems; missing for one FH+Pain and two FH−Pain participants
Lower score is morning preference; missing for one FH+Pain participant
3.2 Emotional Go/NoGo Task Behavior
A MANOVA indicated that there was no significant effect of Emotion, Group, or Emotion × Group interaction on task performance variables, including Hits, Correct Rejections, Reaction Time, and D-Prime (Table 2). During a post-scan computerized exit questionnaire, FH+Pain youth did not differ from their peers on ratings of valence or arousal of the faces they had seen during the fMRI task (Table 3).
Table 2.
Task Performance. Means and (standard deviations).
| FH+Pain | FH−Pain | |
|---|---|---|
| N | 8 | 8 |
| Hitsa | ||
| Happy | 58.37 (1.60) | 57.75 (1.16) |
| Scared | 52.87 (6.60) | 57.87 (2.53) |
| Calm | 55.75 (5.26) | 57.5 (1.41) |
| Correct Rejectionsb | ||
| Happy | 20.12 (5.33) | 22.5 (4.14) |
| Scared | 22.12 (4.82) | 21.86 (3.64) |
| Calm | 20.37 (3.41) | 21.75 (3.28) |
| Reaction Time (ms) | ||
| Happy | 629.59 (100.76) | 540.77 (98.81) |
| Scared | 710.83 (195.59) | 596.59 (152.1) |
| Calm | 605.14 (219.6) | 546.82 (120.54) |
| D-Prime | ||
| Happy | 2.96 (1.16) | 3.15 (.68) |
| Scared | 2.60 (.96) | 3.10 (.65) |
| Calm | 2.62 (1.07) | 2.90 (.52) |
Out of 60 total Hits possible for each emotion.
Out of 26 total Correct Rejections possible for each emotion.
Table 3.
Valence and Arousal Ratings of Faces. Means and (standard deviations).
| FH+Pain | FH−Pain | |
|---|---|---|
| N | 8 | 8 |
| Valence*a | ||
| Happy | 5.85 (1.82) | 6.18 (.89) |
| Scared | 3.81 (1.0) | 4.11 (.81) |
| Calm | 3.96 (1.26) | 4.78 (1.42) |
| Arousal^b | ||
| Happy | 3.69 (1.43) | 3.10 (2.04) |
| Scared | 4.07 (1.82) | 4.26 (2.49) |
| Calm | 2.59 (1.22) | 1.94 (1.01) |
Scale of 1-9; 1 = Unhappy, 9 = Happy
Scale of 1-9; 1 = Bored/Calm, 9 = Excited/Nervous
Happy Valence > Scared/Calm Valence, p < 0.05
Calm Arousal < Happy/Scared Arousal, p < 0.05
3.3 Group Differences in Brain Response to Emotional Faces
FH+Pain youth had significantly different brain activity from their FH−Pain peers in widespread areas of the brain, including the occipital, parietal, and frontal lobes, as well as subcortical areas, such as the right thalamus, left amygdala, and right parahippocampal gyrus. In these regions, FH+Pain youth showed less brain activity to Happy vs. Calm Go faces than FH−Pain youth (Figure 2/Table 4). To better dissociate the directionality of brain response driving significant group differences in the contrast of interest, a mixed model ANOVA was performed with Happy and Calm Go BOLD response as the within-subject factor, and family history status as the between-subjects factor. This analysis indicated that in all 10 clusters of significant group differences to positively valenced faces, an interaction was present (Table 5). Two of these significant interactions are highlighted in Figure 2. For example, simple contrasts showed that in the left amygdala (peak voxel in the uncus), FH+Pain youth showed deactivation during Happy Go faces compared with FH−Pain peers (t14 = 3.02, p < 0.01). However, an opposite pattern of brain activity was present in response to Calm Go faces, such that activity in the left amygdala was greater in FH+Pain, compared with FH−Pain youth (t14 = −2.17, p < 0.05). Thus, different patterns of brain response to Happy and Calm Go faces in each group drove a significant group difference in the contrast of interest.
Figure 2. Significant differences in brain response to positively valenced faces between FH+Pain and FH−Pain youth.
