Abstract
Background
Data are limited on effects of household or community support persons (“care buddies”) on enrolment into and adherence to pre-antiretroviral HIV care. We assessed the impact of care buddies on adherence to HIV clinic appointments, HIV progression and conduct of daily life among pre-ART HIV-infected individuals in Rakai, Uganda.
Methods
1209 HIV infected pre-ART patients aged ≥15 years were randomized to standard of care (SOC) (n = 604) or patient-selected care buddy (PSCB) (n= 605) and followed at 6 and 12 months. Outcomes were adherence to clinic visits; HIV disease progression and self-reported conduct of daily life. Incidence and prevalence rate ratios and 95% confidence intervals (95%CI) were used to assess outcomes in the intent-to-treat and as-treated analyses.
Results
Baseline characteristics were comparable. In the ITT analysis both arms were comparable with respect to adherence to CD4 monitoring visits (adjPRR 0.98, 95%CI 0.93-1.04, p=0.529) and HIV progression (adjPRR=1.00, 95%CI 0.77-1.31, p=0.946). Good conduct of daily life was significantly higher in the PSCB than the SOC arm (adjPRR 1.08, 95%CI 1.03-1.13, p=0.001). More men (61%) compared to women (30%) selected spouses/partners as buddies (p<0.0001.) 22% of PSCB arm participants discontinued use of buddies.
Conclusion
In pre-ART persons, having care buddies improved the conduct of daily life of the HIV infected patients but had no effect on HIV disease progression and only limited effect on clinic appointment adherence.
Keywords: HIV, pre-ART, patient-selected care buddy, Uganda, randomized controlled trial
INTRODUCTION
Improving the outcomes of HIV/AIDS treatment programs in resource-limited settings requires successful and timely linkage of diagnosed HIV infected patients to pre-antiretroviral therapy (pre-ART) care and retention in pre-ART care until ART initiation1. In line with the Uganda Ministry of Health HIV treatment guidelines, pre-ART patients are provided with services including cotrimoxazole for the prophylaxis of opportunistic infections, a basic care package (health and nutritional education, counseling on living with HIV, insecticide-impregnated bed nets for malaria prevention and clean water vessels with hypochlorite disinfectant for prevention of diarrheal diseases), psychosocial support and laboratory monitoring which have been shown to improve reported quality of life, reduce mortality and may delay progression to ART eligibility2-3.
It is critical that patients fully utilize these care services if these benefits are to be realized, but discontinuation from pre-ART care is particularly high1, 4-12(e.g. 70% of HIV positive patients did not remain in care within one year of enrolling4) and can result in increased morbidity, mortality and faster disease progression4,7-8,13. Non-adherence to pre-ART HIV care has also been associated with detectable viremia and an AIDS-defining CD4 count14. Many studies have assessed interventions that support adherence to ART15-18, but there is limited assessment of interventions to support pre-ART patients. Interventions have included the use of patient-nominated treatment supporters 15-16, 19, a strategy acceptable to patients and cost-effective to the health sector20. In the Nigeria randomized study by Taiwo et al (2010), the intervention group of patients with self-selected ART treatment partners achieved significantly higher virologic suppression than the control group, although benefits did not persist beyond six months16.
Interventions that delay ART eligibility can reduce expenditures on costly ART which is especially important, given the decline in global funding for ART 21-23. We therefore conducted a randomized controlled trial to assess the impact of trained patient-selected care buddies (intervention) on adherence to care, HIV disease progression and conduct of daily life among pre-ART HIV-infected patients in Rakai, Uganda.
METHODS
Study setting
Participants were recruited from 17 community-based Rakai Health Sciences Program (RHSP) HIV/AIDS clinics located in Rakai District, Uganda. The clinics served about 6000 HIV+ patients, about half of whom were receiving pre-ART care including regular CD4 screening to determine eligibility for ART and a basic care package. ART eligibility was based on Uganda Ministry of health (MOH) ART initiation guidelines. During the first fifteen months of this study (October 2010 to January 2012), ART was initiated at a CD4 count of <250cells/ul, then changed to <350 cells/ul under new MoH guidelines (February 2012). In the first time period, CD4 cell count monitoring in pre-ART patients was conducted every 3 months if the most recent CD4 count was 251-350 cells/ul, and every 6 months if the most recent CD4 count was ≥350 cells /ul. After the CD4 criterion was changed to <350 cells/ul, monitoring was conducted every 6 months for all pre-ART patients. All HIV-related services were funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) and were provided free of charge to the patient.
