Abstract
Despite considerable advancements, the development of effective cancer screening tools based on serum biomarker measurements has thus far failed to achieve a meaningful clinical impact. The incremental progress observed over the course of serum biomarker development suggests that further refinements based on novel approaches may yet result in a breakthrough. The use of urine as an analytical biofluid for biomarker development may represent such an approach. The unique characteristics of urine including a high level of stability, ease of sampling, and an inactive and low-complexity testing matrix offer several potential advantages over the use of serum. A number of recent reports have demonstrated the utility of urine in the identification of novel cancer biomarkers and also the improved performance of biomarkers previously evaluated in serum. In this review, advancements related to the use of urine biomarkers within the settings of ovarian, breast, and pancreatic cancer are presented and discussed. Findings regarding the identification of specific urine biomarkers for each disease are highlighted along with comparative analyses of urine and serum biomarkers as diagnostic tools.
Keywords: Urine biomarkers, Serum biomarkers, Early detection, Ovarian cancer, Breast cancer, Pancreatic cancer, Screening
INTRODUCTION
Efforts to identify and validate biomarkers present in the bodily fluids of ovarian cancer patients are ongoing. Investigators hope to utilize these findings in the development of minimally invasive tests to predict tumorigenesis, disease recurrence, or treatment response. The bulk of this work has focused on blood, given its systemic exposure and extensive availability through tissue banks. The analysis of blood, through the use of either serum or plasma, carries with it several inherent limitations which have hindered the development of clinically useful biomarker assays. Foremost among these limitations is the relatively high level and complex nature of the protein repertoire found in blood. Components of the blood matrix, including clotting and other serological factors, carrier proteins, immunoregulatory proteins, and active enzymes, among others, all have the capacity to interefere with biomarker measurements. The clotting process itself, employed during the preparation of serum, has been shown to involve enzymatic activity which results in the cleavage of unrelated proteins of interest (1, 2). The invasive nature of blood testing also limits accessibility to repeated measurements and presents a risk of infection to both the patient and healthcare professionals, along with the added cost of minimizing this risk.
Recently, urine has been proposed as an alternative biofluid for analytical biomarker studies on the basis that the systemic nature of such testing might be preserved while several of the limitations inherent to blood testing could be eliminated. Urine is available in larger quantities than blood through less invasive means, allowing for repeated measurements aimed at patient surveillance or establishment of assay reproducibility. The urinary proteome is proposed to contain over 100,000 peptides, with 5000 of those present at high frequency (3), and studies have shown that this proteome is stable for hours at room temperature, days at 4°C, and years at −20°C (4). The urinary proteome is a direct product of renal filtration and consists of low-molecular-weight, soluble peptides which are highly amenable to proteomic analysis and may represent disease-specific cleavage processes (5). Renal filtration also results in a less complex matrix than that of blood, containing fewer factors known to interfere with biomarker assays (6). The use of urine as a diagnostic biofluid does present unique challenges including a high variability in protein concentrations due to differences in fluid intake. However, this barrier has been overcome successfully through normalization based on levels of creatinine or other common urinary peptides (7, 8). What remains in the development of urine-based analytical platforms is evidence that systemic disease-specific biomarkers are released into this biological compartment in a manner which can be reliably measured.
Traditionally, investigations focused on urinary biomarkers have been limited to those related to disorders of the urogenital system, although it is estimated that only 70% of the urinary proteome originates from the kidneys or urinary tract, with the remaining 30% resulting from the glomerular filtration of blood plasma (9). Urine therefore can be considered a systemic biofluid with expanded clinical applications. Such applications may include the early detection of various human malignancies, including those originating in tissues beyond the genitourinary system. Here, recent findings regarding the development of urine biomarkers aimed at the detection of ovarian, breast, and pancreatic cancer are reviewed. Findings regarding specific urine biomarkers are presented within each disease setting along with a discussion of the comparative performance of urine, serum, and combination biomarker panels.
OVARIAN CANCER
A number of significant findings have been reported through the analysis of urine obtained from ovarian cancer patients. Several early reports characterized the use of urinary gonadotropic peptide (UGP) as a general biomarker of gynecological malignancy (10–12). The combination of UGP and serum CA 125 proved particularly useful in the diagnosis of ovarian serous carcinomas, providing a sensitivity/specificity ratio of 86%/89% (11). More recently, several other biomarkers including HE4 (13), mesothelin (14), Bcl-2 (15), and angiostatin (16) were found to be differentially present in the urine of ovarian cancer patients and controls. In their respective studies, urinary HE4 was found to discriminate ovarian cancer patients from controls at a level similar to that of serum HE4, while urinary mesothelin outperformed its serum counterpart. Urine levels of angiostatin and Bcl-2 were associated with both early- and late-stage ovarian cancer in comparison to healthy and benign controls. Both markers were elevated in ovarian cancer patients regardless of tumor grade, stage, size, histological subtype, creatinine levels, age, or menopausal status, and displayed complementation with serum CA 125 measurements. Proteomic-based studies performed by several independent research groups have identified a number of additional urinary biomarkers and biomarker panels offering diagnostic potential for ovarian cancer (6, 17–20). Notable among these findings are a 3-biomarker panel which, in combination with CA 125, could discriminate malignant from benign pelvic masses with an area under the receiver-operating characteristic (ROC) curve (AUC) of 0.96 (19), and the combination of glycosylated eosinophil-derived neurotoxin and C-terminal osteopontin (OPN) fragments which provided a sensitivity of 94% at a specificity of 72% for early-stage ovarian cancer compared with benign controls (6).
