SUMMARY
Type 2 diabetes and other noncommunicable diseases are a growing public health challenge globally. An estimated 285 million people, corresponding to 6.4% of the world’s adult population, has diabetes, which is expected to reach 552 million by the International Diabetes Federation in 2030. A much larger segment of the world’s population, approximating 79 million individuals in the USA alone, has prediabetes. Globally, a relatively small percentage of those with diabetes or prediabetes are diagnosed with the potential for developing chronic complications. To address this epidemic, governments, in concert with the private sector, need to set policies that promote healthy nutritional and agricultural policies, favor modifications in the environment that encourage greater physical activity and make prevention affordable for all citizens at high risk. The public health sector has the charge of translating evidence-based findings into practical, accessible and cost-effective programs and monitoring the process to continuously improve prevention initiatives. The clinical sector has the formidable challenge of screening and identifying those at high risk and referring them to accredited intervention programs. There is a need to explore additional cost-effective interventions that are customized to meet individual needs that can be offered at the community and clinical levels. Thus, all three sectors, government, public health and clinical, each have a critical role in this process and by working in a partnership, ought to create the necessary synergies essential for making substantial forays in the prevention of Type 2 diabetes.
As Type 2 diabetes and other noncommunicable diseases (NCD) are a growing public health challenge globally, the World Economic Forum foresees a severe disaster impacting global economic growth [1,2]. An estimated 285 million people, corresponding to 6.4% of the world’s adult population has diabetes, with the prevalence varying from 10.2% in the western Pacific to 3.8% in the African region. This is expected to reach 552 million by 2030, corresponding to 7.8% of the adult population with the African region expected to experience the greatest increase. In total, 70% of cases occur in low- and middle-income countries (LMIC) resulting from consumption of high-calorie foods as well as decreasing physical activity. Diabetes in LMICs affects an increasing number of younger individuals especially in their productive years.
Dysglycemia characterized by glucose levels not meeting conventional criteria for diabetes but yet higher than normal constitutes the population with prediabetes [3,4], a condition that may exist for many years before Type 2 diabetes occurs. In the USA, approximately a quarter (and as high as 50–80% in LMICs) of individuals with diabetes and only 7% of those with prediabetes are diagnosed with the potential for developing chronic complications due to delayed detection and timely diagnosis of prediabetes [5,101]. A substantial number of individuals (up to 70%) with prediabetes may develop diabetes in subsequent years [6], with an average annual risk approximating 5–10% compared with well below 1% in normoglycemic individuals [7]. Furthermore, prediabetes is associated with a 10–40% increased risk of cardiovascular complications, as well as stroke and microvascular disease [8,9]. Early recognition of prediabetes in high-risk individuals is, therefore, crucial as lifestyle modification in particular, as well as medication, have been shown to be effective in reducing the progression to diabetes in seminal studies conducted across diverse cultures [8–11]. Details of the various diabetes prevention programs have been recently summarized including publications describing sustainability of their effects [12].
This paper will review the definition of prediabetes and surrounding controversy, subsequently consider current community interventions for the prevention of Type 2 diabetes and then describe global healthcare policies and formulate recommendations for further efforts at prevention.
Definition of prediabetes
American Diabetes Association
The American Diabetes Association (ADA) [2,13–15] clinical guidelines define prediabetes as either impaired fasting glucose (IFG; fasting plasma glucose [FPG] = 5.6–6.9 mmol/l) or impaired glucose tolerance (IGT; glucose = 7.8–11.1 mmol/l during a 2-h oral glucose tolerance test [OGTT]). FPG is the preferred test for screening as it is convenient and the receiver operator curve (ROC) analysis indicates that a FPG cut-point of 5.6 mmol/l gives the best combination of sensitivity and specificity for predicting future diabetes. Diagnosis of diabetes is based on a FPG of ≥7.0 mmol/l and/or a postchallenge glucose (120 min) level of ≥11.1 mmol/l, or a casual plasma glucose of ≥11.1 mmol/l in the presence of symptoms with an abnormal result requiring repeat measurement in an asymptomatic individual [12].
The ADA recommends glycated hemoglobin (HbA1c) as another screening option for prediabetes [14,15]. A HbA1c level between 5.7 and 6.4% identifies those at risk for diabetes whereas those with a level of 6.0–6.5% are at particularly high risk. Diabetes is diagnosed by a HbA1c value ≥6.5%, as most epidemiological studies and recently in the DETECT-2 analysis [16] indicated that there is an increased risk for retinopathy with HbA1c levels approximating 6.5% (comparable to the risk for corresponding FPG [≥7.0 mmol/l] and 2 h plasma glucose [≥11.1 mmol/l]).
WHO
WHO [17,102] has recommended that the diagnostic cut-point for IFG should be maintained at 6.1 mmol/l [2] (vs 5.6 mmol/l suggested by the Expert Committee [10] and the ADA [15]). This decision was based on concerns about the substantial increase in IFG prevalence by lowering the cut-point to 5.6 mmol/l and the associated impact on individuals and healthcare systems [18]. WHO advocated maintaining established IGT criteria with the recommendation that individuals with IFG undergo an OGTT to exclude IGT or diabetes.
The WHO recommended HbA1c >6.5% as a diagnostic threshold for diabetes whereas a lower value does not exclude diabetes using glucose-based criteria. The WHO also indicated that there is currently ‘insufficient evidence’ to make any formal recommendation on the interpretation of HbA1c levels below 6.5% [17].
Caveats of current definitions
The early identification of prediabetes permits intensive management to delay the progression to diabetes and to potentially prevent the development of chronic complications [19]. However, there is a lack of consensus as to which screening procedure is most appropriate [20]. For decades, the diagnosis of prediabetes has been based upon FPG and/or 2-h glucose levels after an OGTT. Advantages of the FPG include its ease and inexpensiveness with universally available automated instruments. Similarly, OGTT serves as another option for diagnosing prediabetes, the 2-h glucose level being a better predictor of cardiovascular morbidity and mortality than the FPG [21,22]. Nevertheless, both glucose-based measurements are subject to methodological limitations, particularly in terms of biologic and analytic variability. Moreover, OGTT (which is time consuming and expensive) has relatively poor reproducibility [21,22].