Patient eligibility for enrolment into study
Study eligibility criteria included age ≥15 years, receiving HIV care from RHSP HIV clinics, the ability and willingness to acquire a “care buddy” and disclose HIV sero-status to that person, and written consent to be randomized to the “Patient-selected care buddy” (intervention group) or the standard of care (non-intervention group).
Procedures
Sample size calculation
The statistical software PASS 2008 was used for sample size and power estimation, assuming a power of 80% at a two-sided α =0.05 level of significance to detect various group differences. Calculation was based on baseline CD4 count stratification of 251-350 and 351+.For the 251-350 CD4 group, assuming the proportion becoming eligible for ART in the standard of care arm, Pc=30%, and proportion becoming eligible for ART in the patient-selected care buddy (PSCB) group Pi=15% over the 12 months of follow up, adjusting for a non-response or loss to follow up rate of 10%, and a design effect of 1.5, 200 patients were needed per group for this study. For the category of patients with CD4 351+ cells, assuming Pc=20% and Pi=10% over the 12 months, a sample size of 199 per group was required, adjusting for non-response rate or loss to follow up of 10% and a design effect of 1.5, this resulted in 332 patients per group; making a total of 532 per arm. A design effect was included because study participants were drawn from 17 HIV clinics.
Patient Screening and Randomization into study
Between October 2010 and August 2011, all ART-ineligible pre-ART HIV care patients attending RHSP HIV clinics were screened for study eligibility. Based on the most recently available CD4 count results, patients were stratified into groups of CD4 count 251-350 and CD4 count >350 cells/ul, so as to enable stratified block randomization to improve comparability of CD4 counts between arms at baseline. To ensure random allocation to study arm, we used varying block sizes of four (2-per arm) and six (3-per arm), in sealed opaque envelopes in batches of 12 for distribution among the multiple enrollment clinics. Patients selected an envelope from a box of 12 envelopes without replacement for assignment of the study arm. The randomization blocks and numbers were generated by STATA statistical software.
Study intervention and standard of care arm
Standard of care (SOC)
Patients enrolled for pre-ART care received general health education, clinical monitoring, CD4 testing monitoring and other clinically indicated laboratory investigations, treatment and prevention for opportunistic infections in addition to cotrimoxazole prophylaxis.
Patient-selected Care Buddy intervention (PSCB)
In addition to standard of care services (as above), pre-ART patients randomized to the PSCB arm were asked to choose a care buddy aware of the patient’s HIV status who resided in the same household or in close proximity to the patient. Patient- selected care buddies attended at least two HIV health education sessions providing information on HIV, the importance of HIV-infected persons adhering to clinic visits and to prescribed medications and care, and moral and social support required by persons living with HIV. The buddy training sessions lasted 2-3 hours, were didactic in nature, followed by group discussions, and were conducted by trained RHSP nurses. PSCBs were asked to remind participants to take their prophylactic medication and adhere to clinic appointments and were encouraged to keep track of the patient’s clinic appointment dates. PSCBs received a soft drink and snack during training and a transport refund of up to ~$6 per session. Participants who lost a buddy in the first six months of follow-up were offered an opportunity to identify a replacement buddy. Pre-ART patients did not receive any additional reminders from RHSP clinic staff.
Data collection
Data collected included routine clinic and laboratory data, and interviewer-administered questionnaires which included the patient’s socio-demographic characteristics (age, education, occupation and marital status, distance from the participant’s home to the HIV clinic). Three study visits were conducted at baseline, six and twelve month follow-up, at locations close to the patients’ clinics. At the two follow-up visits, data were also collected on retention of the initial care buddy for those in PSCB arm. Since individuals interact freely in their communities, we also assessed utilization of services of a trained buddy by patients in the SOC arm (this constituted cross-over). PSCB arm participants who had lost a buddy in the first six months were asked whether they were willing to replace the buddy. Other key data included self-reported adherence to clinic appointments and cotrimoxazole use, conduct of daily life (participants’ perception of their general health, pain and ability to perform activities of daily living), sexual behaviors (sexual activity, condom use and number of sexual partners), and use of components of the basic care package. Outcome measures such as progression to ART eligibility and appointment adherence were obtained from the RHSP HIV clinic. Routine clinic data included date of clinic visit, number of cotrimoxazole pills dispensed, pill count and self report adherence to drugs, blood samples for CD4 testing, patient health status (e.g. opportunistic infections, WHO staging) and laboratory results. CD4 counts were assessed by flow cytometry using a FACSCalibur (Becton Dickinson, San Jose, CA, USA). The RHSP Quality Control Department provided oversight of data quality issues throughout the data collection period. All data were entered in Microsoft Visual Fox Pro version 9 databases.
