Abstract
Objective
We sought to validate the clinicopathologic implications and prognostic significance of ATR (ataxia telangiectasia mutated and Rad3-related) mutation in patients with endometrioid endometrial cancer and defective DNA mismatch repair enrolled in a cooperative group molecular staging study of endometrial cancer
Methods
After pathology review, only endometrioid tumors with high neoplastic cellularity (≥70%) and high quality DNA for molecular analyses were included. MSI (microsatellite instability) typing was performed and the target sequence in exon 10 of ATR was evaluated by direct sequencing in all MSI-high tumors. Associations between ATR mutations and clinicopathologic variables were assessed using contingency table tests. Differences in overall survival (OS) and disease-free survival (DFS) were evaluated by univariate analyses and multivariable Cox proportional hazard models.
Results
A total of 475 eligible cases were identified. Of 368 MSI+ cases, the sequence of interest could be successfully genotyped in 357 cases. ATR mutations were exclusively identified in 46 tumors with high level microsatellite instability (MSI+) (12.9%, p<0.001) and were associated with higher tumor grade (p=0.001). ATR mutations were not associated with OS (HR 1.16; 95% CI, 0.58–2.32; p=0.68) or DFS (HR 0.61; 95%CI, 0.25–1.50; p=0.28).
Conclusion
Truncating mutations in exon 10 of ATR occur exclusively in tumors with evidence of defective DNA mismatch repair. We were not able to confirm the prognostic value of these mutations in patients with endometrioid endometrial cancer.
Keywords: ATR, Mutation, Endometrial, Cancer, Survival
INTRODUCTION
Endometrial cancer is the fourth most common malignancy affecting American women. The incidence and mortality associated with this disease have increased over the last decade [1]. Despite an initially anticipated good prognosis, some patients will present with advanced stages or experience disease recurrence or progression. Several clinicopathologic models have been proposed to identify patients at risk of recurrence and death from endometrial cancer. These strategies have the ultimate objective of identifying individuals who would most benefit from postoperative therapeutic interventions.
The clinical utility of various clinical, surgical and pathologic risk assessment models for patients with endometrial cancer remains sub-optimal. Therefore, attention is being aimed at identifying molecular signatures that could predict clinical outcomes and potentially guide the development of targeted therapies. Multiple molecular alterations have been described in the histogenesis and progression of endometrial cancer. Traditionally, PTEN loss, defects in DNA mismatch repair as well as mutations in KRAS2, CTNNB1, RB and TP53 appeared characteristic in endometrioid tumors. Recent work has demonstrated that beyond histologic types, it is possible to categorize endometrial cancers into four categories: POLE ultramutated (POLE codes for the central catalytic subunit of DNA polymerase epsilon), copy-number low, copy-number high and interestingly a group of microsatellite instability hypermutated tumors [2,3].
An estimated 10–30% of endometrial cancers exhibit microsatellite instability (MSI), a quantifiable phenotype of tumors with deficient DNA mismatch repair [3–8]. It has been proposed that tumors with defective DNA mismatch repair accumulate deleterious mutations. ATR is a serine/threonine-specific protein kinase that is involved in sensing DNA damage and activating the DNA damage checkpoint, leading to cell cycle arrest. Somatic mutations have been identified in exon 10 of ATR in endometrioid endometrial tumors with DNA mismatch repair defects [9–10]. These insertion/deletion variants involve the A10 mononucleotide of exon 10 of ATR and result in early stop codons. The truncate ATR product has been shown to provide a survival advantage to cancer cells. These mutations in exon 10 of ATR are independent prognostic markers of disease-free and overall survival among patients with endometrioid endometrial cancer [11,12].
We sought to validate the clinicopathologic implications and prognostic significance of ATR mutation in patients with endometrioid endometrial cancer and defective DNA mismatch repair enrolled in a cooperative group molecular staging study of endometrial cancer.
METHODS
Study Participants and Clinical Data
The objectives and specifics of Gynecologic Oncology Group’s GOG0210: A Molecular Staging Study of Endometrial Carcinoma (NCT00340808) have been previously reported [13,14]. Briefly, women undergoing surgical staging of newly diagnosed endometrial cancer were enrolled in this protocol. The study was approved by the institutional review boards of all participating institutions and all patients consented for participation. Clinical data, tumor and biospecimens (e.g. blood and urine) for biomarker research were collected at the time of surgery. Eligibility, clinical reports and pathology was centrally reviewed by NRG/GOG for each case. A total 3,838 subjects were enrolled between September 2003 and September 2007 (when enrollment criteria were restricted). Of those, 2,715 evaluable cases had endometrioid endometrial tumors and were evaluated by the GOG Tissue Bank for potential inclusion in the present study.