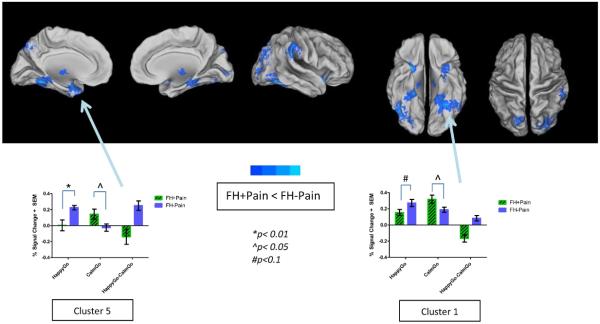
Brain response to Happy vs. Calm Go faces was reduced in FH+Pain youth compared with their FH−Pain peers, as indicated by areas in cool colors surface-mapped on the Population-Average, Landmark- and Surface-based (PALS-B12) template brain, multiple comparison corrected, (p/α<0.05/0.05). These nine clusters are labeled on the maps with names corresponding to regions where the peak coordinate of differences in brain response was located, and include occipital, parietal, frontal, thalamic, and limbic brain regions. Brain activity is bar graphed from two of these clusters (Clusters 1 and 5) as examples of patterns seen in both the contrast of Happy vs. Calm Go faces, and the simple effects of those contrasts. In both regions group differences suggest blunted activity to Happy faces, but increased activity to Calm faces in FH+Pain youth, compared with their FH−Pain peers. L = left, R = right.
Table 4.
Group Differences in Brain Response to Positively and Negatively Valenced Faces and Inhibitory Control in Emotional vs. Non-emotional Contexts
| Peak Anatomic Location |
Regions Included |
# Voxelsa | x | y | z | t statisticb | Cohen’s dc |
|---|---|---|---|---|---|---|---|
|
| |||||||
| FH+Pain vs. FH−Pain | |||||||
|
| |||||||
| Go | |||||||
|
| |||||||
| Happy > Calm | |||||||
|
| |||||||
| None | |||||||
|
| |||||||
| Happy < Calm | |||||||
|
| |||||||
| 1. Left Fusiform Gyrus | Cerebellum, PHG, MTG | 162 | −50 | −47 | −13 | −2.76 | 1.48 |
| 2. Right Fusiform Gyrus |
LG, MOG, IOG, Declive |
97 | 38 | −65 | −10 | −2.73 | 1.46 |
| 3. Right IPL | SMG | 47 | 65 | −35 | 30 | −3.12 | 1.67 |
| 4. Right Cuneus | MOG, SOG, MTG | 46 | 32 | −86 | 27 | −2.78 | 1.49 |
| 5. Left Uncus | Amyg, STG, PHG | 44 | −23 | 2 | −25 | −2.75 | 1.47 |
| 6. Right Culmen | PHG, Fusiform Gyrus |
43 | 32 | −35 | −19 | −2.79 | 1.49 |
| 7. Right IFG | STG, PHG | 39 | 26 | 14 | −16 | −2.83 | 1.51 |
| 8. Right Thalamus | PHG, MB, STN | 39 | 2 | −11 | −7 | −2.91 | 1.56 |
| 9. Left Precuneus | 30 | −17 | −74 | 48 | −2.60 | 1.39 | |
| 10. Right PHG | Thalamus, SN, STN, MB |
27 | 14 | −23 | −13 | −2.78 | 1.49 |
|
| |||||||
| Go | |||||||
|
| |||||||
| Scared > Calm | |||||||
|
| |||||||
| None | |||||||
|
| |||||||
| Scared < Calm | |||||||
|
| |||||||
| 1. Left SFG | MFG | 41 | −17 | 71 | 6 | −2.83 | 1.51 |
| 2. Left PCC | PHG, LG, Culmen | 35 | −5 | −50 | 6 | −3.09 | 1.65 |
| 3. Right Precuneus | 35 | 26 | −68 | 33 | −2.81 | 1.50 | |
| 4. Right Declive | 34 | 2 | −80 | −22 | −2.61 | 1.40 | |
| 5. Right SFG | MFG, MeFG | 33 | 20 | 8 | 54 | −2.85 | 1.52 |
|
| |||||||
| Calm NoGo | |||||||
|
| |||||||
| Happy > Calm | |||||||
|
| |||||||
| None | |||||||
|
| |||||||
| Happy < Calm | |||||||
|
| |||||||
| 1. Right Postcentral | IPL, Cingulate | ||||||
| Gyrus | Gyrus, Precentral Gyrus |
150 | 35 | −35 | 63 | −2.58 | 1.38 |
| 2. Right IFG | Insula, STG | 80 | 47 | 14 | −1 | −2.50 | 1.34 |
|
| |||||||
| Calm NoGo | |||||||
|
| |||||||
| Scared > Calm | |||||||
|
| |||||||
| 1. Right MTG | PCC, Cuneus, Precuneus |
26 | −29 | −68 | 30 | 3.01 | 1.61 |
| 2. Right Cingulate Gyrus |
PCC | 25 | 5 | −29 | 27 | 2.62 | 1.40 |
|
| |||||||
| Scared < Calm | |||||||
|
| |||||||
| None | |||||||
PHG = parahippocampal gyrus, MTG = middle temporal gyrus, LG = lingual gyrus, MOG = middle occipital gyrus, IOG = inferior occipital gyrus, SMG = supramarginal gyrus, SOG = superior occipital gyrus, STG = superior temporal gyrus, MB = mammillary body, STN = subthalamic nucleus, SN = substantia nigra, MFG = middle frontal gyrus, MeFG = medial frontal gyrus, IPL = inferior parietal lobule, PCC = posterior cingulate cortex, IFG = inferior frontal gyrus, SFG = superior frontal gyrus
Voxel sizes were determined by examining voxel count reports of the voxel/cluster thresholded maps using Analysis of Functional NeuroImage’s (AFNI’s) Graphic User Interface cluster report tool.
