Abstract
Objective
New tools, including light emitting diode (LED) fluorescence microscopy and the molecular assay Xpert MTB/RIF® offer increased sensitivity for TB in persons with HIV but come with higher costs. Using operational data from rural Malawi we explored the potential cost-effectiveness of on-demand screening for TB in low-income countries of sub-Saharan Africa.
Design & Methods
Costs were empirically collected in four clinics and one hospital using a micro-costing approach, through direct interview and observation from the national TB program perspective. Using decision analysis newly diagnosed persons with HIV were modeled as being screened by one of three strategies: Xpert, LED or standard of care (i.e., at the discretion of the treating physician).
Results
Cost-effectiveness of TB screening among persons newly diagnosed with HIV was largely determined by two factors: prevalence of active TB among patients newly diagnosed with HIV and volume of testing. In facilities screening at least 50 people with a 6.5% prevalence of TB, or at least 500 people with a 2.5% TB prevalence, screening with Xpert is likely to be cost-effective. At lower prevalence – including that observed in Malawi – LED microscopy may be the preferred strategy, whereas in settings of lower TB prevalence or small numbers of eligible patients, no screening may be reasonable (such that resources can be deployed elsewhere).
Conclusions
TB screening at the point of HIV diagnosis may be cost-effective in low-income countries of sub-Saharan Africa, but only if a relatively large population with high prevalence of TB can be identified for screening.
Keywords: Costs and Benefits, Cost Effectiveness, Diagnostic tests, Tuberculosis, screening, Decision analysis
Introduction
Tuberculosis (TB) is the leading cause of death among people living with HIV and AIDS (PLWHA) worldwide1. Although antiretroviral therapy (ART) has greatly reduced the burden of TB mortality among PLWHA, the first six months after initiating ART remain a period of high risk for TB-associated mortality, likely due to prevalent subclinical TB at the time of ART initiation 2, 3. Intensified case finding (ICF) for TB is increasingly recommended for those newly diagnosed with HIV as a tool to reduce TB mortality 4, 5. Unfortunately, the test most widely used for TB ICF, namely sputum smear microscopy (SSM), has less than 40% sensitivity among PLWHA6, 7. New tools, including light emitting diode (LED) fluorescence microscopy and the molecular assay Xpert MTB/RIF® (“Xpert”, Cepheid, Inc., Sunnyvale, CA, USA) offer increased sensitivity over traditional SSM and can be performed in under 2 hours. However, these diagnostic tests – especially if performed, at the point of care – are much more expensive than standard light microscopy, which is often performed in batches in centralized laboratories. We therefore use cost and operational data from a trial of LED microscopy and Xpert for point-of-treatment TB screening among people newly diagnosed with HIV in rural Malawi as a model to explore the potential cost-effectiveness of on-demand screening for TB in low-income countries of sub-Saharan Africa.
Methods
Data Collection
We collected costs and operational data from a randomized trial of point-of-care screening for TB among people receiving a new HIV diagnosis in rural Malawi. The parent study is an ongoing cluster-randomized trial of Xpert versus LED microscopy for TB screening in 12 rural clinics, with both tests performed by nurses or trained assistants on the day of HIV diagnosis before the patient leaves the clinic. Consenting participants are initially evaluated by asking for any of four symptoms: cough of any duration, weight loss, fevers, and night sweats; those with any symptom are screened with LED microscopy or Xpert. Those diagnosed with TB are started immediately on treatment. Symptomatic patients who tested negative for TB were asked to return in one month, at which time they were screened for symptoms and tested a second time with either Xpert or LED microscopy if still symptomatic.
Here, we use data from a cost and operational analysis performed at four study sites and the district hospital that serves the corresponding region, as a model evaluation for other similar sites in low-income countries of sub-Saharan Africa. These data were collected using a unit-based or “ingredients” approach and included comprehensive budgetary reviews, interviews and logs of study staff (two staff members per clinic), direct observation of procedures, and prospective documentation of start-up costs. Other costs (e.g., TB treatment, ART) were obtained from Malawian notifications and published literature and were varied in sensitivity analysis. For this analysis, we also used study data on the prevalence of TB among eligible individuals. Diagnosis of TB in the study was made using SSM, LED microscopy or Xpert.
