Abstract
Amotivation in schizophrenia is a central predictor of poor functioning, and is thought to occur due to deficits in anticipating future rewards, suggesting that impairments in anticipating pleasure can contribute to functional disability in schizophrenia. In healthy comparison (HC) participants, reward anticipation is associated with activity in frontal–striatal networks. By contrast, schizophrenia (SZ) participants show hypoactivation within these frontal–striatal networks during this motivated anticipatory brain state. Here, we examined neural activation in SZ and HC participants during the anticipatory phase of stimuli that predicted immediate upcoming reward and punishment, and during the feedback/outcome phase, in relation to trait measures of hedonic pleasure and real-world functional capacity. SZ patients showed hypoactivation in ventral striatum during reward anticipation. Additionally, we found distinct differences between HC and SZ groups in their association between reward-related immediate anticipatory neural activity and their reported experience of pleasure. HC participants recruited reward-related regions in striatum that significantly correlated with subjective consummatory pleasure, while SZ patients revealed activation in attention-related regions, such as the IPL, which correlated with consummatory pleasure and functional capacity. These findings may suggest that SZ patients activate compensatory attention processes during anticipation of immediate upcoming rewards, which likely contribute to their functional capacity in daily life.
Keywords: fMRI, Reward, Punishment, Motivation, Schizophrenia
Highlights
-
•
Schizophrenia patients show frontal–striatal impairment during reward anticipation.
-
•
Patients recruit additional inferior parietal lobes (IPL) during reward anticipation.
-
•
IPL activation may contribute to motivation and functional enhancements in SZ.
1. Introduction
Motivation is the process that drives a person to act towards a desired outcome. Motivational impairments are a cardinal feature of schizophrenia, are present in the earliest phases of the illness and are a significant predictor of impaired real-world functioning (Foussias and Remington, 2010; Schlosser et al., 2014). Current clinical and neuroimaging data indicate that these motivational impairments may be related to neural and behavioral deficits in anticipating future rewards (Juckel et al., 2006; Simon et al., 2010). Specifically, growing evidence indicates that schizophrenia patients may not be able to use anticipation of future rewards to modulate subsequent goal-directed behavior, suggesting impairments in frontal–striatal interactions and dopaminergic transmission between these regions (Abi-Dargham, 2003; Barch and Dowd, 2010; Gold et al., 2008; Strauss et al., 2015). Thus, the question remains as to whether recruitment of impaired frontal–striatal systems may enhance motivation and goal-directed behavior in schizophrenia patients or whether recruitment of other intact networks may be more useful targets for potentiating goal-directed functions in the real-world. The present fMRI study investigates this question.
The reward circuitry is well established in healthy participants (HC). Abundant neuroimaging evidence indicate that, in healthy participants, the motivation to receive upcoming monetary rewards is associated with anticipatory activity in frontal–striatal networks, including the medial prefrontal cortex (extending to the anterior cingulate cortex, mPFC/ACC), caudate, putamen, ventral striatum and nucleus accumbens (Barch and Dowd, 2010; Knutson et al., 2001; Murray et al., 2008). Specifically, data suggest that activity in the ventral striatum (VS), particularly within the nucleus accumbens, during immediate reward anticipation on a Monetary Incentive Delay (MID) task (Knutson et al., 2000) predicts arousal, positive affect, and, most importantly, real-world goal oriented consummatory behavior (e.g. products purchased and money spent at a shopping mall) (Knutson et al., 2001, 2003, 2007).