HbA1c reflecting average plasma glucose over the previous 8–12 weeks, when compared with glucose testing, avoids the problem of day-to-day variability of glucose values and the need to fast. Moreover, HbA1c measurement also has superior methodological attributes when compared with blood glucose, such as minimal intraindividual variability [21,22]. Therefore, as standardization procedures have improved, the availability of HbA1c as a single, nonfasting blood test could facilitate successful population-level screening programs, as has also been recently demonstrated by the WEQAYA study and by others [23,24].
On the other hand, the use of HbA1c implies that stringent quality assurance tests are in place and assays are standardized to criteria aligned with international reference values and that there are no conditions present that preclude its accurate measurement. Indeed, HbA1c may be affected by a variety of genetic, hematological and illness-related factors, with hemoglobinopathies being the most common, as well as certain anemias and disorders associated with red cell turnover such as malaria [17–20,25,26,102]. Preliminary data from the FIN-D2D study also suggest that the HbA1c value is age dependent. Thus, in healthy populations screened with OGTT, in older individuals compared with younger ones, a particular HbA1c value implied slightly lower fasting glucose but relatively higher 2-h glucose levels, a finding that needs to be verified in different populations [27]. In addition, limited availability and cost in many countries may not allow HbA1c testing as a practical option for screening purposes.
Several studies indicate that the HbA1c range of 5.7–6.4% permits adequate screening for prediabetes and is an excellent predictor of risk for progression to diabetes and/or development of cardiovascular disease [19–21,28,29]. However, the use of HbA1c as a screening test remains controversial [30]. Many individuals diagnosed with prediabetes based on glucose testing, in particular IFG, are reclassified as normoglycemic when a HbA1c determination is used. Therefore, Mann et al. [31] and others [32–34] stressed that the use of HbA1c alone, in part due to lesser sensitivity, might overlook a large number of individuals with prediabetes. Alternatively, the use of HbA1c may also lead to the reclassification of a considerable number of subjects without IFG to having prediabetes [31].
As the evolution to diabetes follows a continuum [35], risk of diabetes and/or cardiovascular complications could extend below the current recommended threshold criteria for diagnosis. Contradictory data, in particular with regard to the sensitivity of HbA1c, limit the ability to accurately diagnose prediabetes. This, in conjunction with divergent global definitions of prediabetes, infuses discordance in healthcare policy with regard to precisely defining the appropriate target population who will benefit most from prevention strategies. Threshold criteria for defining risk with greater accurately should be based on outcomes from future epidemiological studies. Glucose and HbA1c criteria for diagnosing dysglycemic conditions appear to be discrepant as they may be defining different populations at risk for progression to diabetes. Therefore, the combination of FPG and HbA1c may represent a more adequate strategy for identifying individuals at risk [36,37].
Community interventions & policies for the prevention of Type 2 diabetes
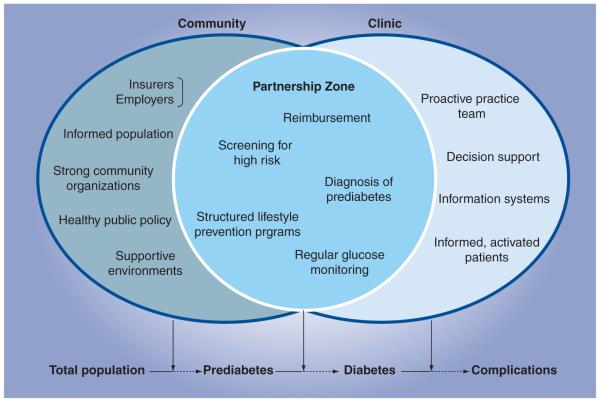
Healthcare systems are generally focused on care and not prevention. Most European countries have national health insurances that finance care after an International Classification of Diseases 10/11 diagnosis has been made. Preventing Type 2 diabetes requires complementary clinical and public health strategies at the community level [38,39]. The clinical sector plays a significant role in identifying risk status, referring individuals at high risk to community-based lifestyle programs, providing nutrition counseling, prescribing medication when required and treating those who develop diabetes. On the other hand, the public health sector has a major role in monitoring diabetes risk, mobilizing partnerships to establish diabetes prevention services for individuals at high risk and assuring the quality of these programs. Furthermore, the public health sector needs to examine policies that support risk reduction by facilitating lifestyle modification and changes to community environments that make it easier to practice healthy behaviors. The synergies of clinical and community public health sectors are represented in Figure 1.
Figure 1. Prevention of Type 2 diabetes: the community–civic partnership model.
Provided by CDC, Division of Diabetes Translation. Elements in the clinical component are adapted from the Chronic Care Model, MacColl Institute for Healthcare Innovation. The elements listed in this figure are not intended to be all-inclusive, but to provide information on the kinds of elements contributed by each sector and shared across sectors.
As progression from low to high risk for diabetes occurs as a continuous process, effective interventions along this continuum are theoretically needed. The evidence for diabetes prevention, however, mostly involve those at high risk (i.e., with prediabetes [40]) and as large-scale diabetes prevention programs have focused on the latter, it has not been established whether intervening in lower risk individuals will be equally effective.
Several translational research studies conducted in real-world settings have implemented modified versions of the lifestyle intervention from clinical trials, such as the US Diabetes Prevention Program (US DPP) [8] and Finnish Diabetes Prevention Program [11]. The structured lifestyle intervention includes an initial series of sessions (usually delivered weekly) that help participants learn skills to reduce caloric intake, increase physical activity and problem solve to achieve weight loss, which is followed by a series of maintenance sessions (usually delivered monthly).
A major focus of translational research is how to best utilize limited resources for delivering lifestyle intervention to significantly decrease the incidence of and health costs associated with Type 2 diabetes. Uusitupa et al. summarized published implementation studies conducted in various countries with different designs and outcome measures and described prevention activities in Finland [41]. The Finnish program, demonstrated that it was possible to prevent Type 2 diabetes in real life, primary healthcare settings [41,42]. Methods for recruiting high-risk subjects were simple and easy to use. Lifestyle changes and risk reduction of diabetes required a modest number of visits to health centers or occupational healthcare outpatient clinics. Moderate weight loss in very high-risk individuals was especially effective in reducing the risk of diabetes and reduction of cardiovascular risk [42]. The Life! Program in Australia, is based on the successful exchange of results from randomized trials and implementation trials in Finland and Australia. The latter represents yet another example of translating prevention research into a large-scale intervention in adults over 50 years of age at high risk of developing diabetes using community-based facilitators [43,44].