We coupled cost and operational data with data from the literature on diagnostic accuracy and likely patient outcomes to populate a decision analytic model of point-of-treatment TB screening among people obtaining a new diagnosis of HIV in different settings of TB prevalence and patient volume. The primary outcome was the incremental cost-effectiveness ratio (ICER), defined as the incremental cost per disability adjusted life year (DALY) averted comparing universal screening for TB (among people receiving a new HIV diagnosis) with Xpert or LED microscopy to a standard of care in which clinical judgment alone is used to refer patients for standard SSM.
Model assumptions
Screening for TB among persons newly diagnosed with HIV is not the current standard of care in many settings. Therefore, in addition to the Xpert and LED scenarios in which we assumed all patients newly diagnosed with HIV and at least one TB symptom would be screened, we also considered a standard of care scenario in which such patients are screened only at the discretion of the treating physician we assumed this was equivalent the probability of a future diagnosis of TB through the routine health system estimated at 66%8. Treatment regimens were indicated based on the diagnostic test result; patients diagnosed by smear, LED microscopy, or Xpert with a negative test for rifampin resistance were put on first-line therapy (two months of isoniazid, rifampin, ethambutol, and pyrazinamide, followed by four months of isoniazid and rifampin). Those diagnosed with rifampin-resistant TB by Xpert would initiate second-line therapy. Patients with false negative Rif resistant results were assumed to start first-line therapy but have lower probability of success while false positive Rif resistant patients started second-line therapy, however the effects of unnecessary treatment are not explicitly modeled, apart from the costs. Patients with underlying multidrug-resistant (MDR) TB treated with first-line therapy were assumed to have worse outcomes. We assumed that 15% of patients testing positive would not complete a sufficient course of therapy to achieve cure9. Patients who were false negatives or lost to follow-up had the opportunity of a future TB diagnosis with sputum smear microscopy through the routine health system 8, 10, 11. Treatment failure and untreated TB were assumed to be universally fatal in this population of HIV-infected individuals.
According to Malawian national guidelines, we assumed that patients with new HIV diagnoses received ART immediately if they had a CD4+ T-cell count ≤350 cells/mm3 or a diagnosis of active TB. Those with CD4+ count >350 cells/mm3 were assumed to delay initiation of ART12. Model parameter values for tree probabilities, effectiveness measures and costs are included in Table 1. ART costs and DALYs averted were calculated over the individuals’ lifetime assuming a life expectancy of 59.2 years for HIV-infected individuals on ART13.
Table 1.
Cohort model inputs including costs, effectiveness measures and tree probabilities
| Model Parameter Inputs | Parameter Value | Range (Min, Max) |
Data Source |
|---|---|---|---|
|
Cost Parameters | |||
| Cost of 1st Line TB Treatment | $185 | (154, 236) | 20,21 |
| Cost of 2nd Line TB Treatment | $1759 | (1353, 2351) | 20,21 |
| Lifetime cost of ART started immediately | $2563 | (0, 4000) | CHAI Treatment costs for HIV (MATCH study) 22 |
| Cost of a symptom screen | $0.20 | (0, 1) | Chepetsa costing study |
| Cost of standard smear (1000 tests/year at peripheral lab) | $4.06 | (1, 10) | Chepetsa costing study |
|
Effectiveness parameters | |||
|
(Including effectiveness of ART) | |||
| DALY – 1st line treatment success | −1.53 | (−2.53, −0.5) | 14,23 |
| DALY – 2nd line treatment success | −1.9878 | (−2.99, −0.99) | 14,23 |
| DALY – Death | −23.8967 | (−27.8967, −29.90) | 9,13,14 |
| DALY – No TB, ART initiation (delayed and immediate) | −1.2710 | (−2.2710, −0.27) | 9,13,14 |
|
Cohort proportions | |||
| Probability of active TB among patients newly diagnosed with HIV in Malawi | .024 | (0.01, 0.06) | Chepetsa, facility report |
| Probability that symptomatic patients would receive smear results without screening | .40 | (0,1) | |
| Probability that CD4+ <350 at time of screening | .60 | (0.5, 0.75) | 24 |
| Probability that missed TB case is later diagnosed with TB outside of screening | 0.66 | (0.61, 0.71) | 8 |
| Probability of loss to follow up during TB treatment | .15 | (0.1, 0.21) | 23 |
| Probability of Rifampicin resistance among patients with TB | .004 | (0.0014, 0.01) | 8 |