Only cases of histologically confirmed endometrioid endometrial adenocarcinoma with viable tissue and high quality DNA available were included in the present study (N=475). Despite initial assumptions anticipating a high yield among these endometrioid endometrial cases, upon tissue bank pathology review for the present and other GOG-210 related studies, it was noted that unfortunately less than 60% of endometrioid endometrial adenocarcinomas had high neoplastic cellularity (≥70%) tissue available for molecular analyses.
Tissue processing, MSI Typing and ATR Genotyping
DNA was extracted in a semi-automated fashion using a Maxwell 16 nucleic acid purification system (Promega Corporation; Madison, WI). DNA concentration and purity was measured by nanodrop spectrophotometry.
MSI typing was performed as previously described using five National Cancer Institute consensus microsatellite markers (BAT25, BAT26, D2S123, D5S346 and D17S250) [15]. Multiplex analysis of PCR products generated using fluorescent primers relied on ABI 3130 genetic analyzer and GeneMapper software v4.0 (Applied Biosystems Inc; Foster City, CA). Standard definitions for MSI were applied as previously described [12,15]. Cases were designated as MSI-high (MSI+) if novel PCR bands were present in at least two of the five consensus panel markers. Cases were designated as MSI-low if a novel PCR band was identified in at least one of the five consensus panel markers and as microsatellite stable if there was no evidence of MSI in any of the five markers.
DNA aliquots of all tumors with evidence of MSI underwent ATR mutation analysis. The A10 mononucleotide repeat of exon 10 of ATR (#ENSG00000175054) was amplified by PCR (433-bp amplicon) using the Deep VentR high-fidelity DNA polymerase (New England Biolab, Ipswich, MA) with the following primers: 5′-CACGGCATGTTTTATCTGACA-3′ (forward) and 5′-TCAGGTATGCCCCATTTAGG-3′ (reverse) [Tm = 63 ºC]. Amplification products were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA) and sequenced unidirectionally with the ABI Prism BigDye Terminator chemistry version 1.1 (Applied Biosystems, Foster City, CA). Sequencing was carried out at the Washington University School of Medicine’s Protein and Nucleic Acid Chemistry Laboratory.
All ATR sequences were analyzed using Sequencher DNA analysis software (v4.9; Ann Arbor, MI) and putative insertion/deletion variants involving the A10 mononucleotide of interest in exon 10 of ATR were visually inspected to confirm the presence of mutations. Ambiguous sequence reads were resolved by repeat sequencing reaction and/or repeat PCR to generate sequencing template. Non-informative specimens were analyzed at least three times.
Statistical Analyses
The primary objective of this study was to validate the clinicopathologic associations and prognostic significance of ATR mutation in endometrioid endometrial cancer cases with defective DNA mismatch repair.
The relationship between ATR mutation status and covariates was assessed using Chi-square test, Fisher’s exact test or Student’s t-test as appropriate. Overall survival (OS) was defined as the time (in months) from study enrollment to death due to any cause. Disease-free survival (DFS) was defined as the time from date of enrollment to date of recurrence, progression or death due to disease. Survivors were censored at the date of last contact. Relative to DFS, patients who did not die of disease were censored at the date of death. The Kaplan-Meier product limit method was used to estimate OS and DFS. Differences in OS and DFS by ATR mutation status were evaluated using the log-rank test. Univariate and multivariate Cox proportional hazard regression models were fitted to assess the effects of known covariates and ATR mutation status on OS and DFS. Covariates that were significant on univariate analysis (p-value < 0.2) were included in the corresponding multivariate model after adjusting for known prognostic factors. All analyses were two-sided and significance was set at a p-value of 0.05. Statistical analyses were performed using either SAS (Cary, NC) versions 9.2 or R.
Previous studies proposed ATR mutation to be an independent prognostic variable for both OS (Hazard Ratio [HR], 3.88; 95% confidence interval [CI], 1.64 to 9.18; p = 0.002) and DFS (HR, 4.29; 95% CI, 1.48 to 12.45; p = 0.007) [12]. Assuming proportional hazard rates and previously observed differences attributable to ATR mutations and recurrence rates, we anticipated that 424 MSI+ cases with an ATR mutation rate of 8.7% (37 ATR mutated cases) would result in minimal detectable hazard ratios of 2.45 with 80% power and significance set at 0.05 (two-sided).
RESULTS
A total of 475 endometrioid endometrial adenocarcinomas with high quality DNA were included in the present study. Patient demographic and clinicopathologic characteristics for those patients are presented in Table 1.
Table 1.