t values of the clusters were determined by extracting t-values using AFNI’s 3dROIstats tool from the corresponding t statistical sub-brick of the resulting voxel/cluster thresholded maps.
Cohen’s d for independent samples t-tests was calculated using the following formula: Cohen’s d = 2t/√df (Rosenthal & Rosnow, 1991). All of the effect sizes reported are considered large (Cohen’s d > 0.8).
Table 5.
Group × Emotion Interactionsa in Brain Regions of Significant BOLD Activity Differences between FH+Pain and FH−Pain Youth
| Peak Anatomic Location | Group × Emotion Interactions | |||
|---|---|---|---|---|
|
| ||||
| FH+Pain vs. FH−Pain | F(1,14) | MSE | p | partial η2 |
| Go: Happy < Calm | ||||
|
| ||||
| Left Fusiform Gyrus | 19.56 | .006 | .001 | .583 |
| Right Fusiform Gyrus | 14.32 | .022 | .002 | .506 |
| Right IPL | 25.72 | .006 | .00017 | .648 |
| Right Cuneus | 11.15 | .017 | .005 | .443 |
| Left Uncus | 12.08 | .025 | .004 | .463 |
| Right Culmen | 27.55 | .004 | .00012 | .663 |
| Right IFG | 14.13 | .016 | .002 | .502 |
| Right Thalamus | 18.64 | .006 | .001 | .571 |
| Left Precuneus | 9.92 | .032 | .009 | .397 |
| Right PHG | 11.42 | .009 | .004 | .449 |
|
| ||||
| Go: Scared < Calm | ||||
|
| ||||
| Left SFG | 14.13 | .074 | .002 | .502 |
| Left PCC | 17.06 | .007 | .001 | .549 |
| Right Precuneus | 10.31 | .005 | .006 | .424 |
| Right Declive | 11.40 | .028 | .005 | .449 |
| Right SFG | 10.96 | .004 | .005 | .439 |
|
| ||||
| Calm NoGo: Happy < Calm | ||||
|
| ||||
| Right Postcentral Gyrus | 11.21 | .017 | .005 | .445 |
| Right IFG | 8.55 | .039 | .011 | .379 |
|
| ||||
| Calm NoGo: Scared > Calm | ||||
|
| ||||
| Right MTG | 19.39 | .004 | .001 | .581 |
| Right Cingulate Gyrus | 10.83 | .006 | .005 | .436 |
These interactions were extracted from mixed model analyses of variance (ANOVAs) in which “Group” was the between-subjects factor, and “Emotion” (Happy, Scared, Calm) vs. baseline brain response were the within-subjects factors. The ANOVAs were conducted to examine which of the emotional conditions vs. baseline were driving the significant group differences in the contrasts of interest. In all of these clusters, a significant interaction between Group and Emotion emerged.
Five areas of significant group difference emerged for the contrast examining Scared vs. Calm Go brain activity between FH+Pain and FH−Pain youth (Figure 3/Table 4). These differences were in bilateral superior frontal gyrus (SFG), right precuneus, left posterior cingulate cortex, and right cerebellar declive. Mixed model ANOVAs found significant Emotion × Group interactions in these clusters (Table 5). Bar graphs to illustrate one of the significant interactions are presented in Figure 3. Simple contrasts suggested that in the right SFG, FH+Pain youth showed deactivation to Scared Go faces, compared with FH−Pain youth, who had positive brain activity in this area (t14 = 4.18, p < 0.01), while there were no differences in BOLD activity to Calm Go faces between the groups.
