Abstract
Race and ethnicity shape the experience of pain in adults, with African Americans typically exhibiting greater pain intensity and evoked pain responsiveness than Non-Hispanic Whites. However, it remains unclear whether there are racial differences in conditioned pain modulation (CPM) and if these are present in youth. CPM refers to a reduction in perceived pain intensity for a test stimulus during application of a conditioning stimulus and may be especially relevant in determining risk for chronic pain. The present study assessed CPM to evoked thermal pain in 78 healthy youth (ages 10 to 17), 51% of whom were African American and 49% were Non-Hispanic White. African-American youth reported lower mean conditioning pain ratings than Non-Hispanic White youth, controlling for mean pre-conditioning pain ratings, which is consistent with stronger CPM. Multilevel models demonstrated stronger CPM effects in African-American than Non-Hispanic White youth, as evident in more rapid within-person decreases in pain ratings during the conditioning phase. These findings suggest that diminished CPM likely does not account for the enhanced responsiveness to evoked thermal pain observed in African-American youth. These results may have implications for understanding racial differences in chronic pain experience in adulthood.
Perspective
This study evaluated conditioned pain modulation to evoked thermal pain in African-American and Non-Hispanic White youth. Findings could have implications for the development of personalized chronic pain treatment strategies that are informed by race and ethnicity.
Keywords: race, pain, CPM, DNIC, adolescents
Race and ethnicity shape the experience of pain in both clinical and experimental settings. African-American adults report greater pain unpleasantness [47] and pain intensity [13] as well as greater evoked pain responsiveness than Non-Hispanic Whites [42]. However, the mechanisms contributing to a higher prevalence of chronic pain in African Americans compared to Non-Hispanic Whites [39] remain unclear. Responses to experimental pain stimuli distinguish certain clinical pain populations from healthy controls, correlate with changes in clinical pain, and could reflect preexisting risk markers for the onset of chronic pain [17]. The present study sought to determine whether racial differences in descending pain inhibition are present in youth without chronic pain.
Conditioned pain modulation (CPM), also called diffuse noxious inhibitory controls or “pain inhibits pain,” refers to a reduction in perceived pain intensity for a test stimulus during application of a conditioning stimulus to a remote area of the body. Diminished CPM is believed to reflect dysfunction of descending endogenous pain modulatory systems [55] and has been observed in individuals with fibromyalgia [25,27], irritable bowel syndrome [24,60], temporomandibular disorder [24], and in chronic headache [38]. In addition, diminished CPM in healthy individuals has been linked to increased bodily pain and poorer physical functioning [16]. Hence, diminished CPM has been investigated as a potential mechanism, or a biomarker, of risk for developing chronic pain [55,61].
A recent review of racial differences in evoked pain responses highlighted a paucity of studies examining CPM [42]. Although diminished CPM was reported in African Americans compared with Non-Hispanic Whites in one study of healthy adults [5], other studies reported no racial differences in CPM in healthy young adults [19] or in middle-aged and older adults [45]. A study of older adults with knee osteoarthritis found significant racial differences in CPM, such that Non-Hispanic Whites did not evince CPM and African-Americans reported higher pain ratings during application of the conditioning stimulus, which is consistent with pain facilitation [10]. These inconsistencies suggest that it is premature to draw firm conclusions regarding the existence of racial differences in CPM. A second gap in the literature is that few evoked pain studies have been conducted in healthy youth [2,31,36]. One study found that African-American youth reported lower evoked pain intensity than Non-Hispanic White youth [31], and we have recently reported diminished temporal summation of second pain in African-American compared to Non-Hispanic White youth [37]. To our knowledge, whether racial differences in CPM are present in youth without chronic pain has not been examined.
A prospective study of pain-free individuals revealed that diminished CPM measured pre-operatively predicted increased risk for developing chronic post-operative pain [63]; this finding suggests that individual differences in CPM efficiency could help to identify those at risk for developing chronic pain. If healthy African-American youth demonstrate impaired CPM relative to Non-Hispanic White youth, this might suggest that racial differences in pain responsiveness emerge relatively early in life and may be linked to differences in descending endogenous pain inhibitory systems and predict differential risk for developing chronic pain in the future. Given prior work showing greater evoked pain responsiveness in African Americans compared to Non-Hispanic Whites, our primary hypothesis in the current cross-sectional study was that African-American youth would exhibit diminished CPM compared to Non-Hispanic White youth.
Method
Participants
Participants were recruited from the Adolescent and Young Adult Health Clinic at the Monroe Carrell Jr. Children’s Hospital at Vanderbilt University and from a research recruitment website through the Vanderbilt Kennedy Center. The Adolescent and Young Adult Clinic provides primary care for youth living in Metropolitan Nashville and surrounding counties, including routine annual physical examinations. Study procedures were approved by the Meharry Medical College and Vanderbilt University Institutional Review Boards. All subjects and their parents provided written informed assent and consent, respectively, prior to beginning study procedures.
