Abstract
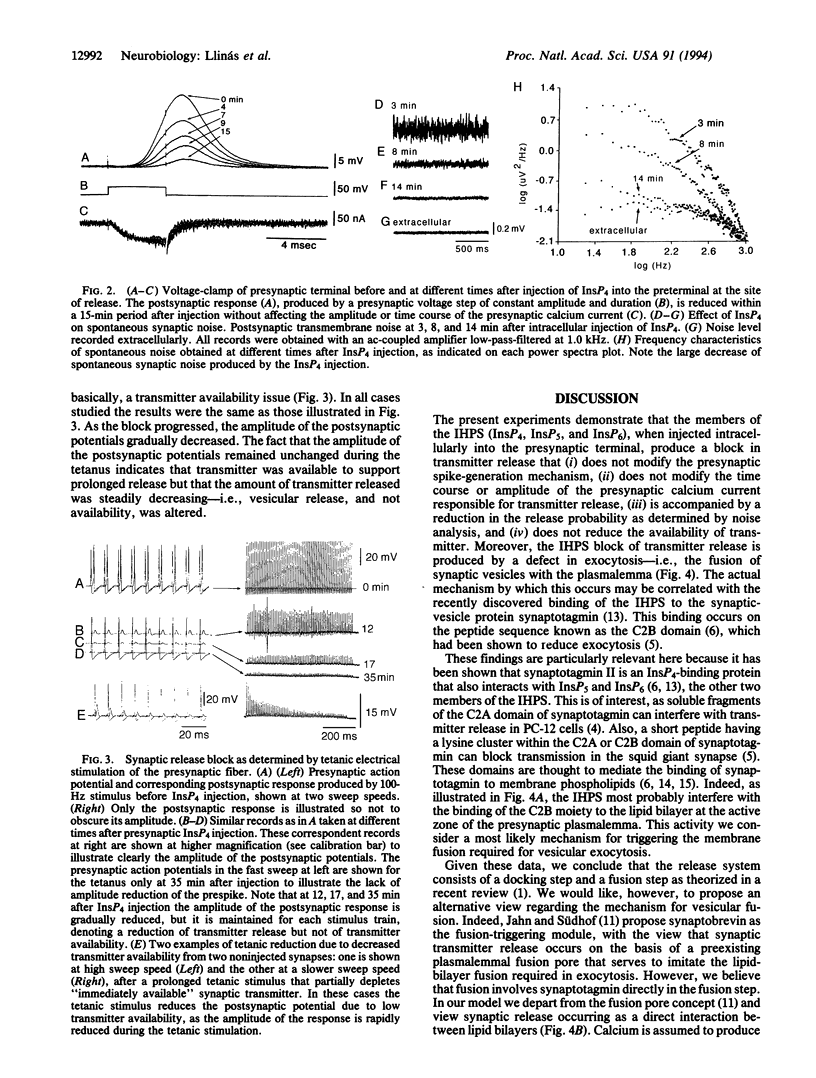
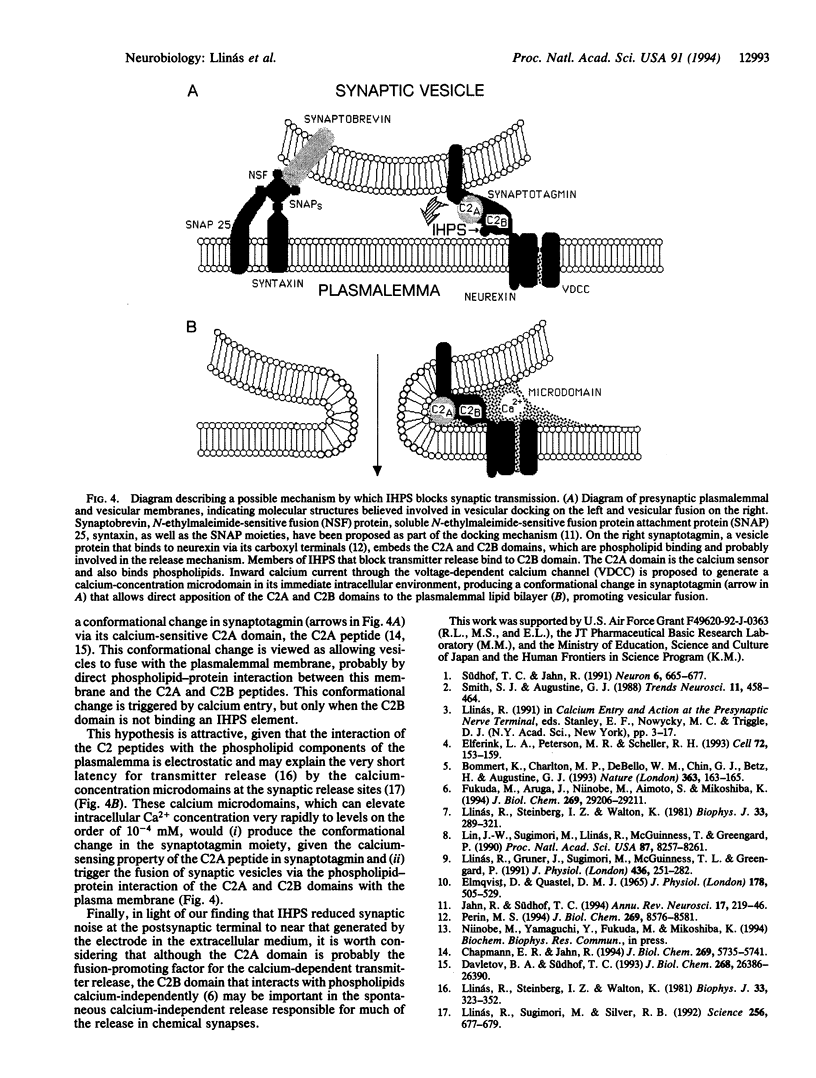
Presynaptic injection of inositol 1,3,4,5-tetraphosphate, inositol 1,3,4,5,6-pentakisphosphate, or inositol 1,2,3,4,5,6-hexakisphosphate--which we denote here the inositol high-polyphosphate series (IHPS)--is shown to block synaptic transmission when injected into the preterminal of the squid giant synapse. This effect is not produced by injection of inositol 1,4,5-trisphosphate. The synaptic block is characterized by a time course in the order of 15-45 min, depending on the injection site in the preterminal fiber; the fastest block occurs when the injection is made at the terminal release site. Presynaptic voltage clamp during transmitter release demonstrates that IHPS block did not modify the presynaptic inward, calcium current. Analysis of synaptic noise at the postsynaptic axon shows that both the evoked and spontaneous transmitter release are blocked by the IHPS. Tetanic stimulation of the presynaptic fiber at frequencies of 100 Hz indicates that block is accompanied by gradual reduction of the postsynaptic response, demonstrating that the block interferes with vesicular fusion rather than with vesicular docking. These results, in combination with the recently demonstrated observation that the IHPS bind the C2B domain in synaptotagmin [Fukada, M., Aruga, J., Niinobe, M., Aimoto, S. & Mikoshiba, K. (1994) J. Biol. Chem. 269, 29206-29211], suggest that IHPS elements are involved in vesicle fusion and exocytosis. In addition, a scheme is proposed in which synaptotagmin triggers transmitter release directly by promoting the fusion of synaptic vesicles with the presynaptic plasmalemma, in agreement with the very rapid nature of transmitter release in chemical synapses.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman E. R., Jahn R. Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J Biol Chem. 1994 Feb 25;269(8):5735–5741. [PubMed] [Google Scholar]