Ali et al. have recently published a systematic review and meta-analysis of 28 US translation studies based on the US DPP demonstrating that 12 months subsequent to the intervention, there was an average weight loss of 4% from baseline, which was similar regardless of whether the intervention was delivered by a healthcare professional or lay educator [45]. Furthermore, with each additional lifestyle session attended, weight loss increased by 0.26%.
In addition to demonstrating durability of treatment effect, it is also critical that healthcare costs associated with community-based lifestyle intervention for preventing Type 2 diabetes are assessed. Evidence from a simulation model projecting the costs and benefits of a nationwide community-based lifestyle intervention suggest that such a program would represent an efficient use of healthcare resources [46]. Despite considerable initial investment, within 25 years, the program would prevent or delay approximately 885,000 cases of Type 2 diabetes in the USA and produce savings of US$5.7 billion nationwide. Although cost savings would occur in both younger and older individuals, greater health and economic gains would be achieved if directed at those under the age of 65 years.
Reduction in the incidence of Type 2 diabetes on a population level requires collaboration among community-based organizations, insurance payers, healthcare and public health professionals, academia and others. In 2010, the US Congress authorized the CDC to establish the National Diabetes Prevention Program (National DPP) to translate and systematically scale the US DPP for individuals at high risk. The National DPP brings together the groups listed above and unifies delivery of proven lifestyle change programs in communities throughout the country. The National DPP consists of four components, as outlined below.
Training
The CDC established the Diabetes Training and Technical Assistance Center (DTTAC) at Emory University (Atlanta, GA, USA) to help increase the work force by providing training to lifestyle coaches and those who train lifestyle coaches. There are other organizations, such as the Young Men’s Christian Association (YMCA), that provide training so DTTAC also serves to coordinate training functions [103].
Program recognition
The CDC Diabetes Prevention Recognition Program (DPRP) [104] assures program quality, consistency, provides a registry of recognized programs and implements standardized reporting on performance of recognized programs.
Intervention sites
The YMCA and UnitedHealth Group (UHG) are the first to participate in the National DPP and are collaborating on instituting community-based prevention programs in which the YMCA delivers the lifestyle change program while the UHG provides third-party reimbursement for its beneficiaries. This is a new payment model in which an insurer reimburses a community-based organization based on performance. With implementation of the DPRP, more organizations are involved in program delivery and reimbursement.
Health marketing
Participant engagement and healthcare provider referrals are important for program success. CDC and others, such as the Diabetes Prevention and Control Alliance, are testing various marketing strategies to enhance program participation.
Implementing lifestyle change programs to prevent diabetes is not without challenges. In order to achieve the desired health outcomes on a large scale, the areas discussed in the following sections are among those that need to be addressed.
Identification of individuals at increased risk
The preponderance of evidence for diabetes prevention is derived from initiatives focusing on those at increased risk (i.e., with prediabetes) for diabetes [47] in which the target population was identified based on risk stratification and outcomes were measurable. Measuring risk, however, is more challenging when defined at the population level, and therefore validated instruments assessing risk gradients are required [48,49]. An example is a questionnaire based on the FINDRISK studies that has been implemented in several countries [49]. A two-step screening procedure starting with the FINDRISK questionnaire followed by a glucose test for those identified at increased risk might be the most cost-effective approach [49,50].
Standardization of lifestyle intervention
Policy development requires utilization of evidence-based, standardized lifestyle intervention recommendations that are customized to reflect cultural and individual circumstances. Furthermore, diabetes prevention is strongly related to an increase in physical activity and a reduction in fat and increased fiber consumption. Standardized recommendations for diabetes prevention can therefore be related to physiologically based core goals and increased effectiveness of policy development. This is exemplified by The European IMAGE project that has pioneered guidelines with a practice toolkit [51,52], as has NICE [53]. The National DPP is achieving this through the DPRP, which requires a standardized curriculum that recognized organizations can obtain gratis from the DPRP website. Alternatively, curricula can be evaluated to ensure that required content areas and program length are met.
Education & training of personnel
Implementation of diabetes prevention initiatives, although not requiring medical specialists per se, requires skilled personnel. There is a growing need for the development and implementation of training curricula for prevention personnel so that they may effectively instruct individuals at risk in sustained lifestyle change [54]. Curricula have been developed in Europe [55] and the USA [105], although it will take time until a critical mass has been trained. The number of skilled personnel will depend on the organizational structure in which they will work.
Monitoring
The public health sector can play an important role in continuous evaluation and monitoring to ensure successful implementation of diabetes prevention programs. Furthermore, this is vital for quality assurance and benchmarking of standardized procedures. Scientific outcome evaluation indicators and measurement recommendations (e.g., body weight, waist circumference, HbA1c and total energy intake) have been developed to monitor the effectiveness and efficiency of the programs [56]. Recent experience demonstrates that monitoring alone, as a function of quality management, is a driver for increasing the quality of intervention programs [57]. The CDC DPRP, as part of the National DPP, serves this monitoring function for diabetes prevention programs in the USA.
In addition to targeting high-risk individuals through lifestyle change programs in the community, general population level policy requires implementation of evidence and practice-based policies in modifying the environment and infrastructure to improve nutrition, decrease weight, increase physical activity and facilitate tobacco cessation. In some places, complementary strategies targeting those at high risk and the general population is not occurring and in some cases, only one segment of a population is involved. Some countries assign responsibilities for diabetes prevention to agricultural ministries in which activities are often restricted to aspects of food production, and therefore do not establish a prevention initiative. As LMICs often lack the financial resources to manage diabetes, they therefore may only develop strategies for lifestyle education as well as physical activity in schools [48]. Effective policies for food procurement and production, as well as strategies promoting healthy lifestyles in children, have important potential to contribute to diabetes prevention and should continue to be examined.
Global health policy & perspectives from the ground
As previously described, screening procedures for diabetes and prediabetes are fraught with complexity and inaccuracy, often making diagnosis difficult except in the most obvious circumstances. Nonetheless, based on fasting glucose or HbA1c levels, 35% of US adults aged 20 years or older were estimated to have prediabetes in 2005–2008 (50% of those aged 65 years or older). Thus, 79 million Americans aged 20 years or older are estimated to have prediabetes in 2010 [106].