By contrast, when schizophrenia patients perform the MID task, several studies have consistently revealed reduced ventral striatum (VS) activity during reward anticipation (Juckel et al., 2006; Kirsch et al., 2007; Schlagenhauf et al., 2008). Yet, prior behavioral research indicate that while patients with schizophrenia reveal specific deficits in anticipating signals leading to future rewarding outcomes, they experience similar levels of consummatory (in the moment) pleasure to HC participants (Gard et al., 2007; Herbener et al., 2008; Kring and Moran, 2008). However, it must also be noted that not all studies have found trait differences between schizophrenia and HC participants in self-reported anticipatory pleasure ratings (assayed with the TEPS anticipatory scale) (Strauss et al., 2011); indeed some behavioral studies have shown that deficits in schizophrenia patients' ability to respond to future rewarding stimuli occur only when the stimuli are not presently available compared to when the rewarding cues are more immediately present (Heerey et al., 2011). Together, these findings suggest that additional studies are needed that use anticipatory pleasure ratings from the TEPS anticipatory scale with neuroimaging measures of immediate reward anticipation, to determine the precise neural underpinnings of amotivation in schizophrenia. To this end, the purpose of this study is to use fMRI in schizophrenia to explicitly examine the relationship between neural activity during the anticipation of an immediate reward (assayed when participants anticipate winning money in response to a WIN $ cue) in relation to self-reports of real world motivated behavior (both in-the-moment pleasure as represented by the TEPS consummatory scale, and future representations of pleasure, as measured by the TEPS anticipatory scale). Therefore, it is possible that neural activation patterns during current representation of rewarding cues in schizophrenia may not directly be associated with future representations of reward (measured with the TEPS-Anticipatory Scale), but rather with more intact consummatory pleasure levels (assayed here with the TEPS-Consummatory Scale), as is shown in healthy participants (Knutson et al., 2007). It also must be noted that while the process of anticipating pleasure can enhance motivation and preparation for upcoming future rewarding events, high levels of consummatory (in the moment) pleasure can also increase neural reward motivational processes to repeat a rewarding activity (Trémeau et al., 2010). In the present study, we therefore hypothesized that it was possible for neural signal during immediate reward anticipation on the MID task to be correlated with either the TEPS-Anticipatory scale, the TEPS-Consummatory scale or with both scales.
Since deficits in anticipatory pleasure are related to deficits in motivation and functional outcome (Gard et al., 2007), and since amotivation in schizophrenia has been shown to be a central predictor of poor functioning, these findings suggest that deficits in anticipating pleasure can contribute to functional disability in schizophrenia (Foussias and Remington, 2010; Gard et al., 2009). However, thus far, no one has investigated the explicit link between deficits during anticipation of immediate rewards, real world motivated behavior (both in-the-moment and the future), and real-world functioning, and whether and how additional intact neural networks (rather than impaired frontal–striatal circuits) may mediate motivated behavior in schizophrenia.
To this end, we examined whole-brain activation in SZ patients and HC participants during the anticipatory phase of presentation of stimuli that predicted immediate monetary gain (reward) and loss (punishment), as well as during the outcome phase when participants were notified as to whether they had won money or lost money (Knutson et al., 2000). Previous research has shown that activation within the ventral striatum during anticipation of an immediate reward is negatively correlated with negative symptoms, such as apathy (i.e., lack of motivation), as well as with positive symptoms, while striatal activation during reward outcome is negatively correlated with depressive symptoms (Juckel et al., 2006; Nielsen et al., 2012; Simon et al., 2010). These findings suggest that lower striatal activation during anticipation of an immediate reward may contribute to patients' lack of motivation as well as to the development of psychotic symptoms while lower striatal activation during reward outcome may contribute to depressive symptoms (Simon et al., 2010).
In the present study, we examine whole-brain neural activation in relation to clinical symptoms (assayed with the Positive and Negative Symptom Scale), motivation (assayed with the Behavioral Activation Scale); both anticipatory pleasure ratings of future representations of reward and in the moment consummatory pleasure ratings (assayed with the TEPS) (Gard et al., 2007) and with real-world functional capacity (UCSD Performance-based Skills Assessment) (Patterson et al., 2001). Given that prior meta-analyses have indicated that SZ patients do not reveal deficits during “in the moment” emotional experiences (Cohen and Minor, 2010), we predicted that we would not observe overall group differences in neural activation when SZ patients were notified that they had won money. However, in view of the previous studies mentioned above, we hypothesized that SZ patients would reveal hypoactivation in VS specifically during immediate reward anticipation. To our knowledge, this is the first study to investigate whether frontal–striatal dysregulation during immediate reward anticipation may require recruitment of additional intact networks such as those within parietal regions in schizophrenia, that may predict better motivation (assayed with BAS-Drive scale), consummatory pleasure (TEPS) and real-world functioning (assayed with the UCSD Performance-based Skills Assessment).