Figure 3. Significant differences in brain response to negatively valenced faces between FH+Pain and FH−Pain youth.
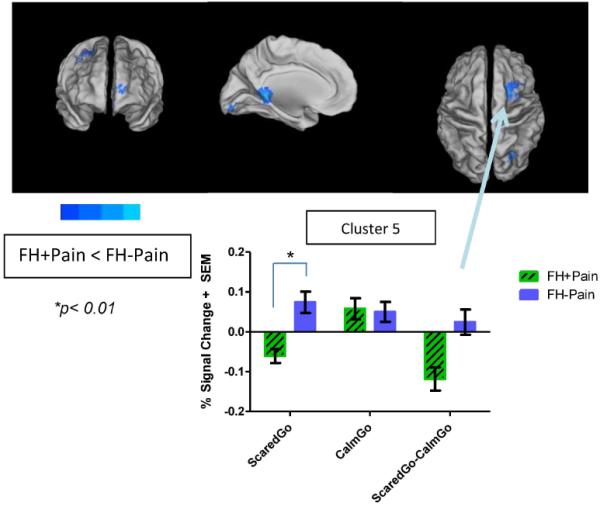
Brain response to Scared vs. Calm Go faces was reduced in FH+Pain youth compared with their FH−Pain peers, as indicated by areas in cool colors surface-mapped on the Population-Average, Landmark- and Surface-based (PALS-B12) template brain, multiple comparison corrected, (p/α<0.05/0.05). These five clusters are labeled on the maps with names corresponding to regions where the peak coordinate of differences in brain response was located, and include frontal, occipital, parietal, and cingulate cortex. Brain activity is bar graphed from one of these clusters (Cluster 5) as an example of patterns seen in both the contrast of Scared vs. Calm Go faces, and the simple effects of those contrasts. In this region group differences suggest blunted activity to Scared faces, but no significant differences in activity to Calm faces in FH+Pain youth, compared with their FH−Pain peers. L = left, R = right.
Furthermore, we conducted an additional analysis to examine whether any significant group differences in brain response were present between FH+Pain and FH−Pain youth when processing Calm Go faces, but no significant clusters were present between groups (multiple comparison corrected, p/α < 0.05).
3.4 Group Differences in Brain Response during Response Inhibition within Emotional Contexts
FH+Pain youth showed two areas of significant deactivation during response inhibition in positively valenced vs. non-emotional contexts, compared with their peers. These two clusters were comprised of regions that are considered part of the fronto-parietal and salience networks, including the inferior parietal lobule, inferior frontal gyrus (IFG), and insula (Figure 4). Similarly to the previous clusters, Group × Emotion interactions were found in these regions (Table 5). One of the interactions is illustrated in Figure 4, in which simple effects indicate that significant deactivation was present during response inhibition in FH+Pain youth in the positively valenced context compared with FH−Pain peers.
Figure 4. Significant differences in brain response during inhibitory control in both positively and negatively valenced contexts between FH+Pain and FH−Pain youth.
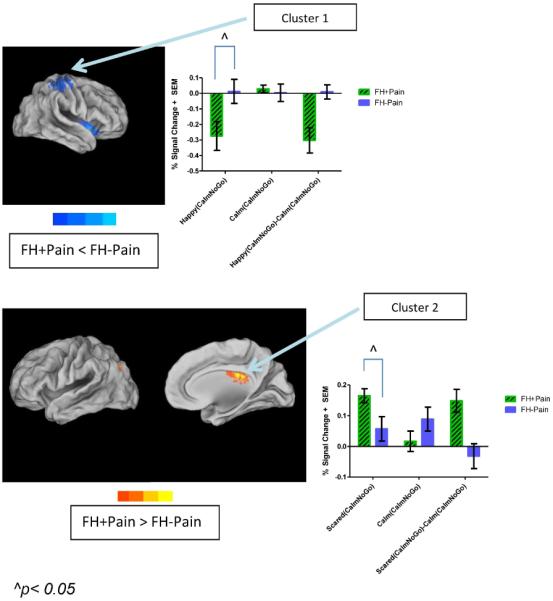
Brain response to Happy(CalmNoGo) vs. Calm(CalmNoGo) faces was reduced, while brain activity to Scared(CalmNoGo) vs. Calm(CalmNoGo) faces was increased in FH+Pain youth compared with their FH−Pain peers. This is indicated by areas in cool, and warm colors, respectively, surface-mapped on the Population-Average, Landmark- and Surface-based (PALS-B12) template brain, multiple comparison corrected, (p/α<0.05/0.05). All clusters are labeled on the maps with names corresponding to regions where the peak coordinate of differences in brain response was located. In the top panel, this includes the postcentral gyrus and inferior frontal gyrus. Brain activity is bar graphed from two of these clusters (Cluster 1), with simple effects indicating reduced inhibitory control brain response in positively valenced emotional contexts in FH+Pain youth compared with their FH−Pain peers. In the bottom panel, brain activity is increased in middle temporal and cingulate gyri, suggesting reduced suppression of some areas of the default mode network during cognitive control in FH+Pain youth, compared with their FH−Pain peers. L = left, R = right.