Translating prevention initiatives to policy
Although the US DPP research study in 2002 [8], upon which translational models have been developed (see above), demonstrated the inarguable benefit of lifestyle modification in preventing the progression from IFG and/or IGT to Type 2 diabetes, the majority of the US population with prediabetes remains undiagnosed and untreated [6,58]. Although the reasons for this are unknown, national policies, such as public and provider education programs, need to be developed, especially as dissemination of national guidelines are generally ineffective in changing clinical practice [59]. There exists an urgent need to translate evidence from prevention initiatives into policy and affordable, feasible programs [59,60] in order to detect individuals at risk with appropriate referral to lifestyle intervention programs. This is particularly essential because once individuals with prediabetes progress to diabetes, management of the latter remains inadequate. In the USA where US$132 billion is spent annually on diabetes care, simultaneous control of glucose levels, blood pressure and lipid levels is achieved in less than 10% of individuals with diabetes [61,62]. Furthermore, effective prevention strategies should constitute a major approach particularly in view of the lengthy preclinical phase characterizing the transition from prediabetes to Type 2 diabetes that thereby provide an extended window for intervention [62].
Examining current public health policies with regard to diabetes prevention is vital, given the enormous economic and social burdens diabetes creates, as it most often affects individuals in the prime of their lives, reducing their productivity aside from driving direct healthcare expenditures. It has been estimated that the proportion of cardiovascular disease attributable to diabetes has increased over the past 50 years, highlighting the need for increased efforts at prevention and aggressively addressing cardiovascular risk factors among those with diabetes [63]. Indirect costs, such as decreased income, premature retirement and unemployment, can be even more costly than the direct expenditures associated with the condition [64]. Indeed, global costs of diabetes approximated US$500 billion in 2010 and are anticipated to reach US$745 billion in 2030.
Diabetes prevention & global health policies
Effective global public health policies are crucial for addressing diabetes and other NCDs, especially as they account for the preponderance of deaths worldwide and constitute a ‘slow-motion disaster’ [65]. Although NCDs are by definition noninfectious, Type 2 diabetes has also been elegantly characterized as an infectious disease by Matthews and Matthews in their 2010 Banting Memorial Lecture [66], and as such might be ‘eradicated’ in the same fashion. Calorie excess serves as a transmissible agent, propagated by inadequate food labeling and poorly regulated advertising vectors embedded within a reservoir of fast-food outlets providing cheap calories. Sedentary lifestyles provide a predisposing toxic milieu in which limited physical activity works in concert with consumption of excess calories leading to weight gain, obesity and increased risk of diabetes [66]. Analogous to an infectious pandemic, breaking the cycle of transmission in the case of diabetes must involve political will and decisive legislation and support by the medical community [66].
Whether diabetes is characterized as communicable or noncommunicable, both perspectives commonly point to the critical need for action. Thus, the increasing toll associated with NCDs led to a 2-day high-level meeting of the UN General Assembly in September 2011, creating an awareness of the enormity of the global problem. This meeting was constituted by unprecedented participation of global leadership from Heads of State, the WHO, nongovernmental organizations and member states. This was a very positive step for NCDs as well as global collaboration as the high-level meeting resulted in a political declaration calling for collaborative effort to reduce risk factors and strengthen national policies to prevent and control NCDs. While recognizing the NCD epidemic, the conference did not elucidate deadlines or targets or a system of accountability and neither was funding allocated for treatment or prevention. However, targets are under discussion and will be released before the end of 2012. Although the UN Summit did not address all of the expectations, it provided diabetes and NCDs with a global platform [67]. It was left to governments to “customize the implementation” of their commitments.
The International Diabetes Federation has been instrumental in providing an overall framework representing the global diabetes community [67]. In addition to improving the health outcomes of individuals with diabetes and addressing discrimination of individuals with diabetes, prevention of Type 2 diabetes constitutes a key objective of the plan. Prevention and treatment are not considered to be alternative options as they are both equally important. The UN and its agencies are advised to work with national governments to reorient health systems to a preventative model addressing health in all polices, such as urban design and housing, workplace design, food production, healthy nutrition and physical activity. Concerted action at the international and national policy levels will, therefore, be required to advance science-driven health initiatives in these areas and translation into practice [68,69], while meticulously avoiding overzealous and well-meaning policy initiatives of hitherto unproven benefit. These recommendations are congruous with the three pivotal levers for change described by Yach et al. [70]:
■ Raising the profile of chronic disease in the mind and on the agenda of policy makers;
■ Providing policy makers with evidence to support the case for prevention;
■ Advocating the need for widespread health system change.
These should also include entire government systems beyond healthcare and involve global corporations and labor unions, as well as nongovernmental organizations. Furthermore, chronic disease alliances need to be formulated including industry and academia [60,71,107], such as the Oxford Health Alliance, the Global Alliance for Chronic Diseases and the Global Partnerships Forum. Political commitment and action are critically required at high global and national levels particularly as prevention of diabetes and its complications are dramatically underfunded and as major gaps exist between findings from clinical trials and their implementation in clinical and public health practice [71].
Research funding agencies tend to favor medical and surgical solutions over health promotion and health systems interventions and policies [71]. Furthermore, although research has shown that improving diets has greater potential to improve quality-adjusted life years, reduce morality and medical costs than medications, funding favors medications [72,107]. The WHO has called for research into prevention and intervention implementation in addition to integrating prevention efforts in national programs and engaging government and corporate sectors [72,73]. The WHO priority areas for diabetes prevention, control and research include community-based primary prevention models focusing on nutrition, physical activity, urbanization and transportation [73]. Indeed, multifaceted approaches to diabetes management have been effective in secondary prevention but a similar response to primary prevention is lacking, thereby “exposing primary prevention as the weak link in the public health response to diabetes” [74]. Detecting prediabetes and undiagnosed diabetes may be the link and stimulus to reorient systems toward preventive care. Hence, if efforts are not made in primary prevention, the increasing rate of diabetes will obscure or negate achieved successes in secondary prevention [74].