Exclusion criteria were as follows: chronic pain (defined as daily clinical pain ≥ three months in duration), use of prescription opioid analgesics, learning difficulties requiring full-time special education services, sunburn or painful dermatological conditions at the time of the laboratory assessment, and pregnancy. All females who reported having had menarche provided urine samples for a pregnancy test prior to the pain-testing protocol (no female participants were excluded due to pregnancy).
Measures
Demographic Information
Participants provided information on age, sex and race by self-report.
Pubertal maturation
Tanner staging was conducted based on pictorial representations of genital/breast development provided by youth self-report [32,33]. Tanner stages 1 and 2 reflect development up to the onset of puberty and Tanner stages 3 to 5 reflect post-pubertal development. A dichotomous score was derived for each participant (stages 1 or 2 = 0; stages 3 to 5 = 1). Menarchal status of females was determined by self-report.
Socioeconomic status (SES)
SES was calculated using the Hollingshead four-factor index (Hollingshead, 1975), which is a composite of parents’ education and occupation.
Somatic symptoms
The Children’s Somatization Inventory revised form (CSI) [56] was used to determine the perceived severity of somatic symptoms (e.g., headache, dizziness, nausea, back pain) in the past two weeks. Participants reported how much they were bothered by 24 somatic symptoms on a 5-point scale ranging from “not at all” (0) to “a whole lot” (4). Items were summed and total scores ranged between 0 and 96. In this sample, coefficient alpha for the CSI was .84.
Pain catastrophizing
The Pain Catastrophizing Scale for Children (PCS-C) [9,51] is a 13-item self-report questionnaire assessing the degree to which youth catastrophize about their pain (0 = not at all; 4 = very much). Items were summed and total scores ranged between 0 and 52, with higher scores indicating greater catastrophizing. In this sample, coefficient alpha for the PCS-C was .90.
Functional disability
The Functional Disability Inventory (FDI) [7,58] was used to determine the perceived impact of general physical health on psychosocial and physical functioning. Participants reported the degree of difficulty they would have performing 15 specific activities due to their physical health on a 5-point scale ranging from 0 to 4. Items were summed and total scores ranged between 0 and 60 (higher scores indicate greater disability). In this sample, coefficient alpha for the FDI was .91.
Anxiety and depressive symptoms
The short form PROMIS depressive and anxiety symptom scales were used to determine the severity of depressive and anxiety symptoms in the past week [22]. Participants reported how frequently they experienced 8 anxiety and 8 depressive symptoms on 5-point scales ranging from “never” (0) to “almost always” (5). Items were summed and total scores could range from 0 to 32 for each scale. In this sample, coefficient alphas were .79 and .92 for the PROMIS anxiety and depressive symptoms scales, respectively.
Pain Testing Procedures
The ‘test stimulus’ for the CPM thermal pain protocol was a thermal pain stimulus delivered by a thermode (30 × 30 mm) applied to the ventral forearm of the participant’s non-dominant arm and administered via a computerized Medoc TSA-II Neurosensory Analyzer using commercially available software (TPS-CoVAS version 3.19, Medoc Inc., Ramat Yishay, Israel). The ‘conditioning stimulus’ for the CPM protocol was a Boekel General Purpose Water Bath (Boekel Scientific, Feasterville, PA) maintained at a steady temperature of 46.5°C in accordance with previously-established guidelines [63]. Perceived pain intensity was rated by participants on a 0 to 10 scale (0 = “no pain” and 10 = “worst imaginable pain”). First, for each participant, the thermode temperature eliciting a pain rating between 5 and 7 was determined (hereafter referred to as “P-6”). To determine the P-6 temperature, the thermode was applied to the non-dominant ventral forearm in sequences of 15 second pulses at 45 °C, 46 °C, and 47 °C, and at additional lower or high temperatures as warranted until the P-6 was identified. Then, after the thermode was moved to a non-overlapping location on the non-dominant ventral forearm, three pre-conditioning pain ratings in response to the forearm thermal test stimulus at the P-6 temperature applied continuously for a 30-second period were obtained at 10 second intervals. Next, participants took a 10-minute break from the pain testing protocol and completed height and weight measurements. After the break, participants immersed their dominant hand in the hot water bath (conditioning stimulus) for 60 seconds. During this immersion, participants provided three water bath pain ratings at 0, 10 and 20 seconds. At 30 seconds, a single 30 second heat pulse at the P-6 temperature (test stimulus) was applied to the non-dominant arm with the thermode and participants provided three conditioning pain ratings at 40, 50 and 60 seconds.