| Probability of death among TB patients with HIV given treatment failure, missed diagnosis, or loss to follow-up | 1 | (0.63, 1) | Assumption 25 |
| Probability of treatment success, MDR-TB treated with 1st line drugs | .47 | (0.42, 0.52) | 10 |
| Probability of Treatment success, smear-negative TB treated with 1st line therapy | .8 | (0.72, 0.88) | 8 |
| Probability of Treatment success, smear positive TB treated with 1st line therapy | .87 | (0.78, 0.96) | 8 |
| Probability of treatment success, 2nd line | .80 | (0.7, 0.9) | 11 |
|
Diagnostic Parameters | |||
| Sensitivity of Xpert for RIF resistance | .976 | (0.94, 0.99) | 26,27 |
| Sensitivity of Xpert for LED-negative TB | .718 | (0.29, 0.79) | 26–28 |
| Sensitivity of Xpert for LED-positive TB | .977 | (0.92, 0.99) | 26,27 |
| Sensitivity of LED for TB among Smear negatives | 0.095 | (0.09, 0.2) | 8,25,29 |
| Sensitivity of LED for TB among Smear positives | 1 | (0, 1) | 29 |
| Specificity of GXP | .992 | (0.98, 0.996) | 26,27 |
| Specificity of GXP for RIF resistance | 1 | (0.9, 1) | 26,27 |
| Specificity of LED | 0.944 | (0.92, 0.96) | 30 |
| Sensitivity of standard smear among LED positive people with HIV | .37 | (0.36, 0.7) | 8,25 |
| Specificity of smear among people with HIV | .8 | (0, 1) | |
Abbreviations: TB: active tuberculosis disease, GXP: Gene Xpert, LED: light emitting diode fluorescence microscopy, MDR: multi drug resistant tuberculosis, ART: antiretroviral therap
Economic Methods
Unit costs for diagnosis and treatment included labor costs, material costs and overhead costs. Overhead costs were allocated based on discussions with experienced clinic staff and direct measurements of dimensions. Items such as building space, water utilization, housekeeping, and cleaning supplies were allocated based on proportional space required (approximately 1% of total clinic overhead costs allocated to Xpert or LED annually). Overhead staff costs and mobile airtime costs were allocated based on estimated staff time required to devote to Xpert or LED and were approximately 5% of overall overhead costs in these categories. Direct observations of supervisory staff were estimated based on interview, as actual allocation of study staff in this research setting was not reflective of typical operating scenarios. Staff time in reference to performing the symptom screen, LED and/or Xpert tests and follow-up was directly observed in time-motion studies. Equipment, supervisory costs and start-up costs were calculated for one year, and were allocated based on patient volume. Specific labour and consumable costs were estimated per individual test. Start up costs included recruitment and advertising costs for project and field managers, microscopy or Xpert training, petty cash and postage costs. We assumed these were costs incurred yearly due to high staff turnover, and were allocated based on patient volume. As patient volume increased, start-up costs per test decreased. Costs were measured from the healthcare perspective and inflated to 2010 US dollars (2010 selected as the year because of a devaluation of the Malawian currency in May 2012 during the period of data collection). DALYs were calculated without age weighting using standardized disability weights from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 201014. To calculate the years of life lost (YLL) resulting from a TB death, we took the life expectancy of PLWHA in Malawi with a mean CD4 count at first presentation and the mean age at presentation of 31 years. All costs and DALYs were discounted at 3% per year, with sensitivity analysis for 0–7%15.
Sensitivity & Uncertainty analyses
We performed one-way sensitivity analysis on all model parameters by varying their values across broad yet plausible ranges. Upon finding that two parameters (testing volume and TB prevalence) were key drivers of cost-effectiveness, we conducted a two-way sensitivity analysis to include these parameters, reporting our primary results as a function of these two inputs. A probabilistic uncertainty analysis was performed using 10,000 Monte Carlo simulations of parameter values simultaneously drawn from beta distributions with upper and lower values as shown in Table 1 and a uniform alpha (shape) parameter of four. We report 95% uncertainty ranges as the 2.5th and 97.5th percentiles of those simulations. Scenario analyses were performed to specifically evaluate cost-effectiveness under conditions of high, medium, and low test volume; with and without ART; and across varying levels of symptom-driven diagnosis of TB in the standard of care. The decision analysis was performed using TreeAge Pro Version 2013 (TreeAge Software Inc., Williamstown, MA).
