Abstract
Objective
To compare the value and effectiveness of different prioritization strategies of pre-exposure prophylaxis (PrEP) in New York City (NYC).
Design
Mathematical modeling utilized as clinical trial is not feasible.
Methods
Using a model accounting for both sexual and parenteral transmission of HIV we compare different prioritization strategies (PPS) for PrEP to two scenarios—no PrEP and PrEP for all susceptible at-risk individuals. The PPS included PrEP for all MSM, only high-risk MSM, high-risk heterosexuals, and injection drug users, and all combinations of these four strategies. Outcomes included HIV infections averted, and incremental cost effectiveness (per-infection averted) ratios. Initial assumptions regarding PrEP included a 44% reduction in HIV transmission, 50% uptake in the prioritized population and an annual cost per person of $9,762. Sensitivity analyses on key parameters were conducted.
Results
Prioritization to all MSM results in a 19% reduction in new HIV infections. Compared to PrEP for all persons at-risk this PPS retains 79% of the preventative effect at 15% of the total cost. PrEP prioritized to only high-risk MSM results in a reduction in new HIV infections of 15%. This PPS retains 60% of the preventative effect at 6% of the total cost. There are diminishing returns when PrEP utilization is expanded beyond this group.
Conclusions
PrEP implementation is relatively cost-inefficient under our initial assumptions. Our results suggest that PrEP should first be promoted among MSM who are at particularly high-risk of HIV acquisition. Further expansion beyond this group may be cost-effective, but is unlikely to be cost-saving.
Keywords: Mathematical models, Prevention of bloodborne transmission, Antiretroviral therapy, Prevention of sexual transmission, Cost effectiveness studies
INTRODUCTION
Evidence suggests that pre-exposure prophylaxis (PrEP) using antiretroviral therapy (ART) is an efficacious tool to reduce HIV transmission. In 2010 the iPrEx study demonstrated that daily oral tenofovir-emtricitabine (TDF-FTC) led to a 44% reduction in HIV incidence overall in men who have sex with men (MSM) [1]. In two other studies conducted in sub-Saharan Africa, similar PrEP regimens among heterosexual persons demonstrated a 62%–75% reduction in HIV incidence [2, 3]. As a result of these findings, the United States Food and Drug Administration (FDA) approved the use of TDF-FTC for the indication of reducing the risk of sexually acquired HIV infection [4]. More recently, PrEP has been demonstrated to have similar efficacy in injection drug users [5]. In addition, both the U.S. Centers for Disease Control and World Health Organization have issued clinical guidelines for the usage of PrEP in the United States and abroad for these populations [6–10].
While PrEP may be efficacious in preventing new HIV infections, its costs are substantial. Several prior studies have evaluated the cost-effectiveness of PrEP specifically among men who have sex with men (MSM) each reaching different conclusions. Desai and colleagues first estimated that prioritizing PrEP to high-risk MSM (~5% of all susceptible MSM) in New York City (NYC) would cost $32,000 per quality adjusted life year (QALY) gained and could avert nearly 9% of new HIV infections within MSM [11]. Other studies have suggested that PrEP use within the MSM population more generally would not necessarily be considered cost-effective based on historical guidelines and definitions of cost-effectiveness [12, 13], although prioritization to the higher risk portions of the MSM community were associated with gains in value [14–16].
Previous mathematical models of PrEP implementation captured the dynamics of HIV transmission and PrEP’s impact on transmission among MSM. We used a previously developed epidemic model of both sexual and injection drug use transmission to simulate PrEP use among various populations [17]. We sought to examine and compare both the effectiveness and value of PrEP implementation among different communities at risk of HIV acquisition (prioritization strategies) including both those addressed in previous models (e.g. MSM) as well as those previously unaddressed, such as injection drug users and high-risk heterosexuals in New York City (NYC), a metropolitan area highly impacted by the HIV epidemic.
METHODS
Overview
This mathematical model integrates equilibrium results from a Monte Carlo simulation of HIV progression with a deterministic compartmental model of HIV transmission [17]. The model incorporates both sexual transmission and transmission through needle-sharing during injection drug use. The probability of transmission between partners is adjusted to account for the infected partner’s gender (in the case of sexual transmission), viral load, and treatment status (on antiretroviral treatment or not). The considered time horizon is 20 years.
Costs of PrEP (including drugs, monitoring, and care) were estimated on an incremental basis in 2012 US Dollars. Benefits were measured as number and percentage of infections averted (as compared to the counterfactual scenario where no PrEP is available, but other prevention mechanisms currently employed are in place).
Economic value was evaluated through determination of cost-per-infection averted ratios and incremental cost effectiveness ratios for each PrEP implementation prioritization strategy. For the purposes of this analysis, a threshold of $360,000 per infection averted was selected as cost-saving (since the downstream medical costs averted from preventing a new infection would offset the programmatic costs of preventing that new infection) [18]. A cost-per-infection averted ratio between $0.36 million – $1 million was considered as likely cost-saving because a more recent evaluation of the lifetime costs of care for individuals with HIV/AIDS suggests higher costs [19]. More complete details of model specification, initial population structure and parameterization have been published elsewhere [17].