Patient demographic and clinicopathologic characteristics
| Characteristic | No. of Patients (n=475) | % |
|---|---|---|
| Age (years) | ||
| < 40 | 6 | 1.3 |
| 40 – 49 | 35 | 7.4 |
| 50 – 59 | 143 | 30.1 |
| 60 – 69 | 162 | 34.1 |
| 70 – 79 | 97 | 20.4 |
| ≥ 80 | 32 | 6.7 |
| BMI (kg/m2) | ||
| <18.5 | 2 | 0.4 |
| 18.5 – 24.9 | 77 | 16.2 |
| 25.0 – 29.9 | 95 | 20.0 |
| 30.0 – 34.9 | 112 | 23.6 |
| ≥ 35.0 | 188 | 39.6 |
| Not specified | 1 | 0.2 |
| Ethnicity | ||
| Hispanic | 7 | 1.5 |
| Non-Hispanic | 396 | 83.4 |
| Unknown/Not specified | 72 | 15.2 |
| Race | ||
| African American | 27 | 5.7 |
| Caucasian | 434 | 91.4 |
| Other | 9 | 1.9 |
| Unknown/Not specified | 5 | 1.1 |
| Stage (FIGO 1988) | ||
| I | 322 | 67.8 |
| II | 48 | 10.1 |
| III | 92 | 19.4 |
| IV | 13 | 2.7 |
| Tumor grade | ||
| 1 | 168 | 35.4 |
| 2 | 205 | 43.2 |
| 3 | 102 | 21.5 |
| LVSI | ||
| No | 319 | 67.2 |
| Yes | 146 | 30.7 |
| Not reported | 10 | 2.1 |
| Myometrial invasion | ||
| None | 61 | 12.8 |
| Inner half | 261 | 55.0 |
| Outer half | 120 | 25.3 |
| Serosa | 15 | 3.2 |
| Not reported | 18 | 3.8 |
| Adjuvant treatmenta | ||
| No | 250 | 52.6 |
| Yes | 225 | 47.4 |
| Disease status | ||
| No evidence of disease | 395 | 83.2 |
| Recurred/progressed | 80 | 16.8 |
| Causes of death | ||
| Alive | 391 | 82.3 |
| Treatment | 2 | 0.4 |
| Disease | 45 | 9.5 |
| Other | 23 | 4.8 |
| Unknown | 14 | 3.0 |
Chemotherapy (n=48), radiation (118), radiation plus chemotherapy (56), and other (3).
A high rate of MSI-high prevalence (N=368, 77.5%) was noted. Of the remaining 107 cases, 30 (6.3%) were characterized as MSI low and 77 (16.2%) as microsatellite stable. Random examples of microsatellite stable and all MSI-low cases underwent ATR genotyping. As expected, we found no evidence of ATR mutations among MSI stable and MSI-low cases. Of 368 MSI+ cases, the sequence of interest could be successfully genotyped in 357 cases. ATR mutations were exclusively identified in 46 MSI+ tumors (12.9%, p<0.001). Figure 1 demonstrates representative examples of ATR genotypes. ATR mutations were associated with higher tumor grade (FIGO grade 2–3) and were observed in 2.5% and 13.9% of low (FIGO grade 1) and high grade (FIGO grade 2–3) tumors respectively (p=0.001). ATR mutation was not associated with recurrence (p=0.36).
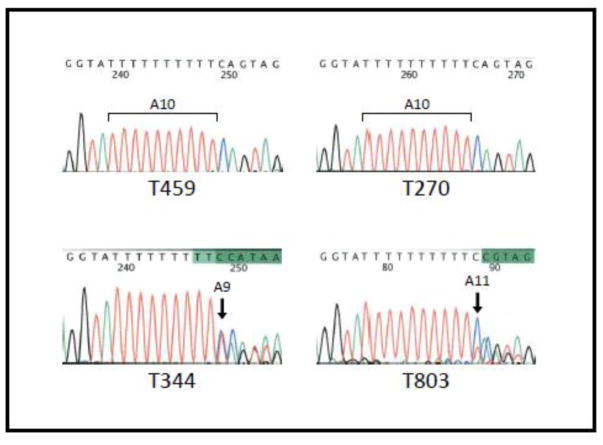
Figure 1.
Representative examples of ATR genotypes (A10 mononucleotide repeat of exon 10) in the study cohort. Tumors T459 (MSI-low) and T270 (MSI+) demonstrate wild type A10 mononucleotide repeat. Tumors T344 and T803 are MSI+ and demonstrate deletion (A9) and insertion (A10) respectively. Arrows indicate site of insertion/deletion. A: adenine, C: cytosine, T: thymine and G: guanine.
Table 2 illustrates demographic and clinicopathologic characteristics of the study cohort stratified by ATR mutation status.
Table 2.