A major review by Popkin and colleagues states that improving global diets is ‘imperative’ to prevent and control obesity, diabetes and other NCDs [75]. Realistic policy interventions must be directed at making healthy choices easier rather than coercing individuals to make healthy choices [76]. Taxation is one option to change health behavior [77] but may not be a popular approach [78]. The pricing and availability of healthy foods needs attention. Pricing is best addressed across all major food categories, and supported by the need for better agricultural and food policies to address diabetes [79]. Strong industry collaboration has made significant progress on several key areas, such as reformulating to reduce sugar, innovating smaller package sizes, labeling calories and sugar, restricting marketing to children, withdrawing full-calorie sodas from schools and investment in activity programs, such as the Healthy Weight Commitment Foundation and the International Food and Beverage Alliance [80].
Focusing on improving health through modifying the environment for the entire population is more desirable than health education or promotion campaigns. Policies to align agricultural policy with nutrition and health goals will allow the agriculture and food industries, both with great influence on health, to contribute to the prevention and control of diabetes and other NCDs [80,81].
Ground level proposals for the primary prevention of diabetes
While confronting NCDs in general and diabetes in particular at the highest levels of national and international governmental agencies is absolutely necessary to promote shifts in healthcare delivery, this process will clearly take time. Since the crisis is well upon us, current strategies involving community resources described above are crucial and need further expansion. Narayan et al. has stated that, “the fight against diseases is global and … solutions can emerge from anywhere” [68]. So, what additional options can be undertaken to identify and refer individuals at risk for progression to diabetes? Evidence-based approaches involving health promotion, obesity prevention and policies to improve the behaviors and environment on a population basis need to be reinforced with the identification and referral of the almost one-third of adults with prediabetes to effective lifestyle change programs [74]. Investment in research to better understand what kinds of policies at environmental levels work to reduce obesity and diabetes risk is needed. Currently, few interventions tested at the population or environmental levels are proven to be beneficial.
Novel approaches to training are required to meet the global demands of caring for patients with chronic conditions [82]. The healthcare system needs to transition from a reactive to a proactive perspective with regard to prevention and approach this issue on a population basis beyond caring for the individual patient [82]. Healthcare workers will, therefore, need to develop a broad approach to patient care considering the entire continuum from community prevention to palliative care [82]. Establishing new core competencies will require restructuring of training to include knowledge, skills and abilities designed to prepare 21st century health workers to address current challenges [82].
Ground level proposals, for primary prevention, listed in Box 1, complement community programs such as the National DPP. A limiting factor in prevention is the inability to recognize metabolic disorders early in their course, because these tend to be subtle and often are not considered given the significant challenges of primary care practitioners in managing ever-increasing patient volumes in the context of decreased reimbursement. Providing adequate compensation for prevention visits during which individuals at high risk would be advised to engage in lifestyle modification and are provided referrals for nutritional counseling and community-based programs is essential and would help reduce ‘clinical inertia’. The latter appears to be increasingly undertaken by third-party insurers. The development of accredited, cost-effective hospital-based initiatives should be encouraged to supplement prevention programs in the community. The CDC DPRP recognizes any organization that meets the program standards. All healthcare professionals, regardless of specialty, should be instructed to refer individuals at risk to their physician for further evaluation and referral.
Box 1. Ground level proposals for the primary prevention of diabetes.
■ Broaden emphasis on prevention in medical and graduate training and extend to allied healthcare providers.
■ Integration of graduate programs in public health (MPH) with medical and allied healthcare curricula with reference to prevention.
■ Medical and scientific societies – especially primary care – need to embrace platforms for prevention.
■ Continuing medical education offerings should include prevention modules (e.g., webinars, scientific meetings and so on).
■ Delivery of community-based prevention programs that are supported by third-party reimbursement with referrals from healthcare professionals.
■ Increase dialog between practice managers and health insurers for reimbursement and incentivizing visits for primary prevention and nutrition.
■ Community screening for prediabetes/diabetes in high-risk populations.
■ Establish hospital and academically based prevention and treatment referral programs.
■ Promote collaboration between academic medical centers with local/regional public health agencies.
■ Public outreach seminars on diabetes prevention.
Continuing medical education courses, utilizing web-based and other formats, as well as satellite symposia held at scientific congresses, should be focused particularly on pediatricians, primary care and family physicians. Clinical case presentations should be utilized to illustrate screening, diagnosis and treatment principles. Given the enormous population at risk, community-based strategies involving local health departments should also be considered. The overwhelming burden of screening and treatment may, therefore, be mitigated and achieved by integrating diabetes prevention programs within extant hospitals or community clinics garnered with necessary training and duly recognized.
As current medical school curricula offer basic public health principles, perhaps because it tends not to adequately focus on chronic disease management [83], consideration should be given to the development of more extensive prevention modules in collaboration with public health faculty. This should enhance life-long awareness of the importance of prevention and may also provide motivation to pursue a career in public health practice and research.
Conclusion & future perspective
It is the responsibility of the medical community to reach out to the broader public raising awareness of the current epidemic and offering basic instruction in the importance of lifestyle modification while encouraging referral for those at risk. Governments, in concert with the private sector, need to set policies that promote healthy nutritional and agricultural policies, favor modifications in the environment that encourage greater physical activity and make prevention affordable for all citizens at high risk. The public health sector has the charge of translating evidence-based findings into practical, accessible and cost-effective programs and monitoring the process to continuously improve prevention initiatives. The clinical sector has the formidable challenge of screening and identifying those at high risk and referring to accredited intervention programs. There is a need to explore additional cost-effective interventions that are customized to meet individual needs that can be offered at the community and clinical levels. Thus, all three sectors, government, public health and clinical, each have a critical role in this process and by working as a partnership, ought to create the necessary synergies essential for making substantial forays in the prevention of diabetes.
Practice Points.
■ Prediabetes, characterized by an extended period of increasing dysglycemia, may exist for many years before Type 2 diabetes is diagnosed.
■ Prediabetes is defined globally by glucose criteria, although fasting threshold levels differ in accordance with American Diabetes Association (ADA) or WHO recommendations.
■ HbA1c threshold ≥6.5% has been adopted globally for defining diabetes. Prediabetes is defined by the ADA, but not the WHO, with HbA1c levels 5.7–6.4%, the range between 6.0–6.4% conferring high-risk status. Contradictory data with regard to sensitivity may indicate that the ability to accurately diagnose prediabetes is limited.
■ HbA1c may be affected by genetic, hematological and illness-related factors that may limit its accuracy.