PrEP prioritization strategies (PPS)
Several independent PPS were considered and compared to a base case scenario where no PrEP was available and a scenario where PrEP was available for all HIV-negative persons for whom PrEP might be considered a prevention option (including MSM, IDUs and heterosexuals at substantial risk for HIV acquisition) [9] (Table 1). Not all prioritized groups represent mutually exclusive categories and some represent subsets of larger populations.
Table 1.
PrEP prioritization strategies
| Abbreviation | Description |
|---|---|
| None (base case)Δ | No PrEP available |
| HR HET | High-risk†, susceptible (i.e. HIV negative) heterosexuals |
| MSM | Any susceptible MSM |
| HR MSM | High-risk†, susceptible MSM only |
| IDU | Susceptible injection drug users only |
| All at riskΔ (“best” case) | Any susceptible person from all the above categories |
population that consists of injection drug users and or those participating in multiple, concurrent sexual partnerships
considered as comparator strategies
Three parameters—uptake among prioritized population, effectiveness, and cost-- determined the impact, and value of PrEP in our model (Table 2). We assumed a 44% risk reduction in the probability of HIV acquisition as a result of sexual contact between discordant partners as our base case estimate of PrEP efficacy corresponding to the estimates obtained in the iPrEx trial [1]. This was selected as the global point estimate as MSM are a key population at risk in NYC, accounting for the largest proportion of new infections within the city [20]; the generalizability of the results of other PrEP randomized controlled trials to the heterosexual population of NYC is of serious concern [2, 3, 21]; and this estimate is generally a more conservative assumption than other estimates from these international trials. However, we explored a range of effectiveness estimates in sensitivity analysis from between 25% to 75%.
Table 2.
Key input parameters to computer simulation
| Parameter description | Point estimate | Range | Source | |||
|---|---|---|---|---|---|---|
| PrEP characteristics | ||||||
| Relative risk reduction on sexual transmission of HIV associated with PrEP use | 44% | 25–75% | [1–3] | |||
| Relative risk reduction on parenteral transmission of HIV associated with PrEP use | 49% | 25–75% | [5] | |||
| Uptake of PrEP in prioritized population | 50% | 10–90% | [22, 24, 26, 39–41] | |||
| Cost of PrEP per person per year | $9,672 | $4,836–19,345 | [11, 14, 16] | |||
| Population characteristics | ||||||
| Proportion of population who are abstinent | 21% | 15–25% | [42] | |||
| Proportion of men who are MSM (exclusively or non-exclusively) | 6% | 4–10% | [28] | |||
| Probability of monogamous relationship (if sexually active) | ||||||
| Men who have sex with men (MSM) | 56% | 44%–75% | [28] | |||
| Men who have sex with women (MSW) | 78% | 76%–84% | [28] | |||
| Probability of multiple partnerships (if sexually active) | ||||||
| MSM | 44% | 25%–64% | [28] | |||
| MSW | 22% | 16%–24% | [28] | |||
| WSM | 9% | 7–10% | [28] | |||
| Proportion of population that are injection drug users (IDU) | 1.4% | 1.0–1.9% | [43] | |||
| Proportion of IDU that have unsafe injection practices | 32% | 23%–50% | [44] | |||
| Shared injections per year (if unsafe IDU) | 70 | 25–100 | Expert opinion | |||
| Sex acts (per partnership) per year | 89 | 50–100 | [45] | |||
| HIV related characteristics | ||||||
| Probability of annual HIV test | 31% | 10%–50% | [28] | |||
| Probability of adherence to ART | 63% | 50–75% | [46] | |||
Uptake was assumed to be 50% under our initial assumptions which corresponds to an approximate midpoint value from a number of studies evaluating willingness to use PrEP across different populations and settings [22–27]. Additionally, PrEP uptake was assumed to be immediate and continued for the entirety of the simulation time horizon (20 years). Finally, annual PrEP cost was derived from previous literature estimates. We used $9,672/year as our base case estimate, the midpoint between two published estimates [11, 14]. These estimates included not only drug costs but costs associated with screening and monitoring as well. Annual total costs for PrEP were aggregated using a “pre-purchasing” perspective—total cost of intervention equals per unit cost times the total number of persons in the prioritized population. Costs were considered from a health care payer perspective and are expressed in 2012 US dollars. We did not discount costs or benefits. Of note, men who have sex with men exclusively represent 4.8% of the male population, while 0.8% of men have sex with both genders (Table 2) [28].
Analysis plan
We simulated a base case scenario where no PrEP was available starting from 2010, as well as a best case where all at-risk susceptible adults would be able to use PrEP and estimated the number of new HIV infections during the next twenty years. We then examined scenarios where PrEP was implemented among the different groups outlined above to determine the impact on HIV infections averted, and cost-per-infection averted as compared to base case and determine the proportion of maximal effect and cost each PPS had in comparison to the all at-risk case. We compared our initial estimates of PrEP effectiveness and cost under each of the utilization strategies. We additionally sought to define specific scenarios that would result in a cost-saving implementation of PrEP (cost-per-infection averted <$0.36 million) by varying the three defined PrEP characteristics within each PPS across the plausible ranges of values we considered.