Patient demographic and clinicopathologic characteristics stratified by ATR mutation status a
| Characteristic | ATR Mutation | Total | p-value b | |
|---|---|---|---|---|
| Absent | Present | |||
| Ethnicity | ||||
| Hispanic | 6 | 1 | 7 | 0.423 |
| Non-Hispanic | 351 | 36 | 387 | |
| Unknown/Not specified | 61 | 9 | 70 | |
| Race | ||||
| African American | 25 | 1 | 26 | 0.412 |
| Caucasian | 380 | 44 | 424 | |
| Other | 9 | 0 | 9 | |
| Unknown/Not specified | 4 | 1 | 5 | |
| Stage (FIGO 1988) | ||||
| I | 280 | 33 | 313 | 0.955 |
| II | 43 | 4 | 47 | |
| III | 83 | 8 | 91 | |
| IV | 12 | 1 | 13 | |
| Tumor grade | ||||
| Low grade (grade 1) | 158 | 4 | 162 | 0.001 |
| High grade (grade 2 or 3) | 260 | 42 | 302 | |
| LVSI | ||||
| No | 283 | 28 | 311 | 0.557 |
| Yes | 126 | 17 | 143 | |
| Not reported | 9 | 1 | 10 | |
| Myometrial invasion | ||||
| None | 57 | 3 | 60 | 0.396 |
| Inner half | 228 | 28 | 256 | |
| Outer half | 104 | 11 | 115 | |
| Serosa | 12 | 3 | 15 | |
| Not reported | 17 | 1 | 18 | |
| Adjuvant treatment | ||||
| No | 221 | 23 | 244 | 0.757 |
| Yes | 197 | 23 | 220 | |
N=464 (11 MSI-high cases without ATR data were excluded)
Fisher’s Exact Test
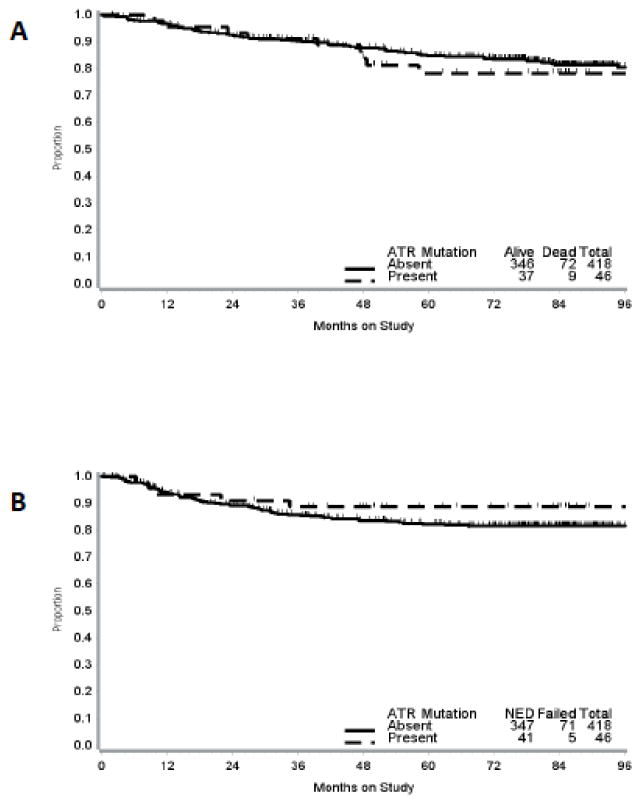
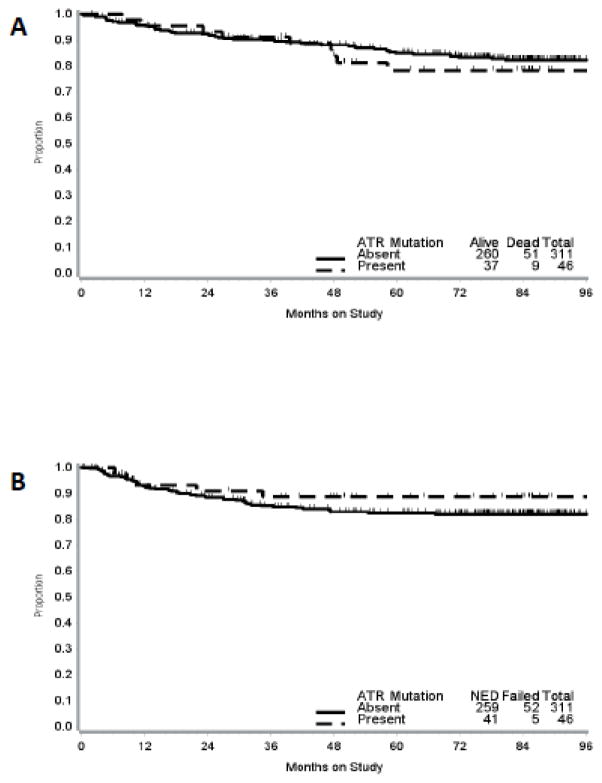
The median follow-up time for the study cohort was 79 months (range, 0.2 – 122.5 months). Kaplan-Meier estimates for OS and DFS stratified by ATR mutation status and MSI status are presented in Figures 2 and 3.
