Abstract
Alcohol consumption increases the incidence of multiple types of cancer. However, how chronic alcohol consumption affects tumor progression and host survival remains largely unexplored. Using a mouse B16BL6 melanoma model, we studied the effects of chronic alcohol consumption on s.c. tumor growth, iNKT cell antitumor immune response, and host survival. The results indicate that although chronic alcohol consumption inhibits melanoma growth, this does not translate into increased host survival. Immunizing mice with a melanoma cell lysate does not significantly increase the median survival of water-drinking, melanoma-bearing mice, but significantly increases the median survival of alcohol-consuming, melanoma-bearing mice. Even though survival is extended in the alcohol-consuming mice after immunization, the mean survival is not different from the immunized mice in the water-drinking group. Immunization with tumor cell lysate combined with α-galatosylceramide activation of iNKT cells significantly increases host survival of both groups of melanoma-bearing mice compared to their respective non-immunized counterparts; however, the median survival of the alcohol-consuming group is significantly lower than that of the water-drinking group. Alcohol consumption increases NKT cells in the thymus and blood and skews NKT cell cytokine profile from Th1 dominant to Th2 dominant in the tumor-bearing mice. In summary, these results indicate that chronic alcohol consumption activates the immune system, which leads to the inhibition of s.c. melanoma growth and enhances the immune response to immunization with melanoma lysate. With tumor progression, alcohol consumption accelerates iNKT cell dysfunction and compromises antitumor immunity, which leads to decreased survival of melanoma-bearing mice.
Keywords: Alcohol, Melanoma, NKT cells, Survival
1. Introduction
Alcohol and its metabolite, acetaldehyde, are carcinogenic, and in 2012 were listed as group 1 carcinogens by the International Agency for Research on Cancer. Chronic alcohol consumption increases the incidence of multiple types of cancer including melanoma [1-9]. The effects of alcohol consumption on cancer can be divided into two equal important aspects. One is how alcohol consumption induces cancer. The study of this aspect is focused on the investigation of the molecular mechanisms associated with alcohol-mediated carcinogenesis. Extensive studies have been conducted and profound progress has been made in this research area during the past decade [10-13]. The second aspect addresses how alcohol consumption affects tumor progression and the survival of cancer patients. Compared to studies on how alcohol consumption induces cancer, research on how alcohol consumption affects the progression of established cancer has largely been unexplored until recently [14-16]. Epidemiological studies indicate that alcohol consumption consistently decreases the survival of patients with upper aerodigestive cancers and Non-Hodgkin Lymphoma [5, 15-17]; however, the underlying mechanism is not known.
The progression of cancer is affected by multiple factors, and the immune system plays an important role. Tumor immunotherapy alone or combined with chemotherapy or radiotherapy has become one of the most promising approaches to treat cancer [18, 19]. It is well known that chronic alcohol consumption affects the immune system [20]. A large body of data indicates that chronic alcohol consumption in humans and mice activates the immune system, which is reflected in the activation of CD8+ T cells, dendritic cells (DC), and NKT cells, and increased production of pro-inflammatory cytokines such as IFN-γ and TNF-α [21-25]. We found that chronic alcohol consumption along with advancing growth of s.c. melanoma leads to inhibition in the expansion of memory and melanoma-specific CD8+ T cells, acceleration in the decay of Th1 cytokine producing CD8+ T cells [26], and compromised circulation of mature B cells [27]. Chronic alcohol consumption also increases invariant NKT (iNKT) cells and especially IFN-γ-producing iNKT cells [28] [29]. iNKT cells are specific T cells that recognize lipid antigens and produce a broad spectrum of cytokines upon activation [30]. Activation of iNKT cells can enhance the antitumor immune response [31]. Continuous activation of iNKT cells leads to iNKT cell anergy and inhibits antitumor immune responses [32].
How these functional changes in the immune system related to chronic alcohol consumption affect tumor progression and host survival and especially how they affect the response of the host to immunization and cancer immunotherapy are not known. Herein, we designed a series of experiments to determine not only how chronic alcohol consumption affects B16BL6 melanoma progression and host survival but also the response to immunization with melanoma lysates with and without activation of iNKT cells with α-galactosylceramide (αGalCer), a potent agonist of iNKT cells. We also studied how chronic alcohol consumption affects iNKT cells in B16BL6 melanoma-bearing mice. We found that chronic alcohol consumption inhibits melanoma growth but does not increase host survival. Immunization of the mice with melanoma lysate significantly increases the survival of chronic alcohol-consuming mice, but not water-drinking mice. Compared to their water-drinking counterparts, alcohol consumption significantly decreases the survival of the melanoma-bearing mice immunized with tumor cell lysate combined with activation of iNKT cells.
2. Material and Methods
2.1. Animals and alcohol administration
Female C57BL/6 mice at 6-7 weeks of age were purchased from Charles River Laboratories (Wilmington, MA). After arrival, mice were single housed in plastic cages with micro-filter tops in the Wegner Hall Vivarium, which is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. Mice were allowed free access to Purina 5001 rodent laboratory chow and sterilized Milli-Q water. After one week of acclimation, mice were randomly divided into two groups. The control group was continuously provided with chow and Milli-Q water. The treatment group was provided with chow and 20% (w/v) alcohol diluted from 190-proof Everclear (St. Louis, MO) with sterilized Milli-Q water. Mice consume at least 30% of their caloric intake from alcohol [33]. They were used for experiments 3-6 months after starting alcohol consumption, since during this time period alterations in immune parameters induced by chronic alcohol consumption are relatively stable [34]. All studies were approved by the Institutional Animal Care and Use Committee at Washington State University.
2.2. Antibodies and reagents
The following PE, FITC and PerCP labeled anti-mouse monoclonal antibodies were purchased from BioLegend: anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-NK1.1 (PK136), anti-NKG2A (16A11), anti-IFN-γ (XGM1.2), anti-IL-4 (11B11). CD1d/PBS57-tetramer-PE was synthesized by NIH tetramer facility (Atlanta, Georgia). αGalCer was purchased from Avanti Polar Lipids, Inc.
2.3. Tumor cell culture and inoculation
The highly invasive and metastatic B16BL6 melanoma cell line originally obtained from the Mason Research Institute, Worcester, MA, was used in the study. Tumor cells were cultured in Dulbecco's modified Eagles medium supplemented with 10% FBS, 1% penicillin and streptomycin in a humidified incubator with 5% CO2 at 37°C. Cells were harvested when they reached 50-70% confluence. Each mouse was inoculated with 2×105 cells (in 200 μl PBS) subcutaneously in the right side of the hip.
2.4. Immunization of mice with tumor cell lysate
A melanoma whole cell lysate was used to immunize mice. In vitro cultured tumor cells were harvested and resuspended in PBS. The cells were washed three times with PBS at 5-10 × 106 cells/50 ml PBS before they were frozen at -70°C. The frozen cells were thawed on ice and passed through a syringe fitted with a 27 gauge needle. The crude cell lysate was refrozen, thawed and passed through the syringe two additional times. The resulting cell lysate from 2×105 cells in 200 μl of PBS was injected into the peritoneal cavity of the mice and again 7 days later to enhance the immune response. Tumor cells were inoculated s.c. on day 14 after the first dose of tumor cell lysate.