Results
Results under observed conditions
Using primary costing data from the parent trial we calculated the cost per test for LED microscopy and Xpert (including overhead costs, equipment, consumables and salaries). Cost per test was heavily influenced by test volume; therefore, three scenarios were separately considered: low volume of 50 tests/year, observed volume of 100 tests/year, and high volume of 1000 tests/year. Cost per test at observed volume was US$90.5 for Xpert and US$21.4 for LED; under high volume conditions these fell to US$24.8 for Xpert and US$4.05 for LED (Table 2). Volume-driven price reductions primarily reflect equipment costs for microscopes and Xpert systems (i.e., same equipment can be used to run more tests), but lower overhead costs also play an important role. These variations in test cost also strongly influenced initial estimates of cost-effectiveness, as shown in Table 3. Relative to the standard of care, both LED microscopy and Xpert – as performed at low volume – carried an incremental cost-effectiveness of over $1800 per DALY averted, above the per-capita gross domestic product of most low-income countries in sub-Saharan Africa. However, if higher patient volume could be achieved, incremental cost-effectiveness improved to $500–$700 per DALY averted, and Xpert became more cost-effective than LED microscopy.
Table 2.
Unit cost of Xpert and LED by input type and annual test volume
| Input Type | Costs per Test (US$ 2010) | |||||
|---|---|---|---|---|---|---|
| LED | Xpert | |||||
| 50 /yr | 100/yr | 1000/yr | 50/yr | 100/yr | 1000/yr | |
| Overhead* | $21.99 | $10.99 | $1.10 | $22.8 | $11.4 | $1.14 |
| Equipment** | $13.77 | $6.89 | $0.68 | $120.63 | $60.32 | $6.04 |
| Staff | $2.67 | $1.89 | $1.19 | $2.57 | $1.79 | $1.09 |
| Consumables & Reagents *** | $1.02 | $1.02 | $1.02 | $16.44 | $16.44 | $16.44 |
| Training costs | $1.22 | $0.61 | $0.06 | $1.07 | $0.57 | $0.06 |
| Total | $40.7 | $21.4 | $4.05 | $163.5 | $90.5 | $24.8 |
Approximately 75% of overhead costs are supervisory staff costs assuming 1 day/week at clinic to provide monitoring and ongoing training, 18% of overhead costs for Xpert and 14% of overhead costs for LED comprises building space, remaining 7% and 11% includes water, mobile airtime, other overhead staff costs, housekeeping and cleaning supplies.
Approximately 77% of equipment costs are the Xpert machine and computer, 20% include the solar panel. Approximately 87% of LED equipment costs are due to the microscope, and remaining equipment costs are for the battery.
The Xpert cartridge accounts for 58% of Xpert consumables.
Table 3.
Influence of test volume on cost-effectiveness estimates
| Screening with LED | Screening with Xpert | |||||
|---|---|---|---|---|---|---|
| Test Volume (tests/year) | 50 | 100 | 1000 | 50 | 100 | 1000 |
| Observed screening conditions: (prevalence of TB: 2.4%) | ||||||
| •Cost per person screenedincremental to std of care | $116 ($43 to $298) |
$78 (CS to $261) |
$45 (CS to $226) |
$343 ($302 to $516) |
$199 ($123–$371) |
$69 ($16 to $240) |
| •DALYs averted per person screened * | 0.064 (0.05 to 0.075) |
0.122 (0.10 to 0.138) |
||||
| ICER** ($ per DALY averted) | $1808 ($567 to $$6023) |
$1216 (CS to $5313) |
$699 (CS to $4782) |
$2809 ($2191 to $5039) |
$1615 ($898 to $3644) |
$564 ($113 to $2386) |
| ICER** (Reference standard is LED) | $2,205 ($507 to $2379) |
|||||
| Unfavorable to screening: (low prevalence of TB: 1%) | ||||||
| •Cost per person screened incremental to std of care | $112 ($29 to $287) |
$336 ($290 to $501) |
||||
| •DALYs averted per person screened * | 0.027 (0.022 to 0.028) |
0.051 (0.045 to 0.054) |
||||
| •ICER** ($ per DALY averted) | $4190 ($1036 to $12909) |
$6606 ($5398 to $11105) |
||||
| Favorable to screening: (high prevalence of TB: 6%) | ||||||
| •Cost per person screened incremental to std of care | $55 (CS to $229) |
$91 ($31 to $261) |
||||
| •DALYs averted per person screened incremental to std of care | 0.16 (0.095 to 0.226) |
0.305 (0.21 to 0.399) |
||||
| •ICER** ($ per DALY averted) | $347 (CS to $2416) |
$298 ($77 to $1241) |
||||
Uncertainty ranges calculated using probabilistic sensitivity analysis, monte-carlo simulations over 10,000 runs.