Data Analytic Plan
Changes from average pre-conditioning pain rating to average conditioning pain rating in the overall sample was examined using paired-samples t tests in SPSS 22 for Windows (SPSS Inc. Headquarters, Chicago, Illinois, USA). Next, racial differences in mean conditioning pain ratings controlling for mean pre-conditioning pain ratings (i.e., baseline corrected change) were examined using analysis of covariance (ANCOVA). Power in the present study to detect racial differences in mean conditioning pain ratings was 0.71. Finally, based on descriptive evidence in the sample for steadily declining pain ratings across the three conditioning ratings and potentially important individual differences in patterns of change during CPM procedures, we conducted exploratory analyses to examine within-individual changes in pain ratings from mean pre-conditioning baseline across the three conditioning trials. A series of multilevel models (MLM) were specified using hierarchical linear models (HLM 6) [43] consisting of a within-person (i.e., level-1) sub-model describing how each individual’s pain ratings changed over successive trials, and a between-person (i.e., level-2) sub-model describing how these changes varied across individuals [4,49].
The Level 1 model was as follows:
The Level 2 model was as follows:
In this equation, Painti indicates the numerical pain rating (0 to 10) at trial t for person i, Trial denotes the pain rating (baseline, conditioning 1-3), and Race denotes African-American (0) or Non-Hispanic White (1). Of primary interest was the interaction between race and trial (β11). Normality assumptions were checked using a Q-Q plot of residuals.
Results
Information on demographic and clinical features, and pain responses for the two groups is provided in Table 1. The sample was relatively balanced in terms of both race, and gender, consisting of 20 African-American males (26%), 20 African-American females (26%), 18 Non-Hispanic White males (22%), and 20 Non-Hispanic White females (26%). No racial differences were observed in sex distribution, age, menarchal status, pubertal status, somatic symptoms, pain catastrophizing, functional disability due to physical health, anxiety symptoms, or depressive symptoms (Table 1). P-6 temperatures were significantly lower for African-American than Non-Hispanic White youth, suggesting greater evoked pain sensitivity in the former group.
Table 1.
| African-American (n = 40) |
Non-Hispanic White (n = 38) |
African-American vs. Non-Hispanic White |
|
|---|---|---|---|
| N (%) | N (%) | X 2 | |
|
|
|||
| Sex | 0.05 | ||
| Male | 20 (50) | 18 (47) | |
| Female | 20 (50) | 20 (53) | |
| Postmenarchal females | 19 (95) | 17 (85) | 1.11 |
| Tanner stagea | 0.13 | ||
| 1-2 | 3 (8) | 4 (11) | |
| 3-5 | 34 (92) | 34 (89) | |
|
|
|||
| M (SD) | M (SD) | t | |
|
|
|||
| Age | 14.6 (2.0) | 15.0 (1.6) | 1.19 |
| SES | 34.5 (13.0) | 40.0 (12.7) | 1.88 |
| Somatic symptoms | 7.3 (5.9) | 9.8 (8.8) | 1.45 |
| Pain catastrophizing | 13.0 (10.0) | 12.0 (8.8) | 0.49 |
| Functional disability | 3.6 (6.6) | 3.7 (7.0) | 0.07 |
| Anxiety symptoms | 4.2 (4.0) | 4.2 (4.2) | 0.02 |
| Depressive symptoms | 3.6 (4.4) | 3.8 (5.4) | 0.22 |
| CPM taskb | |||
| P-6 temperature | 46.3 (1.8) | 47.3 (1.3) | 2.81* |
| Pre-conditioning | 5.6 (1.5) | 6.2 (1.2) | 1.84 |
| CPM trial 1 | 5.4 (2.0) | 5.7 (1.7) | 0.22 |
| CPM trial 2 | 4.2 (2.2) | 5.1 (1.7) | 1.91 |
| CPM trial 3 | 3.4 (2.2) | 4.7 (1.5) | 2.82* |
Three African-American participants chose not to answer this question.
Four participants (3 African-Americans, 1 Non-Hispanic White) did not complete the CPM task due to equipment failure (n = 1) and request to discontinue the task (n = 3).
p < .01.
Note: SES = socioeconomic status; CPM = conditioned pain modulation.
Four participants (1 African American, 3 Non-Hispanic Whites) were excluded from CPM analyses due to equipment failure (n = 1) and request to discontinue the task because these participants indicated the water temperature itself was intolerable (n = 3). These four participants did not differ significantly from the remaining sample on any of the demographic or clinical features. Preliminary MLM analyses revealed that none of the following predictors were associated with changes in pain ratings across trials: age (p > .10); the age X race interaction (p > .10); sex (p > .30); the sex X race interaction (p > .30); pain catastrophizing (p > .10); and functional disability (p > .08). However, child somatization was associated with changes in pain ratings as indicated by a significant somatization X trial interaction (b = .18, SE = .07, p = .01). Simple slope analyses revealed that pain ratings declined for youth with lower (− 1 SD) somatization scores (b = −.46, SE = .10, p < .01), consistent with a CPM effect, but did not change significantly for youth with higher (+ 1 SD) somatization scores (b = .08, SE = .29, p = .78). Including somatization scores as predictors of intercept and slope parameters in subsequent MLM analyses did not significantly alter findings reported below for race effects.