We then conducted simulations of every mutually exclusive combination of the PPS (n=12) (e.g., a combination of MSM and HR MSM would be considered non-mutually exclusive whereas a combination of MSM and IDU would be considered mutually exclusive) under initial effectiveness and cost assumptions. We sought to identify the combination(s) of PPS delivering the greatest health benefit, given a plausible budget scenario by calculating the incremental cost-effectiveness ratio (ICER) of all possible combinations of strategies. ICERs measure the additive benefit of each strategy compared with its next best alternative and interpret this benefit together with its additive cost. We plotted the incremental cost of each combination against infections averted (efficient frontier) to highlight strategies that are not preferred due to their inability to deliver the greatest benefit regardless of budget [29]. We conducted one way sensitivity analyses on key model parameter inputs (Table 2) varying these across their considered ranges and calculating cost-per-infection averted ratios for PrEP under these assumptions.
RESULTS
Comparator scenarios
Under base case conditions our model predicts the occurrence of 58,024 new HIV infections over a twenty-year time horizon. Under best case conditions, the model predicts that 13,953 (24%) of these new infections would be averted which represents the maximal effect expected. The cost-per-infection-averted under the best case scenario is $11 million. The total estimated budgetary cost is $7600 million annually.
Under extremely hypothetical conditions where PrEP was available for all susceptible individuals (i.e. the entire HIV negative population of NYC) the model predicts that 16,886 (29%) of these new infections would be averted. The cost-per-infection-averted under this scenario is more than $54 million. The total estimated budgetary cost for implementation of PrEP throughout the entire population is $52,000 million annually.
Impact and cost-effectiveness of PrEP using different PPS
Prioritization to all MSM prevents 19% of new HIV infections overall compared to the absence of a PrEP intervention (Figure 1); 92% of all infections averted under this prioritization strategy would be among MSM. The cost-per-infection-averted for prioritization to all MSM is estimated at $1.6 million. The estimated budgetary cost for PrEP scale up among all MSM is $1000 million annually. This PPS retains 79% of the maximal estimated effectiveness of PrEP (i.e. all at-risk) at only 15% of the total cost.
Figure 1. a,b. Cost-effectiveness analysis of all mutually exclusive prioritization strategies (PPS) for PrEP in NYC.

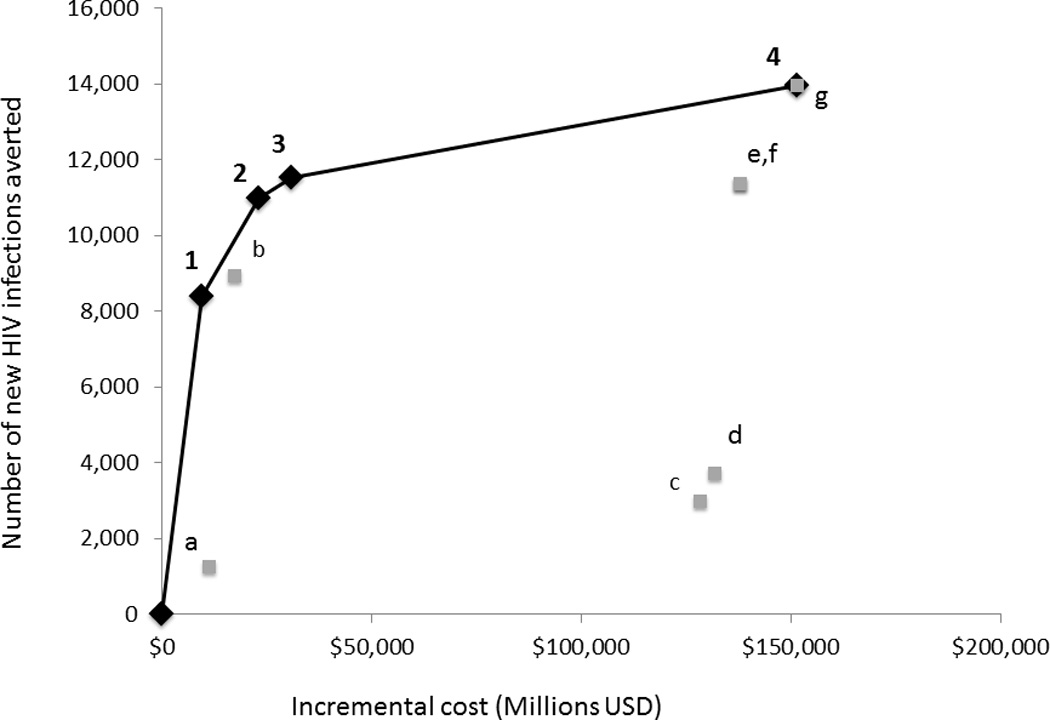
a. All independent PPS listed. Incremental cost effectiveness ratios (ICER) represent incremental cost-per-infection-averted. If PPS resides on efficient frontier it is labeled with (#) which corresponds to number in figure b. All other PPS are labeled with lower case letters. b. Incremental costs and number of HIV infections averted are plotted for each PrEP PPS with the origin corresponding to the base case of no PrEP. Under each PrEP scenario, persons initiate PrEP immediately and continue PrEP for 20 years.