2.5. αGalCer administration
αGalCer, which activates iNKT cells, was used to boost the immune response induced by immunization with the melanoma tumor lysate. αGalCer was dissolved in DMSO at 1 mg/ml to make stock solution and stored at -20°C. It was diluted in PBS from the stock solution to form a working solution at 20 μg/ml and 4 μg in 200 μl was injected i.p. 30 min before the first injection of tumor cell lysate.
2.6. Measurement of tumor growth
Tumor size was measured from day 8 through day 30 after tumor inoculation. The longest (length) and the shortest (width) diameters of the tumor were measured by calipers every other day. The tumor size was calculated using the following formula established by Feldman et al. [35]: where V is volume, a is the length, b is the width.
2.7. Measurement of tumor metastasis
Tumor metastasis to the lung, draining inguinal and axillary lymph nodes (LN), which are the LN on the same side with the tumor, and control inguinal and axillary LN, which are the LN on the opposite side of the tumor, were examined at necropsy. Since most of the draining LN were fully covered by metastatic tumor and it was hard to identify the number of colonies, we classified the severity of the metastasis into 4 categories: 0, 1, 2, and 3. If there was no visible metastatic tumor colony, it was designated as 0; if there was some visible metastatic tumor colonies, it was designated as 1; if half or more of the surface of the lymph node was covered by metastatic tumors, it was designated as 2; if the whole lymph node was took over by the metastatic tumors, it was designated as 3.
2.8. Leukocyte isolation and cell phenotype analysis
Leukocytes from thymus, spleen and blood were isolated following our previous protocols [34]. Cell phenotypes were analyzed by flow cytometry using BD Biosciences CellQuest software as described [27].
2.9. Cytokine intracellular staining of IFN-γ and IL-4-producing iNKT cells
Splenocytes and PBL were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin and streptomycin. Two million cells per ml were stimulated with 150 ng/ml αGalCer, or 50 ng/ml PMA + 500 ng/ml Ionomycin. Cells also were treated with 5ug/ml Brefeldin A to inhibit extracellular transport of the cytokines. Cells were cultured in a humidified incubator with 5% CO2 at 37°C for 4 hr. Cells were harvested and placed on ice. Anti-CD16/32 was added to block nonspecific binding to Fc receptors. Cell surface staining was conducted with anti-CD3-PerCP and CD1d/PBS57tetramer-PE at room temperature for 30 min. After washing with FACS buffer (PBS+0.1%BSA+0.1% NaN3), cells were fixed and permeabilized using a BD Cytofix/Cytoperm kit according to the manufacturer's instructions. Then cells were stained with anti-IFN-γ or IL-4 monoclonal antibody. Cytokine producing cells were analyzed by flow cytometry and CellQuest software.
2.10. Statistical analysis
Microsoft Excel and GraphPad Prism software were used to analyze the data. Student's t-test, and one way ANOVA with Tukey's multiple comparison test were used to test the difference between and among groups as appropriate. The log-rank (Mantel-Cox) test was used to analyze differences in survival between/among experimental groups. Differences between groups were considered significant at p< 0.05.
3. Results
3.1. Chronic alcohol consumption inhibits B16BL6 melanoma growth
We previously reported that chronic alcohol consumption increases IFN-γ-producing memory T cells, NK cells and NKT cells in the non-melanoma injected mice [25, 28, 29, 34]. The percentage of IFN-γ-producing T cells in the alcohol-consuming mice continues to be elevated up to 11 days after s.c. melanoma inoculation as compared to water-drinking mice [26]. It is well-known that the immune system plays an important role in the control of tumor growth. Since its activation should inhibit melanoma growth, we determined if this were the case in the alcohol-consuming mice. We found that tumor volume was significantly lower in the alcohol-consuming mice than in the water-drinking mice from day 20 to day 30 after tumor inoculation (Fig. 1A). The tumor weight (Fig. 2D) determined at necropsy of mice dying from tumor also was lower, reflecting inhibition of tumor growth in the alcohol-consuming group relative to the water-drinking mice.
Fig. 1.
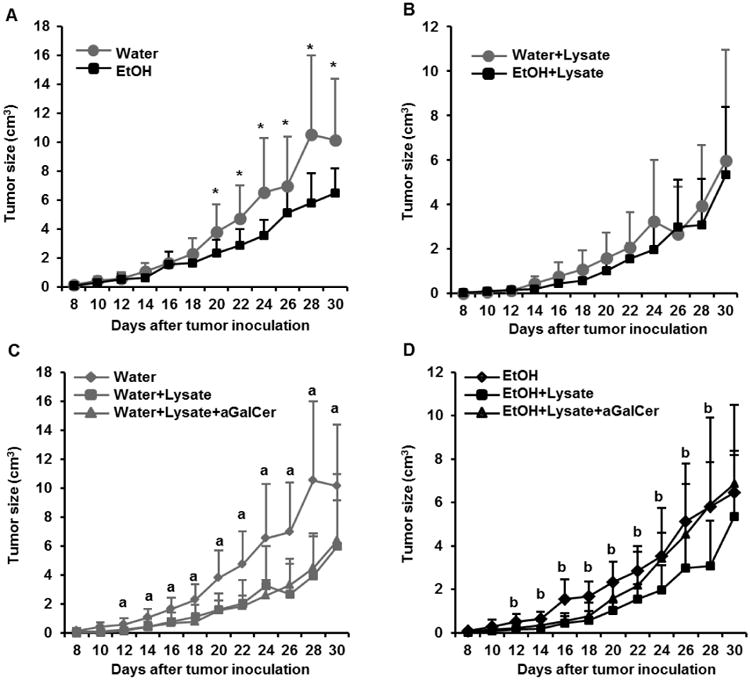
Effects of chronic alcohol consumption on B16BL6 melanoma growth in non-immunized mice, mice immunized with melanoma cell lysate, and mice treated with αGalCer prior to immunization with melanoma lysate. A. Melanoma growth in water-drinking mice and alcohol-consuming mice as a function of time after tumor inoculation. B. Comparison in tumor growth between water-drinking mice and alcohol-consuming mice immunized with B16BL6 melanoma cell lysate as described in Materials and Methods. C. Comparison of tumor growth in water-drinking mice immunized with melanoma lysate alone and after pretreatment with αGalCer as described in the Material and Methods. D. Comparison of tumor growth in alcohol-drinking mice immunized with melanoma lysate alone and after pretreatment with αGalCer as described in the Material and Methods. Each group contained 10 mice. Two-tailed Student's t-test was used to analyze the difference between two groups. One-way ANOVA was used to test the difference among three or more groups. *Tumor size different from EtOH group, p<0.05. a, Tumor size in non-immunized water-drinking mice different (p<0.05) from immunized mice and immunized mice stimulated with αGalCer, p<0.05. There is no difference in the tumor size between immunized water-drinking mice and immunized-water-drinking mice stimulated with αGalCer, p>0.05. b, Tumor size in non-immunized, alcohol-consuming mice different from immunized mice, p<0.05, but not different from the immunized mice stimulated with αGalCer p>0.05. The tumor size in immunized alcohol-consuming mice different from immunized alcohol-consuming mice stimulated with αGalCer (p<0.05). Water =water-drinking mice; EtOH=alcohol-drinking mice; Water+Lysate=water-drinking mice immunized with melanoma cell lysate; EtOH+Lysate=alcohol-consuming mice immunized with melanoma cell lysate; Water+Lysis+αGalCer= water-drinking mice immunized with melanoma cell lysate and stimulated with αGalCer. EtOH+Lysis+αGalCer= alcohol-consuming mice immunized with melanoma cell lysate and stimulated with αGalCer.