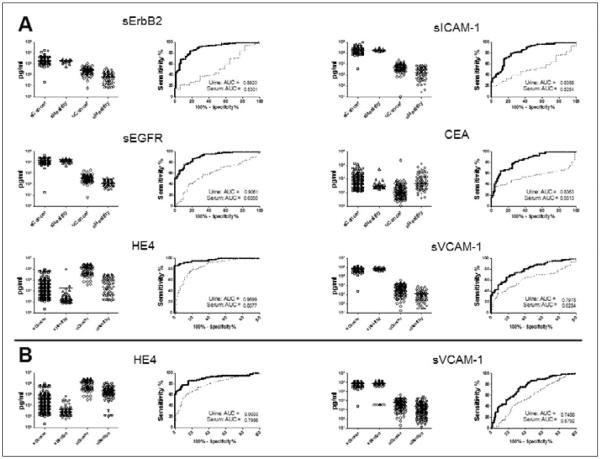
Our group has previously reported on the use of serum biomarker panels for the discrimination of ovarian cancer patients from healthy controls and women with benign pelvic masses (21, 22). In order to evaluate the comparative performance of urine and serum biomarkers in these diagnostic settings, we analyzed levels of 15 biomarkers found to be significant in the previous studies in urine obtained from 108 patients diagnosed with ovarian cancer, 118 women with benign pelvic conditions, and 72 healthy controls (Tab. I). Fourteen of these biomarkers including HE4, CYFRA 21-1, EGFR, ErbB-2, IL-2R, sICAM-1, CEA, Eotaxin-1, sVCAM-1, CA 15-3, tPAI-1, CA 125, MMP-9, and MPO were detectable in urine, with CA 72-4 being the lone undetectable marker. The diagnostic performance of each urine biomarker was compared to our previously reported findings in serum (Fig. 1). In a ROC analysis of cancer patients and healthy controls, several urine biomarkers outperformed their serum counterparts from our previous study. Notably, this list included CEA, HE4, and sVCAM-1, 3 of the 4 markers included in the highest performing panel from that study. In the same comparison, CA 125 provided a significantly higher level of discrimination in serum compared to urine (data not shown). In the comparison of ovarian cancer patients and benign controls, sVCAM-1 and HE4 performed significantly better in urine (Fig. 1), while EGFR was most effective in serum (data not shown). The performance of multimarker panels in the discrimination of the ovarian cancer and control groups was evaluated using the previously described Metropolis-Monte Carlo (MMC) algorithm (22) (Fig. 2). A urine biomarker panel comprised of HE4, CEA, and CYFRA 21-1 was identified as the optimal model for the classification of ovarian cancer cases and healthy controls. This panel significantly outperformed serum CA 125 measured in the same experimental group, and the addition of serum CA 125 to the urine biomarker panel resulted in a significant improvement in sensitivity. For the classification of benign versus malignant pelvic disease, HE4 alone measured in urine provided the highest diagnostic value, exceeding serum CA 125 and any combination of urine biomarkers. The combination of urine HE4 and serum CA 125 provided greater diagnostic utility. Overall, these results demonstrated that certain urine biomarkers and multimarker panels are capable of outperforming similar serum-based tests for diagnostic purposes and the combined use of urine and serum biomarker testing may provide a superior means of patient classification.
TABLE 1.
PATIENT CHARACTERISTICS FOR URINE BIOMARKER ANALYSES
| Group | Age (years) | Gender | Histology | Stage |
|---|---|---|---|---|
| Ovarian | ||||
| Healthy | Range: 49–85 | Female (n=72) | ||
| N=72 | Median: 62 | |||
| Benign | Range: 47–86 | Female (n=118) | Non-malignant neoplasms (n=41) | |
| N=118 | Median: 62 | Benign cysts (n=36) | ||
| LMP tumors (n=15) | ||||
| Other benign lesions (n=26) | ||||
| Cancer | Range: 48–87 | Female (n=108) | Serous (n=72) | I (n=5) |
| N=108 | Median: 63 | Endometrioid (n=7) | II (n=5) | |
| Mucinous (n=3) | III (n=85) | |||
| Mixed (n=19) | IV (n=13) | |||
| Undifferentiated/Unknown (n=7) | ||||
| Breast | ||||
| Healthy | Range: 49–84 | Female (n=90) | ||
| N=90* | Median: 67 | |||
| Cancer | Range: 49–92 | Female (n=74) | Ductal (n=63) | I–II (n=74) |
| N=74* | Median: 63 | Lobular (n=11) | ||
| Pancreatic | ||||
| Healthy | Range: 22–65 | Male (n=16) | ||
| N=45 | Median: 46 | Female (n=29) | ||
| Benign | Range: 32–85 | Male (n=24) | Benign cyst (n=4) | |
| N=44 | Median: 60 | Female (n=20) | Pancreatitis (n=10) | |
| Other (n=30) | ||||
| Cancer | Range: 42–91 | Male (n=36) | PDAC (n=55) | I (n=2) |
| N=55 | Median: 66 | Female (n=19) | II (n=18) | |
| III (n=13) | ||||
| IV (n=22) |
Includes training and validation sets; LMP, low malignant potential; PDAC, pancreatic ductal adenocarcinoma
Fig. 1.
Comparative performance of urine and serum biomarkers in ovarian cancer. Urines obtained from 118 patients diagnosed with ovarian cancer, 118 women with benign pelvic conditions, and 72 healthy controls were evaluated using Luminex multiplexed immunoassays for 15 biomarkers found to be informative in 2 previous analyses in serum. Biomarker level distributions in urine and serum are presented along with ROC curves and AUC values (solid line: urine; dashed line: serum). A. Urine biomarker levels from ovarian cancer patients and healthy controls are compared with serum biomarker levels from a previous report (22). B. Urine biomarker levels from ovarian cancer patients and benign controls are compared with serum biomarker levels from a previous report (21). Biomarkers demonstrating significantly elevated AUC levels, based on 95% confidence intervals, in urine versus serum are shown.
Fig. 2.
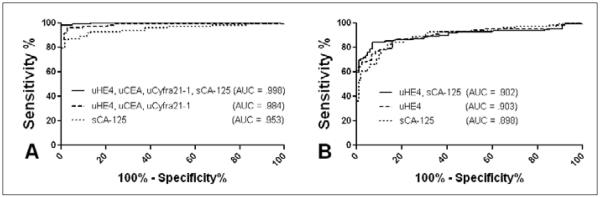
Performance of multimarker panels for the discrimination of ovarian cancer patients from controls. The top performing urine biomarker panels for the discrimination of ovarian cancer patients from healthy (A) and benign (B) controls were identified using the Metropolis-Monte Carlo (MMC) algorithm. Panel performance was compared to serum CA 125 measured in the same group. The impact of adding serum CA 125 to the top urine biomarker panel was evaluated for each comparison. s, serum; u, urine.
The minimally acceptable positive predictive value of 10% (1 case identified for every 10 individuals tested) required for effective ovarian cancer screening necessitates diagnostic tools which provide a high level of sensitivity and specificity (23, 24). Currently used tools such as CA 125 testing in blood and imaging procedures such as transvaginal ultrasound have failed to perform to this standard (25). Although many additional blood-based biomarkers for ovarian cancer have been identified and evaluated, little progress has been achieved in the development of diagnostic tests. Our preliminary study demonstrates that several previously identified serum biomarkers of ovarian cancer provide greater levels of diagnostic utility when evaluated in urine, and suggests that urine biomarker panels may provide levels of sensitivity and specificity for the discrimination of ovarian cancer patients from healthy controls approaching those required for routine screening. Several recent reports investigating the efficacy of biomarker panels within this setting have identified the combination of CA 125 and HE4 as an effective diagnostic tool capable of discriminating benign from malignant pelvic masses with high sensitivity and specificity (21, 26). This combination later showed efficacy in a prospective study (27) and was subsequently incorporated into a scoring model termed the Risk of Ovarian Malignancy Algorithm (ROMA) (28). Our results indicate that the incorporation of urine HE4 into this model may further improve the diagnostic efficacy.