Ethical review
The protocol was reviewed and approved by the Makerere University Higher Degree Ethics and Research Committee, and the Uganda National council for Science and Technology All adults provided written informed consent and minors under 18 years provided assent with parent/guardian consent. The study was registered on clinical trials.gov under identifier number NCT02135003.
Statistical analysis
Statistical analyses used Stata Version 13 (Stata Corporation, 4905 Lakeway Drive, College Station, TX 77845, USA). We assessed the comparability between study arms at enrollment. The primary outcomes included adherence to clinic appointments, HIV disease progression as measured by progression to ART eligibility and ability to conduct activities of daily living. The primary assessment of outcomes used intent-to-treat analysis where all participants were analyzed by their allocated study arms. We also conducted an as-treated analysis where patients were analyzed according to the SOC or PSCB support they actually received.
Adherence to a clinic appointment was defined as attendance for a CD4 blood draw within one month of the scheduled date, for patients scheduled to return in three months or attendance within two months for patients scheduled to return in six months. Adherence for the entire 12 months follow up period was defined as “adherent” if a patient adhered to both the 0-6 month visit and 6-12 month clinic visit.
To determine adherence to HIV care appointments, CD4 blood draw were used instead of cotrimoxazole refill visits because patients could procure the drug in other locales, such as pharmacies and drug stores, and a missed refill visit did not necessarily indicate non-adherence. We estimated the proportion of participants who adhered to their CD4 appointments by study arm, and estimated unadjusted and adjusted prevalence risk ratios (adjPRR) of adherence to CD4 appointment using “modified” Poisson via generalized linear models with a family (Poisson) and link (log) with robust standard errors24 and accounted for clustering of the clinics. Covariates adjusted for included baseline CD4 count, age, sex, occupation, marital status and travel distance to the clinic.
We determined HIV progression by the incidence of ART eligibility per 100 person years for the whole interval 0-12 months and intervals of 0-6, and 6-12 months. Incidence rate ratios of ART eligibility were estimated in PSCB relative to SOC arm using Poisson multivariable regression. Adjusted incident rate ratios and their 95% confidence intervals (95% CI) of ART eligibility were estimated including all covariates with p-values <0.2 in the unadjusted analyses and potential confounders.
At the start of the study, the MOH guidelines recommended that ART be initiated at a CD4 count below 250 cells/ul and this was the ART eligibility criterion until February 1st 2012, when the cut-off threshold was increased to <350 cells/ul. The ART eligibility outcome was assessed using the ART initiation guidelines current at the time of the follow-up visits.
Conduct of daily life was assessed by participants’ perception of their general health and ability to conduct the activities of daily living (vigorous physical activities e.g. digging, splitting firewood; moderate physical activities like washing clothes; light physical activities like kneeling, bending or carrying light items and routine home activities like feeding oneself, dressing up and walking to the latrine/toilet), using a Likert scale (1:excellent, 2:very good, 3:good, 4:poor, and 5:very poor). The conduct of daily life was regarded as good if the general health and ability to conduct activities of daily life ranged from good to excellent, or poor if it was described as either poor or very poor. The proportions of participants reporting good conduct of daily life was estimated by study arm and unadjusted and adjusted prevalence risk ratios and 95% CI estimated using “modified” Poisson via generalized linear models with a family (Poisson) and link (log) with robust standard errors, adjusted for baseline CD4 count, age, sex, occupation and marital status.