Fig. 2.
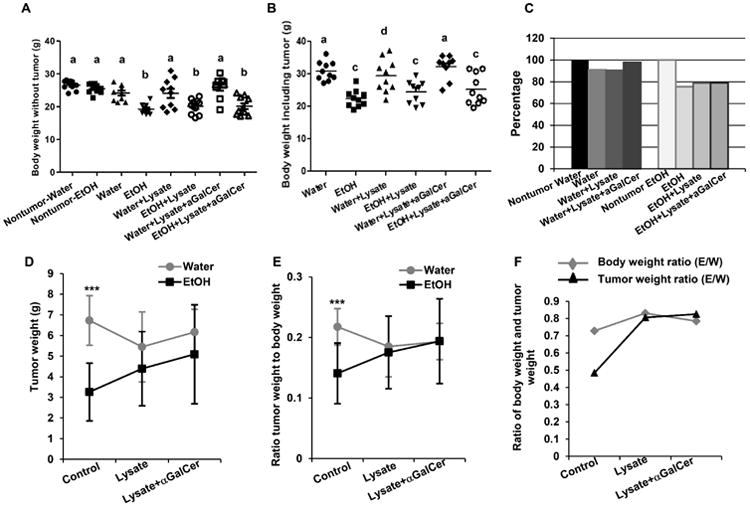
Effects of chronic alcohol consumption on body weight and melanoma tumor weight at necropsy in non-immunized mice, mice immunized with melanoma lysate, and in mice pretreated with αGalCer prior to immunization. A. Body weight of mice before tumor injection or body weight with tumor removed in the indicated mice. B. Body weight including tumor in the indicated mice tumor bearing mice. C. Percentage of body weight with tumor removed at necropsy relative to the body weight of mice before tumor injection. D. Tumor weight in the indicated mice. E. Ratio of tumor weight to body weight in the indicated mice. F. Comparison of ratio of body weight and tumor weight of alcohol-consuming mice as compared to water-drinking mice at necropsy. Each group contained 10 mice. One way ANOVA with Tukey's multiple comparison test was used to determine the differences among the experimental groups. ***p<0.001. a, Different from alcohol-consuming tumor-bearing mice, alcohol-consuming tumor-bearing mice immunized with tumor cell lysate, and alcohol-consuming tumor-bearing mice pretreated with αGalCer prior to immunization, p<0.01. b, Different from non-tumor injected alcohol-consuming mice, non-tumor injected water-drinking mice, water-drinking tumor-bearing mice immunized with melanoma lysate, and water-drinking tumor-bearing mice pretreated with αGalCer prior to immunization, p<0.01. c, Different from water-drinking tumor-bearing mice, and immunized water-drinking tumor-bearing mice pretreated with αGalCer, p<0.01. d, different from alcohol-consuming tumor-bearing mice only, p<0.05. Nontumor-Water=non-tumor injected water-drinking mice; Nontumor-EtOH=non-tumor injected alcohol-consuming mice; Water =water-drinking tumor-bearing mice; EtOH=alcohol-drinking tumor-bearing mice; Water+Lysate=water-drinking tumor-bearing mice immunized with melanoma cell lysate; EtOH+Lysate=alcohol-consuming tumor-bearing mice immunized with melanoma cell lysate; Water+Lysis+αGalCer= water-drinking tumor-bearing mice immunized with melanoma cell lysate and stimulated with αGalCer. EtOH+Lysis+αGalCer= alcohol-consuming tumor-bearing mice immunized with melanoma cell lysate and stimulated with αGalCer; E/W=ratio of ETOH to Water group.
Alcohol consumption itself does not decrease the body weight in the non-tumor-bearing mice (Fig. 2A). The final body weight of mice including the tumor and also the final body weight with the tumor removed at necropsy is lower in the alcohol-consuming mice than in the water-drinking mice (Fig.2A, 2B). The final body weight with the tumor removed decreased 10% compared to the body weight before tumor inoculation in water-drinking mice, while the body weight decreased 25% in the alcohol-consuming mice (Fig. 2C). The loss in body weight primarily results from the loss of body fat and skeletal muscle (data not shown) in the alcohol-consuming, tumor-bearing mice [36]. The ratio of tumor weight to body weight is much lower in the alcohol-consuming, tumor-bearing mice than in the water-drinking, tumor-bearing mice (Fig. 2E). The degree of tumor weight decrease (calculated as the ratio of tumor weight in alcohol-consuming mice to the tumor weight of water-drinking mice) was greater than the degree of body weight decrease (calculated as the ratio of body weight in alcohol-consuming mice to the water-drinking mice) in the alcohol-consuming mice compared to the water-drinking mice (Fig. 2F), indicating that the effect of alcohol consumption on the inhibition of tumor growth exceeds the effect that alcohol has on the body weight loss.
3.2. Immunization with tumor cell lysate inhibits tumor growth in both alcohol-consuming mice and water-drinking mice. Immunization plus αGalCer activation of iNKT cells inhibits tumor growth only in water-drinking mice
We examined the effect that immunization with a melanoma lysate alone and immunization preceded by activation of iNKT cells with αGalCer had on tumor growth. The results indicated that immunization significantly inhibits tumor growth in both the water-drinking (Fig. 1C) and alcohol-consuming mice (Fig. 1D) compared to respective non-immunized mice. However, there was no difference in tumor size between the immunized water-drinking and immunized alcohol-consuming mice (Fig. 1B). αGalCer stimulation of iNKT cells did not have any additional effect on tumor size in the immunized water-drinking mice (Fig. 1C) and abrogated the effect of immunization but not the inhibitory effect of alcohol 20 days after tumor inoculation in the alcohol-consuming mice (Fig. 1D). However, immunization with tumor cell lysate alone or in combination with αGalCer treatment did not significantly alter the final body weight of the water-drinking or alcohol-consuming mice inoculated with melanoma compared to their non-immunized counterparts (Fig. 2A, 2B, 2C). While immunization alone tended to decrease final tumor weight in the water-drinking group, although it was not statistically significant (Fig. 2D). There were no responders in the combined lysate + αGalCer group. However there was a tendency for the mean tumor weight to be higher in the treated alcohol-consuming mice (Fig. 2D).