2. Materials and methods
2.1. Participants and procedures
This study represents the baseline imaging data from the imaging component of our NIMH-funded RO1 of a double-blind randomized clinical trial of cognitive training in schizophrenia (ClinicalTrials.gov NCT02105779). Thirty-seven clinically stable volunteer schizophrenia patients (SZ: mean age = 45.14; mean education = 14.55 years; mean IQ = 102.11; mean illness duration = 25.40 years), who were willing to undergo two imaging sessions, were recruited from the parent study. All patients were stratified by age, education, gender, and symptom severity and then randomly assigned to either social computerized cognitive training, or to a control computerized cognitive training condition without the social training, performed for 80 h. Informed consent was obtained from all subjects. Schizophrenia patients were scanned using fMRI while they performed the Monetary Incentive Delay Task (used to assay reward/punishment processing) at baseline and after 80 h of intervention. We report here the results at baseline of our reward/punishment fMRI experiment investigating frontal–striatal cortical systems in schizophrenia patients when compared to 20 healthy comparison participants (HC), matched at a group level on age, gender, and education (Table 1). SZ participants also underwent clinical and neuropsychological assessments (Table 2). fMRI data from two SZ participants were later excluded due to very poor signal to noise ratio, resulting from excessive motion during the scan.
Table 1.
Demographics and behavioral measures (mean, SD) of healthy comparison (HC) and schizophrenia (SZ) participants.
| Baseline | HC (N = 20) | SZ (N = 37) |
|---|---|---|
| Age | 43.72 (SD = 13.32) | 45.14 (SD = 9.97) |
| Education | 13.63 (SD = 2.11) | 14.55 (SD = 1.58) |
| Gender | 14M, 6F | 25M, 12F |
| TEPS Anticipatory pleasure | 46.00 (SD = 4.55) | 43.09 (SD = 7.85) |
| TEPS Consummatory pleasure | 39.65 (SD = 4.74) | 38.74 (SD = 7.78) |
| MID total accuracy | 25.95 (SD = 4.95) | 22.42 (SD = 6.93) |
| MID win accuracy | 13.18 (SD = 2.63) | 11.56 (SD = 3.51) |
| MID no lose accuracy | 12.77 (SD = 2.43) | 10.86 (SD = 3.67) |
Table 2.
Medication profile, clinical symptoms, BIS–BAS and UPSA functional outcome scores (mean, SD) in schizophrenia (SZ) participants.
| SZ (N = 37) | |
|---|---|
| 1st generation (N) | 8 |
| 2nd generation (N) | 32 |
| Multiple (N) | 8 |
| No antipsychotic (N) | 0 |
| Other psychiatric medication | |
| Antidepressants or mood stabilizers (N) | 23 |
| Benzodiazepines (N) | 9 |
| Mean chlorpromazine (CPZ) equivalents | 374.96 (SD = 555.62) |
| Mean cogentin equivalents | 1.07 (SD = 1.51) |
| Overall clinical symptom severity (PANSS) | 2.14 (SD = 0.55) |
| Positive symptom severity (PANSS) | 2.55 (SD = 1.03) |
| Negative symptom severity (PANSS) | 2.06 (SD = 0.89) |
| BAS-Drive | 2.65 (SD = .75) |
| BAS-Fun seeking | 2.78 (SD = .56) |
| BAS-Reward responsiveness | 3.56 (SD = .36) |
| BIS | 3.05 (SD = .61) |
| UPSA | 73.01 (SD = 12.58) |
2.2. Clinical and neuropsychological assessments
Eligibility diagnosis for schizophrenia was determined using the Structured Interview for the DSM (SCID) (First et al., 2002). Symptom severity in schizophrenia was assessed with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). Neurocognition was assessed using the MATRICS Consensus Cognitive Battery (MCCB, Nuechterlein et al., 2008) which assesses seven cognitive domains: attention/vigilance, speed of processing, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition, and provides a global cognition score across all measures. Associations were conducted between reward anticipation and the MCCB measures of global cognition and reasoning and problem solving (Neuropsychological Assessment Battery — Mazes Test). Recent research indicate that neurocognitive function predicts real-world functional outcome in patients with schizophrenia but this is largely mediated by functional capacity (e.g. the ability to perform functionally relevant, everyday living skills such as writing a check, following a bus schedule), as measured with the UCSD Performance-based Skills Assessment (UPSA) (Bowie et al., 2006). Therefore, we thought that the UPSA would be the most valid and reliable measure to assess functional capacity in schizophrenia, related to the skills essential to the individual's ability to function independently in the community (Patterson et al., 2001). The Behavioral Inhibition and Behavioral Activation Scales (BIS/BAS), which are a self-report measure of reward sensitivity, were used to assess aversive and appetitive motivation in schizophrenia (Carver and White, 1994). Examples of these items, respectively, are: “I worry about making mistakes” and “When I want something, I usually go all-out to get it.” The Temporal Experience of Pleasure Scale (TEPS) was used to measure individual dispositions in reward responsivity during real-world experiences of future anticipatory pleasure and in-the-moment consummatory pleasure (Gard et al., 2007). Examples of anticipatory pleasure items include: “Looking forward to a pleasurable experience is in itself pleasurable” and consummatory pleasure items include: “A hot cup of coffee or tea on a cold morning is very satisfying to me.” The SCID, PANSS, UPSA, and BIS/BAS were administered only to SZ patients. The TEPS was administered to both HC and SZ participants on the day of scanning.