Additionally, FH+Pain youth showed two areas of significantly greater activation during response inhibition in negatively valenced contexts vs. non-emotional contexts, compared with FH−Pain peers. One of these clusters was the posterior cingulate cortex (PCC) (peak voxel in the cingulate gyrus), a brain region that is classified as part of the DMN, which is generally suppressed during cognitively demanding tasks. While neither adolescent group showed complete suppression of this region during response inhibition, the interaction (Figure 4/Table 5) illustrates that suppression was least present during response inhibition in negatively valenced contexts for FH+Pain youth.
Hierarchical regressions were used to determine if Group significantly predicted brain response in all of the reported clusters, above and beyond maternal MDD status. To conduct these regressions, maternal MDD was entered into the first block of the regression model, followed by Group status in the second block. When controlling for maternal MDD, family history of chronic pain was a better predictor of brain response in all clusters, with the exception of one region, in which maternal MDD status significantly explained brain activity (right precuneus cluster during response inhibition in negatively valenced contexts).
Finally, no significant group differences in BOLD response were present between FH+Pain and FH−Pain adolescents when correctly inhibiting during Calm NoGo faces in non-emotional contexts (when Go faces were also Calm; multiple comparison corrected, p/α < 0.05).
4. Discussion
The goal of the current pilot study was to investigate the neural correlates associated with risk for developing chronic pain due to family history. The findings from an emotion-cognition fMRI task indicate that despite no group differences in task performance, reduced brain activity in visual processing and affective brain regions, such as the amygdala, in response to positively valenced emotional faces was present in youth at heightened risk for the development of pain. Reduced brain activity was also seen in FH+Pain youth in fronto-parietal regions in response to negatively valenced faces, compared with FH−Pain youth. Furthermore, group differences were seen during response inhibition, such that positively valenced contexts elicited reduced BOLD activity in brain regions involved in salience detection and executive functioning, such as the insula and inferior parietal lobe, respectively. On the other hand, negatively valenced contexts resulted in less suppression of DMN areas, such as the PCC, in FH+Pain youth, compared with their low-risk peers.
To our knowledge, the findings from this pilot study are the first to suggest neurobiological phenotypes associated with familial risk for developing chronic pain. Blunted affective and occipital response to positively valenced faces could suggest altered responses to pleasant stimuli in FH+Pain youth. Previous research has shown that processing of emotional faces requires attention, such that the amplitude of amygdalar activity is significantly decreased to unattended emotional faces 42, suggesting that FH+Pain youth may not attend to positively valenced information to the same extent as their peers. Lower levels of positive affect reported in chronic pain patients 64 could be related to premorbid neural characteristics whereby high-risk individuals show decreased neural response to positive affective stimuli. Reduced response to appetitive, positive emotional information could decrease their resilience for managing aversive pain-related emotional responses 65.
FH+Pain youth also displayed reduced brain activity in prefrontal brain areas when viewing negatively valenced stimuli, which may indicate less efficient top-down regulation of brain activity to emotional stimuli. During adolescence, reduced inhibitory brain response in the face of emotional information could imply difficulties with emotion regulation 13, and may suggest early markers of a risk phenotype that could predispose these youth to problems with negative affect regulation.