BREAST CANCER
Effective screening has the potential to significantly ease the considerable public health burden imposed by breast cancer, which carries a 5-year survival rate of 97% when the disease is localized at diagnosis (29). However, screening efforts have been hampered by the limited effectiveness of mammography, which demonstrates an overall sensitivity of 75% (30), ranging from 54–58% in women younger than 40 years to 81–90% in postmenopausal women (31–33). A number of groups have investigated the use of circulating tumor markers as screening tools in breast cancer, but a clinically useful test has yet to emerge. Numerous serum tumor markers have been described for breast cancer, including members of the MUC family of mucin-associated glycoproteins (e.g., CA 15-3, BR 27.29, MCA, CA 549, CA 125, CA 72-4, and CEA), oncoproteins (e.g., HER-2/c-erbB-2), cytokines and growth factors (e.g., HGF, IL-6, and VEGF), and cytokeratins (e.g., TPA, TPS, and CYFRA 21-1) (34–42). Unfortunately, the diagnostic utility of all presently known serum tumor markers has been hampered by low sensitivity for early-stage disease and an overall lack of specificity.
In the past several years, the use of urine in studies aimed at the identification and development of breast cancer biomarkers has resulted in a number of diverse reports. Urine metabolite profiling through the use of a variety of techniques and strategies has produced several notable findings. Slupsky et al (20) utilized nuclear magnetic resonance spectroscopy to identify urine metabolite patterns specifically associated with early-stage breast and ovarian cancer. Elsewhere, 2 groups demonstrated the usefulness of metabolite panels consisting of 5 and 4 biomarkers, respectively, in the classification of breast cancer cases from controls (43, 44). The latter study identified their metabolite panel on the basis of genome-wide expression signatures. A separate investigation identified a panel of 31 nucleosides in urine which could discriminate breast cancer patients from controls with a sensitivity of 88% at a specificity of 90% (45). Melatonin, particularly the 6-sulfoxymelatonin derivative, has received significant attention as a urine biomarker of breast cancer. This biomarker has been shown to be useful in patient discrimination in both premenopausal (46) and postmenopausal (47, 48) women. Several other urine biomarkers, including N1,N12-diacetylspermine, ADAM-12, MMP-9, and several phytoestrogens have been evaluated with encouraging results, including the ability to outperform current serum biomarkers of breast cancer, CA 15-3 and CEA (49–51).
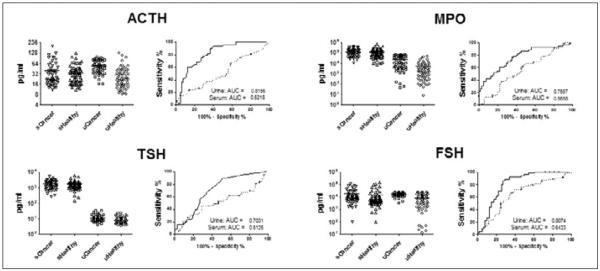
Our group recently reported on our analysis of serum proteins in healthy postmenopausal women and women diagnosed with breast cancer and benign breast lesions (52). Our bioinformatic approach identified a 3-biomarker panel consisting of MIF, MMP-9, and MPO that was able to distinguish healthy women from breast cancer patients with an AUC of 0.82 (sensitivity 60% at specificity 90%); however, benign and malignant cases were indistinguishable. More recently, we have conducted an analysis of the same proteins in urines obtained from 74 postmenopausal patients with early stage breast cancer and 90 age-matched healthy controls (Tab. I). On an individual basis, 4 biomarkers including ACTH, MPO, TSH, and FSH demonstrated significantly higher diagnostic capacities in urine in comparison to serum (Fig. 3). A multivariate analysis of the urine biomarker data identified a 3-biomarker panel consisting of FSH, MPO, and osteocalcin (OC) which provided a sensitivity of 86% at a specificity of 91% in the training set and a sensitivity of 75% at 93% specificity in a blinded validation set (Fig. 4), a 15% improvement in sensitivity over the serum results. Based on these results and the collective work of others it would appear that the advancement of biomarker-based diagnostics within the setting of breast cancer may rely more heavily on urine than serum going forward.
Fig. 3.
Comparative performance of urine and serum biomarkers in breast cancer. Urines obtained from 74 postmenopausal women diagnosed with stage I breast cancer and 90 age-matched healthy controls were evaluated using Luminex multiplexed immunoassays for 98 biomarkers previously tested in serum. Urine biomarker results were compared with serum biomarker results from a previous analysis (52). Biomarker level distributions in urine and serum are presented along with ROC curves and AUC values (solid line: urine; dashed line: serum). Biomarkers demonstrating significantly elevated AUC levels, based on 95% confidence intervals, in urine versus serum are shown. s, serum; u, urine.
Fig. 4.
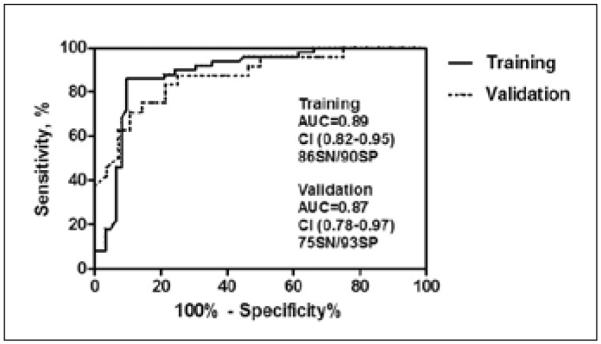
Performance of a urine multimarker panel in the discrimination of stage I breast cancer patients from controls. The top performing urine biomarker panel for the discrimination of stage I breast cancer patients from healthy controls was identified using the Metropolis-Monte Carlo (MMC) algorithm. The performance of this 3-biomarker panel consisting of FSH, MPO, and OC in distinct training and validation sets is presented according to ROC analysis. AUC, area under the curve; CI, confidence interval; SN, sensitivity; SP, specificity.
PANCREATIC CANCER
The devastatingly high mortality rate associated with pancreatic cancer indicates unmet areas of need in all aspects of the clinical management of this disease. One such area includes the development of effective screening tools to aid in early diagnosis. The benefits of improved screening are clear as the overall 5-year survival rises from <5% to 20–30% for patients with small, resectable, early-stage cancers (53–56). Current screening practices rely on the use of expensive and invasive procedures such as computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS). In addition to the expense and invasiveness inherent to these procedures, they are also associated with limited sensitivity and a high degree of overuse in response to vague symptoms such as abdominal pain. A biomarker-based test that could complement or replace the use of CT or MRI, resulting in the more efficient referral of patients for invasive EUS, would offer considerable clinical value to physicians. CA 19-9, the most frequently studied biomarker for pancreatic cancer, had a median sensitivity of 79% (70–90%) and a median specificity of 82% (68–91%) in symptomatic patients (57). A number of other serum biomarkers including TPA/TPS, MIC-1, IGFBP-1, haptoglobin, SAA, TIMP-1, OPN, HE4, and NGAL have been evaluated, but none of these have proven to be superior to CA 19-9 (58–64). Several groups have also reported on the performance of multimarker combinations of these markers (65–68), including several reports involving the use of combined protein and nucleic acid panels (69–71). These multiplexed biomarker approaches have demonstrated improved sensitivity and specificity for the detection of pancreatic cancer. Multiplexed biomarker panels have also proven successful in the discrimination of benign from premalignant pancreatic lesions (72, 73).