Race and Conditioned Pain Modulation
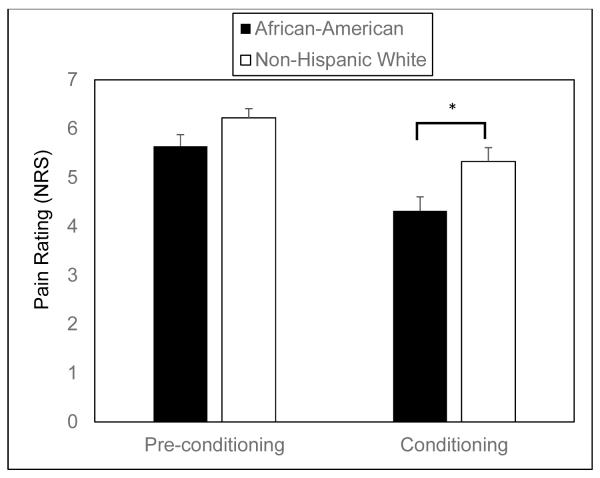
In the overall sample, participants’ average conditioning pain rating declined significantly from their average pre-conditioning pain rating (mean pre-conditioning = 5.9; mean conditioning = 4.8; t = 5.4, p < .01). African-American and Non-Hispanic White youth did not differ in their average pre-conditioning pain ratings (t = 1.8, p = .07), but African Americans reported lower average conditioning pain ratings than Non-Hispanic Whites (t = 2.4, p = .02) (Figure 1). ANCOVA revealed that race was significantly associated with mean conditioning pain ratings after controlling for mean pre-conditioning pain ratings [F = 4.0, p = .049], which is consistent with a stronger CPM effect in African-American than Non-Hispanic White youth.
Figure 1.
Mean pain ratings (± standard error of the mean) for pre-conditioning and conditioning trials for African Americans and Non-Hispanic Whites.
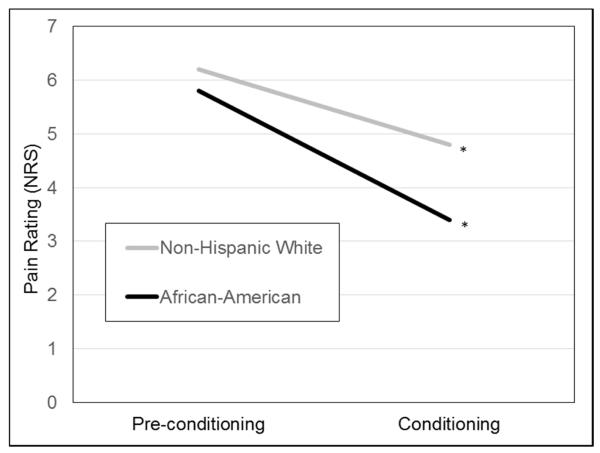
MLM analyses examined whether race influenced within-person changes in pain ratings from baseline (average pre-conditioning rating) across the three conditioning trials. The race X trial interaction was significant (b = .33, SE = .13, p = .02) (Figure 2). Simple slope analyses revealed that pain ratings declined more rapidly for African-American youth (b = −.80, SE = .09, p < .01) than for Non-Hispanic White youth (b = −.47, SE = .10, p < .01). Thus, CPM effects were observed in both racial groups but were elicited more strongly and rapidly for African-American youth.
Figure 2.
Multilevel model testing the race by trial interaction as a predictor of test stimulus pain ratings. *p < .001.
Discussion
African-American adults report greater chronic pain intensity and pain-related disability as well as lower tolerance for evoked noxious stimuli compared to Non-Hispanic White adults with comparable pain conditions [13]. Moreover, African-American adults without chronic pain exhibit greater sensitivity to evoked pain than Non-Hispanic White adults, though the magnitude of racial differences varies across stimulus modalities [42]. Diminished CPM, which is thought to reflect dysfunction of descending endogenous pain modulatory systems [55], has been proposed as a risk factor for chronic pain [63]. The present study sought to determine whether African-American and Non-Hispanic White youth without chronic pain differed in their CPM efficiency. Diminished CPM is found in many [6,14,21,25,27,38,40,60] - but not all [28,29] – chronic pain conditions. Contrary to our hypothesis, African-American youth demonstrated enhanced CPM compared to Non-Hispanic White youth. Weaker CPM in Non-Hispanic White youth could not be explained by lower pre-conditioning pain ratings (i.e., a floor effect), nor could it be explained by other factors that have received attention as potential moderators of CPM effects, including sex differences, age, pubertal or menarchal status, SES, somatic symptoms, pain catastrophizing, pain-related functional disability, and symptoms of anxiety or depression [55]. Of note, CPM effects were found for youth with lower – but not higher – somatization scores. If racial differences in CPM were found to endure into adulthood, they would suggest that greater pain unpleasantness and pain severity reported by African-American adults [13,47] cannot be attributed to impaired CPM efficiency.