Many of the findings seen during brain activity in the presence of response inhibition parallel previously reported studies of chronic pain in adults, suggesting that some underlying phenotypes may, in fact, be premorbid. For example, FH+Pain youth show reduced IFG and insular activity in the current study, and atypical brain activity in fronto-parietal brain areas has also been seen during a go/nogo task in fibromyalgia patients, who exhibit reduced IFG activity during cognitive control 22. Many task-related and functional connectivity studies suggest that the insula, part of the salience network, is atypical in chronic pain patients 28, 29, 39, 41, and given atypical insular activity in the current sample of high-risk youth, premorbid neurobiological vulnerabilities for developing chronic pain may be present. It is possible that emotional contexts may suppress attention to stimuli in cognitively demanding situations, and thus interfere with executive functioning in high-risk youth in emotionally salient circumstances.
It is interesting that differences were present during response inhibition that varied by the valence of the emotional context that response inhibition was embedded in. Multiple studies of adults with chronic pain suggest altered DMN functioning, such that brain regions involved in self-referential processing and rumination show atypical functional connectivity 4, 29, 34, 39, 41, 54. Heightened functional connectivity of the medial prefrontal cortex (MPFC) with other DMN regions has been associated with pain rumination 34. Further, enhanced BOLD response of both the MPFC and PCC has been found in adults with chronic pain during inhibitory 62 and visual attentions tasks 4, compared with healthy controls. In the current study, reduced suppression of DMN regions, such as the PCC, during inhibition could suggest that negative emotional information may be interfering with the ability to efficiently reduce activity of this region. Negative affect regulation may disrupt DMN activity in high-risk youth, and could be a potential target for early intervention aimed at managing the development of pain symptoms.
While the current pilot study presents novel findings on the neurobiological underpinnings of pain heritability, limitations warrant mention. First, this pilot study contained a relatively small sample of youth with and without a family history of chronic pain. A larger sample would have afforded greater power to examine the neurobiological mechanisms associated with pain heritability. Additionally, any non-significant differences may have been due to a lack of power rather than null effects. Therefore, future studies should be aimed at targeting the recruitment of high-risk youth. Second, due to the small sample size, sex differences in brain response could not be analyzed. Given sex differences in the prevalence of chronic pain conditions, with higher rates in females than males 30, it will be important to examine risk by sex interactions. Third, the majority of FH+Pain youth had biological mothers who met criteria for MDD, a characteristic that was not mirrored in FH−Pain youth. Because of the discrepancy in the presence of MDD in biological mothers, it is difficult to tease apart the effects of MDD heritability as opposed to chronic pain heritability. However, results remained significant in all but one cluster when controlling for maternal history of depression diagnoses, thus strengthening support for a neurobiological mechanism specific to pain heritability. Fourth, while there were no task-related behavioral differences between the groups, we did detect differences in brain activity between FH+Pain and FH−Pain youth. It is possible that in emotionally heated situations outside of the laboratory, bottom-up processing may interfere with inhibitory control to a greater extent than in a “cold” laboratory setting. Despite the negative behavioral findings, the neural markers that differentiated the groups may, in fact, be critical neurobiological phenotypes of risk that could predict future onset of chronic pain. These striking group differences even in the absence of pain symptoms should be further explored with other neuroimaging modalities, such as resting state functional connectivity, to better understand network organization in FH+Pain youth. Fifth, there were no measures of pain sensitivity or tolerance collected in this group of adolescents, which could have provided important information on altered pain processing in FH+Pain youth. Future studies with this population will include pain sensitivity and conditioned pain modulation testing, as these may provide important information regarding the heritability of pain processing. Sixth, FH+Pain youth in the study were limited to those with biological mothers with chronic pain and information on paternal pain history is incomplete. A more complete assessment of both biological parents’ pain history should be included in future studies to better characterize the extent and degree of family history risk in FH+Pain youth. Finally, due to the cross-sectional design, this study is unable to determine whether youth will develop self-reported chronic pain. A longitudinal investigation of FH+Pain youth will be necessary to examine whether baseline brain response is indicative of future chronic pain.
While this study has some limitations, there are several strengths that should be highlighted. First, many of the findings fit well with previous reports of brain activity in adults with chronic pain, lending support to the hypothesis that some phenotypes associated with a chronic pain condition may be present prior to the onset of significant pain symptoms. Second, while a greater number of biological mothers met criteria for MDD in the FH+Pain youth than in FH−Pain peers, the fact that the groups were matched on the degree of familial depression, when other relatives were taken into account, suggests that the present effects were not driven by overall greater depression risk due to family history of MDD. Third, the effect sizes found in the study were quite large (Cohen’s d > 0.8), suggesting robust findings even in a fairly small sample. Fourth, to our knowledge this is the first study to examine brain activity in youth with a family history of chronic pain in an effort to identify neurobiological markers of pain risk. The novel findings present possible future neurobiological targets for pain management in high-risk youth. Future work will examine brain activity in a larger sample of FH+Pain youth, as well as resting state functional connectivity characteristics in this cohort to identify intrinsic differences in brain network organization that may be altered before the onset of pain symptoms.