If PrEP were prioritized to only those MSM who were considered to be at highest risk of HIV acquisition through their multiple, concurrent sexual partnerships or because of injection drug use, the model predicted that the intervention could avert 15% of new HIV infections in the population (Figure 1). The cost-per-infection-averted for prioritization to only high-risk MSM is estimated at $1.1 million. The estimated budgetary cost for PrEP scale up to all high-risk MSM is $467 million annually. This PPS retains 60% of the maximal estimated effectiveness of PrEP at 6% of the total maximal cost.
PrEP prioritized to IDUs alone results in 2% of new infections being averted over twenty years. The average annual cost is $610 million and the cost-per-infection averted is estimated to be more than $9 million. PrEP prioritized to only higher-risk heterosexuals, as defined earlier (Table 1) results in 5% of new infections averted with an average annual cost of $6500 million and a cost-per-infection averted estimated to be $43 million. Compared with PrEP for all at-risk persons, with a cost of $7600 million annually, these two strategies retain 8% and 21% of the maximal effect of PrEP at 8% and 85% of the annual cost respectively.
Combinations of PrEP PPS and their relative value
When all mutually exclusive combinations of PPS were compared, the following PPS or combinations of PPS were found to provide the greatest value for resource expended (i.e. lie on cost-effectiveness frontier)—prioritization to HR MSM; prioritization to all MSM; prioritization to all MSM and all IDU; and prioritization to all high-risk heterosexuals, all MSM and all IDU—the remaining PPS or PPS combinations (n=7) were dominated by these five (Figure 1).
Increasing access to PrEP beyond HR MSM leads to diminishing returns as the incremental cost-per-infection-averted for a PPS of only HR MSM is estimated to be $1.1 million whereas for a PPS of all MSM, the cost-per-infection averted was $2.1 million when compared to no PrEP and the incremental cost-effectiveness ratio (ICER) compared to HR MSM was $5.2 million. Furthermore, expanding access to all those populations considered at-risk (e.g. high-risk heterosexuals, MSM, and IDU) results in a cost-per-infection averted ratio of $10.9 million when compared to no PrEP, and an ICER of $49.6 million when compared to the next most cost-effective PPS (All MSM and all IDU).
Sensitivity analysis
One way sensitivity analyses revealed that the operating characteristics of PrEP implementation, including uptake, effectiveness and cost, had a profound impact on the value of PrEP, as measured by cost-per-infection averted (>75% difference in cost-per-infection averted) across all PPS (see supplementary material). Only the assumed number of sex acts per partnership (annually), exhibits this magnitude of influence across more than one PPS. Among the different PPS, if the cost of PrEP is reduced by 50% ($4,836 annually) and uptake of PrEP is at least 50%, prioritization to all MSM could potentially reach the threshold of cost-savings (Figure 2). In addition, under the most optimistic assumptions (uptake 90%, cost $4836 annually, effectiveness 75%) prioritization to all MSM could prevent nearly 50% of new infections (as compared to 19% under initial assumptions). If uptake of PrEP is 70–100%and cost is approximately 50% of initial estimates, prioritization to high-risk MSM would achieve cost-savings (i.e. cost-per-infection averted of ≤$0.36 million) while preventing as many as 40% of new HIV infections (Figure 2). Under no scenario investigated was prioritization to high-risk heterosexuals alone cost-saving.
Figure 2. Effectiveness and value of PrEP as a function of prioritization strategy (PPS) and PrEP characteristics.