The present findings are consistent with one previous study demonstrating CPM (but no sex differences) in children and adolescents [53]. Overall, youth exhibited a 17% reduction in mean pain ratings during the conditioning phase, which is somewhat lower than the median CPM reduction of 29% reported by a review of studies conducted with healthy young adults [41]. This study adds to a nascent literature on racial differences in CPM. Whereas two studies of healthy adults did not find racial differences in CPM [19,45], a third study reported diminished CPM in African-American compared with Non-Hispanic White adults [5]. Reconciling discrepancies between these studies of race and CPM is difficult due to varying methodologies: conditioning stimuli included cold pressor tasks [19,45] and ischemic pain [5], and test stimuli included electrical pain [5], pressure pain [19] and thermal pain [45]. At least two possible explanations for these discrepancies highlight directions for future research. First, maturational changes of endogenous pain modulatory systems could impact studies examining racial differences in CPM. The strength of CPM appears to increase from childhood to adolescence [53] and begins to decline by middle age [15,26,46,59]. Greater variability in CPM responses in youth compared to older adults, when CPM effects may become muted by age-related declines in pain inhibition [45], could affect detection of racial differences.
A second possible explanation for discrepancies in this literature stems from our observation that commonly-used mean pain ratings derived from multiple test stimuli administrations [19,30] could mask increases in CPM during the conditioning phase. Temporal changes in CPM during thermal pain have been previously reported for middle-age adults with chronic fatigue syndrome and healthy controls: whereas healthy controls exhibited CPM effects after a 15-second immersion in hot water (46 °C), patients with chronic fatigue syndrome only exhibited CPM effects after a 2-minute immersion [35]. In addition, increases in CPM effects during conditioning in healthy individuals were reported in a study using a carbon dioxide laser pain test stimulus and thermal conditioning stimulus [23] and in a study using a pressure pain test stimulus and an ischemic pain conditioning stimulus [54]. However, excellent reliability of repeated pressure pain assessments during conditioning (intra-class coefficients ≥ .80) has also been reported, supporting the use of mean conditioning pain ratings [19]. The present study demonstrated significant decreases in test stimulus pain ratings over a 30-second hot water bath immersion, with more rapid decreases observed for African-American than Non-Hispanic White youth. Future studies examining racial differences in CPM effects may benefit from adopting a methodological approach that permits modeling of within-person changes in pain ratings during the conditioning phase.
Yarnitsky has argued that a pro-nociceptive pain modulation profile could be “expressed by either decreased inhibition or enhanced summation, or both” [61]. If findings from our research group showing enhanced CPM and decreased temporal summation of second pain in African-American youth [37] are replicated by others, an intriguing possibility emerges: that African-American youth display an anti-nociceptive pain modulation profile. Such a profile conflicts with evidence of enhanced evoked pain responsiveness and greater clinical pain severity in African-American adults compared to Non-Hispanic White adults [13]. In addition, the absence of racial differences in somatic symptoms or pain-related functional disability in the current sample is somewhat surprising given evidence that experimental pain responses and clinical pain are often linked in healthy individuals [14]. We are unaware of epidemiological studies investigating racial differences in rates of chronic pain conditions in children or adolescents, though one large study of adolescents (n = 6,072) reported higher prevalence of recurrent headaches (> once per week) in white (32.1%) compared to African-American adolescents (24.3%) [44], and another study of adolescent girls (n = 8,370) reported no racial differences in headache or backache but greater frequency of stomachaches occurring more than once per week in Non-Hispanic African-American girls compared to other racial groups [18]. Given evidence that diminished pre-operative CPM predicted greater post-operative pain in adults [63], prospective studies of healthy youth are needed to determine whether racial differences in evoked pain responses – particularly CPM - predict different trajectories of pain experiences across the transition into adulthood.