The current pilot study found altered brain activity during both emotional processing and cognitive control in emotional contexts in FH+Pain youth compared with their peers. These findings lend initial support to the hypothesis that the mechanisms contributing to pain heritability may be detected during adolescence, prior to the onset of pain symptomology. This information could aid our understanding of neural phenotypes that could be targeted for pain management and prevention, and towards reducing the severity of pain symptoms upon their onset in high-risk individuals.
Perspective.
This is the first study to examine neurobiological markers of pain risk in adolescents with a family history of chronic pain. These findings may aid in the identification of neural phenotypes related to vulnerability for the onset of pain in at-risk youth.
Highlights.
Emotional processing brain activity is altered in FH+Pain youth.
Emotional context affects inhibitory control brain response in FH+Pain youth.
Neural markers of chronic pain risk may be present in the absence of pain symptoms.
Acknowledgements
Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at nimtottenham@ucla.edu for more information concerning the stimulus set.
Support for this study was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23 HD064705 - Wilson), the Medical Research Foundation of Oregon Grant (PI: Wilson, Co-I: Nagel), and the National Institute on Alcohol Abuse and Alcoholism (R01 AA017664 - Nagel).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors have no conflicts of interest.
References
- 1.Anttila P, Metsahonkala L, Helenius H, Sillanpaa M. Predisposing and provoking factors in childhood headache. Headache. 2000;40:351–356. doi: 10.1046/j.1526-4610.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 2.Bair MJ, Poleshuck EL, Wu J, Krebs EK, Damush TM, Tu W, Kroenke K. Anxiety but not social stressors predict 12-month depression and pain severity. Clin J Pain. 2013;29:95–101. doi: 10.1097/AJP.0b013e3182652ee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carskadon MA, Acebo C. A self-administered rating scale for pubertal development. J of Adolesc Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- 6.Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, Martinez-Jauand M, Birbaumer N, Chialvo DR, Montoya P. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74:55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS. Parametric analysis of fMRI data using linear systems methods. NeuroImage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 8.Corp. I . IBM SPSS Statistics for Windows. IBM Corp.; Armonk, NY: Version 20.0. Released 2011. [Google Scholar]
- 9.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 10.Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 11.Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol Clin Exp Res. 2014;38:1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Currie C, Samdal O, Boyce W, Smith R. Health Behaviour in School-aged Children: A WHO cross-national study (HBSC): Research protocol for the 2001/2002 survey. Child and Adolescent Research Unit, University of Edinburgh; Edinburgh: 2001. [Google Scholar]
- 13.Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectr. 2001;6:60–72. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- 14.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 15.Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152:1104–1113. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Ellis LK, Rothbart MK. Revision of the Early Adolescent Temperament Questionnaire. Poster presented at the Biennial Meeting of the Society for Research in Child Development; Minneapolis, Minnesota. 2001. [Google Scholar]
- 17.Erpelding N, Sava S, Simons LE, Lebel A, Serrano P, Becerra L, Borsook D. Habenula functional resting-state connectivity in pediatric CRPS. J Neurophysiol. 2014;111:239–247. doi: 10.1152/jn.00405.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fearon P, Hotopf M. Relation between headache in childhood and physical and psychiatric symptoms in adulthood: national birth cohort study. BMJ. 2001;322:1145. doi: 10.1136/bmj.322.7295.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischl B. FreeSurfer. NeuroImage. 2012;62:774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 21.Gatchel RJ, Okifuji A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain. 2006;7:779–793. doi: 10.1016/j.jpain.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Glass JM, Williams DA, Fernandez-Sanchez ML, Kairys A, Barjola P, Heitzeg MM, Clauw DJ, Schmidt-Wilcke T. Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J Pain. 2011;12:1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: a World Health Organization Study in Primary Care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 25.Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocking LJ, Morris AD, Dominiczak AF, Porteous DJ, Smith BH. Heritability of chronic pain in 2195 extended families. Eur J Pain. 2012;16:1053–1063. doi: 10.1002/j.1532-2149.2011.00095.x. [DOI] [PubMed] [Google Scholar]
- 27.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT.: 1957. [Google Scholar]