The tumor weight to body weight ratios among the treatment and control groups were not different (Fig. 2E). Also, no differences were found in the degree of decrease in body weight and tumor weight between the two treatment groups of water-drinking mice and alcohol-consuming mice immunized with tumor cell lysate alone or combined with αGalCer activation (Fig. 2F). These results indicated that alcohol consumption leads to accelerated tumor growth in mice treated with the tumor cell lysate and αGalCer during the latter stages of tumor progression.
3.3. Chronic alcohol consumption does not affect the survival of melanoma-bearing mice. Immunization with tumor cell lysate increases the survival of alcohol-consuming tumor-bearing mice but not the respective water-drinking mice compared to their non-immunized counterparts
Chronic alcohol consumption activates the immune system and inhibits tumor growth. These effects should favor increased survival; however, this did not occur (Fig. 3 A). While immunization with tumor cell lysate did not significantly increase the survival of water-drinking, tumor-bearing mice (Fig. 3B), it significantly increased the survival of alcohol-consuming, tumor-bearing mice (Fig. 3C) compared to respective non-immunized tumor-bearing mice. The median survival of immunized water-drinking tumor-bearing mice and immunized alcohol-consuming tumor-bearing mice was similar (Fig. 3D).
Fig. 3.
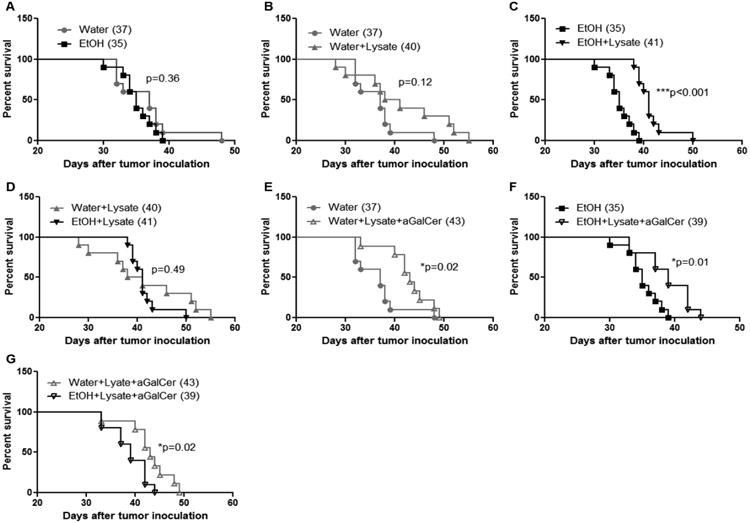
Effects of chronic alcohol consumption on the survival of non-immunized mice, mice immunized with melanoma lysate, and mice pretreated with αGalCer prior to immunization. A. Kaplan-Meier plot indicating survival of non-immunized water-drinking mice and alcohol-consuming mice. B. Kaplan-Meier plot indicating survival of non-immunized water- and immunized water-drinking mice. C. Kaplan-Meier plot indicating survival of non-immunized and immunized alcohol-consuming mice. D. Kaplan-Meier plot indicating survival of immunized water-drinking mice and immunized alcohol-consuming mice. E. Kaplan-Meier plot indicating survival of non-immunized water-drinking mice and immunized water-drinking mice stimulated by αGalCer. F. Kaplan-Meier plot indicating survival of non-immunized and immunized alcohol-consuming mice also stimulated with αGalCer. G. Kaplan-Meier plot comparing survival of water-drinking and alcohol-consuming mice stimulated with αGalCer and immunized with melanoma lysate. Each group contained 10 mice. Numbers in the parenthesis are median survival time in days. P values are from the Log-rank (Mantel-Cox) test. Water =water-drinking mice; EtOH=alcohol-drinking mice; Water+Lysate=water-drinking mice immunized with melanoma cell lysate; EtOH+Lysate=alcohol-consuming mice immunized with melanoma cell lysate; Water+Lysis+αGalCer= water-drinking mice immunized with melanoma cell lysate and stimulated with αGalCer. EtOH+Lysis+αGalCer= alcohol-consuming mice immunized with melanoma cell lysate and stimulated with αGalCer.
3.4. Immunization and αGalCer stimulation significantly increases the survival of water-drinking mice and alcohol-consuming mice compared to their non-immunized counterparts; however, αGalCer stimulation significantly decreases the survival of the immunized alcohol-consuming mice compared to their water-drinking counterparts
It is well documented that iNKT cells have antitumor effects [37], and agonists activating these cells also have been used as immune adjuvants to boost antitumor immunity [38]. We found that alcohol consumption increases IFN-γ-producing iNKT cells in non-tumor injected mice [28] [29]. Herein, we used αGalCer to activate iNKT cells as an adjuvant to boost the antitumor immune response to the melanoma cell lysate. We found that the combined treatment significantly increased survival compared to the non-immunized tumor-bearing, water-drinking (Fig. 3E) and alcohol-consuming mice (Fig. 3F). While αGalCer had a modest effect on increasing survival compared to the lysate-injected water-drinking mice (+3 days), survival was reduced (-2 days) in the comparable alcohol-consuming mice (Compare Fig. 3D with Fig. 3G). This finding along with the fact that the combined treatment did not have an effect on tumor growth in the alcohol consuming mice (Fig.1D) indicates that chronic alcohol consumption hinders the combined immunotherapeutic effect by interfering with the immuno-adjuvant effects of iNKT cells.
3.5. Effects of chronic alcohol consumption and immunization on melanoma metastasis in the lung and LN
Chronic alcohol consumption inhibited tumor growth, but it also enhanced body weight loss and did not increase survival. One possibility was that the enhanced body weight loss and decreased survival result from increased metastasis. To examine this hypothesis we determined the metastasis to the lung, draining inguinal and axillary LN, and control inguinal and axillary LN. Lung and LN are the most common sites of melanoma metastasis. We found that chronic alcohol consumption did not increase metastasis to the lung and LN (Fig. 4). Tumor cell lysate immunization alone did not affect melanoma metastasis to lung and LN in either water-drinking or alcohol-consuming groups (Fig. 4). Cell lysate immunization combined with αGalCer stimulation significantly enhanced the severity of melanoma metastasis to the control axillary LN in water-drinking mice compared to all other groups of mice (Fig. 4E). the combined immunization also significantly enhanced lung metastasis in the water-drinking group than in cell lysate alone immunized alcohol-consuming group or in the combination immunized alcohol-consuming group (Fig. 4A), and significantly increased draining axillary LN metastasis in the water-drinking group compared to the combination immunized alcohol-consuming group (Fig. 4C).
Fig. 4.