- Davletov B. A., Südhof T. C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993 Dec 15;268(35):26386–26390. [PubMed] [Google Scholar]
- Elferink L. A., Peterson M. R., Scheller R. H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993 Jan 15;72(1):153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Elmqvist D., Quastel D. M. A quantitative study of end-plate potentials in isolated human muscle. J Physiol. 1965 Jun;178(3):505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Aruga J., Niinobe M., Aimoto S., Mikoshiba K. Inositol-1,3,4,5-tetrakisphosphate binding to C2B domain of IP4BP/synaptotagmin II. J Biol Chem. 1994 Nov 18;269(46):29206–29211. [PubMed] [Google Scholar]
- Fukuda M., Aruga J., Niinobe M., Aimoto S., Mikoshiba K. Inositol-1,3,4,5-tetrakisphosphate binding to C2B domain of IP4BP/synaptotagmin II. J Biol Chem. 1994 Nov 18;269(46):29206–29211. [PubMed] [Google Scholar]
- Jahn R., Südhof T. C. Synaptic vesicles and exocytosis. Annu Rev Neurosci. 1994;17:219–246. doi: 10.1146/annurev.ne.17.030194.001251. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Sugimori M., Llinás R. R., McGuinness T. L., Greengard P. Effects of synapsin I and calcium/calmodulin-dependent protein kinase II on spontaneous neurotransmitter release in the squid giant synapse. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8257–8261. doi: 10.1073/pnas.87.21.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Silver R. B. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992 May 1;256(5057):677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- Perin M. S. The COOH terminus of synaptotagmin mediates interaction with the neurexins. J Biol Chem. 1994 Mar 18;269(11):8576–8581. [PubMed] [Google Scholar]
- Smith S. J., Augustine G. J. Calcium ions, active zones and synaptic transmitter release. Trends Neurosci. 1988 Oct;11(10):458–464. doi: 10.1016/0166-2236(88)90199-3. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991 May;6(5):665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]