■ It is essential to identify individuals at high risk for developing Type 2 diabetes during this period to potentially obviate the development of diabetes and forestall the development of complications that may occur with prediabetes as well as with diabetes.
■ Evidence-based clinical trials in high-risk individuals have proven the benefit of lifestyle intervention consisting of weight reduction, dietary modification and exercise, which is greater than with pharmacologic treatment, in dramatically reducing the evolution to diabetes.
■ Translational research studies implementing modified versions of lifestyle interventions from clinical trials have been demonstrated to be effective in real-world settings.
■ Government, public health and clinical sectors each have a critical role in preventing Type 2 diabetes by setting policies that promote healthy nutritional and agricultural policies, favor modifications in the environment that encourage greater physical activity and by identifying and referring individuals at high risk to affordable, accredited intervention programs.
Footnotes
Disclaimer
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
■ Websites
- 1.IDF . Diabetes Atlas Fourth Edition. International Diabetes Federation; Brussels, Belgium: 2009. [Google Scholar]
- 2.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E, Gastaldelli A, Fozzo P. Pathophysiology of prediabetes. Med. Clin. N. Am. 2011;95:327–339. doi: 10.1016/j.mcna.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Nathan DM, Davidson MB, De Fronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 5.Geiss L, James C, Gregg E, Albright A, Williamson D, Cowie C. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am. J. Prev. Med. 2010;38(4):403–409. doi: 10.1016/j.amepre.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan KM, Williamson DF. Prevention of Type 2 diabetes: risk status, clinic, and community. J. Gen. Int. Med. 2009;25(2):154–157. doi: 10.1007/s11606-009-1148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■■ Very good perspective and overview of the subject. Concise and makes the point that economic and health consequences of diabetes will not be reduced until individuals with prediabetes are identified and offered access to lifestyle intervention at reasonable cost.
- 8.Diabetes prevention program research group: reduction in the incidence of Type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw J, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Pan X, Li G, Hu Y, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and diabetes study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 11.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran A, Snehalatha C. Diabetes prevention programs. Med. Clin. N. Am. 2011;95(2):353–372. doi: 10.1016/j.mcna.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 13.The Expert Committee on the diagnosis and classification of diabetes mellitus: follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 14.American diabetes association: standards of medical care in diabetes – 2012. Diabetes Care. 2012;35(1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The International Expert Committee: International Expert Committee Report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colagiuri S, Lee CMY, Wong TY, Balkau B, Shaw JE, Borch-Johnsen KB. For the DETECT-2 collaboration writing group. Glycemic thresholds for diabetes-specific retinopathy. Diabetes Care. 2011;34(1):145–150. doi: 10.2337/dc10-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Res. Clin. Pract. 2011;93(3):299–309. doi: 10.1016/j.diabres.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 18.Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26(12):3329–3330. doi: 10.2337/diacare.26.12.3329. [DOI] [PubMed] [Google Scholar]
- 19.Buysschaert M, Bergman M. Definition of prediabetes. Med. Clin. N. Am. 2011;95(2):289–297. doi: 10.1016/j.mcna.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Buysschaert M, Bergman M. Diabetes Prevention – from Science to Practice. Wiley Books; 2012. Diagnosis of prediabetes and diabetes prevention. (In Press) [Google Scholar]
- 21.Sacks DB. A1c versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–523. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echouffo-Tcheugui JB, Ali MK, Griffin AS, et al. Screening for Type 2 diabetes and dysglycemia. Epidemiol. Rev. 2011;33:63–87. doi: 10.1093/epirev/mxq020. [DOI] [PubMed] [Google Scholar]
- 23.Sacks DB, Arnold M, Bakris GL, et al. Position statement executive summary: guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34(6):1419–1423. doi: 10.2337/dc11-9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajat C, Harrison O, Siksek ZA. Diagnostic testing for diabetes using HbA1c in the Abu Dhabi population. Weqaya: the Abu Dhabi cardiovascular screening program. Diabetes Care. 2011;34(9):2400–2402. doi: 10.2337/dc11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverman RA, Thakker U, Ellman T, et al. Hemoglobin A1c as a screen for previously undiagnosed prediabetes and diabetes in an acute-care setting. Diabetes Care. 2011;34(9):1908–1912. doi: 10.2337/dc10-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colagiuri S. Glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus – practical implications. Diabetes Res. Clin. Pract. 2011;93(3):312–313. doi: 10.1016/j.diabres.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Saltevo JT, Kautiainen H, Niskanen L, et al. Ageing and associations of fasting plasma glucose and 2h plasma glucose with HbA1c in apparently healthy population. ‘FIN-D2D’ study. Diabetes Res. Clin. Pract. 2011;93(3):344–349. doi: 10.1016/j.diabres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Bonora E, Kiechi S, Mayr A, et al. High-normal HbA1c is a strong predictor of Type 2 diabetes in the general population. Diabetes Care. 2011;34(4):1038–2011. doi: 10.2337/dc10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradhan AD, Rifai N, Buring JE, Ridker PM. Hemoglobin A1c predicts diabetes but not cardiovascular disease in non-diabetic women. Am. J. Med. 2007;120(8):720–727. doi: 10.1016/j.amjmed.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloomgarden Z. A1C: recommendations, debates and questions. Diabetes Care. 2009;32(12):141–147. doi: 10.2337/dc09-zb12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann DM, Carson AP, Shimbo D, Fonseca V, Fox CS, Muntner P. Impact of A1c screening criterion on the diagnosis of pre-diabetes among U.S. adults. Diabetes Care. 2010;33(10):2190–2195. doi: 10.2337/dc10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■ Using A1c as the prediabetes criterion would reclassify 50 million Americans. Clinicians and health systems need to understand the issues in using A1c or IFG in diagnosing prediabetes.