RESULTS
Enrolment characteristics
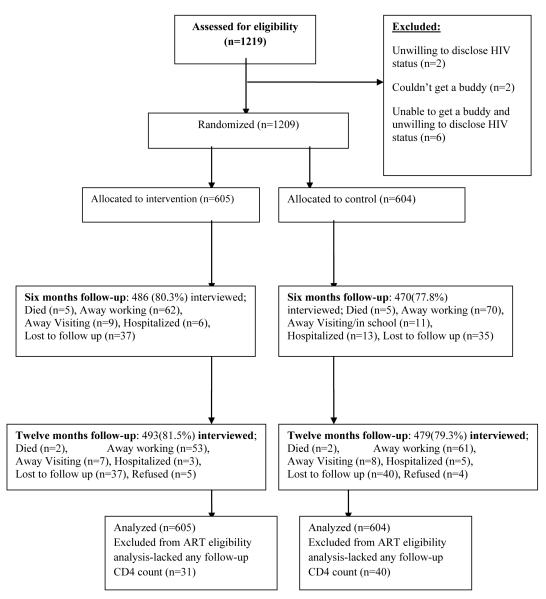
Figure 1 shows the study CONSORT/ trial profile25. A total of 1219 pre-ART patients were screened, of whom 1209 (99.1%) met study eligibility criteria, provided written consent/ assent and were randomized to the PSCB arm (n=605) and SOC arm (n=604). Ten patients were ineligible because they were unwilling to disclose their HIV status or unable to identify a buddy. Table 1 shows participant baseline characteristics which were comparable between study arms. In the intervention arm, selected buddies were mainly spouses/partners or children; a significantly higher proportion of men (61%) compared to women (30%) selected their spouses/partners as buddies (p<0.001.)
Figure 1.
Trial profile
Table 1.
Participant baseline characteristics
| Characteristic | Standard of care | Patient-selected care buddy arm (PSCB) n/N (%) |
P-value | ||
|---|---|---|---|---|---|
| (SOC) | n/N (%) | ||||
| n/N | % | n/N | % | ||
| Overall | 604 /604 | 100 | 605/605 | 100 | - |
| Age (years) | |||||
| Mean (SD) | 37.0 | 8.8 | 37.6 | 9.1 | 0.272 |
| Median (IQR) | 36.0 | 11.0 | 36.0 | 12.0 | |
| Age group | |||||
| 15-29 | 107/604 | 17.8 | 102/605 | 16.9 | 0.439 |
| 30-34 | 147/604 | 24.3 | 149/605 | 24.6 | |
| 35-39 | 147/604 | 24.3 | 135/605 | 22.3 | |
| 40-44 | 86/604 | 14.2 | 91/605 | 15.0 | |
| 45+ | 117/604 | 19.4 | 128/605 | 21.2 | |
| Sex | |||||
| Female | 433/604 | 71.7 | 436/605 | 72.1 | 0.884 |
| Male | 171/604 | 28.3 | 169/605 | 27.9 | |
| Baseline CD4 category | |||||
| 251-350 | 84/604 | 13.9 | 89/605 | 14.7 | 0.690 |
| 351-499 | 148/604 | 24.5 | 159/605 | 26.3 | |
| 500+ | 372/604 | 61.6 | 357/605 | 59.0 | |
| Marital status | |||||
| Not married | 285/604 | 47.2 | 282/605 | 46.6 | 0.842 |
| Married | 319/604 | 52.8 | 323/605 | 53.4 | |
| Main occupation | |||||
| Agriculture/housework | 416/604 | 68.9 | 430 /605 | 71.1 | 0.404 |
| Trading | 66/604 | 10.9 | 72 /605 | 11.9 | |
| Mobile occupation | 8/604 | 1.3 | 5/ 605 | 0.8 | |
| Bar/restaurant worker | 20/604 | 3.3 | 19/605 | 3.1 | |
| Other work | 94/604 | 15.6 | 79/605 | 13.1 | |
| Education level | |||||
| Primary or lower | 506/604 | 83.8 | 526 /605 | 86.9 | 0.119 |
| Secondary or higher | 98 /604 | 16.2 | 79 /605 | 13.1 | |
|
Travel distance to HIV
clinic (km) |
|||||
| 0-1 | 60/604 | 10.0 | 69/605 | 11.4 | 0.333 |
| 2-3 | 138/604 | 22.8 | 134/605 | 22.1 | |
| 4-5 | 109/604 | 18.0 | 93/605 | 15.4 | |
| 5+ | 148/604 | 24.5 | 175/605 | 29.0 | |
| Don’t Know | 149/604 | 24.7 | 134/605 | 22.1 | |
|
Travel time to clinic
(minutes) |
|||||
| <30 | 94/604 | 15.6 | 101/605 | 16.7 | 0.425 |
| 30-59 | 208/604 | 34.4 | 176/605 | 29.1 | |
| 60+ | 287/604 | 47.5 | 317/605 | 52.4 | |
| Don’t Know | 15/604 | 2.5 | 11/605 | 1.8 | |
Participant retention
At 6 months, retention in the PSCB arm was 80.3% (486/605), similar to the SOC arm 77.8% (470/604, p=0.283). Retention at 12 months was 81.5% (493/605) in the PSCB arm and 79.3% (479/604) in the SOC arm (p=0.392). Three participants (1 in SOC and 2 in the PSCB arm) did not have any follow-up information.