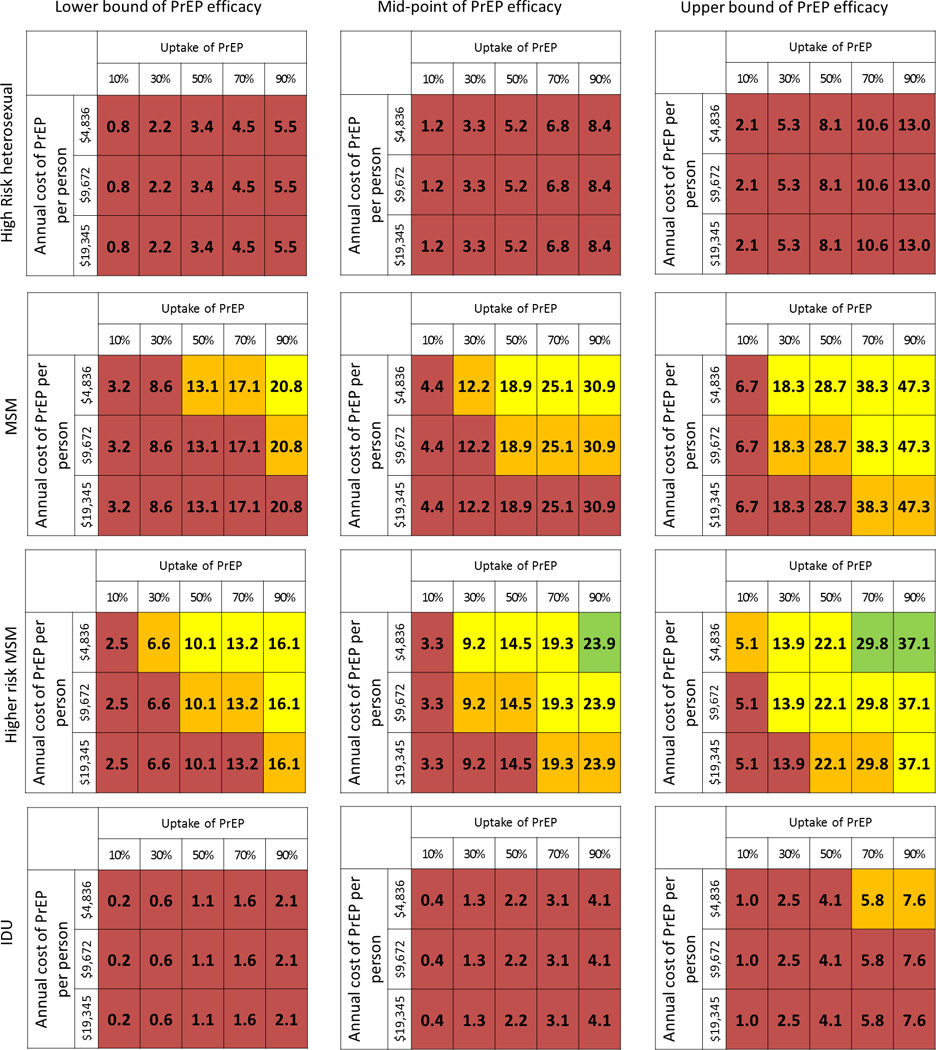
Tables organized as different prioritization strategies (PPS) (top bottom); different PrEP effectiveness assumptions (left
bottom); different PrEP effectiveness assumptions (left right). Number in each cell is percentage of new HIV infections averted. Green cell—Cost-per-infection-averted <$360,000[cost-saving] [47]; Yellow cell—cost-per-infection-averted $360K–$1M [potentially cost-saving][19]; Orange cell—cost-per-infection averted $1M–$2M [likely not cost-saving]; Red cell—cost-per-infection averted >$2M [not cost saving]. Lower bound of PrEP effectiveness = 25% risk reduction of transmission; mid-point PrEP effectiveness = 44% risk reduction; Upper bound of PrEP effectiveness = 75% risk reduction
right). Number in each cell is percentage of new HIV infections averted. Green cell—Cost-per-infection-averted <$360,000[cost-saving] [47]; Yellow cell—cost-per-infection-averted $360K–$1M [potentially cost-saving][19]; Orange cell—cost-per-infection averted $1M–$2M [likely not cost-saving]; Red cell—cost-per-infection averted >$2M [not cost saving]. Lower bound of PrEP effectiveness = 25% risk reduction of transmission; mid-point PrEP effectiveness = 44% risk reduction; Upper bound of PrEP effectiveness = 75% risk reduction
If PrEP effectiveness were assumed to be 25%, there is no scenario under which PrEP would be considered cost-saving. However, if it were prioritized to high-risk MSM, provided at lower cost, utilized by a majority of the community (50–100%), and estimated to be equally effective as initial estimates, it may still be cost-effective (Figure 2). Although an improvement in effectiveness to 75% would increase the overall impact of PrEP, it would not alter which PPS would potentially be cost-saving under certain conditions (i.e. only HR MSM).
DISCUSSION
Our results indicate that expansion of PrEP usage within NYC could have a significant impact on HIV prevention, although prioritization strategies and cost considerations must be taken into account. We have shown that 24% of new HIV infections could be averted with modest assumptions about PrEP uptake and effectiveness when utilized within the larger community at-risk of HIV acquisition in NYC. We have previously reported on less expensive combination prevention strategies that are able to avert similar proportions of HIV infections over a similar time frame [17]. Prioritizing PrEP to high-risk populations would be reasonable to produce health gains in an economically feasible and efficient manner [30].
In our study, when we prioritize PrEP to all MSM, the model predicted that 19% of new HIV infections could be averted within the population at a cost-per-infection-averted of $2.1 million. In addition, if we further constrain PrEP roll-out to those MSM who are at highest risk of HIV acquisition, the model predicts that fewer infections are averted overall (15%), but at a cost-per infection averted of $1.1 million. While not cost-saving, this resource investment may fall within current standards of cost-effectiveness. Indeed, our results suggest that PrEP may have favorable value when offered to HIV-uninfected individuals with a risk of infection approximating those of high-risk MSM, meaning an infectivity rate (probability of infection per year) of at least 4% per year.