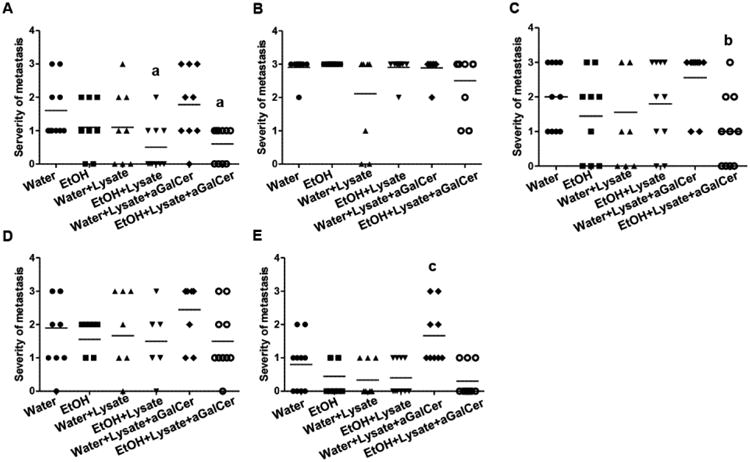
Severity of metastasis to the lung, draining and control LN at necropsy. A. Severity of metastasis to the lung of the indicated groups of mice. B. Severity of metastasis to the draining inguinal LN of the indicated groups of mice. C. Severity of metastasis to the draining axillary LN of the indicated groups of mice. D. Severity of metastasis to the control inguinal LN of the indicated groups of mice. E. Severity of metastasis to the control axillary LN of the indicated groups of mice. One way ANOVA with Tukey's multiple comparison test was used to determine the differences among the experimental groups. Each group contained 6-10 mice. a, different from water-drinking tumor-bearing mice immunized with cell lysate and stimulated with αGalCer, p<0.05. b, different from water-drinking tumor-bearing mice immunized with cell lysate and stimulated with αGalCer, p<0.05. c, different from water-drinking tumor-bearing mice (p<0.05), alcohol-consuming tumor-bearing (p<0.01), water-drinking tumor-bearing mice immunized with cell lysate alone (p<0.001), alcohol-consuming tumor-bearing mice immunized with cell lysate alone (p<0.001) and alcohol-consuming tumor-bearing mice immunized with cell lysate and stimulated with αGalCer (p<0.001).
3.6. Chronic alcohol consumption increases iNKT cells in the thymus and blood of melanoma-bearing mice
Alcohol consumption increases IFN-γ producing iNKT cells in non-tumor injected mice, and this should favor antitumor immune responses. However, the experiments above suggest that alcohol interferes with the antitumor immune response associated with iNKT cells in the melanoma-bearing mice. To examine this we quantified the percentage and numbers of iNKT cells in the thymus and blood of the melanoma-bearing mice. We found that chronic alcohol consumption increased the percentage and number of iNKT cells in the thymus but not in the blood of non-tumor mice. iNKT cells still elevated in the thymus of tumor-bearing, alcohol-consuming mice compared to their water-drinking counterparts (Fig. 5A and 5B). Both the percentage and number of NKT cells in the blood of tumor-bearing mice, however, were increased dramatically in the alcohol-consuming mice (Fig. 5C, 5D). Most of these NKT cells were CD4+ (Fig. 5C, 5D).
Fig. 5.
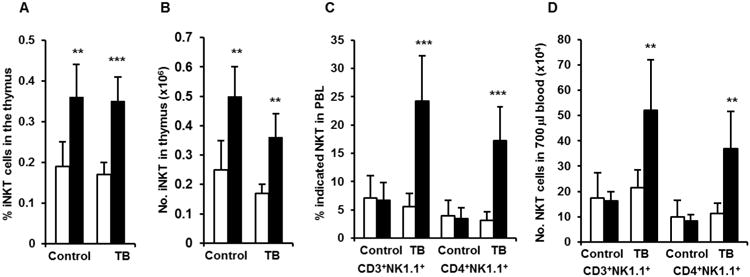
Effects of chronic alcohol consumption on NKT cells in the thymus and blood of non-tumor injected mice and tumor-bearing mice after 2 weeks of tumor inoculation. A. (Percentage) and B. (Number) of iNKT cells in the thymus of non-tumor injected mice (Control) and tumor-bearing mice (TB). C (Percentage) and D. (Number) of CD3+NK1.1+ NKT cells and CD4+NK1.1+ NKT cells in the blood of non-tumor injected mice (Control) and tumor-bearing mice (TB). Values are mean± SD for groups containing 8-10 mice. Each experiment was repeated at least twice with similar results. Open bars are water- drinking mice and black bars are alcohol-consuming mice. Control=mice not injected with melanoma. TB=tumor-bearing mice injected with melanoma. Two-tailed Student's t-test was used to analyze the difference between groups. **Different from water-drinking mice within group, p<0.01; ***Different from water-drinking mice within group, p<0.001.
3.7. Chronic alcohol consumption decreases NK1.1-iNKT cells, and increases NKG2A+ iNKTcells in melanoma-bearing mice
NKT cells originate and develop in the thymus, and they continue to mature further in the peripheral lymphoid and non-lymphoid organs. One of the important features of NKT cell maturation is the acquisition of the NK1.1 cell marker. (i.e. immature NKT cells are NK1.1- and mature NK cells are NK1.1+) [30]. NKT cell maturation requires the activation of T cell receptor by its specific lipid ligand. We previously found that chronic alcohol consumption increases NK1.1+ iNKT cells in non-tumor bearing mice [28]. It is interesting to know if this effect persists in the melanoma-bearing mice. We found that alcohol consumption decreased the percentage of NK1.1- iNKT cells in the spleen and PBL of melanoma-bearing mice (Fig. 6A, 6C). These results suggest that alcohol consumption enhances iNKT cell maturation even in tumor-bearing mice. Chronic alcohol consumption increases inhibitory receptor NKG2A+ iNKT cells in non-tumor bearing mice [28]. We found that the percentage of inhibitory receptor NKG2A+ NKT cells also increased significantly in the PBL, spleen, and thymus of melanoma-bearing, alcohol-consuming mice (Fig. 6B, 6D).
Fig. 6.
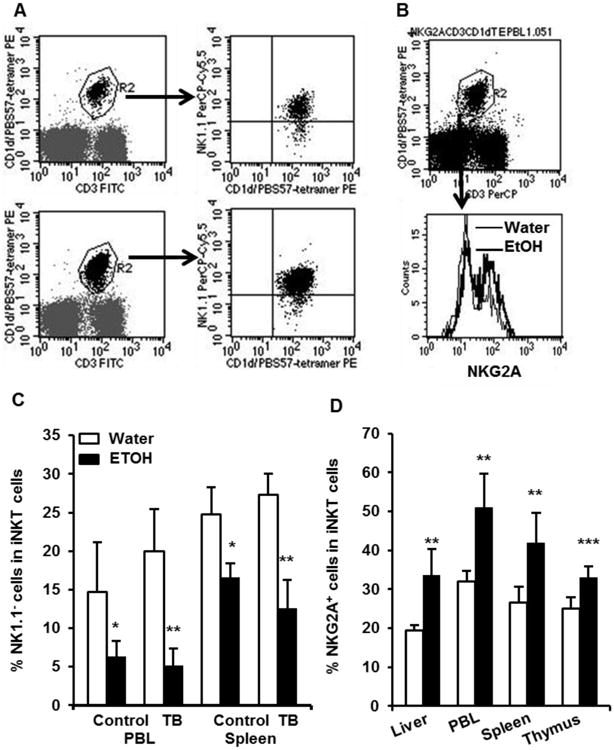
Effects of chronic alcohol consumption on the expression of NKG2A and NK1.1 in the iNKT cells of melanoma-bearing mice. A. Dot plots showing NK1.1- iNKT cells (right lower quadrant in the right panel) in the gated iNKT cells (CD3+/PBS57-tetramer+, R2 region in the left panel). The upper panel is from the PBL of water-drinking, tumor-bearing mice. The lower panel is from the PBL of alcohol-consuming, tumor-bearing mice. B. Dot plot showing gated iNKT cells (upper panel, R2) and histogram showing NKG2A+ cells in gated iNKT cells. C. Percentage of NK1.1- iNKT cells in the total iNKT cells in the PBL and spleen of non-tumor injected mice (Control) and tumor-bearing mice (TB). D. Percentage of NKG2A+ iNKT cells in the total iNKT cells in the indicated tissue and organs. Values are mean ± SD for groups containing 6-10 mice. Each experiment was at least repeated twice with similar results. Two tailed Student's t-test was used to analyze the difference between groups. **Different from water- drinking mice within group, p<0.01; ***Different from water-drinking mice within group, p<0.001. Water=water-drinking mice. EtOH=alcohol-consuming mice.