Crossovers
Twenty two percent of participants (134/605) in the PSCB arm lost a trained buddy without replacement during the 12 months, whereas 10.1% (61/604) of patients in the SOC arm utilized services of trained buddies (p<0.001). Adherence to having a buddy in either arm was ascertained through self-report (questionnaire).
Exposure to buddies
Participants were in touch with their buddies through physical or phone contact. Adherence to having a buddy in either arm was ascertained through self-report (questionnaire). Among participants in the PSCB arm, 71% reported daily contact with their buddy, while 10% reported occasional and 19% rare contact.
Table 2 shows the Prevalence risk ratios of i) adherence to clinic appointments and ii) Conduct of daily life for both ITT and AT analyses.
Table 2.
Prevalence risk ratios of adherence to clinic appointments and Conduct of daily life
| Outcome | Intervention group | Standard of care group |
Unadjusted PRR (95% CI) |
Adj. PRR* (95% CI) |
p-value | ||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| INTENT TO TREAT ANALYSIS | |||||||
| ADHERENCE TO APPOINTMENTS | |||||||
| 0-6 months follow-up interval | |||||||
| Adhered to appointments | 535/605 | 88.4 | 527/604 | 87.3 | 1.01(0.97,1.06) | 1.01(0.98,1.05) | 0.443 |
| 6-12 months follow-up interval | |||||||
| Adhered to appointments | 497/598 | 83.1 | 502/597 | 84.1 | 0.99(0.95,1.03) | 0.98(0.94,1.03) | 0.510 |
| 0-12 months (overall) follow-up | |||||||
| Adhered to appointments | 458/605 | 75.7 | 465/604 | 77.0 | 0.98(0.93,1.03) | 0.98(0.93,1.04) | 0529 |
| CONDUCT OF DAILY LIFE | |||||||
| 0-6 months follow-up | |||||||
| Good | 368/555 | 66.3 | 306/556 | 55.0 | 1.08(1.02,1.13) | 1.10(1.05,1.15) | <0.001 |
| 6-12 months follow-up | |||||||
| Good | 362/491 | 73.7 | 313/478 | 65.5 | 1.08(1.02,1.15) | 1.07(1.02,1.12) | 0.005 |
| 0-12 months follow-up | |||||||
| Good | 469/568 | 82.6 | 412/568 | 72.5 | 1.05(1.00,1.11) | 1.08(1.03,1.13) | 0.001 |
| AS-TREATED ANALYSIS | |||||||
|
ADHERENCE TO
APPOINTMENTS |
|||||||
| 0-6 months follow-up interval | |||||||
| Adhered to appointments | 415/435 | 95.4 | 647/725 | 89.2 | 1.14(1.10,1.19) | 1.10(1.06,1.14) | <0.001 |
| 6-12 months follow-up interval | |||||||
| Adhered to appointment | 416/461 | 90.2 | 583/704 | 82.8 | 1.15(1.01,1.20) | 1.11(1.07,1.15) | <0.001 |
| 0-12 months (overall) follow-up | |||||||
| Adhered to appointments | 387/474 | 81.7 | 536/704 | 76.1 | 1.27(1.04,1.55) | 1.06(1.00,1.12) | 0.041 |
| CONDUCT OF DAILY LIFE | |||||||
| 0-6 months follow-up | |||||||
| Good | 339/435 | 77.9 | 335/512 | 65.4 | 1.07(1.03,1.10) | 1.07(1.03,1.11) | <0.001 |
| 6-12 months follow-up | |||||||
| Good | 339/461 | 73.5 | 336/508 | 66.0 | 1.09(1.02,1.17) | 1.09(1.02,1.16) | 0.021 |
| 0-12 months follow-up | |||||||
| Good | 440/538 | 81.8 | 434/591 | 73.4 | 1.14(1.10,1.20) | 1.14(1.10,1.19) | <0.001 |
Adjusted for baseline CD4 count, age, sex, occupation, marital status and travel distance to the clinic.