When PrEP is prioritized to MSM, which represent approximately 3% of the entire population in our model, 79% of the estimated benefit of the PrEP intervention (number of infections averted) is retained at 15% of the total cost. When PrEP is prioritized to only MSM considered at highest risk (~1.5% of the total population), 60% of the overall effect is retained at less than 6% of the total cost of expansion of PrEP to the entire at-risk population. Furthermore, our findings indicate that under certain prioritization strategies (e.g. PrEP for high-risk MSM only), there may scenarios in which programmatic PrEP implementation could be considered cost-saving, such as if annual costs of PrEP were 50% of base case estimates (which may be achievable with public health pricing and or with the advent of generic formulations of tenofovir and emtricitabine) and effectiveness were assumed to be similar to the upper range of findings in published PrEP randomized controlled trials [3].
Expanding access beyond high-risk MSM provides more benefit in terms of HIV infections averted, however, there are diminishing returns associated with this strategy. A PPS strategy where PrEP is implemented across those communities at highest risk of HIV acquisition including high-risk heterosexuals, IDUs and MSM results in a 24% reduction in new HIV infections but with a cost-per-infection-averted of more than $10 million or an ICER of more than $49 million. However, it should be noted that there likely is a level of risk (e.g. heterosexuals in discordant partnerships) for which PrEP would achieve high value among heterosexuals, but we did not explore these very high echelons of heterosexual risk in our analysis because they are not important drivers of the epidemic in New York City.
Interestingly, we found that PrEP usage among injection drug users in NYC may neither have as significant an impact nor provide the same value for resources expended as prioritization to the MSM population. This is likely related to the assumptions we made based on limited data regarding the prevalence of IDU, the prevalence of HIV within the IDU population, the transmission risk associated with IDU activity, and the partnership mixing between populations at risk (non-random mixing).
It is apparent that PrEP provides the greatest benefit in reducing HIV transmission for the resource expended when used in certain populations that are at highest risk for acquisition. However, risk based assessment for HIV acquisition has significant obstacles to success [31, 32]. The ultimate effectiveness of PrEP will require improvements in identifying and reaching individuals at higher risk and/or use of different approaches such as community-based prioritization. Such a strategy could potentially prioritize specific neighborhoods where concentrations of high-risk individuals reside (and the providers that serve them) with appropriate messaging, education and resources. Further work on the comparison between these two (or other) approaches could be of value for policy makers when determining how to roll-out PrEP.
Our results align with results from other published models on the impact and value of PrEP. Desai and colleagues found that ~9% of infections would be averted amongst MSM living in NYC with a coverage of 25% of HR MSM (assuming 50% would be fully adherent to daily PrEP and assuming effectiveness of 50%) [11]. When we match these assumptions as closely as possible, we arrive at a similar measure of PrEP’s effectiveness (12% infections averted). In a follow-up study, Koppenhaver, et al found a 61% decrease in HIV infections when PrEP coverage is expanded to include all MSM and was associated with a cost-per-infection-averted of ~$0.9 million [14]. Again, when we match these assumptions closely, we estimate that 53% of HIV infections would be averted at a cost-per-infection-averted of $1.2 million.
Similarly, Juusola and colleagues recently reported results suggesting 51% of new HIV infections would be averted under a scenario where all MSM received PrEP [16]. In addition, they found that the value of PrEP was noticeably higher when prioritized to high-risk MSM rather than all MSM given similar rates of coverage and adherence ($52,443/QALY vs. $216,480/QALY). This is in concordance with our findings of improved value as measured by cost-per-infection averted under the more focused prioritization strategy. Similar factors and model inputs in these studies play a key role in the determination of economic value between these published reports and ours [33].
Our analysis has a number of important limitations. There are critical elements relating to the norms of people of various sexual identities and behavioral patterns, including differential condom use with casual partners, serosortive and seroadaptive practices, and alterations in risk behavior as a result of HIV awareness, that are not explicitly accounted for in our computer simulation due to inherent choices and trade-offs made between model complexity and transparency. Many of these elements may act to reduce individual level risk for HIV acquisition and therefore our results may overestimate the actual health benefits of PrEP assuming that PrEP use itself does not further modify these practices [34–37]. Furthermore, we made assumptions about the likelihood of mixing between different sexual risk groups (e.g. individuals who participate in multiple, concurrent partnerships, individuals who are monogamous) though we undertook sensitivity analysis to determine the impact that these assumptions may have had on our results. We did not stratify the effect of PrEP on HIV transmission by the type of sexual partnership or positioning (e.g. anal vs. vaginal intercourse, insertive vs. receptive anal intercourse) but rather used a conservative, fairly generalizable global estimate of effectiveness. Furthermore we did not account for potential improvements over time in PrEP uptake and or costs resulting from increased awareness of this modality and perhaps easier, cheaper regimens becoming available.