The low prevalence and vague symptomatic presentation of pancreatic cancer dictate that a high level of performance will be required for any biomarker-based screening test to achieve clinical implementation. While the search for additional informative biomarkers is ongoing, the use of urine as an alternative to serum for biomarker analysis may result in advancements in the near future. Relatively few studies have been conducted to date regarding the use of urine biomarkers in the early diagnosis of pancreatic cancer. In one early report, UGP, a biomarker implicated in several malignant settings, was evaluated in patients with cancers of digestive organs, where it was found to be elevated in 61.5% of pancreatic cancers (74). More recent reports such as that by Weeks et al (75) suggest that urine may be a useful medium for biomarker analysis. Using proteomic methods the authors identified numerous altered peptides in patients diagnosed with pancreatic ductal adenocarcinoma (PDAC) in comparison with control individuals. Szajda et al (76, 77) in a series of reports identified several enzymes involved in carbohydrate metabolism as potential urine biomarkers of pancreatic cancer. Urine diacetylspermine was identified as a novel tumor marker for pancreatobiliary carcinoma with a sensitivity/specificity level similar to that of serum CA 19-9 (78).
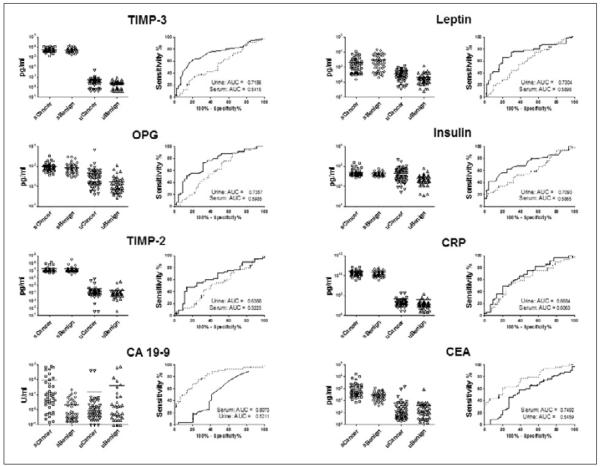
We performed an analysis of 20 pancreatic-cancer-related biomarkers in sera and urines obtained from 55 patients diagnosed with PDAC and 44 patients diagnosed with a spectrum of benign pancreatic diseases (Tab. I). Our biomarker panel consisted of ApoA1, OC, osteoprotegrin (OPG), OPN, ACTH, insulin, PTH, leptin, MPO, CRP, SAA, sICAM-1, transglutaminase II, TIMP 1–4, CA 19-9, CA 125, and CEA. The performance of each biomarker in urine and serum was evaluated by ROC analysis (Fig. 5). On the basis of AUC 95% confidence intervals, CA 19-9 was the only marker which differed significantly, with a higher performance in serum. Among the remaining non-significant markers, 10 demonstrated higher AUC values in urine while 8 were higher in serum. Transglutaminase II was not detectable in urine. The mixed performance of this biomarker set in urine and serum led us to hypothesize that the development of diagnostic biomarker panels might benefit most from the combined use of urine and serum factors. In order to broaden our approach, urine samples were tested for an additional 198 biomarkers. A set of urines obtained from 45 healthy control individuals was included in this analysis. Multivariate analysis utilizing the MMC algorithm demonstrated that a urine 3-biomarker panel in combination with serum CA 19-9 outperformed the best serum biomarker panel for the discrimination of PDAC from benign disease (Fig. 6). This panel did not outperform a serum biomarker panel consisting of CA 19-9, CEA, and TIMP-1, identified in our recent report (68), in terms of sensitivity (72% vs 76%) at 90% specificity. However, our previous study utilized a much larger patient cohort and candidate biomarker array. For the discrimination of patients with PDAC from healthy controls, a urine biomarker panel consisting of human growth factor (HGF) and stem cell factor (SCF) performed optimally with a sensitivity of 93% at a specificity of 90%. This panel did outperform the serum biomarker panel consisting of CA 19-9, sICAM-1, and OPG, which provided a sensitivity/specificity ratio of 88%/90% in our previous report (68). Thus, similar to our observation regarding ovarian cancer, the combined use of urine and serum biomarkers may provide the most robust means of improvement for the development of novel screening tools.
Fig. 5.
Comparative performance of urine and serum biomarkers in pancreatic cancer. Paired sera and urines obtained from 55 patients diagnosed with pancreatic ductal adenocarcinoma and 44 patients diagnosed with a spectrum of benign pancreatic conditions were evaluated using Luminex multiplexed immunoassays for 20 biomarkers. Biomarker level distributions in urine and serum are presented along with ROC curves and AUC values (solid line: urine; dashed line: serum).
Fig. 6.
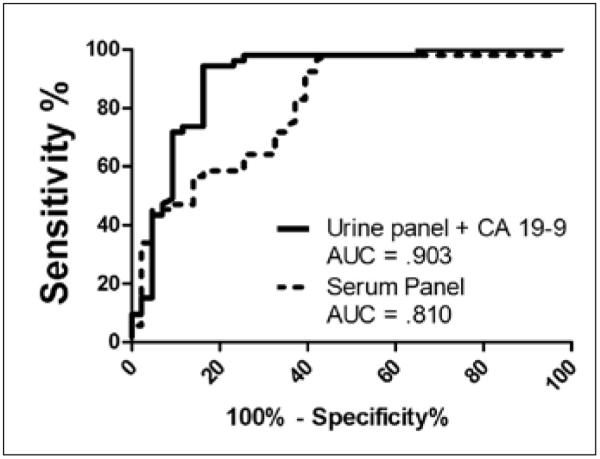
Performance of multimarker panels for the discrimination of pancreatic cancer patients from benign controls. The top performing urine and serum biomarker panels for the discrimination of pancreatic cancer patients from benign controls were identified using the Metropolis-Monte Carlo (MMC) algorithm. The best urine panel consisted of CA 125, insulin, and H-FABP while the top serum biomarker panel consisted of sICAM-1, TIMP-1, TIMP-4, CA 125, and CA 19-9. The addition of serum CA 19-9 to the urine panel resulted in optimal performance.