- 32.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care. 2010;33(3):562–568. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1c-based diagnostic criteria. Diabetes Care. 2010;33(10):2184–2189. doi: 10.2337/dc10-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan V, Vijayachandrika V, Gokulakrishnan K, et al. A1c cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care. 2010;33(3):515–519. doi: 10.2337/dc09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes Metab. Res. Rev. 2010;26(1):5–6. doi: 10.1002/dmrr.1013. [DOI] [PubMed] [Google Scholar]
- 36.Bonora E, Tuomilehto J. The pros and cons of diagnosing diabetes with A1c. Diabetes Care. 2011;34(5):S184–S190. doi: 10.2337/dc11-s216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heianza Y, Hara S, Arase Y, et al. HbA1c : 5.7–6.4% and impaired fasting plasma glucose for diagnosis of prediabetes and risk of progression to diabetes in Japan (TOPICS 3): a longitudinal cohort study. Lancet. 2011;378(7):147–155. doi: 10.1016/S0140-6736(11)60472-8. [DOI] [PubMed] [Google Scholar]
- 38.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 39.Albright A, Williamson DF. Community approaches to diabetes prevention. In: LeRoith D, editor. Prevention of Type 2 Diabetes: from Science to Therapies. Springer; NY, USA: 2011. [Google Scholar]
- 40.Tuomilehto J, Schwarz P, Lindstrom J. Long-term benefits from lifestyle interventions for Type 2 diabetes prevention: time to expand the efforts. Diabetes Care. 2011;34(Suppl. 2):210–214. doi: 10.2337/dc11-s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uusitupa M, Tuoilehto J, Puska P. Are we really active in the prevention of obesity and Type 2 diabetes at the community level? Nut. Metab. Cardiovas. Dis. 2011;21(5):380–389. doi: 10.1016/j.numecd.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Saaristo T, Moilanen L, Korpi-Hyovalti E, et al. Lifestyle intervention for prevention of Type 2 diabetes in primary healthcare. One-year follow-up of the Finnish National Diabetes Prevention Program (FIN-D2D) Diabetes Care. 2010;33(10):2146–2151. doi: 10.2337/dc10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oldenburg B, Absetz P, Dunbar JA, Reddy P, O’Neil A. The spread and uptake of diabetes prevention programs around the world: a case study from Finland and Australia. Transl. Behav. Med. 2011;1(2):270–282. doi: 10.1007/s13142-011-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddy P, Rankins D, Timoshanko A, Dunbar JA. Life! in Australia: translating prevention research into a large-scale intervention. Br. J. Diabetes Vasc. Dis. 2011;11(1):193–197. [Google Scholar]
- 45.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the diabetes prevention program? Health Aff. 2012;31(1):67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- ■■ Very thorough and well carried out systematic review and meta-analysis of translation studies based on the Diabetes Prevention Program research study that provides important information about how to lower program costs and maintain effectiveness.
- 46.ZHuo X, Zhang P, Gregg EW, et al. A Nationwide community-based lifestyle program could delay or prevent Type 2 diabetes cases and save $5.7 billion in 25 years. Health Aff. 2012;31(1):50–60. doi: 10.1377/hlthaff.2011.1115. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz PEH, Greaves CJ, Yates T, Davies MJ. Nonpharmacological interventions for the prevention of Type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012;8(6):363–373. doi: 10.1038/nrendo.2011.232. [DOI] [PubMed] [Google Scholar]
- ■■ Up-to-date and comprehensive reivew of identifying individuals at risk and implementation of evidence-based lifestyle strategies. Also addresses barriers to implementation.
- 48.Schwarz PEH, Greaves C, Reddy P, Dunbar J, Schwarz J. Diabetes Prevention in Practice. TUMAINI Institute for Prevention Management; Dresden, Germany: 2010. [Google Scholar]
- 49.Schwarz PE, Li J, Lindstrom J, Tuomilehto J. Tools for predicting the risk of Type 2 diabetes in daily practice. Horm. Metab. Res. 2009;41(2):86–97. doi: 10.1055/s-0028-1087203. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz PE, Li J, Reimann M, et al. The Finnish diabetes risk score is associated with insulin resistance and progression towards Type 2 diabetes. J. Clin. Endocrinol. Metab. 2009;94(3):920–926. doi: 10.1210/jc.2007-2427. [DOI] [PubMed] [Google Scholar]
- 51.Paulweber B, Valensi P, Lindström J, et al. A European evidence-based guideline for the prevention of Type 2 diabetes. Horm. Metab. Res. 2010;42(Suppl. 1):S3–S36. doi: 10.1055/s-0029-1240928. [DOI] [PubMed] [Google Scholar]
- 52.Lindstrom J, Neumann A, Sheppard KE, et al. Take action to prevent diabetes – the IMAGE toolkit for the prevention of Type 2 diabetes in Europe. Horm. Metab. Res. 2010;42(4 Suppl. 1):S37–S55. doi: 10.1055/s-0029-1240975. [DOI] [PubMed] [Google Scholar]
- 53.NICE . NICE Public Health Guidance 35: Preventing Type 2 Diabetes: Population and Community-Level Interventions in High-Risk Groups and the General Population. NICE; London, UK: 2011. [Google Scholar]
- 54.Greaves CJ, Sheppard KE, Abraham C, et al. Systematic review of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11(1):119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kronsbein P, Fischer MR, Tolks D, et al. IMAGE – development of a European curriculum for the training of prevention managers. Br. J. Diabetes Vasc. Dis. 2011;11(4):163–167. [Google Scholar]
- 56.Pajunen P, Landgraf R, Muylle F, et al. For the image study group. Quality indicators for the prevention of Type 2 diabetes in Europe-IMAGE. Horm. Metab. Res. 2010;42(Suppl. 1):S56–S63. doi: 10.1055/s-0029-1240976. [DOI] [PubMed] [Google Scholar]
- 57.Rothe U, Muller G, Schwarz PE, et al. Evaluation of a diabetes management system based on practice guidelines, integrated care, and continuous quality management in a Federal State of Germany: a population-based approach to healthcare research. Diabetes Care. 2008;31(5):863–868. doi: 10.2337/dc07-0858. [DOI] [PubMed] [Google Scholar]
- 58.Karve A, Hayward RA. Prevalence, diagnosis, and treatment of impaired glucose tolerance in nondiabetic U.S. adults. Diabetes Care. 2010;33(11):2355–2359. doi: 10.2337/dc09-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Narayan KM, Ali MK, Koplan JP. Global noncommunicable diseases – where worlds meet. N. Engl. J. Med. 2010;363(13):1196–1198. doi: 10.1056/NEJMp1002024. [DOI] [PubMed] [Google Scholar]
- 60.Siminerio L, Mbanya JC. Translating diabetes research into global communities. Diabetes Res. Clin. Pract. 2011;93(3):443–445. doi: 10.1016/j.diabres.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 61.McGlynn EA, Asch SM, Adams J, et al. The quality of healthcare delivered to adults in the United States. N. Engl. J. Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 62.Colagiuri R, Colagiuri S, Yach D, Pramming S. The answer to diabetes prevention: science, surgery, service delivery, or social policy? Am. J. Public Health. 2006;96(9):1562–1569. doi: 10.2105/AJPH.2005.067587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fox CS, Coady S, Sorlie PD, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham heart study. Circulation. 2007;115(12):1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 64.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat. Med. 2006;12(1):62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- ■ Thoughtfully discusses the economic burden of obesity and diabetes on healthcare systems. Roadmaps for navigating these vast problems will vary amongst countries given differences in political, economic and social structures. Inaction leading to reversal of improved life expectancy should provide motivation for action.