Figure 2.
Kaplan-Meier curves for overall survival (A) and disease-free survival (B) by ATR mutation status. Vertical bars represent censored cases.
Figure 3.
Kaplan-Meier curves for overall survival (A) and disease-free survival (B) by ATR mutation status among patients with MSI+ tumors. Vertical bars represent censored cases.
On univariate analysis (Table 3) mutations in ATR were not associated with OS (HR, 1.16; 95% CI, 0.58 to 2.32; p = 0.68). Conversely, older age (HR, 1.03; 95% CI, 1.01 to 1.05; p = 0.006), FIGO grade 3 (HR, 2.77; 95% CI, 1.58 to 4.84; p < 0.001), advanced stage (III/IV) (HR, 2.11; 95% CI, 1.35 to 3.29; p = 0.001), lymphovascular space invasion (LVSI) (HR, 2.46; 95% CI, 1.59 to 3.81; p < 0.001), outer half myometrial invasion (HR, 3.17; 95% CI, 1.33 to 7.54; p = 0.009) and serosal invasion (HR, 8.65; 95% CI, 3.08 to 24.33; p < 0.001) were all associated with worse OS. BMI, race, MSI status and use of adjuvant treatment were not associated with OS. Variables that approached significance (p<0.2) were incorporated in the multivariate model (Table 4). After controlling for confounding factors, the effects of age (HR, 1.03; 95% CI, 1.01 to 1.05; p = 0.01), high FIGO grade (HR, 2.01; 95% CI, 1.21 to 3.32; p = 0.007) and deep myometrial invasion (HR, 2.67; 95% CI, 1.54 to 4.62; p < 0.001) on OS remained statistically significant.
Table 3.
Univariate analyses
| Characteristic | Overall Survival | Disease-free Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | 1.030 | 1.008 – 1.051 | 0.006 | 1.030 | 1.009 – 1.052 | 0.005 |
| BMI (kg/m2) | 1.009 | 0.985 – 1.033 | 0.482 | 0.991 | 0.966 – 1.017 | 0.507 |
| Race | ||||||
| Caucasian | 1.0 | - | - | 1.0 | - | - |
| African American | 1.454 | 0.633 – 3.340 | 0.378 | 0.980 | 0.358 – 2.684 | 0.969 |
| MSI | ||||||
| Negative | 1.0 | - | - | 1.0 | - | - |
| Positive | 0.783 | 0.460 – 1.334 | 0.368 | 0.874 | 0.498 – 1.532 | 0.638 |
| ATR mutation | ||||||
| Absent | 1.0 | - | - | 1.0 | - | - |
| Present | 1.159 | 0.579 – 2.318 | 0.677 | 0.605 | 0.245 – 1.498 | 0.278 |
| Tumor grade | ||||||
| 1 | 1.0 | - | - | 1.0 | - | - |
| 2 | 1.281 | 0.733 – 2.240 | 0.385 | 1.302 | 0.753 – 2.250 | 0.345 |
| 3 | 2.767 | 1.582 – 4.841 | <0.001 | 2.243 | 1.262 – 3.987 | 0.006 |
| Tumor grade | ||||||
| 1 | 1.0 | - | - | 1.0 | - | - |
| 2 or 3 | 1.750 | 1.059 – 2.892 | 0.029 | 1.597 | 0.970 – 2.627 | 0.066 |
| Stage | ||||||
| IA | 1.0 | - | - | 1.0 | - | - |
| IB/IC | 1.759 | 0.695 – 4.451 | 0.233 | 1.679 | 0.662 – 4.261 | 0.275 |
| II | 1.825 | 0.597 – 5.579 | 0.291 | 1.610 | 0.511 – 5.074 | 0.416 |
| III/IV | 3.476 | 1.349 – 8.960 | 0.010 | 3.360 | 1.301 – 8.680 | 0.012 |
| Stage | ||||||
| Early (stage I or II) | 1.0 | - | - | 1.0 | - | - |
| Late (stage III or IV) | 2.105 | 1.347 – 3.289 | 0.001 | 2.146 | 1.360 – 3.385 | 0.001 |
| LVSI | ||||||
| No | 1.0 | - | - | 1.0 | - | - |
| Yes | 2.464 | 1.593 – 3.810 | <0.001 | 2.020 | 1.302 – 3.135 | 0.002 |
| Myometrial invasion | ||||||
| None | 1.0 | - | - | 1.0 | - | - |
| Inner half | 1.148 | 0.479 – 2.751 | 0.758 | 1.062 | 0.470 – 2.402 | 0.884 |
| Outer half | 3.172 | 1.334 – 7.544 | 0.009 | 2.286 | 1.004 – 5.205 | 0.049 |
| Serosa | 8.654 | 3.079 – 24.326 | <0.001 | 5.804 | 2.035 – 16.555 | 0.001 |
| Adjuvant treatment | ||||||
| No | 1.0 | - | - | 1.0 | - | - |
| Yes | 1.368 | 0.889 – 2.106 | 0.154 | 1.476 | 0.947 – 2.299 | 0.085 |
Table 4.