2.3. Monetary Incentive Delay (MID) task
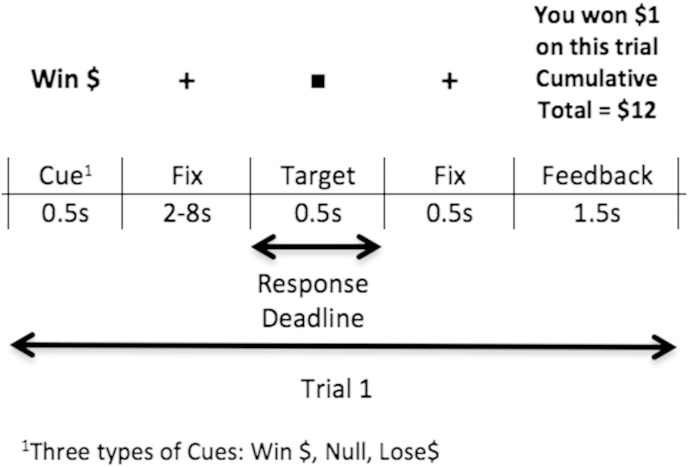
We used a standard Monetary Incentive Delay (MID) task, as described by Knutson et al. (2001), to assay the neural patterns associated with immediate anticipation and outcome of monetary reward (gain) and punishment (loss) in SZ and HC participants (Knutson et al., 2001). The MID paradigm is a Reaction Time (RT) task in which each trial has a pre-established monetary value. At the beginning of each trial, a cue (i.e., marking the onset of an anticipatory period) indicates the amount of money at stake on that trial: Win cues indicate potential monetary gain, Null cues indicate no monetary gain/no outcome, and Lose cues indicate potential monetary loss (Fig. 1). When the cue is presented, participants anticipate making a speeded response to the target (a white square), which is presented on the screen after a variable fixation interval (i.e., the anticipation period, duration from 2, 4, 6 or 8 s, randomized across all trials). Such variable delays were used to jitter the events and optimize deconvolution of the fMRI signal from successive events. After participants respond to the target, they receive feedback on how they performed on that trial in terms of receiving rewarding, punishing or neutral feedback. Specifically, on Win trials, participants are informed as to whether or not they won money; on Null trials, participants receive neutral feedback that they did not win/lose money; and on Lose trials, participants are informed as to whether or not they lost money on that trial. After the feedback prompt, there is a variable inter-trial-interval (ITI), jittered between 2, 4, 6, or 8 s randomized across all trials, after which the next cue is presented (marking the onset of the next anticipatory period). Participants succeed on the trial if the response happens within a fixed time window. For example, for each participant, performance level was titrated at 68% accuracy, such that the response window determining success was based on the participant's mean RT within one standard deviation of the mean averaged across performance based on the previous run. Responses to the first run were roughly titrated at 68% accuracy based on a previous practice run. Responses to the white square were captured for the total target presentation duration (i.e., 0.5 s); however, participants were specifically instructed to respond as fast as possible as soon as the target appeared. Early responses prior to target onset were not considered and participants were instructed not to make multiple responses. Each run consisted of 60 trials: 20 Win trials, 20 Null trials and 20 Loss trials, pseudorandomized. Participants completed 3 runs altogether, with each run lasting for a total time of 12 min and 24 s. Participants were provided with money based on their maximum earnings on their best performance run. Overall accuracy for each participant was calculated by computing the total number of Win trials and No-Loss trials, averaged across the three runs.
Fig. 1.
Illustration of one MID trial.