3.8. Chronic alcohol consumption induces an IL-4 dominant cytokine profile in melanoma-bearing mice
We previously found that alcohol consumption increases IFN-γ-producing NKT cells in non-tumor injected mice [29] and in vivo activation of NKT cells with αGalCer increases the plasma concentration of IFN-γ, but decreases the plasma concentration of IL-4 [28]. It is also reported that the cytokine profile of NKT cells skews from IFN-γ dominant toward IL-4 dominant in advanced prostate cancer patients. The skewing of NKT cell cytokine profile toward IL-4 is associated with poor prognosis and decreased survival [39]. Herein, we examined if chronic alcohol consumption alters the cytokine profile of iNKT in the melanoma-bearing mice at the late stage of tumor growth. We found that alcohol consumption significantly increased the percentage of IL-4-producing iNKT cells, and decreased the percentage of IFN-γ-producing iNKT cells relative to water-drinking mice after three weeks of tumor cell inoculation (Fig. 7A). The ratio of IL-4-producing to IFN-γ-producing iNKT cells was >2-fold higher in the alcohol-consuming compared to the water-drinking mice (Fig. 7B). Thus, the crosstalk between alcohol and melanoma induces a switching from a Th-1 dominant profile observed in non-tumor injected mice to a Th-2 dominant cytokine profile in tumor-bearing mice.
Fig. 7.
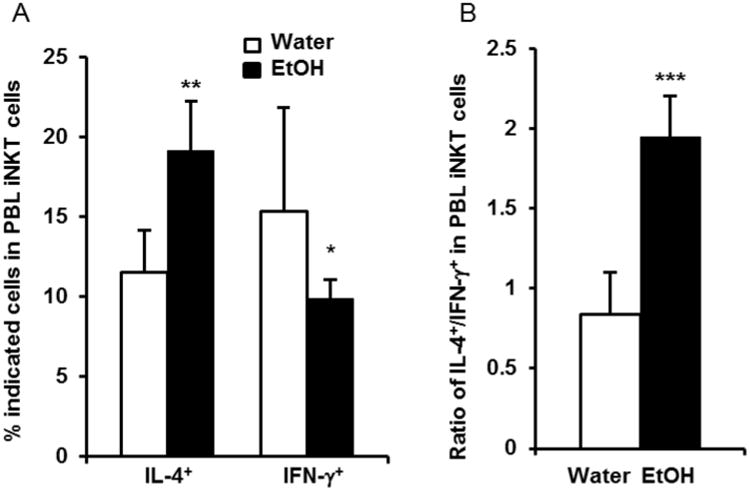
Chronic alcohol consumption increases IL-4-producing iNKT cells in melanoma-bearing mice. PBL from tumor-bearing mice were isolated three weeks after tumor inoculation and stimulated with αGalCer for 4 hr. IL-4 and IFN-γ-producing iNKT cells were analyzed by flow cytometry. A. Percentage of IL-4 and IFN-γ-producing iNKT cells in the blood iNKT cells. B. Ratio IL-4-producing iNKT cells to IFN-γ-producing iNKT cells in the blood of water-drinking mice and alcohol-consuming mice. Each group contained 6-10 mice. Each experiment was repeated twice with similar results. Values are mean± SD. Two tailed Student's t-test was used to analyze the difference between groups. *Different from Water, p<0.05; **Different from Water, p<0.01; ***Different from Water, p<0.001. Water=water-drinking mice. EtOH=alcohol-consuming mice.
4. Discussion
The data in the present study clearly indicate that chronic alcohol consumption inhibits B16BL6 melanoma growth; however, this does not translated into increased host survival. Immunization of the mice with a melanoma cell lysate significantly decreases the tumor growth in both alcohol-consuming and water-drinking mice (Fig. 1C, 1D); however survival is increased only in the alcohol-consuming mice compared to respective non-immunized mice (Fig. 3C) and not the water-drinking mice (Fig. 3B). αGalCer treatment facilitated the response of water-drinking mice to melanoma lysate resulting in increase of survival (Fig 3E). αGalCer treatment was ineffective in enhancing survival, and even decreases survival after immunization in the alcohol-consuming mice. Alcohol interacting in vivo with melanoma during growth significantly increases iNKT cells in the blood, and these cells exhibit an immunoinhibitory Th2 cytokine-dominant profile, which favors tumor progression. These results support the concept that chronic alcohol consumption affects the antitumor immune response in two opposing phases. The first phase associated with immune stimulation promotes antitumor immune responses and decreased tumor growth, and the second phase resulting from the continuous interaction between alcohol and melanoma leads to immune inhibition and augmented tumor progression and decreased survival. The inhibition of melanoma growth as a function of chronic alcohol consumption likely results from the initial activation of antitumor immune responses in mice before they are inoculated with melanoma. Because melanoma-bearing mice consuming alcohol have a lower body weight at necropsy, one could argue that this also is responsible for the decrease observed in tumor growth and the final tumor weight at necropsy relative to mice consuming water. However, it is previously reported that the decrease in body weight in the alcohol consuming mice inoculated with melanoma tumors only is evident during the last few days of life [36]. Moreover, if decreased body weight were the only factor in determining tumor growth, the ratio of body weight decrease to the tumor weight decrease should be similar. The results indicate that the degree of tumor weight decrease is much greater than the degree of body weight decrease in the alcohol-consuming mice compared to the water-drinking mice (Fig. 2F). Thus, additional factors are involved. This supports our contention that the primed immune response in the alcohol-consuming mice initially plays an important role in inhibiting tumor growth and that this is reflected in a slower growth.
Immunization with a melanoma cell lysate will activate the immune system [40]. Immunization with the lysate did not affect final body weight (Fig. 2A), but significantly inhibited tumor growth in both water-drinking mice (Fig. 1C) and alcohol-consuming mice (Fig. 1D). These results further support an important role for the immune system in the control of B16BL6 melanoma growth.