Incremenal to standard of care;
ICER: Incremental cost-effectiveness ratio: Cost ($) per DALY averted, calculated incremental to cost/DALY averted with standard of care unless otherwise noted. CS: Cost saving compared with reference arm
Drivers of cost-effectiveness
To identify the factors most likely to drive cost-effectiveness estimates across different settings, we performed a one-way sensitivity analysis of all model parameters. Parameters that influenced model results by more than 10% are presented in the tornado diagram in Figure 1. Cost-effectiveness of intensified TB case finding among individuals newly diagnosed with HIV was largely determined by two factors: prevalence of active TB among patients newly diagnosed with HIV and volume of testing (which, as above, strongly influenced the unit cost of both Xpert and LED microscopy).
Figure 1. Tornado diagram depicting one-way sensitivity analysis of model parameters.
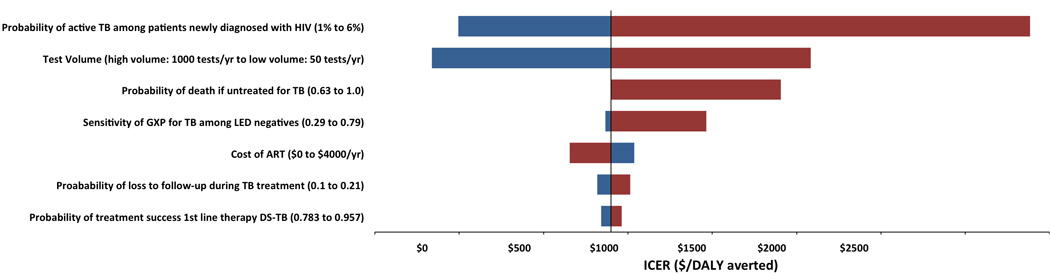
ICER calculated for screening arm with Xpert in refernce to standard of care. Bars in blue represent parameter values that increase, while bars in red reflect parameter values that decrease. Spread of the bars reflects the variability in ICER found when parameter was varied across range of interest. Bars on the left indicate ICERs were smaller, and bars on the right represent ICERS that were larger compared with default estimates.
We present the impact of these two factors on cost-effectiveness in Figure 2, assuming a willingness to pay of $1417 USD per DALY averted, corresponding to the average per capita gross domestic product (GDP) of low-income countries in sub-Saharan Africa16. Scenarios in which LED microscopy was preferred to the standard of care include screening populations of 50 people per year with an expected TB prevalence of >3%, 100 people per year with an expected TB prevalence of >2%, or 500 people per year with an expected prevalence of >1.8%. Xpert was preferred to LED in screening populations of 50/year if prevalence were >6.5%, 100/year if prevalence were >3.5%, or 500/year if prevalence were >2.5%. More detailed results are given for “favorable,” observed, and “unfavorable” scenarios in Table 3. The incremental cost-effectiveness of LED microscopy and Xpert relative to the standard of care varied by 12-fold and 20-fold across these scenarios, respectively – a range far greater than that induced by varying all other model parameters simultaneously (as seen in the corresponding uncertainty ranges).
Figure 2. Impact of test volume and TB prevalence on cost-effectiveness of TB screening with Xpert and LED.