2.4. MID task: affect and arousal assessments
Immediately after the fMRI scan, participants rated their affect and arousal retrospectively on how they had felt when they viewed each condition on the MID task (i.e., Win, Lose, Null). Affect was rated on a Likert scale of 1–7, labeled on each end from “Negative” to “Positive”, and arousal was similarly rated on a Likert scale of 1–7, labeled on each end from “Not aroused” to “Very aroused”. Participants were explained that “arousal” was defined as feeling “activated, charged or energized, physically or mentally.”
2.5. MID task: behavioral statistical analyses
We first conducted between-group one-way ANOVAs in SPSS to examine between-group differences for overall accuracy (only correct trials) and RT (for both correct and incorrect trials) (Fig. 2). Correct trials only applied to the Win and No-Lose conditions. We next conducted between-group 2 × 2 repeated-measures ANOVA on accuracy to examine the main effects of condition (Win/No-Lose accuracy), main effects of group (HC/SZ), and group by condition (Win/No-Lose) interaction effects. We also conducted between-group 2 × 3 repeated-measures ANOVA on overall RT to examine the main effects of condition (Win/No-Lose/Null RT), main effects of group (HC/SZ), and group by condition (i.e., Win/No-Lose/Null) interaction effects.
Fig. 2.
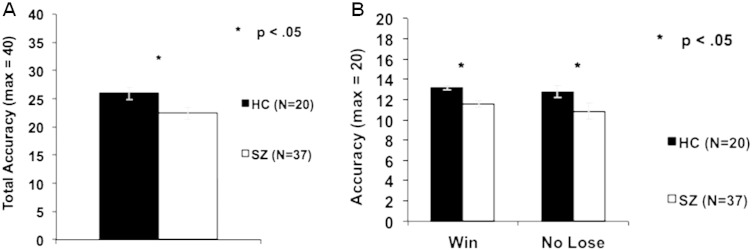
Behavior: Mean accuracy in HC and SZ participants. A. One-way ANOVA reveals a significant group difference in overall accuracy.B. One-way ANOVAs reveal a significant group difference in the No Lose condition as well as in the Win condition.
2.6. MID task: fMRI acquisition
Visual stimuli were presented with MATLAB and back-projected onto an LCD projector. Participants viewed the screen using a mirror attached to the head coil and made finger-press responses on a fiber-optic response pad. fMRI was acquired on a 3 Tesla Tim Trio Siemens scanner and twelve channel head coil, using an Echo-planar sequence (TR = 2.4 s, 35 slices, 306 volumes, TE = 30 ms, FOV = 230 mm; matrix = 64 × 64).
2.7. MID task: fMRI statistical analyses
Image analysis was performed using SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Slice timing was performed in interleaved order, where the first slice was used as the reference. Images were realigned to correct for motion artifacts using a 6-parameter affine transformation, normalized to a standard stereotaxic space (Montreal Neurological Institute Template) using a 12 parameter affine/non-linear transformation, and spatially smoothed with an 8 mm Full-width half-maximum (FWHM) Gaussian kernel. Data were submitted to a General Linear Model analysis. For each participant (i.e., first-level analysis), we fit a reference canonical hemodynamic response function (hrf) to each event within the trial (i.e., cue, the fixation period between cue and target, target, and feedback) (see Fig. 1).
We then computed the following contrasts of interest:
-
(i)
for immediate reward anticipation, we contrasted signal during the anticipation to win money with no outcome trials (i.e., Win cue plus fixation vs. Null cue plus fixation) by modeling the entire anticipatory period of the cue and fixation-cross presentation duration;
-
(ii)
for immediate punishment anticipation, we contrasted signal during the anticipation to lose money with no outcome trials (i.e., Lose cue plus fixation vs. Null cue plus fixation), by modeling the anticipatory period of the cue and fixation-cross presentation duration
-
(iii)
for reward outcome, we contrasted signal when participants were notified that they won money (monetary gain) compared to when they did not win money on Win trials, by modeling the duration of the feedback prompt
-
(iv)
for punishment outcome, we contrasted fMRI signal when participants were notified that they lost money (monetary loss) compared to not losing money on Lose trials by modeling the duration of the feedback prompt.