Adherence to CD4 monitoring appointments
In the ITT analysis, adherence to appointments for CD4 blood draws did not differ significantly between study arms during the 0-6 months follow up (adjPRR=1.01, 95% CI: 0.98-1.05.p=0.443); in the 6-12 months interval (adjPRR= 0.98, 95% CI: 0.94-1.03,p=0.510), and overall 0-12 months interval (adjPRR= 0.98, 95% CI: 0.93-1.04,p=0.529).
However, in the AT analysis, adherence to scheduled appointments was significantly greater in the PSCB arm compared to the SOC arm in the first six months follow-up (adj PRR=1.10, 95% CI: 1.06-1.14, p<0.001), in the 6-12 month follow-up (adj PRR= 1.11, 95% CI: 1.07-1.15,p<0.001), and in the overall (0-12 months) follow-up period (adjPRR=1.06, 95% CI: 1.00-1.12, p=0.041).
HIV disease progression
Tables 3 shows the incidence of ART eligibility by study group and follow up-interval for the ITT analysis.
Table 3.
Incidence of ART eligibility by study group and follow up- interval (ITT analysis)
| Intervention (PSCB) |
Standard of care (SOC) |
Unadjusted IRR(95% CI) |
Adj IRR* (95% CI) |
p value | |
|---|---|---|---|---|---|
| 0-6 month follow-up interval | |||||
| 0-6 months follow-up | |||||
| Number at risk | 535 | 527 | |||
| Incident events/100 person yrs | 36/277 (13.0) | 43/289 (14.9) | 0.87(0.57,1.34) | 0.83 (0.57,1.20) | 0.324 |
| 6-12 months follow-up | |||||
| Number at risk | 485 | 480 | |||
| Incident events/100 person yrs | 47/209 (22.5) | 44/206 (21.4) | 1.05(0.70,1.57) | 1.02 (0.68,1.53) | 0.935 |
| 0-12 months follow-up | |||||
| Number at risk | 574 | 564 | |||
| Incident events/100 person yrs | 83/547(15.2) | 87/558(15.6) | 0.97(0.74,1.29) | 1.00 (0.77,1.31 ) | 0.946 |
Adjusted for age, sex, occupation and baseline CD4 count
In the intent to treat (ITT) analysis, patients in the PSCB arm were less likely to have CD4 decline to ART eligibility during the first six months, but this difference was not statistically significant. (Adj. IRR 0.83, 95%CI 0.57- 1.20, p=0.324); there was no difference in ART eligibility in the 6th to 12 months follow-up (Adj. IRR 1.02, 95%CI 0.68-1.53, p=0.935), or during the overall 12 month follow-up period (Adj. IRR 1.00 95%CI 0.77-1.31, p=0.946). Similarly, there were no differences in ART eligibility between the two study arms in the as-treated analysis. A total of 71 patients (31/605 (5.1%) in the PSCB arm and 40/604 (6.6%) in the SOC arm; p=0.27) did not have any follow-up CD4 counts and hence did not contribute person time to this analysis.
Conduct of daily life
In the ITT analysis, a statistically significantly higher proportion of participants in the PSCB arm reported good conduct of daily life compared to the SOC arm participants, in the first 6 months of follow-up (adjPRR= 1.10, 95% CI: 1.05-1.15, p<0.001); second six months; (adjPRR= 1.07, 95% CI: 1.02-1.12, p=0.005) and overall 0-12 month follow-up (adjPRR= 1.08, 95% CI: 1.03-1.13, p=0.001). Similarly, the AT analysis showed significantly higher proportion of participants reporting good conduct of daily life in the PSCB than the SOC arm, throughout the follow-up period.
Participants in the PSCB arm reported having received care buddy help in form of; reminder to return to the clinic (97%), reminders to take medication (68%), emotional support (47%), food provision (27%), assistance with household chores (13%) and financial assistance (10%).
DISCUSSION
This is the first randomized controlled trial of patient-selected care buddies for HIV infected persons not yet on antiretroviral therapy in a resource-limited setting. Previous studies of treatment partners focused on patients on antiretroviral therapy15-16,19. There was no significant difference in HIV disease progression (ART eligibility) between the study arms. These findings differ from results from another randomized trial of patient-selected ART treatment partners, where the intervention achieved significantly higher virologic suppression than the control, although benefits did not persist beyond six months16. Adherence to clinic appointments for CD4 assessment was similar between arms in the ITT analysis throughout the follow-up, but was significantly higher in the PSCB arm compared to the SOC arm in the AT analysis. These differences between the ITT and AT analysis results may indicate that contamination due to crossovers could have biased the results, hence limiting the power to detect a significant difference between the two arms. Crossover rates could probably have been minimized with a cluster randomized trial at the village level.