We did not account for any increases in morbidity associated with PrEP usage nor did we explicitly include any assumptions about behavioral disinhibition because of limited evidence suggesting these are significant issues related to the use of PrEP [1–3, 38]. Furthermore we assumed immediate uptake of PrEP and we did not account for the possible role that PrEP may have in the development of ART resistance among patients who acquire HIV despite PrEP use or among those who mistakenly start PrEP despite undiagnosed acute HIV infection. Randomized control trial data to date have not demonstrated significantly increased risks of development of resistance among such patients [1]. Our analysis has particular strengths, as well, which include the ability to evaluate the cost and effectiveness of PrEP on both sexual and parenteral transmission of HIV and the ability to account for mixing between risk groups, albeit with substantial abstraction.
Conclusions
We have demonstrated in our analysis that the thoughtful implementation of PrEP among the most at-risk populations, most notably high-risk MSM, could have a significant impact on the HIV epidemic in this setting. In addition, prioritization to high-risk MSM could achieve cost savings under set(s) of assumptions regarding effectiveness and cost that are potentially achievable. Further expansion would provide greater impact; however, the attendant costs may be prohibitive.
Supplementary Material
Acknowledgments
JK, JEM, RSB conceived the study and the analyses. NM, JEM, BC, JK conducted reviews to inform model parameters. KN, AK, CT created the mathematical model and conducted the analyses. JK wrote the manuscript. All authors contributed to analysis interpretation, manuscript editing and preparation.
Footnotes
Disclaimers and conflicts of interest to declare: None
References
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Administration USFaD. FDA Approves First Medication to Reduce HIV Risk. 2012 [Google Scholar]
- 5.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 6.Update to interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection:PrEP for injecting drug users. MMWR Morb Mortal Wkly Rep. 2013;62:463–465. [PMC free article] [PubMed] [Google Scholar]
- 7.Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65–68. [PubMed] [Google Scholar]
- 8.Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR Morb Mortal Wkly Rep. 2012;61:586–589. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevenion. Preexposure prophylaxis for the prevention of HIV infection in the United States-2014: A clinical practice guideline. [Accessed on July 8, 2014];2014 :1–67. http://www.cdc.gov/hiv/pdf/prepguidelines2014.pdf.
- 10.World Health Organization. Geneva: 2012. [Accessed on July 8,2014]. Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: Recommendations for use in the context of demonstration projects. http://apps.who.int/iris/bitstream/10665/75188/1/9789241503884_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 11.Desai K, Sansom SL, Ackers ML, Stewart SR, Hall HI, Hu DJ, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS. 2008;22:1829–1839. doi: 10.1097/QAD.0b013e32830e00f5. [DOI] [PubMed] [Google Scholar]
- 12.Cutler DMRA, Vijan S. The value of medical spending in the United States, 1960–2000. NEJM. 2006;355:920–927. doi: 10.1056/NEJMsa054744. [DOI] [PubMed] [Google Scholar]
- 13.Gold MSJ, Russell L, Weinstein M. Cost-effectiveness in health and medicine. Oxford: Oxford Univerity Press; 1996. [Google Scholar]
- 14.Koppenhaver RT, Sorensen SW, Farnham PG, Sansom SL. The cost-effectiveness of pre-exposure prophylaxis in men who have sex with men in the United States: an epidemic model. J Acquir Immune Defic Syndr. 2011;58:e51–e52. doi: 10.1097/QAI.0b013e31822b74fe. [DOI] [PubMed] [Google Scholar]
- 15.Paltiel AD, Freedberg KA, Scott CA, Schackman BR, Losina E, Wang B, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–815. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156:541–550. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler JMJ, Nucifora K, Mensah N, Kowalski A, et al. Averting HIV infections in New York City: a modeling approach estimating the future impact of additional behavioral and biomedical prevention strategies. PLoS One. 2013 doi: 10.1371/journal.pone.0073269. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schackman BRGK, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 19.Farnham PG, Gopalappa C, Sansom SL, Hutchinson AB, Brooks JT, Weidle PJ, et al. Updates of Lifetime Costs of Care and Quality-of-Life Estimates for HIV-Infected Persons in the United States: Late Versus Early Diagnosis and Entry Into Care. J Acquir Immune Defic Syndr. 2013;64:183–189. doi: 10.1097/QAI.0b013e3182973966. [DOI] [PubMed] [Google Scholar]
- 20.New York City HIV/AIDS annual surveillance statistics. New York: 2012. New York City Department of Health and Mental Hygiene. [Google Scholar]