- 28.Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T. Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache. 2012;52:441–454. doi: 10.1111/j.1526-4610.2011.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA, Harris RE. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 2014 doi: 10.1016/j.jpain.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Junqueira DR, Ferreira ML, Refshauge K, Maher CG, Hopper JL, Hancock M, Carvalho MG, Ferreira PH. Heritability and lifestyle factors in chronic low back pain: Results of the Australian Twin Low Back Pain Study (The AUTBACK study) Eur J Pain. 2014 doi: 10.1002/ejp.506. [DOI] [PubMed] [Google Scholar]
- 32.Kashikar-Zuck S, Goldschneider KR, Powers SW, Vaught MH, Hershey AD. Depression and functional disability in chronic pediatric pain. Clin J Pain. 2001;17:341–349. doi: 10.1097/00002508-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs M. The Children's Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 34.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 2014;34:3969–3975. doi: 10.1523/JNEUROSCI.5055-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurell K, Larsson B, Eeg-Olofsson O. Headache in schoolchildren: association with other pain, family history and psychosocial factors. Pain. 2005;119:150–158. doi: 10.1016/j.pain.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- 37.Manning JS, Jackson WC. Depression, pain, and comorbid medical conditions. J Clin Psychiatry. 2013;74:e03. doi: 10.4088/JCP.12049vs3c. [DOI] [PubMed] [Google Scholar]
- 38.Mikail SF, von Baeyer CL. Pain, somatic focus, and emotional adjustment in children of chronic headache sufferers and controls. Soc Sci Med. 1990;31:51–59. doi: 10.1016/0277-9536(90)90009-h. [DOI] [PubMed] [Google Scholar]
- 39.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 41.Otti A, Guendel H, Wohlschlager A, Zimmer C, Noll-Hussong M. Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC Psychiatry. 2013;13:84. doi: 10.1186/1471-244X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen A, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth and Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 44.Piira T, Pullukat R. Are the children of chronic pain patients more likely to develop pain? Enfance. 2006;58:20–25. [Google Scholar]
- 45.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr., Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 46.Rickard K. The occurrence of maladaptive health-related behaviors and teacher-rated conduct problems in children of chronic low back pain patients. J Behav Med. 1988;11:107–116. doi: 10.1007/BF00848259. [DOI] [PubMed] [Google Scholar]
- 47.Sava S, Lebel AA, Leslie DS, Drosos A, Berde C, Becerra L, Borsook D. Challenges of functional imaging research of pain in children. Mol Pain. 2009;5:30. doi: 10.1186/1744-8069-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schweinhardt P, Kalk N, Wartolowska K, Chessell I, Wordsworth P, Tracey I. Investigation into the neural correlates of emotional augmentation of clinical pain. NeuroImage. 2008;40:759–766. doi: 10.1016/j.neuroimage.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 49.Shelby GD, Shirkey KC, Sherman AL, Beck JE, Haman K, Shears AR, Horst SN, Smith CA, Garber J, Walker LS. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132:475–482. doi: 10.1542/peds.2012-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sherman AL, Bruehl S, Smith CA, Walker LS. Individual and additive effects of mothers' and fathers' chronic pain on health outcomes in young adults with a childhood history of functional abdominal pain. J Pediatr Psychol. 2013;38:365–375. doi: 10.1093/jpepsy/jss131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons LE, Pielech M, Erpelding N, Linnman C, Moulton E, Sava S, Lebel A, Serrano P, Sethna N, Berde C, Becerra L, Borsook D. The Responsive Amygdala: Treatment-induced Alterations in Functional Connectivity in Pediatric Complex Regional Pain Syndrome. Pain. 2014 doi: 10.1016/j.pain.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 53.Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- 54.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talairach J, Tournoux P. Three-Dimensional Proportional System: An Approach to Cerebral Imaging. Thieme; New York: 1988. Coplanar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 56.Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- 57.Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vehof J, Zavos HM, Lachance G, Hammond CJ, Williams FM. Shared genetic factors underlie chronic pain syndromes. Pain. 2014 doi: 10.1016/j.pain.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain. 1998;77:231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 60.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Psychological Corporation; San Antonio, TX.: 1999. [Google Scholar]
- 62.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152:384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 63.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 64.Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, Davis MC. Fibromyalgia: evidence for deficits in positive affect regulation. Psychosom Med. 2005;67:147–155. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–220. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]