Limitations of the present study provide directions for future research. First, despite excellent reviews on methodological factors associated with - and clinical relevance of - CPM assessment [41,55,61], there is still no clear consensus on optimal methods for assessing and operationalizing CPM. Though the present study protocol is partially in line with recent recommendations [62], we were not able to seek replication of results across two types of test stimuli (e.g., thermal and mechanical). Second, it is unclear whether racial differences in CPM observed in the present sample of adolescents reporting low levels of somatic symptoms and functional disability would extend to youth with chronic pain. Third, this study was cross-sectional; prospective studies following youth into emerging adulthood will be necessary to determine whether age and race interact to determine CPM, and to bridge the child and adult experimental pain literature. Fourth, the present study did not assess chronic stress levels or stress response system activity, which have been linked to the development of chronic pain [52] and could impact endogenous pain modulatory systems. African-American youth report higher levels of chronic stress than Non-Hispanic White youth [1,3] and exhibit altered diurnal cortisol rhythm [8,12,34,50]. Of note, recent evidence suggests that a history of moderate adversity may be associated with greater resilience to pain, as evident in lower pain intensity ratings during a cold pressor task [48]. Although African-American youth in the current study exhibited greater evoked pain sensitivity (lower P6 temperatures), contrary to these previously described adversity effects, they nonetheless did display greater endogenous pain inhibition (CPM) consistent with increased pain resilience. Future studies should investigate whether stress, basal hypothalamic-pituitary-adrenal (HPA) activity and/or HPA reactivity to evoked pain can account, in part, for racial differences in CPM and their links to chronic pain over time. Fifth, racial differences in CPM may be shaped by sociocultural factors not assessed in the present study, including attitudes toward – and experiences within - healthcare settings. For example, African Americans report greater financial difficulties associated with chronic pain, more doubt that their pain can be effectively managed with medication, and stronger beliefs that their ethnicity/race affects healthcare access and pain management than Non-Hispanic Whites [20]. Finally, the present study did not include a control condition for the conditioning stimulus and therefore cannot distinguish whether racial differences in repeated pain ratings were due to a CPM effect or habituation.
Despite these limitations, the current study adds to a growing literature on evoked pain responses in youth by demonstrating enhanced CPM in African Americans compared to Non-Hispanic Whites. These findings underscore the importance of dynamic pain assessment modalities such as temporal summation and CPM, particularly in light of recent prospective evidence showing that CPM – but not static pain assessments such as pain threshold – predicted subsequent onset of chronic pain [63]. Early identification of youth at risk for chronic pain is critical given that pain experiences in youth are known to increase the likelihood of developing chronic pain in adulthood [11,57]. The present findings suggest that racial differences in CPM should be considered when developing pain susceptibility profiles designed to improve early detection and intervention for youth at elevated risk for chronic pain.
Highlights.
Although race shapes the experience of pain in adults, less is known about youth.
Conditioned pain modulation (CPM) may be a risk factor for chronic pain.
CPM to evoked thermal pain was assessed in 78 healthy youth.
Stronger CPM effects were observed in African-Americans than Non-Hispanic Whites.
Results suggest no CPM impairment in African-American youth.
Acknowledgments
Research was funded in part by grants from the National Institute of Health (UL1 RR024975/TR000445, U54 RR026140/MD007593, G12 RR003032/MD007586, R01 MH068391, K01 MH101403, RO1 DA017805, R24 DA021471, R01 DA031726, R01 DA037891, R01 HD23264), by NICHD Grant P30HD15052 to the Vanderbilt Kennedy Center for Research on Human Development, by the Endowed Chair in Brain and Behavior Research at Meharry Medical College (Uma Rao) and by the Betsey R. Bush Endowed Professorship in Behavioral Health at the University of Tennessee (Uma Rao). These funding agencies had no further role in the study design, data collection, analysis or interpretation of data, writing of the report, or the decision to submit the manuscript for publication. We gratefully acknowledge all individuals who participated in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no conflicts of interest.