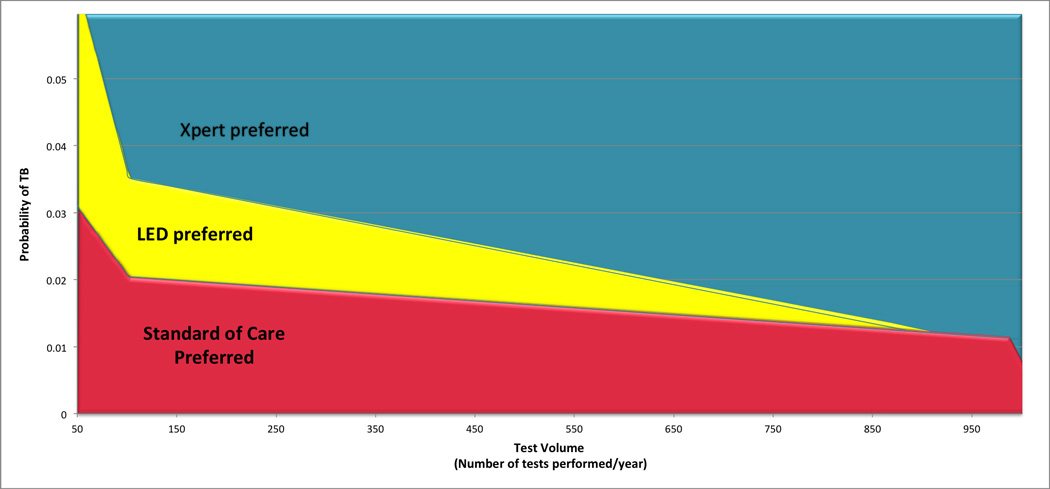
The shaded area corresponds to the combination of values for test volume and TB prevalence where one screening strategy is more cost-effective compared with the others, using a threshold of $1417 USD corresponding to the average GDP per capita of developing countries in Sub-Saharan Africa. For example the values of TB prevalence and test volume that fall in the red shaded area indicate a setting where the standard of care is more cost-effectives compared with LED or Xpert, values in the yellow zone denote settings where LED is more cost-effective, and values in the teal zone correspond to settings where Xpert is preferred.
As Xpert machines are rolled out across many settings for a wide range of patient groups, equipment and implementation costs may be reduced independent of HIV positive patient volume being screened for TB. To explore such scenarios we reduced equipment and maintenance costs for the Xpert machine by half. In this scenario assuming patient volume in this population remains low (50 tests/year) ICERs for Xpert become almost identical to that of LED at $1816 per DALY averted, and at higher patient volumes of 100 or 1000 tests per year, the ICERs for Xpert becomes more cost-effective than LED at $1134 per DALY averted and $500 per DALY averted respectively.
In further sensitivity analyses, we assumed that the average number of DALYs averted from preventing a a death is only half the estimate that we obtained using our mean DALY estimate. In this sensitivity analysis, the effectiveness of the intervention is essentially reduced by half, leading to a doubling of the ICER in all scenarios.
Impact of ART and symptom-driven TB diagnosis
We also considered the isolated costs and cost-effectiveness of LED microscopy and Xpert (i.e., excluding costs and impact of ART). Excluding ART costs only (while retaining the benefit of ART in terms of life expectancy) drove the cost per person screened, and thus incremental cost per DALY averted, down from $1216 to $917 (LED microscopy versus standard of care) and from $1615 to $1486 per DALY averted (Xpert versus standard of care).
Given the paucity of data on symptom-driven TB diagnosis in low-income countries of sub-Saharan Africa, we also varied the probability that TB cases missed by screening will get diagnosed and effectively treated before death from 36% to 80%. In a setting where the percentage of missed cases later become diagnosed through routine services decreases from 66% to 36% (i.e., screening becomes more important to avert eventual death), both LED and Xpert became more cost effective, with the incremental cost per DALY averted falling by about 40% (LED: $704, Xpert: $918). Conversely, if 80% of cases missed by initial screening are ultimately diagnosed through routine services, both LED and Xpert become less cost-effective, with an incremental cost per DALY averted of $1980 (LED) and $2651 (Xpert).
Discussion
Tuberculosis remains the leading cause of death among PLWHA in low-income countries of sub-Saharan Africa, but the cost-effectiveness of novel tests to screen for TB among adults receiving a new diagnosis of HIV remains uncertain. This economic evaluation, using data from a randomized trial in rural Malawi, suggests that the cost-effectiveness of TB screening at the point of HIV diagnosis in these settings depends critically on the volume of people being screened and the TB prevalence in the screened population. In facilities that can screen at least 50 people with a 6.5% prevalence of TB, or at least 500 people with a 2.5% TB prevalence, point-of-diagnosis TB screening with Xpert is likely to be cost-effective at a willingness to pay of per-capita GDP per DALY averted. At somewhat lower prevalence – including that observed in Malawi – LED microscopy may be the preferred screening strategy, whereas in settings of lower TB prevalence or small numbers of eligible patients, no screening may be reasonable such that resources can be deployed to other interventions for PLWHA. These results provide important guidance to low-income countries in sub-Saharan Africa as they contemplate the most appropriate approaches to implementing novel TB diagnostic test for screening among people newly diagnosed with HIV.