The effect on adherence seen in the AT analysis may suggest efficacy of patient-selected care buddies on adherence. In another study, buddies proved useful for reminders and other supportive tasks in the first three months, but were generally less beneficial by six or more months19. A main feature of some programs is the requirement for patients to choose buddies to provide support and reminders for patients to take their medications consistently19,26 . Our findings indicate that it is not necessary for HIV programs to delay HIV care while waiting for a patient to identify a treatment buddy.
It is possible that some care buddy exhaustion occurred, as supported by the high proportion (22%) of intervention arm participants who no longer retained a buddy. Such buddy burnout has also been documented after extended periods of time27.The absence of differences in the incidence of ART eligibility could be explained by the fact that both groups received health education including adherence counseling as part of the routine care.
Patients in the PSCB arm consistently reported better ability to conduct activities of daily life than the SOC arm, as reported in other patient-selected partner studies19.
Buddies potentially improved the conduct of daily life by reminding study participants to return to the clinic, reminders to take medication so as to remain healthy and encouragement to seek timely medical care in case of illness, all of which could contribute to better health. Better health may have resulted in better ability to conduct activities of daily life. Similar interventions improved conduct of daily life among patients on ART28.
This study required the patient to disclose their HIV status to their buddy. We note that women were less likely than men to select their spouse as a care buddy. It is likely that married women did not select their husband because disclosure of HIV status often carries adverse consequences for women, including intimate partner violence, abandonment and divorce29-30. Fear of disclosure has also been identified as a major barrier to adherence31. To some extent, fear of disclosure was a deterrent to participation in the study, as indicated by eight participants, who were excluded because of unwillingness to disclose their status to a buddy.
Study Strength and limitations
This study’s strength is that it is the first randomized controlled trial evaluating the impact of patient-selected care buddies conduct of daily life in HIV infected patients not yet receiving ART. However, the length of follow up was limited to 12 months, and results of a longer intervention are unknown. Study limitations included the high crossover rates, particularly in the PSCB arm which may have diluted study power.
The interviewers were out of touch with the study participants between follow up visits. It is therefore unlikely that the inability to blind the study team to which arms the participants had been allocated to introduced any bias when carrying out the follow up interviews
Conclusion
In pre-ART persons, having care buddies improved the conduct of daily life of the HIV infected patients but had no effect on HIV disease progression and only limited effect on clinic appointment adherence.
ACKNOWLEDGMENTS
The authors would like to thank the study participants, care buddies, research assistants, especially Anna Bwetunge and Maria Gorret Nalubega, RHSP clinic staff, Rakai program quality control team and management of Rakai Health Sciences Program, for their contributions to this study.
The study was conducted through the Rakai Health Sciences Program, a research collaboration between the Uganda Virus Research Institute and researchers at Makerere and Johns Hopkins University. HIV care was supported by the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control, Uganda.
Funding source: This study was funded through a training grant from the Fogarty International Center, NIH grant number 2 D43 TW001508-10 and in part by the African Doctoral dissertation fellowship award. Steven J. Reynolds was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Source of support: Fogarty International Center, NIH grant number 2 D43 TW001508-10 and in part by the African Doctoral dissertation fellowship award.
Footnotes
Conflicts of Interest and Source of Funding: The authors declare no conflict of interest.