References
- [1].Adkins DE, Wang V, Elder GH., Jr Structure and Stress: Trajectories of Depressive Symptoms across Adolescence and Young Adulthood. Soc Forces. 2009;88:31. doi: 10.1353/sof.0.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain. 2010;149:76–88. doi: 10.1016/j.pain.2010.01.011. [DOI] [PubMed] [Google Scholar]
- [3].Boardman JD, Alexander KB. Stress trajectories, health beahviors, and the mental health of black and white young adults. Soc Sci Med. 2011;72:1659–66. doi: 10.1016/j.socscimed.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bryk AS, Raudenbush SW. Applications and data analysis methods. Sage; Thousand Oaks: 1992. Hierarchical linear models. [Google Scholar]
- [5].Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in diffuse noxious inhibitory controls. J Pain. 2008;9:759–66. doi: 10.1016/j.jpain.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cathcart S, Winefield AH, Lushington K, Rolan P. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache. 2010;50:403–12. doi: 10.1111/j.1526-4610.2009.01545.x. [DOI] [PubMed] [Google Scholar]
- [7].Claar RL, Walker LS. Functional assessment of pediatric pain patients: Psychometric properties of the Functional Disability Inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- [9].Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PSC-C): A preliminary validation. Pain. 2003;104:639–46. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- [10].Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL, 3rd, King CD, Glover TL, Sotolongo A, Herbert MS, Schmidt JK, Fessler BJ, Staud R, Redden D, Bradley LA, Fillingim RB. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheum. 2014;66:1800–10. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- [12].DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [13].Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerence: Clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–23. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- [14].Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–54. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- [15].Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: A comparison of DNIC in healthy older and younger adults. Pain. 2003;101:155–65. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- [16].Edwards RR, Ness TJ, Weigent DA, Fillingim RB. Individual differences in diffuse noxious inhibitory controls (DNIC): Association with clinical variables. Pain. 2003;106:427–37. doi: 10.1016/j.pain.2003.09.005. [DOI] [PubMed] [Google Scholar]
- [17].Edwards RR, Sarlani E, Wesselmann U, Fillingim RB. Quantitative assessment of experimental pain perception: Multiple domanins of clinical relevance. Pain. 2005;114:315–19. doi: 10.1016/j.pain.2005.01.007. [DOI] [PubMed] [Google Scholar]
- [18].Ghandour RM, Overpeck MD, Huang ZJ, Kogan MD, Scheidt PC. Headache, stomachache, bachache, and morning fatigue among adolescent girls in the United States. Arch Pediatr Adolesc Med. 2004;158:797–03. doi: 10.1001/archpedi.158.8.797. [DOI] [PubMed] [Google Scholar]
- [19].Goodin BR, Kronfli T, King CD, Glover TL, Sibille K, Fillingim RB. Testing the relation between dispositional optimism and conditioned pain modulation: Does ethnicity matter? J Behav Med. 2013;36:165–174. doi: 10.1007/s10865-012-9411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Green CR, Baker TA, Ndao-Brumblay SK. Patient attitudes regarding healthcare utilization and referral: A descriptive comparison in African- and Caucasian Americans with chronic pain. J Natl Med Assoc. 2004;96:31–42. [PMC free article] [PubMed] [Google Scholar]
- [21].Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. Clin J Pain. 2010;26:104–09. doi: 10.1097/AJP.0b013e3181bff800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Irwin DE, Stucky B, Langer MM, Thissen D, Dewitt EM, Lai JS, Varni JW, Yeatts K, DeWalt DA. An item response analysis of the pediatric PROMIS anxiety and depressive symptoms scales. Qual Life Res. 2010;19(4):595–07. doi: 10.1007/s11136-010-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kakigi R. Diffuse noxious inhibitory control. Reappraisal by pain-related somatosensory evoked potentials following CO2 laser stimulation. J Neurol Sci. 1994:198–05. doi: 10.1016/0022-510x(94)90036-1. [DOI] [PubMed] [Google Scholar]
- [24].King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., 3rd Deficiency in endogenous modulation of prolonged heat pain in patients with irritable bowel syndrome and temporomandibular disorder. Pain. 2009;143:172–78. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- [26].Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–10. doi: 10.1097/AJP.0b013e31806a23e8. [DOI] [PubMed] [Google Scholar]
- [27].Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–96. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- [28].Leffler AS, Hansson P, Kosek E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain. 2002;6:149–59. doi: 10.1053/eujp.2001.0312. [DOI] [PubMed] [Google Scholar]
- [29].Leffler AS, Kosek E, Lerndal T, Nordmark B, Hansson P. Somatosensory perception and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from rheumatoid arthritis. Eur J Pain. 2002;6:161–76. doi: 10.1053/eujp.2001.0313. [DOI] [PubMed] [Google Scholar]
- [30].Locke D, Gibson W, Moss P, Munyard K, Mamotte C, Wright A. Analysis of meaningful conditioned pain modulation effect in a pain-free adult population. J Pain. 2014;15:1190–98. doi: 10.1016/j.jpain.2014.09.001. [DOI] [PubMed] [Google Scholar]