As a threshold of less than three times per-capita GDP per DALY averted is sometimes recommended as “cost-effective”, using the “highly cost-effective” threshold of one GDP per capita particularly in one of the lowest-income countries in Africa (per-capita GDP of $360 in 2010 16), represents a conservative approach.17 Even at the “cost-effective” threshold of three times GDP per capita for Malawi ($1080), the ICER is higher than the threshold; however, , screening for TB would be considered cost-effective in most countries (for example, neighboring Tanzania, per-capita GDP is $525 in 2010).16, 17
Logistical feasibility is an important consideration in rolling out any TB screening strategy. In the rural Malawian setting, sputum smears are sent off-site and performed at centralized laboratories with high sample throughput; in other sub-Saharan African settings, LED microscopy (and even Xpert) at the point of HIV diagnosis may already be available, thereby markedly improving the cost-effectiveness of screening. In such settings, the incremental cost of LED microscopy (or Xpert, where already available) for screening may largely be limited to consumables and some staff costs (e.g., $1–$2 per test for LED microscopy and $16–$17 for Xpert), and incremental cost-effectiveness of TB screening – given that these tests are already available and being used for symptom-driven TB diagnosis – may approach that of the high-volume scenario shown here. Where implementation of TB screening would require new equipment, however, testing volume is a critical consideration. Indeed, in rural settings such as this one, technical and logistical problems (e.g., electrical outages, equipment breakdown, theft) may further reduce the effective testing volume and drive up costs. These findings emphasize that there is no “one size fits all” solution to TB screening in low-income sub-Saharan Africa; settings with high TB prevalence, high patient volumes, or existing capacity for Xpert or LED microscopy may find universal TB screening of symptomatic patients with new HIV diagnoses to be highly cost-effective, whereas small centers with lower TB prevalence and little existing capacity may be justified in deploying their resources elsewhere.
In a similar analysis in South Africa (where Xpert-based TB diagnosis has been scaled up throughout the country, and per-capita GDP is over 10 times that of the average used here), Andrews et al identified the prevalence of active TB as the most influential driver of cost-effectiveness when considering TB screening for people with newly-diagnosed HIV 18. This analysis did not vary test volume as widely and concluded that TB screening was likely to be highly cost-effective in South Africa. Our results suggest where this may also be true in lower-income countries of sub-Saharan Africa.
Other cost-effectiveness analyses of TB diagnosis in southern Africa have found ART costs to be very influential19, 20. In our analysis, by contrast, ART costs were less important because of the restriction of the population to those being newly diagnosed with ART and the corresponding assumption that all members of the population would eventually start ART. Furthermore, the prevalence of TB in this screening population was lower than among individuals seeking diagnosis for cough or other TB symptoms, making the cost of TB screening relatively more expensive compared to the cost of ART (the cost of which continues to decline in the African setting).
As with any model-based economic evaluation, this analysis has certain important limitations. Data on symptom-driven TB diagnosis and outcomes of untreated TB in low-income sub-Saharan Africa are very sparse. In the absence of convincing data, we assumed that 40% of people with TB would be captured at the time of HIV diagnosis, that 66% of people missed by initial screening would eventually be diagnosed before death, and that untreated TB was universally fatal among PLWHA. To the extent that these assumptions are not reflective of specific settings in low-income sub-Saharan Africa, our estimates of cost-effectiveness may be incorrect. On wide variation of these parameters,, untreated TB mortality had little influence, but symptom-driven TB diagnosis patterns were important. Our results should therefore be interpreted with caution where symptom-driven TB diagnosis patterns are very different from those assumed here. Our cost and operational data from rural Malawi may not directly generalize to other countries or urban centers, and care should be taken when generalizing these findings to other countries in sub-Saharan Africa. However, sensitivity analyses suggest that test volume and TB prevalence are likely to be key considerations in most settings. In many settings (including Malawi), the current recommended algorithm suggests Xpert testing only after negative smear result. However, when implementing a test such as Xpert for TB screening (rather than diagnosis) in a rural clinic (as opposed to a centralized lab), it is unlikely to be realistic to have patients wait hours for their smear result, and another two hours for Xpert, particularly given the low probability of smear positivity in this screening patient population, where the prevalence of TB is less than 3% and therefore 98–99% of patients will be smear negative. Finally, our model does not account for potential reduction of TB transmission due to earlier TB diagnosis; thus, our estimates of TB screening cost-effectiveness may be somewhat conservative. Future studies could consider inclusion of transmission and collection of data to inform corresponding assumptions.