REFERENCES
- 1.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS medicine. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mermin J, Ekwaru JP, Liechty CA. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–61. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 3.Burtle D, Welfare W, Elden S, et al. Introduction and evaluation of a ‘pre-ART care’ service in Swaziland: an operational research study. BMJ Open. 2012;2:e000195. doi: 10.1136/bmjopen-2011-000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson BA, Brennan A, McNamara L, et al. Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health. 2010;15(Suppl 1):43–7. doi: 10.1111/j.1365-3156.2010.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulissa A, Jerene D, Bindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS ONE. 2010;5:e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clouse K, Pettifor AE, Maskew M, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary healthcare clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2013;62:e39–466. doi: 10.1097/QAI.0b013e318273ac48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amuron B, Namara G, Birungi J, et al. Mortality and loss to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban South Africa. J Acquir Immune Defic Syndr. 2009;5:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tayler-Smith K, Zachariah R, Massaquoi M, et al. Unacceptable attrition among WHO stages 1 and 2 patients in a hospital based setting in rural Malawi: can we retain such patients within the general health system? Trans R Soc Trop Med Hyg. 2010;104:313–319. doi: 10.1016/j.trstmh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Lessells RU, Mutevedzi PC, Cooke GS, Newell ML. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56:e79–86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2012;15:17383. doi: 10.7448/IAS.15.2.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugglin C, Estill J, Wandeler G, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17:1509–1520. doi: 10.1111/j.1365-3156.2012.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Losina E, Basset IV, Giddy J, et al. The “ART” of Linkage: Pre-Treatment Loss to Care after HIV Diagnosis at Two PEPFAR Sites in Durban, South Africa. PLoS ONE. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Paal L, Shafer LA, Todd J, Mayanja BN, Whitworth JA, Grosskurth H. HIV-1 disease progression and mortality before the introduction of highly active antiretroviral therapy in rural Uganda. AIDS. 2007;21(Suppl 6):s.21–9. doi: 10.1097/01.aids.0000299407.52399.05. [DOI] [PubMed] [Google Scholar]
- 15.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24:1273–80. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taiwo BO, Idoko JA, Welty LJ, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune DeficSyndr. 2010;54:85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 17.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune DeficSyndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rueda S, Park-Wyllie LY, Bayoumi AM, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database Syst Rev. 2006;(3) doi: 10.1002/14651858.CD001442.pub2. CD001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foster SD, Nakamanya S, Kyomuhangi R, et al. The experience of “medicine companions” to support adherence to antiretroviral therapy: quantitative and qualitative data from a trial population in Uganda. AIDS Care. 2010;22(Suppl 1):35–43. doi: 10.1080/09540120903500027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farmer P, Léandre F, Mukherjee J, Gupta R, Tarter L, Kim JY. Community-based treatment of advanced HIV disease: introducing DOT-HAART (directly observed therapy with highly active antiretroviral therapy) Bull World Health Organ. 2001;79:1145–51. [PMC free article] [PubMed] [Google Scholar]
- 21.AVERT. 2012.
- 22.Donald G. McNeil Jr. At Front Lines, AIDS War Is Falling Apart. The New York times. 2010 May 9th; [Google Scholar]
- 23.Zwillich T. Obama administration may flat-line funding for PEPFAR. Lancet. 2009;373:1325. doi: 10.1016/s0140-6736(09)60755-8. [DOI] [PubMed] [Google Scholar]
- 24.Guangyong Z. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Douglas G. Altman, Kenneth F. Schulz, Moher David, et al. The Revised CONSORT Statement for Reporting Randomized Trials: Explanation and Elaboration. [DOI] [PubMed]
- 26.Amuron B, Coutinho A, Grosskurth H, et al. A cluster randomized trial to compare home-based with health facility-based antiretroviral treatment in Uganda: Study design and baseline findings. Open AIDS Journal. 2007;1:21–27. doi: 10.2174/1874613600701010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph R. Ferrari, William Mccown, Josephine Pantano. Experiencing satisfaction and Stress as an Aids Care Provider: The AIDS Caregiver Scale. Eval Health Prof. 1993;16:295–310. doi: 10.1177/016327879301600303. [DOI] [PubMed] [Google Scholar]
- 28.Van Tam V, Larsson M, Pharris A, et al. Peer support and improved conduct of daily life among persons living with HIV on antiretroviral treatment: a randomised controlled trial from north-eastern Vietnam. Health Qual Life Outcomes. 2012;10:53. doi: 10.1186/1477-7525-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karamagi CAS, Tumwine JK, Tylleskar T, Heggenhougen K. Intimate partner violence against women in eastern Uganda: Implications forHIV prevention. BMC Public Health. 2006;6:284. doi: 10.1186/1471-2458-6-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koenig MA, Lutalo T, Zhao F, et al. Domestic violence in rural Uganda: Evidence from a community-based study. Bull World Health Organ. 2003;81:53–60. [PMC free article] [PubMed] [Google Scholar]
- 31.Mills E, Nachega J, Bangsberg D, et al. Adherence to ART: A systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Medicine. 2006;3:e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]