Multivariate analyses
| Characteristic | Overall Survival | Disease-free Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.028 | 1.006 – 1.051 | 0.013 | 1.034 | 1.012 – 1.057 | 0.003 |
| ATR mutation | 0.921 | 0.422 – 2.010 | 0.836 | 0.513 | 0.187 – 1.407 | 0.195 |
| High tumor grade | 2.007 | 1.212 – 3.324 | 0.007 | 1.517 | 0.900 – 2.557 | 0.118 |
| Advanced surgical stage | 1.598 | 0.887 – 2.881 | 0.119 | 1.633 | 0.908 – 2.937 | 0.102 |
| Adjuvant treatment | 0.613 | 0.339 – 1.111 | 0.107 | 0.818 | 0.463 – 1.445 | 0.489 |
| Deep myometrial invasion | 2.667 | 1.539 – 4.622 | <0.001 | 1.778 | 1.023 – 3.091 | 0.041 |
| LVSI | 1.390 | 0.810 – 2.385 | 0.232 | 1.326 | 0.769–2.288 | 0.310 |
ATR mutations were not associated with DFS (HR, 0.61; 95% CI, 0.25 to 1.50; p = 0.28) on univariate analysis. Older age (HR, 1.03; 95% CI, 1.01 to 1.05; p = 0.005), FIGO grade 3 (HR, 2.24; 95% CI, 1.26 to 3.99; p = 0.006), advanced stage (III/IV) (HR, 2.15; 95% CI, 1.36 to 3.39; p = 0.001), lymphovascular space invasion (LVSI) (HR, 2.02; 95% CI, 1.30 to 3.14; p = 0.002), outer half myometrial invasion (HR, 2.29; 95% CI, 1.004 to 5.21; p = 0.049) and serosal invasion (HR, 5.8; 95% CI, 2.04 to 16.56; p = 0.001) were all associated with worse DFS. BMI, race, MSI status and use of adjuvant treatment were not associated with DFS. After controlling for confounding factors, the effects of age (HR, 1.03; 95% CI, 1.01 to 1.06 p = 0.003) and deep myometrial invasion (HR, 1.78; 95% CI, 1.02 to 3.09; p=< 0.04) on DFS remained statistically significant.
COMMENT
Somatic mutations in the mononucleotide repeat A10 in exon 10 of ATR have been identified in endometrioid endometrial tumors with DNA mismatch repair defects [9,12]. ATR is well recognized for its important role in cellular responses to DNA damage via activation of cell cycle checkpoints. ATR belongs to the PIK subfamily and has been recognized for its participation in cellular responses to DNA damage. ATR activates cell cycle checkpoints Chk1 and Chk2 in response to DNA damage and is structurally similar to ATM and other PIK members [16]. Specifically, ATR phosphorylates p53 at both Ser-15 and Ser-37 in vitro and activates the checkpoint kinase Chk1 resulting in G2 arrest in response to ionizing radiation, topoisomerase inhibitors and cytotoxic methylation events [10,11,17].
The functional significance of truncating mutations in exon 10 of ATR has previously been described. These mutations seem to provide a survival advantage in endometrial cancer cell lines through resistance to ionizing radiation and topoisomerase inhibitors [10]. The clinical relevance of such effect has been previously explored in a cohort of 248 endometrioid endometrial carcinomas to test the hypothesis that heterozygous mutations in exon 10 of ATR would be associated with more aggressive phenotypes and worse clinical outcomes. ATR was mutated in approximately 5% of endometrioid endometrial tumors with DNA mismatch repair defects. Importantly, ATR mutation was an independent prognostic variable for both overall survival (HR, 3.88; 95% CI, 1.64 to 9.18; P = 0.002) and disease-free survival (HR, 4.29; 95% CI, 1.48 to 12.45; P = 0.007). Large hazard ratios were observed specifically for MSI cases (3.52 and 3.01 for OS and DFS respectively), suggesting an apparent aggressive tumor behavior in vivo [12].