The present study further supports the concept that chronic alcohol consumption accelerates the dysfunction of the immune system in the melanoma-bearing mice such as skewing iNKT cell cytokine profile from Th1-dominant to Th2 dominant as we reported in the present study, and inhibiting tumor-specific CD8+ T cell expansion and accelerating the decay of IFN-γ-producing CD8+ T cells as previously reported [26]. Although we found that alcohol consumption inhibits tumor growth and that immunization with a melanoma lysate further inhibits the growth of tumor in alcohol-consuming mice (Fig. 1D), the effect of immunization is not long lasting since the final tumor weight in the immunized mice is not different from non-immunized mice and tends to be higher (Fig. 2C). A major difference between the alcohol-consuming and water-drinking mice is that immune functions associated with antitumor activity are augmented in the alcohol-consuming mice prior to immunization and tumor implantation.
It is well documented that continuous activation of the immune system induces anergy and immunosenecence [41-43]. Because chronic alcohol consumption itself activates the immune system in mice, this would predispose these mice to accelerated immunosenecence and anergy. Immunization could further accelerate and amplify this process. Additional evidence supporting this is our previous finding that alcohol consumption accelerates the dysfunction of CD8+ T cells within two weeks after melanoma inoculation [26]. These functional changes in the immune system are reflected in the lack of a survival benefit in the melanoma-bearing mice. Alcohol consumption, which activates the immune system and inhibits tumor growth, should favor antitumor immunity and increase survival. However, this is not what we observed (Fig. 3A). One possible explanation would be that at the early stage after tumor inoculation, alcohol consumption favors antitumor immunity; however, the continuous crosstalk between alcohol and melanoma accelerates immunosenescence and favors tumor progression and decreased host survival. Initially the immune system is activated in alcohol-consuming mice and produces Th1 dominant cytokines. Therefore, immunization with tumor cell lysate in the alcohol-consuming mice with a pre-activated immune system will induce a much stronger antitumor immune response compared to the water-drinking mice without a pre-activated immune system. Indeed, immunization did not significantly increase the survival of the water-drinking tumor-bearing mice (Fig. 3B), but significantly increased the survival of alcohol-consuming tumor-bearing mice compared to the non-immunized alcohol-consuming mice (Fig. 3C). Interestingly, boosting the melanoma lysate immunization with αGalCer significantly increased the survival of water-drinking tumor-bearing mice, but decreased the survival of alcohol-consuming tumor-bearing mice compared to their water-drinking counterparts (Fig. 4G). This result suggests that alcohol consumption reverses antitumor function of iNKT cell to one that is immunoinhibitory in the melanoma-bearing mice as further explained below.
Alcohol consumption increases iNKT cells in the thymus of non-tumor injected and melanoma injected mice, and it also increases NK1.1+ mature iNKT cells and NKG2A+ iNKT cells in the blood of melanoma-bearing mice, suggesting that alcohol consumption alone induces a signal to enhance iNKT cell proliferation and maturation. We call this signal as Signal I. The difference between non-tumor injected and melanoma-bearing mice is that alcohol consumption: 1) increases iNKT cells in the blood, and 2) reverses the iNKT cytokine profile from Th1 dominant to Th2 dominant in the tumor-bearing mice. These results suggest that melanoma or the crosstalk between alcohol and melanoma induces another signal to further activate iNKT. We call this signal as Signal II. It is reported that continuous activation induces iNKT cell anergy and that the anergic iNKT cells retain the function to produce IL-4 but are unable to produce IFN-γ [43, 44]. This phenomenon was also found in advanced prostate cancer patients [39]. We hypothesize that in the melanoma-bearing mice Signal I and Signal II continuously stimulate iNKT cells thus inducing iNKT cell anergy and that the Th2-dominant cytokine profile inhibits antitumor immunity and enhances tumor progression. This is supported by the results as discussed above.
It is well known that 80% of advanced cancer patients experience cancer-associated cachexia (CAC), which is responsible for 25% of cancer-related death [45, 46]. CAC is a syndrome of progressive weight loss, which includes the progressive loss of adipose tissue and skeletal muscle. Multiple factors are involved in CAC. Inflammatory cytokines, such as IFN-α and IL-6, and hormones, such as catecholamine are the major factors that trigger this process. Our results indicated that chronic alcohol consumption significantly enhances CAC in melanoma-bearing mice. The enhanced CAC is unlikely associated with primary tumor weight or tumor metastasis, since chronic alcohol consumption decreased tumor weight and did not increase tumor metastasis to the lung or LN (Fig. 4). It is likely that alcohol consumption synergizes with melanoma-derived cachexia signals to enhance CAC. Immunization with tumor cell lysate alone or combined with αGalCer activation did not affect CAC or the effect of alcohol on CAC (Fig. 2A, 2C), but increased survival, suggesting that immune system may play a more important role than CAC in control of survival of the tumor-bearing host.
In summary, we found that chronic alcohol consumption activates the immune system to inhibit melanoma growth. However, the alcohol and tumor interaction accelerates immunosenecence and promotes tumor progression. Activation of the immune system by alcohol consumption favors immunization to enhance antitumor immunity. However, this enhanced antitumor immunity is not long lasting because the pre-activation of the immune system also accelerates immune exhaustion in the immunized mice, which enhance tumor growth at the late stage of tumor progression. Alcohol consumption interacting with melanoma reverses iNKT cell cytokine profile from Th1 to Th2 dominant, which inhibits antitumor immunity and decreases the survival of tumor-bearing mice. Although the survival of the alcohol-consuming tumor-bearing mice is not different from the survival of the water-drinking tumor-bearing mice, the underlying immune response is significantly different. Finding ways to boost and sustain an activated immune system and to inhibit or prevent immune anergy is critical for cancer immunotherapy in alcoholics and an important area for future research.
Highlights.
Chronic alcohol consumption inhibits melanoma growth but does not increase survival.
As tumor growth alcohol revises iNKT cell cytokines from Th1-dominant to Th2-dominant.
Alcohol decreases tumor-host survival induced by immunization and iNKT cell activation.
Acknowledgments
This project was supported by National Institutes of Health grant K05AA017149, R21AA022098 and by funds provided for medical biological research by the State of Washington Initiative Measure No. 171. Mouse CD1d/PBS57-tetramer was provided by NIH tetramer facility (Atlanta, GA).