CONCLUSIONS
Although the incremental advancement of therapeutic agents and strategies has led to significant progress in cancer care over the course of the last decade, the accurate and timely detection and diagnosis of malignancy remains the single most important prognostic factor for nearly all affected individuals. Dramatic improvements in survival stemming from early detection are well described for each of the human cancers discussed in this review; however, each disease presents a unique set of challenges which has hampered or precluded the implementation of widespread screening. Despite the availability of useful biomarkers such as CA 125 and CA 19-9, the low prevalence associated with both ovarian and pancreatic cancer represents a significant obstacle which researchers and clinicians have yet to overcome. The high cost, morbidity, and patient anxiety associated with the diagnostic procedures employed within the settings of ovarian and pancreatic cancer, including both surgical and invasive imaging procedures, render the high level of false positive results generated through suboptimal screening practices unacceptable. While the increased prevalence of breast cancer allows for more permissive screening requirements, the disappointing performance of currently used methods such as mammography, the high cost of adjunct imaging modalities, and the current lack of useful biomarkers have limited the efficacy of screening strategies. Several avenues exist for the improvement and optimization of biomarker-based diagnostic tools. These include the development of multimodal screening strategies which incorporate biomarker testing and imaging; the discovery and development of novel specific biomarkers; and the use of alternative analytical biofluids displaying advantageous characteristics for biomarker development. The expanded use of urine in biomarker investigations offers the potential to spark improvement within each of these avenues and propel biomarker-based screening tools into broad clinical use.
The use of urine in biomarker analyses raises questions regarding the processing and filtration of serum proteins, particularly those of high molecular weight. This issue is well illustrated through our observations concerning CA 125, which did not productively contribute to diagnostic panel development when measured in urine. A plausible explanation for our observations stems from the considerable size of the CA 125 glycoprotein, estimated at 3–5 MDa, and the estimated molecular weight cutoff associated with glomerular filtration, 30–50 kDa. CA 125 was detectable in urine, suggesting that fragments of the molecule do indeed pass through the glomerulus in a form which can be recognized by the immunoassay; however, the observed results indicate that cleavage processes responsible for such fragmentation are not reliable indicators of serum CA 125 levels. Several other biomarkers included in this investigation were also relatively large, with a molecular weight greater than 100 kDa, including CA 15-3, sEGFR, CEA, sVCAM-1 and MPO. A recent study examining the mechanisms of glomerular filtration concluded that in addition to molecular size, additional factors such as molecular conformation, charge, and deformability account for the ability of an individual molecule to be filtered (79). The authors of that study demonstrate that molecules as large as 350–500 kDa are rapidly cleared intact through the glomerulus. Such a phenomenon may indeed play a role in the detection of the protein biomarkers listed above, but it is also likely that the observed urine biomarker levels represent specific proteolytic cleavage processes. The latter notion suggests that observed increases or decreases in serum biomarker levels in cancer patients relative to controls may not persist in urine. We observed exactly that for CA 125, EGFR, MMP-9, MPO, and sVCAM-1 in our pancreatic cancer study described above.
The advancement of biomarker studies in urine will also require a detailed assessment of sampling strategies and methods of data normalization. The use of spot urines, or urines arbitrarily collected at the time of physician visit, represents a practical approach to urine collection, although this strategy also presents the disadvantages of variation in biomarker measurements resulting from dilution due to fluid intake, sample volume, and varying rates of urine production. As a result, urine biomarker levels are dependent not only on the rate of biomarker production, processing, filtration, and excretion, but also on the urinary flow rate. Normalization based on urine creatinine (UCr), a waste product of muscle metabolism which is excreted in urine via the glomerulus at relatively constant rates, is commonplace in urine biomarker studies (80, 81). However, the appropriateness of creatinine normalization in the study of urine biomarkers of cancer has not been examined in detail. Recent studies have demonstrated that UCr excretion rates vary significantly across individuals in general, across demographic and ethnic populations, and within individuals as a function of time and health status (80, 82–85). Thus, whenever the excretion rate of a particular biomarker of interest does not share a linear relationship with that of UCr, normalization based on UCr levels may confound biomarker measurements (80, 82, 86, 87). UCr excretion rates have also been observed to vary in response to gender, age, dietary protein levels, chronic inflammation, physical activity, and illness (88–93). Given that, within the setting of cancer, many of the factors described above are likely to coalesce, the relevance of UCr normalization in cancer biomarker studies should be further examined. In our studies, UCr normalization was performed and individual biomarker levels were evaluated both as absolute measurements and as ratios based on UCr levels. In general, markers which differed significantly between the case and control groups based on absolute biomarker levels maintained that significance when normalized data were analyzed. Biomarkers displaying the largest magnitude differences between groups, and thus those selected for diagnostic panel development, were impacted the least by creatinine normalization. Going forward, it may become apparent that the stringent performance requirements imposed upon biomarker-based screening tools, which necessitate the selection of highly altered and specific protein factors, will mitigate the need for normalization based on UCr.
The study of serum biomarkers associated with the spectrum of human malignancies has had a profound impact on the overall clinical management of patients, particularly in the areas of screening, diagnosis, prognosis, and monitoring. However, while much has been accomplished, much more remains to be done. Serum biomarker-based tests have thus far failed to overcome the specific challenges posed within each malignant setting and achieve their promised clinical goals. Further advancement will require the development of novel methods and strategies. The use of urine in biomarker analyses has the potential to exceed and complement the results observed in serum. Further development and validation of biomarker testing in both serum and urine should help maximize the clinical impact of these tools.
List of Abbreviations
- ACTH
adrenocorticotropic hormone
- AUC
area under the curve
- CEA
carcinoembryonic antigen
- CRP
C-reactive protein
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- EUS
endoscopic ultrasound
- FSH
follicle stimulating hormone
- HE4
human epididymus protein 4
- HGF
human growth factor
- IGFBP
insulin-like growth factor binding protein
- MCA
mucin-like carcinoma-associated antigen
- MIC-1
macrophage inhibitrory cytokine 1
- MIF
macrophage inhibitory factor
- MMC
Metropolis-Monte Carlo
- MMP
matrix metalloproteinase
- MPO
myeloperoxidase
- MRI
magnetic resonance imaging
- NGAL
neutrophil gelatinase-associated lipocalin
- OC
osteocalcin
- OPG
osteoprotegrin
- OPN
osteopontin
- PDAC
pancreatic ductal adenocarcinoma
- PTH
parathyroid hormone
- ROC
receiver operating characteristic
- ROMA
risk of ovarian malignancy algorithm
- SAA
serum amyloid A
- sICAM
soluble intercellular adhesion molecule
- sVCAM
soluble vascular cell adhesion molecule
- TIMP
tissue inhibitor of metalloproteinase
- TPA
tissue polypeptide antigen
- tPAI-1
tissue plasminogen activator inhibitor 1
- TPS
tissue polypeptide-specific antigen
- TSH
thyroid stimulating hormone
- UCr
urine creatinine
- UGP
urinary gonadotropic peptide
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest statement: The authors declare no competing interests.