In the present study, we attempted to validate those findings and hoped to further characterize the association between ATR mutation status and specific response to adjuvant treatment. GOG-210 represents the largest molecular study of endometrial cancer and as such provided a unique opportunity to accomplish those goals. As expected, we were able to identify ATR mutations only in tumors with evidence of defective DNA mismatch repair. Our sample was enriched for bulky tumors with high neoplastic cellularity (e.g. those identified as adequate for molecular studies after pathology review undoubtedly had more tumor material, less necrosis, etc) and resulted in a selected cohort with a high rate of MSI+ (~78%) with a high rate of ATR mutation (9.9% of all evaluable cases with ATR data and 12.9% of MSI+ cases). Such distribution allowed for adequate power to evaluate the impact of ATR mutation on survival. Unfortunately, we did not find an association between ATR mutation and OS or DFS in this cohort. The discrepancy between our results and previous reported prognostic significance remains elusive. It could be that biologic differences between the tumors analyzed in the former study and those included in this cohort could account for this apparent incongruity. It is also possible that a type-I error accounted for a false positive result in the former study. As expected, well recognized clinicopathologic risk factors such as advanced age, higher stage and FIGO grade, LVSI and deep myometrial invasion were indeed associated with worse survival outcomes.
Current risk stratification models and therapeutic interventions for patients with high risk endometrial cancer are far from optimal. Even in the absence of a definitive prognostic role, further study of ATR in patients with endometrial cancer might be very important. Modulation of ATM and ATR functions is emerging as an attractive adjuvant intervention for cancer radiotherapy and chemotherapy. Furthermore, the intrinsic therapeutic potential of ATR-pathway inhibition is also very promising. ATR or CHEK1 inhibition in ERCC1-deficient cells causes failure to complete cell cycle transitions even after drug removal, suggesting that ATR pathway targeted drugs may offer particular utility in cancers with reduced ATR pathway function by causing synthetic lethality [18–20]. As such, one could foresee therapeutic impact derived from modulation or inhibition of ATR specifically in the subset of endometrial cancer patients with defective DNA mismatch repair.
Highlights.
Exon 10 of ATR represents a mutational hotspot for endometrioid endometrial tumors with defective DNA mismatch repair.
We could not validate the prognostic significance of ATR mutations in endometrioid endometrial tumors.
Acknowledgments
This study was supported by R21 CA155674 (I.Z. and P.J.G.) and Cancer Center Support Grant P30 CA91842 (The Siteman Cancer Center) as well National Cancer Institute grants: NRG Oncology 1 U10 CA180822, Gynecologic Oncology Group (GOG) Administrative Office and the GOG Tissue Bank (CA 27469) and the GOG Statistical and Data Center (CA 37517).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Ohio State University Medical Center, University of Oklahoma Health Sciences Center, Washington University School of Medicine, Women and Infants Hospital, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Case Western Reserve University, University of Minnesota Medical Center – Fairview, Stony Brook University Medical Center, Roswell Park Cancer Institute, University of North Carolina at Chapel Hill, Cooper Hospital University Medical Center, Yale University, Duke University Medical Center, University of Massachusetts Memorial Health Care Western Michigan (Butterworth), Women’s Cancer Center of Nevada, University of Pittsburgh, University of Illinois, University of Iowa Hospitals and Clinics, University of Cincinnati, University of California Medical Center at Irvine – Orange Campus, University of Arkansas Medical Center, Penn State Milton S Hershey Medical Center, University of Virginia, Abington Memorial Hospital, University of Texas Southwestern Medical Center, University of Wisconsin Hospital and Clinics, Northwestern University, University of California at Los Angeles Health System, Indiana University Hospital/Melvin and Bren Simon Cancer Center, University of Chicago, Mayo Clinic, Moffitt Cancer Center and Research Institute, St. Vincent Hospital, Walter Reed National Military Medical Center, Fred Hutchinson Cancer Research Center, University of New Mexico, Fox Chase Cancer Center, The Hospital of Central Conn at New Britain General, Cleveland Clinic Foundation, North Shore University Hospital, Gynecologic Oncology of West Michigan PLLC, University of Alabama at Birmingham, Wake Forest University Health Sciences and Tufts-New England Medical Center.