Conclusion
This analysis demonstrates that test volume and TB prevalence are key drivers of cost-effectiveness when considering screening people newly diagnosed with HIV for TB using LED microscopy or Xpert in low-income sub-Saharan Africa. In settings of high patient volume and TB prevalence – or existing capacity and low logistical barriers – Xpert may be a highly cost-effective method to screen all people with new HIV diagnoses and any TB symptoms. In settings of moderate volume and TB prevalence, LED microscopy may be the preferred option, and in low-volume peripheral centers with high logistical barriers, resources may be better allocated to other interventions (which could include transport of sputum specimens to other centers). Future studies – including primary results from the parent trial – could improve estimates of long-term effectiveness of such TB screening strategies. In assessing the cost-effectiveness of TB screening among PLWHA in low-income countries of sub-Saharan Africa, evaluations should move away from a “one size fits all” approach and toward consideration of key drivers including patient volume, TB prevalence in the screened population, existing capacity, and logistical feasibility.
Acknowledgements
Authors would like to thank all those who made this work possible, including the staff who graciously provided their time and assistance in costing efforts.
This work is supported by grants from the National Institute of Allergy and Infectious Diseases, NIH: R01AI093316, AZ is supported by a fellowship from the Canadian Institutes of Health Research.
DD, RC, EC and MS conceived and designed the experiments; MS, LN and TH collected the data. DD, MS, MK and AZ analyzed the data, AZ wrote the first draft of the manuscript and DD and AZ wrote the manuscript.
Footnotes
Presented at the 45th Union World Conference on Lung Health, Barcelona, Nov. 1, 2014
Conflicts of interest
There are no conflicts of interest.
References
- 1.UNAIDS. 2013 UNAIDS Report on the global AIDS epidemic. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 2.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. Aids. 2008 Oct 1;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn SD, Kranzer K, Edwards DJ, McNally M, Bekker LG, Wood R. Tuberculosis during the first year of antiretroviral therapy in a South African cohort using an intensive pretreatment screening strategy. Aids. 2010 Jun 1;24(9):1323–1328. doi: 10.1097/QAD.0b013e3283390dd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1999 Jun;3(6):457–465. [PubMed] [Google Scholar]
- 5.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA : the journal of the American Medical Association. 2008 Jul 23;300(4):423–430. doi: 10.1001/jama.300.4.423. [DOI] [PubMed] [Google Scholar]
- 6.Getahun H, Harrington M, O'Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007 Jun 16;369(9578):2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 7.Long R, Scalcini M, Manfreda J, Jean-Baptiste M, Hershfield E. The impact of HIV on the usefulness of sputum smears for the diagnosis of tuberculosis. American journal of public health. 1991 Oct;81(10):1326–1328. doi: 10.2105/ajph.81.10.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH. Malawi: Tuberculosis profile 2011. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 9.MacPherson P, Corbett EL, Makombe SD, et al. Determinants and consequences of failure of linkage to antiretroviral therapy at primary care level in Blantyre, Malawi: a prospective cohort study. PloS one. 2012;7(9):e44794. doi: 10.1371/journal.pone.0044794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA : the journal of the American Medical Association. 2000 May 17;283(19):2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 11.Yew WW, Leung CC. Management of multidrug-resistant tuberculosis: Update 2007. Respirology. 2008 Jan;13(1):21–46. doi: 10.1111/j.1440-1843.2007.01180.x. [DOI] [PubMed] [Google Scholar]
- 12.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997 Jun 15;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Annals of internal medicine. 2011 Aug 16;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 14.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 16.World Bank Open Data. The World Bank. 2012
- 17.Marseille E, Larson B, Kazi DS, James KG, Rosen S. Thresholds for the cost–effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews JR, Lawn SD, Rusu C, et al. The cost-effectiveness of routine tuberculosis screening with Xpert MTB/RIF prior to initiation of antiretroviral therapy: a model-based analysis. Aids. 2012 May 15;26(8):987–995. doi: 10.1097/QAD.0b013e3283522d47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews JR, Lawn SD, Dowdy DW, Walensky RP. Challenges in evaluating the cost-effectiveness of new diagnostic tests for HIV-associated tuberculosis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 Oct;57(7):1021–1026. doi: 10.1093/cid/cit412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menzies NA, Cohen T, Lin HH, Murray M, Salomon JA. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS medicine. 2012;9(11):e1001347. doi: 10.1371/journal.pmed.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]