REFERENCES
- 1.Koomen JM, Li D, Xiao LC, et al. Direct tandem mass spectrometry reveals limitations in protein profiling experiments for plasma biomarker discovery. J Proteome Res. 2005;4:972–81. doi: 10.1021/pr050046x. [DOI] [PubMed] [Google Scholar]
- 2.Teisner B, Davey MW, Grudzinskas JG. Interaction between heparin and plasma proteins analysed by crossed immunoelectrophoresis and affinity chromatography. Clin Chim Acta. 1983;127:413–7. doi: 10.1016/0009-8981(83)90170-5. [DOI] [PubMed] [Google Scholar]
- 3.Coon JJ, Zürbig P, Dakna M, et al. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl. 2008;2:964–73. doi: 10.1002/prca.200800024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schaub S, Wilkins J, Weiler T, Sangster K, Rush D, Nickerson P. Urine protein profiling with surface-enhanced laser-desorption/ionization time-of-flight mass spectrometry. Kidney Int. 2004;65:323–32. doi: 10.1111/j.1523-1755.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 5.Decramer S, Gonzalez de Peredo A, Breuil B, et al. Urine in clinical proteomics. Mol Cell Proteomics. 2008;7:1850–62. doi: 10.1074/mcp.R800001-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Ye B, Skates S, Mok SC, et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin Cancer Res. 2006;12:432–41. doi: 10.1158/1078-0432.CCR-05-0461. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer E, Mischak H, Novak J. High resolution proteome/peptidome analysis of body fluids by capillary electrophoresis coupled with MS. Proteomics. 2006;6:5615–27. doi: 10.1002/pmic.200600230. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard P, Leverett R. Constancy of urinary creatinine excretion. J Lab Clin Med. 1958;51:211–8. [PubMed] [Google Scholar]
- 9.Thongboonkerd V, Malasit P. Renal and urinary proteomics: current applications and challenges. Proteomics. 2005;5:1033–42. doi: 10.1002/pmic.200401012. [DOI] [PubMed] [Google Scholar]
- 10.Cole LA, Schwartz PE, Wang YX. Urinary gonadotropin fragments (UGF) in cancers of the female reproductive system. I. Sensitivity and specificity, comparison with other markers. Gynecol Oncol. 1988;31:82–90. doi: 10.1016/0090-8258(88)90273-9. [DOI] [PubMed] [Google Scholar]
- 11.Plebani M, Navaglia F, Basso D, et al. Combined use of urinary UGP and serum CA 125 in the diagnosis of gynecological cancers. Anticancer Res. 1996;16:3833–8. [PubMed] [Google Scholar]
- 12.Walker R, Crebbin V, Stern J, Scudder S, Schwartz P. Urinary gonadotropin peptide (UGP) as a marker of gynecologic malignancies. Anticancer Res. 1994;14:1703–9. [PubMed] [Google Scholar]
- 13.Hellstrom I, Heagerty PJ, Swisher EM, et al. Detection of the HE4 protein in urine as a biomarker for ovarian neoplasms. Cancer Lett. 2010;296:43–8. doi: 10.1016/j.canlet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badgwell D, Lu Z, Cole L, et al. Urinary mesothelin provides greater sensitivity for early stage ovarian cancer than serum mesothelin, urinary hCG free beta subunit and urinary hCG beta core fragment. Gynecol Oncol. 2007;106:490–7. doi: 10.1016/j.ygyno.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson NS, Bermudez Y, Badgwell D, et al. Urinary levels of Bcl-2 are elevated in ovarian cancer patients. Gynecol Oncol. 2009;112:60–7. doi: 10.1016/j.ygyno.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drenberg CD, Saunders BO, Wilbanks GD, et al. Urinary angiostatin levels are elevated in patients with epithelial ovarian cancer. Gynecol Oncol. 2010;117:117–24. doi: 10.1016/j.ygyno.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Abdullah-Soheimi SS, Lim BK, Hashim OH, Shuib AS. Patients with ovarian carcinoma excrete different altered levels of urine CD59, kininogen-1 and fragments of inter-alpha-trypsin inhibitor heavy chain H4 and albumin. Proteome Sci. 2010;8:58. doi: 10.1186/1477-5956-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petri AL, Simonsen AH, Hogdall E, et al. Comparison of proteomic biomarker panels in urine and serum for ovarian cancer diagnosis. Proteomics Clin Appl. 2010;4:304–14. doi: 10.1002/prca.200900042. [DOI] [PubMed] [Google Scholar]
- 19.Petri AL, Simonsen AH, Yip TT, et al. Three new potential ovarian cancer biomarkers detected in human urine with equalizer bead technology. Acta Obstet Gynecol Scand. 2009;88:18–26. doi: 10.1080/00016340802443830. [DOI] [PubMed] [Google Scholar]
- 20.Slupsky CM, Steed H, Wells TH, et al. Urine metabolite analysis offers potential early diagnosis of ovarian and breast cancers. Clin Cancer Res. 2010;16:5835–41. doi: 10.1158/1078-0432.CCR-10-1434. [DOI] [PubMed] [Google Scholar]
- 21.Nolen B, Velikokhatnaya L, Marrangoni A, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010;117:440–5. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yurkovetsky Z, Skates S, Lomakin A, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–66. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs IJ, Menon U. Progress and challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics. 2004;3:355–66. doi: 10.1074/mcp.R400006-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Menon U, Jacobs IJ. Ovarian cancer screening in the general population: current status. Int J Gynecol Cancer. 2001;11(Suppl 1):3–6. doi: 10.1046/j.1525-1438.2001.11(suppl.1)sup1003.x. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald ND, Rosenthal AN, Jacobs IJ. Screening for ovarian cancer. Ann Acad Med Singapore. 1998;27:676–82. [PubMed] [Google Scholar]
- 26.Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–8. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–6. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore RG, Jabre-Raughley M, Brown AK, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010;203:228, e1–6. doi: 10.1016/j.ajog.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Cancer Society Statistics. Accessed at www.cancer.org.