Footnotes
Conflict of Interest
The authors wish to disclose that there are no conflict of interests to disclose other than Dr. Zighelboim who wishes to acknowledge that he does receive NCI grants as noted in the Acknowledgement section of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Binder PS, Mutch DG. Update on prognostic markers for endometrial cancer. Womens Health. 2014;10:277–88. doi: 10.2217/whe.14.13. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risinger JI, Berchuck A, Kohler MF, Watson P, Lynch HT, Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993;53:5100–5103. [PubMed] [Google Scholar]
- 5.Caduff RF, Johnston CM, Svoboda-Newman, Poy EL, Merajver SD, Frank TS. Clinical and pathological significance of microsatellite instability in sporadic endometrial carcinoma. Am J Pathol. 1996;148:1671–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Gurin CC, Federici MG, Kang L, Boyd J. Causes and consequences of microsatellite instability in endometrial carcinoma. Cancer Res. 1999;59:462–6. [PubMed] [Google Scholar]
- 7.Goodfellow PJ, Buttin BM, Herzog TJ, Rader JS, Gibb RK, Swisher E, Look K, Walls KC, Fan MY, Mutch DG. Prevalence of defective DNA mismatch repair and MSH6 mutation in an unselected series of endometrial cancers. Proc Natl Acad Sci U S A. 2003;100:5908–13. doi: 10.1073/pnas.1030231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zighelboim I, Goodfellow PJ, Gao F, Gibb RK, Powell MA, Rader JS, Mutch DG. Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol. 2007;25:2042–8. doi: 10.1200/JCO.2006.08.2107. [DOI] [PubMed] [Google Scholar]
- 9.Vassileva V, Millar A, Briollais L, Chapman W, Bapat B. Genes involved in DNA repair are mutational targets in endometrial cancers with microsatellite instability. Cancer Res. 2002;62:4095–9. [PubMed] [Google Scholar]
- 10.Lewis KA, Mullany S, Thomas B, Chien J, Loewen R, Shridhar V, Cliby WA. Heterozygous ATR mutations in mismatch repair-deficient cancer cells have functional significance. Cancer Res. 2005;65:7091–5. doi: 10.1158/0008-5472.CAN-05-1019. [DOI] [PubMed] [Google Scholar]
- 11.Cliby WA, Lewis KA, Lilly KK, Kaufmann SH. S phase and G2 arrests induced by topoisomerase I poisons are dependent on ATR kinase function. J Biol Chem. 2002;277:1599–606. doi: 10.1074/jbc.M106287200. [DOI] [PubMed] [Google Scholar]
- 12.Zighelboim I, Schmidt AP, Gao F, Thaker PH, Powell MA, Rader JS, Gibb RK, Mutch DG, Goodfellow PJ. ATR Mutation in Endometrioid Endometrial Cancer is Associated with Poor Clinical Outcomes. J Clin Oncol. 2009;27:3091–6. doi: 10.1200/JCO.2008.19.9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrison C, Miecznikowski J, Darcy KM, Dolce JM, Kandel E, Erwin DO, Liu S, Shepherd L, Cohn D, McMeekin DS, Block AW, Nowak NJ, Maxwell L. A GOG210 ACGH study of gain at 1q23 in endometrioid endometrial cancer in the context of racial disparity and outcome. Genes Chromosomes Cancer. 2010;49:791–802. doi: 10.1002/gcc.20782. [DOI] [PubMed] [Google Scholar]
- 14.Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, Cohn DE, Walker JL, Moore RG, Downs LS, Soslow RA, Zaino R. Etiologic heterogeneity in endometrial cancer: Evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013;129:277–84. doi: 10.1016/j.ygyno.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–57. [PubMed] [Google Scholar]
- 16.Cimprich KA, Shin TB, Keith CT, Schreiber SL. cDNA cloning and gene mapping of a candidate human cell cycle checkpoint protein. Proc Natl Acad Sci U S A. 1996;93:2850–5. doi: 10.1073/pnas.93.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. A role for ATR in the DNA damage induced phosphorylation of p53. Genes Dev. 1999;13:152–7. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohni KN, Kavanaugh GM, Cortez D. ATR pathway inhibition is synthetically lethal in cancer cells with ERCC1 deficiency. Cancer Res. 2014;74:2835–45. doi: 10.1158/0008-5472.CAN-13-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fokas E, Prevo R, Hammond EM, Brunner TB, McKenna WG, Muschel RJ. Targeting ATR in DNA damage response and cancer therapeutics. Cancer Treat Rev. 2014;40:109–17. doi: 10.1016/j.ctrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Sultana R, Abdel-Fatah T, Perry C, Moseley P, Albarakti N, Mohan V, Seedhouse C, Chan S, Madhusudan S. Ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase inhibition is synthetically lethal in XRCC1 deficient ovarian cancer cells. PLoS One. 2013;8:e57098. doi: 10.1371/journal.pone.0057098. [DOI] [PMC free article] [PubMed] [Google Scholar]