Footnotes
Conflict of interest statement: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–65. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 2.Boutron MC, Faivre J, Dop MC, Quipourt V, Senesse P. Tobacco, alcohol, and colorectal tumors: a multistep process. Am J Epidemiol. 1995;141:1038–46. [PubMed] [Google Scholar]
- 3.Tanaka K, Tsuji I, Wakai K, Nagata C, Mizoue T, Inoue M, et al. Alcohol drinking and liver cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2008;38:816–38. doi: 10.1093/jjco/hyn108. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Gallicchio L, Boyd-Lindsley K, Tao XG, Robinson KA, Lam TK, et al. Alcohol consumption and the risk of nasopharyngeal carcinoma: a systematic review. Nutr Cancer. 2009;61:1–15. doi: 10.1080/01635580802372633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thrift AP, Nagle CM, Fahey PP, Russell A, Smithers BM, Watson DI, et al. The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer. 2012;131:E759–68. doi: 10.1002/ijc.27420. [DOI] [PubMed] [Google Scholar]
- 6.Le Marchand L, Saltzman BS, Hankin JH, Wilkens LR, Franke AA, Morris SJ, et al. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am J Epidemiol. 2006;164:232–45. doi: 10.1093/aje/kwj115. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DM, Sigurdson A, Doody MM, Rao RS, Linet MS. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control. 2003;14:847–57. doi: 10.1023/b:caco.0000003839.56954.73. [DOI] [PubMed] [Google Scholar]
- 8.Millen AE, Tucker MA, Hartge P, Halpern A, Elder DE, Guerry Dt, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042–51. [PubMed] [Google Scholar]
- 9.Kubo JT, Henderson MT, Desai M, Wactawski-Wende J, Stefanick ML, Tang JY. Alcohol consumption and risk of melanoma and non-melanoma skin cancer in the Women's Health Initiative. Cancer Causes Control. 2013;25:1–10. doi: 10.1007/s10552-013-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 11.Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–5. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 12.Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–8. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 13.Joenje H. Metabolism: alcohol, DNA and disease. Nature. 475:45–6. doi: 10.1038/475045a. [DOI] [PubMed] [Google Scholar]
- 14.Nelson DE, Jarman DW, Rehm J, Greenfield TK, Rey G, Kerr WC, et al. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health. 2013;103:641–8. doi: 10.2105/AJPH.2012.301199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saieva C, Bardazzi G, Masala G, Quartini A, Ceroti M, Iozzi A, et al. General and cancer mortality in a large cohort of Italian alcoholics. Alcohol Clin Exp Res. 2012;36:342–50. doi: 10.1111/j.1530-0277.2011.01626.x. [DOI] [PubMed] [Google Scholar]
- 16.Mayne ST, Cartmel B, Kirsh V, Goodwin WJ., Jr Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev. 2009;18:3368–74. doi: 10.1158/1055-9965.EPI-09-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JB, Fan JH, Liang H, Li J, Xiao HJ, Wei WQ, et al. Attributable causes of esophageal cancer incidence and mortality in China. PLoS One. 2012;7:e42281. doi: 10.1371/journal.pone.0042281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan R, Gabrilovich DI. Novel mechanism of synergistic effects of conventional chemotherapy and immune therapy of cancer. Cancer Immunol Immunother. 2013;62:405–10. doi: 10.1007/s00262-012-1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–32. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook RT, Garvey MJ, Booth BM, Goeken JA, Stewart B, Noel M. Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. JClinImmunol. 1991;11:246–53. doi: 10.1007/BF00918182. [DOI] [PubMed] [Google Scholar]
- 22.Laso FJ, Iglesias-Osma C, Ciudad J, Lopez A, Pastor I, Orfao A. Chronic alcoholism is associated with an imbalanced production of Th-1/Th-2 cytokines by peripheral blood T cells. Alcohol Clin Exp Res. 1999;23:1306–11. [PubMed] [Google Scholar]
- 23.Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Chronic alcohol consumption is associated with changes in the distribution, immunophenotype, and the inflammatory cytokine secretion profile of circulating dendritic cells. Alcohol Clin Exp Res. 2007;31:846–54. doi: 10.1111/j.1530-0277.2007.00377.x. [DOI] [PubMed] [Google Scholar]
- 24.Laso FJ, Iglesias MC, Lopez A, Ciudad J, San Miguel JF, Orfao A. Increased interleukin-12 serum levels in chronic alcoholism. J Hepatol. 1998;28:771–7. doi: 10.1016/s0168-8278(98)80226-2. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78:1070–80. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Meadows GG. Chronic alcohol consumption enhances myeloid-derived suppressor cells in B16BL6 melanoma-bearing mice. Cancer Immunol Immunother. 2010;59:1151–9. doi: 10.1007/s00262-010-0837-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Zhu Z, Meadows GG. Chronic alcohol consumption impairs distribution and compromises circulation of B cells in B16BL6 melanoma-bearing mice. JImmunol. 2012;189:1340–8. doi: 10.4049/jimmunol.1200442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Zhang F, Zhu Z, Luong D, Meadows GG. Chronic alcohol consumption enhances iNKT cell maturation and activation. Toxicol Appl Pharmacol. 2015;282:139–50. doi: 10.1016/j.taap.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Zhu Z, McKinley JM, Meadows GG. IFN-gamma is essential for the inhibition of B16BL6 melanoma lung metastasis in chronic alcohol drinking mice. Clin Exp Metastasis. 2011;28:301–7. doi: 10.1007/s10585-011-9372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–66. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 31.Fujii S, Shimizu K, Okamoto Y, Kunii N, Nakayama T, Motohashi S, et al. NKT cells as an ideal anti-tumor immunotherapeutic. Front Immunol. 2013;4:409. doi: 10.3389/fimmu.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. JImmunol. 2009;182:2816–26. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdallah RM, Starkey JR, Meadows GG. Toxicity of chronic high alcohol intake on mouse natural killer cell activity. Res Commun Chem Pathol Pharmacol. 1988;59:245–58. [PubMed] [Google Scholar]
- 34.Zhang H, Meadows GG. Chronic alcohol consumption perturbs the balance between thymus-derived and bone marrow-derived natural killer cells in the spleen. J Leukoc Biol. 2008;83:41–7. doi: 10.1189/jlb.0707472. [DOI] [PubMed] [Google Scholar]
- 35.Feldman JP, Goldwasser R, Mark S, J S, Orion I. A mathematical model for tumor volume evalution using two-dimension. JAQM. 2009;4:455–62. [Google Scholar]
- 36.Núñez NP, Carter PA, Meadows GG. Alcohol consumption promotes body weight loss in melanoma-bearing mice. Alcohol Clin Exp Res. 2002;26:617–26. doi: 10.1097/00000374-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 38.Hunn MK, Farrand KJ, Broadley KW, Weinkove R, Ferguson P, Miller RJ, et al. Vaccination with irradiated tumor cells pulsed with an adjuvant that stimulates NKT cells is an effective treatment for glioma. Clin Cancer Res. 2013;18:6446–59. doi: 10.1158/1078-0432.CCR-12-0704. [DOI] [PubMed] [Google Scholar]
- 39.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. JImmunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa T, Okada N, Tsujino M, Gao JQ, Hayashi A, Tsutsumi Y, et al. Vaccine efficacy of fusogenic liposomes containing tumor cell-lysate against murine B16BL6 melanoma. Biol Pharm Bull. 2006;29:100–4. doi: 10.1248/bpb.29.100. [DOI] [PubMed] [Google Scholar]
- 41.Crespo J, Sun H, Welling TH, Tian Z, Zou W. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol. 2013;25:214–21. doi: 10.1016/j.coi.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mescher MF, Popescu FE, Gerner M, Hammerbeck CD, Curtsinger JM. Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors. Semin Cancer Biol. 2007;17:299–308. doi: 10.1016/j.semcancer.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, et al. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. JImmunol. 2008;181:6707–10. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 45.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89:381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]