- [31].Lu Q, Zeltzer L, Tsao J. Multiethnic differences in responses to laboratory pain stimuli among children. Health Psychol. 2013;32:905–14. doi: 10.1037/a0032428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Martin CG, Bruce J, Fisher PA. Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Horm Behav. 2012;61:661–68. doi: 10.1016/j.yhbeh.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meeus M, Nijs J, Van de Wauwer N, Toeback L, Truijen S. Diffuse noxious inhibitory control is delayed in chronic fatigue syndrome: An experimental study. Pain. 2009;139:439–48. doi: 10.1016/j.pain.2008.05.018. [DOI] [PubMed] [Google Scholar]
- [36].Meier PM, Berde CB, DiCanzio J, Zurakowski D, Sethna NF. Thermal and vibration sensation and thermal pain detection thresholds in healthy children and adolescents. Muscle Nerve. 2001;24:1339–1345. doi: 10.1002/mus.1153. [DOI] [PubMed] [Google Scholar]
- [37].Morris MC, Walker L, Bruehl S, Hellman N, Sherman AL, Rao U. Race effects on temporal summation to heat pain in youth. Pain. 2015 Feb 13; doi: 10.1097/j.pain.0000000000000129. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pielsticker A, Haag G, Zaudig M, Lautenbacher S. Impairment of pain inhibtion in chronic tension-type headache. Pain. 2005;118:215–223. doi: 10.1016/j.pain.2005.08.019. [DOI] [PubMed] [Google Scholar]
- [39].Plesh O, Adams SH, Gansky SA. Racial/Ethnic and gender prevalence in reported common pains in a national sample. J Orofac Pain. 2011;25:25–31. [PMC free article] [PubMed] [Google Scholar]
- [40].Potvin S, Larouche A, Normand E, de Souza JB, Gaumond I, Marchand S, Grignon S. No relationship between the ins del polymorphism of the serotonin transporter promoter and pain perception in fibromyalgia patients and healthy controls. Eur J Pain. 2010;14:742–46. doi: 10.1016/j.ejpain.2009.12.004. [DOI] [PubMed] [Google Scholar]
- [41].Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [42].Rahim-Williams FB, Riley JL, 3rd, Williams AKK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: Do biology, psychology, and culture matter? Pain Med. 2012;13:522–40. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Raudenbush SW, Bryk A, Cheong YF, Congdon R, du Toit M. HLM 6: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincoln, IL: 2004. [Google Scholar]
- [44].Rhee H. Prevalence and predictors of headaches in US adolescents. Headache. 2000;40:528–38. doi: 10.1046/j.1526-4610.2000.00084.x. [DOI] [PubMed] [Google Scholar]
- [45].Riley JL, 3rd, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15:272–82. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Riley JL, 3rd, King CD, Wong F, Fillingim RB, Mauderli AP. Lack of endogenous modulation but delayed decay of prolonged heat pain in older adults. Pain. 2010;150:153–60. doi: 10.1016/j.pain.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Riley JL, 3rd, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain. 2002;100:291–98. doi: 10.1016/S0304-3959(02)00306-8. [DOI] [PubMed] [Google Scholar]
- [48].Seery MD, Leo RJ, Lupien SP, Kondrak CL, Almonte JL. An upside to adversity? Moderate cumulative lifetime adversity is associated with resilient responses in the face of controlled stressors. Psychol Sci. 2013;24:1181–89. doi: 10.1177/0956797612469210. [DOI] [PubMed] [Google Scholar]
- [49].Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- [50].Skinner ML, Shirtcliff EA, Haggerty KP, Coe CL, Catalano RF. Allostasis model facilitates understanding race differences in the diurnal cortisol rhythm. Development and psychopathology. 2011;23:1167–86. doi: 10.1017/S095457941100054X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychol Assess. 1995;7:524–32. [Google Scholar]
- [52].Tak LM, Cleare AJ, Ormel J, Manoharan A, Kok IC, Wessely S, Rosmalen JGM. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biol Psychol. 2011;87:183–194. doi: 10.1016/j.biopsycho.2011.02.002. [DOI] [PubMed] [Google Scholar]
- [53].Tsao JCI, Seidman LC, Evans S, Lung KC, Zeltzer LK, Naliboff BD. Conditioned pain modulation in children and adolescents: Effects of sex and age. J Pain. 2013;14:558–567. doi: 10.1016/j.jpain.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tuveson B, Leffler A-S, Hansson P. Time dependant differences in pain sensitivity during unilateral ischemic pain provocation in healthy volunteers. Eur J Pain. 2006;10:225–32. doi: 10.1016/j.ejpain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- [55].van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. J Pain. 2010;11:408–19. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- [56].Walker LS, Beck JE, Garber J, Lambert W. The Children’s Somatization Inventory: Psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;234:430–40. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150:568–72. doi: 10.1016/j.pain.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Walker LS, Greene JW. The Functional Disability Inventory: Measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- [59].Washington LL, Gibson SJ, Helme RD. Age-related differences in the endogenous analgesic response to repeated cold water immersion in human volunteers. Pain. 2000;89:89–96. doi: 10.1016/S0304-3959(00)00352-3. [DOI] [PubMed] [Google Scholar]
- [60].Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–01. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–15. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- [62].Yarnitsky D, Boudhassira D, Drewes AM, Fillingim RB, Granot M, Hansson P, Landau R, Marchand S, Matre D, Nilsen KB, Stubhaug A, Treede RD, Wilder-Smith OHG. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. 2014;14:339. doi: 10.1002/ejp.605. [DOI] [PubMed] [Google Scholar]
- [63].Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]