- 65.Rosenbaum L, Lamas D. Facing a ‘Slow-Motion Disaster’ – the UN meeting on noncommunicable disease. N. Engl. J. Med. 2011;365(25):2345–2348. doi: 10.1056/NEJMp1112235. [DOI] [PubMed] [Google Scholar]
- 66.Matthews DR, Matthews PC. Banting memorial lecture 2010. Type 2 diabetes as an ‘infectious’ disease: is this the Black Death of the 21st century? Diabet. Med. 2011;28(1):2–9. doi: 10.1111/j.1464-5491.2010.03167.x. [DOI] [PubMed] [Google Scholar]
- ■■ Scholarly and masterful comparison of two pandemics: bubonic plague and the modern-day equivalent, Type 2 diabetes. Tackling the latter requires similar strategies as for an infectious agent.
- 67.Keeling A. International diabetes federation. In the aftermath of the UN summit on NCDs: the way forward for the global diabetes community. Diabetes Res. Clin. Pract. 2011;93(3):446–447. [Google Scholar]
- 68.Narayan KM, Ali MK, del Rio C, Koplan JP. Global noncommunicable diseases-lessons from the HIV-AIDS experience. N. Engl. J. Med. 2011;365(10):876–878. doi: 10.1056/NEJMp1107189. [DOI] [PubMed] [Google Scholar]
- 69.Hu FB. Globalization of diabetes. The role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yach D, Hawkes C, Gould CL, Hofman KJ. The global burden of chronic diseases. Overcoming impediments to prevention and control. JAMA. 2004;291(21):2616–2622. doi: 10.1001/jama.291.21.2616. [DOI] [PubMed] [Google Scholar]
- 71.Beaglehole R, Yach D. Globalization and the prevention of non-communicable diseases: the neglected chronic diseases of adults. Lancet. 2003;362(9387):903–908. doi: 10.1016/S0140-6736(03)14335-8. [DOI] [PubMed] [Google Scholar]
- 72.WHO . World Health Organization Global Status Report on NCD. WHO Press; Geneva, Switzerland: 2011. [Google Scholar]
- 73.Mendis S, Alwan A. Prioritized Research Agenda for Prevention and Control of Noncommunicable Diseases. WHO; Geneva, Switzerland: 2011. [Google Scholar]
- 74.Gregg EW, Albright AL. The public health response to diabetes – two steps forward, one step back. JAMA. 2009;301(15):1596–1598. doi: 10.1001/jama.2009.519. [DOI] [PubMed] [Google Scholar]
- ■■ Excellent commentary describing the paradox in declining diabetes complications (due to multifaceted public health efforts) in concert with an increasing prevalence of diabetes thereby increasing the risk for complications in the total population. It furthermore makes the point that primary prevention of diabetes may be achieved by clinical–community partnerships described in the present paper.
- 75.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nut. Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 77.Hawkes C. Food taxes: what type of evidence is available to inform policy development? Nut. Bull. 2012;37(1):51–56. [Google Scholar]
- 78.Winkler JT. Why soft drink taxes will not work. Br. J. Nut. 2011 doi: 10.1017/S0007114511006477. doi:10.1017/S0007114511006477. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 79.Nugent R. Bringing agriculture to the table. How agriculture and food can play a role in preventing chronic disease. Chicago Council Global Aff. 2011:1–88. [Google Scholar]
- 80.Yach D, Khan M, Bradley D, Hargrove R, Kehoe S, Mensah G. The role and challenges of the food industry in addressing chronic disease. Global Health. 2010;6(10):1–8. doi: 10.1186/1744-8603-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO . World Health Organization Package Of Essential Noncommunicable (Pen) Disease Interventions For Primary Healthcare In Low-Resource Settings. WHO Press; Geneva, Switzerland: 2010. [Google Scholar]
- 82.Pruitt SD, Epping-Jordan JE. Preparing the 21st century global healthcare workforce. BMJ. 2005;330(7492):637–639. doi: 10.1136/bmj.330.7492.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ■ Prescient and timely paper describes the changes necessary for the healthcare workforce to contend with 21st century challenges. Training should be restructured to include new competencies to manage prevalent health problems including a public health perspective.
- 83.Holman H. Chronic disease-the need for a new clinical education. JAMA. 2004;292(9):1057–1059. doi: 10.1001/jama.292.9.1057. [DOI] [PubMed] [Google Scholar]
- ■■ This commentary addresses the pivotal concern that medical education does not adequately prepare students for the healthcare problems associated with chronic diseases they will face in the future. New learning experiences do not require new construction or equipment but new understandings and behaviors.
- 101.Centers for Disease Control and Prevention . Centers for disease control and prevention. US Department of Health and Human Services; Atlanta, GA: 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 102.Report of a WHO/IDF consultation . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. WHO; Geneva, Switzerland: 2006. http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf. [Google Scholar]
- 103.Diabetes Training and Technical Assistance Center http://dttac.org.
- 104.National Diabetes Prevention Program www.cdc.gov/diabetes/prevention.
- 105.CDC Diabetes Prevention Recognition Program www.cdc.gov/diabetes/prevention/recognition.
- 106.CDC 2011 National Diabetes Fact Sheet www.cdc.gov/diabetes/pubs/estimates11.htm#7.
- 107.Cooper K, Thorpe A, Hodges-Mameltzis Food and health: a report on research and development activity in the United States, European Commission and the United Kingdom. C3 Collaborating for Health. 2011 www.c3health.org/wp-content/uploads/2009/09/Final-RandD-report-for-website-20110328.pdf.