- 21.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooks RA, Landovitz RJ, Kaplan RL, Lieber E, Lee SJ, Barkley TW. Sexual risk behaviors and acceptability of HIV pre-exposure prophylaxis among HIV-negative gay and bisexual men in serodiscordant relationships: a mixed methods study. AIDS Patient Care STDS. 2012;26:87–94. doi: 10.1089/apc.2011.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: a multinational study. PLoS One. 2012;7:e28238. doi: 10.1371/journal.pone.0028238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heffron R, Ngure K, Mugo N, Celum C, Kurth A, Curran K, et al. Willingness of Kenyan HIV-1 serodiscordant couples to use antiretroviral-based HIV-1 prevention strategies. J Acquir Immune Defic Syndr. 2012;61:116–119. doi: 10.1097/QAI.0b013e31825da73f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt M, Murphy DA, Callander D, Ellard J, Rosengarten M, Kippax SC, et al. Willingness to use HIV pre-exposure prophylaxis and the likelihood of decreased condom use are both associated with unprotected anal intercourse and the perceived likelihood of becoming HIV positive among Australian gay and bisexual men. Sex Transm Infect. 2012;88:258–263. doi: 10.1136/sextrans-2011-050312. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi M, Lee E, Tan DH. Awareness of, usage of and willingness to use HIV preexposure prophylaxis among men in downtown Toronto, Canada. Int J STD AIDS. 2011;22:738–741. doi: 10.1258/ijsa.2011.011057. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, Gao L, Li S, Li D, Zhang L, Fan W, et al. Willingness to accept HIV pre-exposure prophylaxis among Chinese men who have sex with men. PLoS One. 2012;7:e32329. doi: 10.1371/journal.pone.0032329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New York City Department of Health and Mental Hygiene. Community Health. 2009 public use dataset.; 2009. [Google Scholar]
- 29.Markowitz H. Portfolio selection. Journal of Finance. 1952;7:77–91. [Google Scholar]
- 30.Owens DK, Qaseem A, Chou R, Shekelle P. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154:174–180. doi: 10.7326/0003-4819-154-3-201102010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein KTLK, Begier E, Koblin B, Karpati A, Murrill C. Same-sex attraction disclosure to health care providers among New York City men who have sex with men. Arch Intern Med. 2008;168:1458–1464. doi: 10.1001/archinte.168.13.1458. [DOI] [PubMed] [Google Scholar]
- 32.Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006;145:416–425. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- 33.Schackman BR, Eggman AA. Cost-effectiveness of pre-exposure prophylaxis for HIV: a review. Curr Opin HIV AIDS. 2012;7:587–592. doi: 10.1097/COH.0b013e3283582c8b. [DOI] [PubMed] [Google Scholar]
- 34.Vallabhaneni S, Li X, Vittinghoff E, Donnell D, Pilcher CD, Buchbinder SP. Seroadaptive practices: association with HIV acquisition among HIV-negative men who have sex with men. PLoS One. 2012;7:e45718. doi: 10.1371/journal.pone.0045718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matser A, Heiligenberg M, Geskus R, Heijman T, Low N, Kretzschmar M, et al. The importance of partnership factors and individual factors associated with absent or inconsistent condom use in heterosexuals: a cross-sectional study. Sex Transm Infect. 2014;90:325–331. doi: 10.1136/sextrans-2013-051087. [DOI] [PubMed] [Google Scholar]
- 36.Marks GCN, Senterfitt W, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for prevention programs. JAIDS. 2005;39:446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Geneva: 2011. [Accessed on July 8,2014]. Prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender people. http://apps.who.int/iris/bitstream/10665/44619/1/9789241501750_eng.pdf. [PubMed] [Google Scholar]
- 38.Marcus JL, Glidden DV, Mayer KH, Liu AY, Buchbinder SP, Amico KR, et al. No Evidence of Sexual Risk Compensation in the iPrEx Trial of Daily Oral HIV Preexposure Prophylaxis. PLoS One. 2013;8:e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lama JRSyRG, Peinado P, Cabello R, Gonzales P, Liu A, et al. Willingness to use preexposure prophylaxis (PrEP) among men who have sex with men (MSM) at high-risk for HIV acquisition in Lima, Peru; XIX International AIDS Conference; Washington DC. 2102. [Google Scholar]
- 40.Sandfort TGJ, Masvawure T, Hoffman S, Cahill S, Candelario N, et al. Knowledge of and attitudes toward PrEP in a New York City sample of sexually active MSM; XIX International AIDS Conference; Washington DC. 2012. [Google Scholar]
- 41.Kandathil SHS, Champeau D. Women Initiated Solution to Prevent HIV/AIDS (The WISH Study): factors associated with intentions to use microbicides and tenofovir; XIX International AIDS Conference; Washington DC. 2012. [Google Scholar]
- 42.Adimora AA, Schoenbach VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. American Journal of Public Health. 2007;97:2230–2237. doi: 10.2105/AJPH.2006.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. Journal of urban health : bulletin of the New York Academy of Medicine. 2008;85:323–351. doi: 10.1007/s11524-007-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.New York City Department of Health and Mental Hygiene. HIV Risk and Prevalence among New York City Injection Drug Users. National HIV Behavioral Surveillance Study. 2009 [Google Scholar]
- 45.Mosher WDCA, Jones J. Sexual Behavior and Selected Health Measures: Men and Women 15–44 Years of Age, United States, 2002. Advance Data. 2005:362. [PubMed] [Google Scholar]
- 46.Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21:1579–1589. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