- 30.Carney PA, Miglioretti DL, Yankaskas BC, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–75. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kopans DB. Clinical breast examination for detecting breast cancer. JAMA. 2000;283:1688. author reply 1689. [PubMed] [Google Scholar]
- 32.Baker LH. Breast Cancer Detection Demonstration Project: five-year summary report. CA Cancer J Clin. 1982;32:194–225. doi: 10.3322/canjclin.32.4.194. [DOI] [PubMed] [Google Scholar]
- 33.Foxcroft LM, Evans EB, Joshua HK, Hirst C. Breast cancers invisible on mammography. Aust N Z J Surg. 2000;70:162–7. doi: 10.1046/j.1440-1622.2000.01763.x. [DOI] [PubMed] [Google Scholar]
- 34.Bartsch R, Wenzel C, Pluschnig U, et al. Prognostic value of monitoring tumour markers CA 15-3 and CEA during fulvestrant treatment. BMC Cancer. 2006;6:81. doi: 10.1186/1471-2407-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gion M, Mione R, Becciolini A, et al. Relationship between cytosol TPS, TPA and cell proliferation. Int J Biol Markers. 1994;9:109–14. doi: 10.1177/172460089400900208. [DOI] [PubMed] [Google Scholar]
- 36.Molina R, Agusti C, Filella X, et al. Study of a new tumor marker, CYFRA 21-1, in malignant and nonmalignant diseases. Tumour Biol. 1994;15:318–25. doi: 10.1159/000217908. [DOI] [PubMed] [Google Scholar]
- 37.Fuckar D, Dekanic A, Stifter S, et al. VEGF expression is associated with negative estrogen receptor status in patients with breast cancer. Int J Surg Pathol. 2006;14:49–55. doi: 10.1177/106689690601400109. [DOI] [PubMed] [Google Scholar]
- 38.Leonard GD, Low JA, Berman AW, Swain SM. CA 125 elevation in breast cancer: a case report and review of the literature. Breast J. 2004;10:146–9. doi: 10.1111/j.1075-122x.2004.21374.x. [DOI] [PubMed] [Google Scholar]
- 39.Danforth DN, Jr, Sgagias MK. Interleukin-1 alpha and interleukin-6 act additively to inhibit growth of MCF-7 breast cancer cells in vitro. Cancer Res. 1993;53:1538–45. [PubMed] [Google Scholar]
- 40.Taniguchi T, Toi M, Inada K, Imazawa T, Yamamoto Y, Tominaga T. Serum concentrations of hepatocyte growth factor in breast cancer patients. Clin Cancer Res. 1995;1:1031–4. [PubMed] [Google Scholar]
- 41.Ohuchi N, Matoba N, Taira Y, et al. Levels of circulating tumor-associated glycoprotein (TAG-72) in patients with carcinoma using a novel tumor marker, CA 72-4. Gan To Kagaku Ryoho. 1988;15:2767–72. [PubMed] [Google Scholar]
- 42.Yousef GM, Diamandis EP. Expanded human tissue kallikrein family--a novel panel of cancer biomarkers. Tumour Biol. 2002;23:185–92. doi: 10.1159/000064027. [DOI] [PubMed] [Google Scholar]
- 43.Kim Y, Koo I, Jung BH, Chung BC, Lee D. Multivariate classification of urine metabolome profiles for breast cancer diagnosis. BMC Bioinformatics. 2010;11(Suppl 2):S4. doi: 10.1186/1471-2105-11-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nam H, Chung BC, Kim Y, Lee K, Lee D. Combining tissue transcriptomics and urine metabolomics for breast cancer biomarker identification. Bioinformatics. 2009;25:3151–7. doi: 10.1093/bioinformatics/btp558. [DOI] [PubMed] [Google Scholar]
- 45.Frickenschmidt A, Frohlich H, Bullinger D, et al. Metabonomics in cancer diagnosis: mass spectrometry-based profiling of urinary nucleosides from breast cancer patients. Biomarkers. 2008;13:435–49. doi: 10.1080/13547500802012858. [DOI] [PubMed] [Google Scholar]
- 46.Schernhammer ES, Berrino F, Krogh V, et al. Urinary 6-sulphatoxymelatonin levels and risk of breast cancer in premenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:729–37. doi: 10.1158/1055-9965.EPI-09-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schernhammer ES, Berrino F, Krogh V, et al. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2008;100:898–905. doi: 10.1093/jnci/djn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schernhammer ES, Hankinson SE. Urinary melatonin levels and postmenopausal breast cancer risk in the Nurses' Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:74–9. doi: 10.1158/1055-9965.EPI-08-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pories SE, Zurakowski D, Roy R, et al. Urinary metalloproteinases: noninvasive biomarkers for breast cancer risk assessment. Cancer Epidemiol Biomarkers Prev. 2008;17:1034–42. doi: 10.1158/1055-9965.EPI-07-0365. [DOI] [PubMed] [Google Scholar]
- 50.Umemori Y, Ohe Y, Kuribayashi K, et al. Evaluating the utility of N1,N12-diacetylspermine and N1,N8-diacetylspermidine in urine as tumor markers for breast and colorectal cancers. Clin Chim Acta. 2010;411:1894–9. doi: 10.1016/j.cca.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Ward H, Chapelais G, Kuhnle GG, Luben R, Khaw KT, Bingham S. Breast cancer risk in relation to urinary and serum biomarkers of phytoestrogen exposure in the European Prospective into Cancer-Norfolk cohort study. Breast Cancer Res. 2008;10:R32. doi: 10.1186/bcr1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jesneck JL, Mukherjee S, Yurkovetsky Z, et al. Do serum biomarkers really measure breast cancer? BMC Cancer. 2009;9:164. doi: 10.1186/1471-2407-9-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87–93. doi: 10.1016/s0361-090x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 54.Lowenfels AB, Maisonneuve P. Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am. 2002;16:1–16. doi: 10.1016/s0889-8588(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 55.Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004;59:99–111. [PubMed] [Google Scholar]
- 56.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 57.Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007;33:266–70. doi: 10.1016/j.ejso.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–8. [PubMed] [Google Scholar]
- 59.Eskelinen M, Haglund U. Developments in serologic detection of human pancreatic adenocarcinoma. Scand J Gastroenterol. 1999;34:833–44. doi: 10.1080/003655299750025273. [DOI] [PubMed] [Google Scholar]
- 60.Hustinx SR, Cao D, Maitra A, et al. Differentially expressed genes in pancreatic ductal adenocarcinomas identified through serial analysis of gene expression. Cancer Biol Ther. 2004;3:1254–61. doi: 10.4161/cbt.3.12.1238. [DOI] [PubMed] [Google Scholar]
- 61.Koopmann J, Rosenzweig CN, Zhang Z, et al. Serum markers in patients with resectable pancreatic adenocarcinoma: macrophage inhibitory cytokine 1 versus CA19-9. Clin Cancer Res. 2006;12:442–6. doi: 10.1158/1078-0432.CCR-05-0564. [DOI] [PubMed] [Google Scholar]
- 62.Slesak B, Harlozinska-Szmyrka A, Knast W, Sedlaczek P, van Dalen A, Einarsson R. Tissue polypeptide specific antigen (TPS), a marker for differentiation between pancreatic carcinoma and chronic pancreatitis. A comparative study with CA 19-9. Cancer. 2000;89:83–8. doi: 10.1002/1097-0142(20000701)89:1<83::aid-cncr12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 63.Zhou W, Sokoll LJ, Bruzek DJ, et al. Identifying markers for pancreatic cancer by gene expression analysis. Cancer Epidemiol Biomarkers Prev. 1998;7:109–12. [PubMed] [Google Scholar]
- 64.Moniaux N, Chakraborty S, Yalniz M, et al. Early diagnosis of pancreatic cancer: neutrophil gelatinase-associated lipocalin as a marker of pancreatic intraepithelial neoplasia. Br J Cancer. 2008;98:1540–7. doi: 10.1038/sj.bjc.6604329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carpelan-Holmstrom M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res. 2002;22:2311–6. [PubMed] [Google Scholar]
- 66.Hayakawa T, Naruse S, Kitagawa M, et al. A prospective multicenter trial evaluating diagnostic validity of multivariate analysis and individual serum marker in differential diagnosis of pancreatic cancer from benign pancreatic diseases. Int J Pancreatol. 1999;25:23–9. doi: 10.1385/IJGC:25:1:23. [DOI] [PubMed] [Google Scholar]
- 67.Faca VM, Song KS, Wang H, et al. A mouse to human search for plasma proteome changes associated with pancreatic tumor development. PLoS Med. 2008;5:e123. doi: 10.1371/journal.pmed.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brand RE, Nolen BM, Zeh HJ, et al. Serum biomarker panels for the detection of pancreatic cancer. Clin Cancer Res. 2011;17:805–16. doi: 10.1158/1078-0432.CCR-10-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dianxu F, Shengdao Z, Tianquan H, et al. A prospective study of detection of pancreatic carcinoma by combined plasma K-ras mutations and serum CA19-9 analysis. Pancreas. 2002;25:336–41. doi: 10.1097/00006676-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Chen J, Chang P, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2009;2:807–13. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Farrell JJ, Zhou H, et al. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 138:949–57. e1–7. doi: 10.1053/j.gastro.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allen PJ, Qin LX, Tang L, Klimstra D, Brennan MF, Lokshin A. Pancreatic cyst fluid protein expression profiling for discriminating between serous cystadenoma and intraductal papillary mucinous neoplasm. Ann Surg. 2009;250:754–60. doi: 10.1097/SLA.0b013e3181bd7f20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haab BB, Porter A, Yue T, et al. Glycosylation variants of mucins and CEACAMs as candidate biomarkers for the diagnosis of pancreatic cystic neoplasms. Ann Surg. 251:937–45. doi: 10.1097/SLA.0b013e3181d7738d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motoo Y, Watanabe H, Yamaguchi Y, et al. Urinary gonadotropin peptide in patients with cancer of digestive organs. Anticancer Res. 1996;16:2041–8. [PubMed] [Google Scholar]
- 75.Weeks ME, Hariharan D, Petronijevic L, et al. Analysis of the urine proteome in patients with pancreatic ductal adenocarcinoma. Proteomics Clin Appl. 2008;2:1047–57. doi: 10.1002/prca.200780164. [DOI] [PubMed] [Google Scholar]
- 76.Szajda SD, Snarska J, Jankowska A, Puchalski Z, Zwierz K. Isoenzymes A and B of N-acetyl-beta-D-hexosaminidase in serum and urine of patients with pancreatic cancer. Hepatogastroenterology. 2008;55:695–8. [PubMed] [Google Scholar]
- 77.Szajda SD, Waszkiewicz N, Chojnowska S, Zwierz K. Carbohydrate markers of pancreatic cancer. Biochem Soc Trans. 2011;39:340–3. doi: 10.1042/BST0390340. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi K, Nakamura M, Shirahane K, et al. Urine diacetylspermine as a novel tumour maker for pancreatobiliary carcinomas. Dig Liver Dis. 2005;37:190–4. doi: 10.1016/j.dld.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Ruggiero A, Villa CH, Bander E, et al. Paradoxical glomerular filtration of carbon nanotubes. Proc Natl Acad Sci U S A. 2010;107:12369–74. doi: 10.1073/pnas.0913667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heavner DL, Morgan WT, Sears SB, Richardson JD, Byrd GD, Ogden MW. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers' spot and 24-h urines. J Pharm Biomed Anal. 2006;40:928–42. doi: 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Suwazono Y, Åkesson A, Alfvén T, Järup L, Vahter M. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10:117–26. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- 82.Alessio L, Berlin A, Dell'Orto A, Toffoletto F, Ghezzi I. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55:99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- 83.Curtis G, Fogel M. Creatinine excretion: diurnal variation and variability of whole and part-day measures. A methodologic issue in psychoendocrine research. Psychosom Med. 1970;32:337–50. doi: 10.1097/00006842-197007000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Hsu J, Johansen KL, Hsu CY, Kaysen GA, Chertow GM. Higher serum creatinine concentrations in black patients with chronic kidney disease: beyond nutritional status and body composition. Clin J Am Soc Nephrol. 2008;3:992–7. doi: 10.2215/CJN.00090108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 1998;32:992–9. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- 86.Jatlow P, McKee S, O'Malley SS. Correction of urine cotinine concentrations for creatinine excretion: is it useful? Clin Chem. 2003;49:1932–4. doi: 10.1373/clinchem.2003.023374. [DOI] [PubMed] [Google Scholar]
- 87.Waikar SS, Sabbisetti VS, Bonventre JV. Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 2010;78:486–94. doi: 10.1038/ki.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Aloul M, Jackson M, Bell G, Ledson M, Walshaw M. Comparison of methods of assessment of renal function in cystic fibrosis (CF) patients. J Cyst Fibros. 2007;6:41–7. doi: 10.1016/j.jcf.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol. 2005;39:15–20. doi: 10.1002/ppul.20138. [DOI] [PubMed] [Google Scholar]
- 90.Neubert A, Remer T. The impact of dietary protein intake on urinary creatinine excretion in a healthy pediatric population. J Pediatr. 1998;133:655–9. doi: 10.1016/s0022-3476(98)70107-6. [DOI] [PubMed] [Google Scholar]
- 91.Penner SB, Bernstein KN, Fine A. Changes in endogenous creatinine clearance in man on a controlled protein diet: Effect of route of administration of a protein load. Clin Invest Med. 1990;13:233–6. [PubMed] [Google Scholar]
- 92.Tan KHV, Mulheran M, Knox AJ, Smyth AR. Aminoglycoside prescribing and surveillance in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:819–23. doi: 10.1164/rccm.200109-012CC. [DOI] [PubMed] [Google Scholar]
- 93.Worsfold M, Davie MWJ, Haddaway MJ. Age-related changes in body composition, hydroxyproline, and creatinine excretion in normal women. Calcif Tissue Int. 1999;64:40–4. doi: 10.1007/s002239900576. [DOI] [PubMed] [Google Scholar]