We isolated activation during immediate reward/punishment anticipation by comparing brain activation for each participant during the Win/Lose Cue with the Null Cue. During both Null cue and Win cue, participants anticipate making a response (i.e., pressing a button to a target). Therefore, the only cognitive process that is different (and, consequently, results in brain activation differences) during the Win Cue versus Null Cue has to be specifically due to anticipation of a reward, rather than due to a preparatory motor response (pressing a button to a target). Our general linear model (GLM) analysis allowed us to extract signal to each trial-type, and to factor out signal due to temporally adjacent events (e.g. to the feedback response), to ensure that signal could be isolated to the event of interest (e.g. reward anticipation). For example, when extracting signal related to anticipation events, we included in the analysis: the target and the feedback responses to factor out signal tied to those outcome events than to the anticipation event.
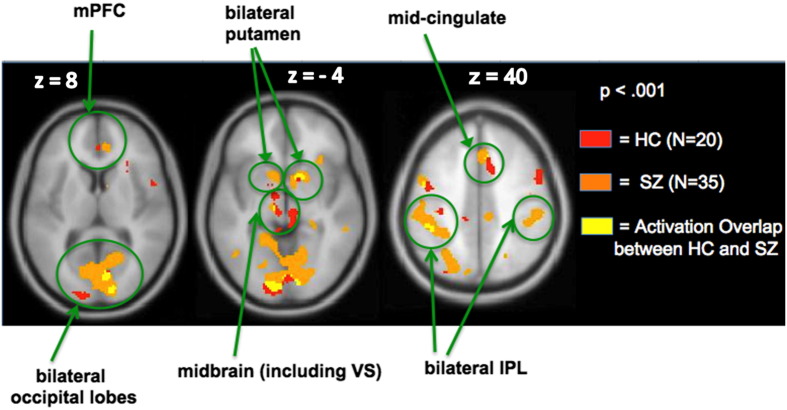
Next, we conducted one sample t-tests to investigate random-effects whole-brain voxelwise analyses for each group for each contrast using a significance threshold of p < 0.001. In order to run conjunction analyses at the group level, we used IMCalc in SPM8 to determine the overlap between groups with the equation i1 + 2 ∗ i2 for each of the four contrasts. The resulting images that had a value of 3 (i.e., illustrated by the yellow voxels as shown in Fig 3, for example) indicated activated voxels that were common to both HC and SZ groups, revealing that the two groups activated the same network for all four contrasts. We also conducted one-way ANOVAs to examine whole-brain voxel-wise between-group differences for each contrast. We did not find any regions that survived multiple comparison correction (at FDR, p < .05). Therefore, we now present regions showing voxel-wise group differences at an uncorrected threshold of p < .001 in the Supplement as these regions have a small cluster extent which limit the reliability of these effects, but may be useful as exploratory analyses for future studies (see Supplementary Table 1). We also conducted a whole-brain voxel-wise t-test in the SZ group in which we included chlorpromazine (CPZ) medication dosage equivalents as a covariate in order to find whether there were regions in the brain where CPZ medication dosage predicted reward anticipatory/outcome activation. Finally, we conducted a whole-brain voxel-wise one-sample t-test in our combined cohort of HC and SZ participants for each contrast. Table 3 shows all the areas that survived multiple comparison correction (FWE, p < .05) in the combined cohort.
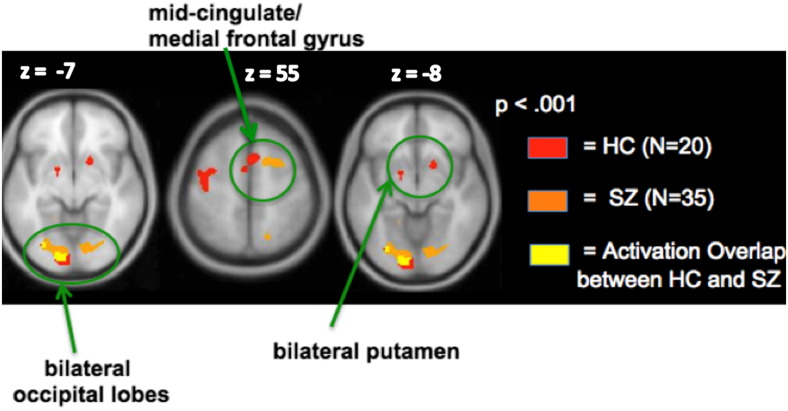
Fig. 3.
Conjunction analyses: immediate Reward anticipation versus Null. Whole-brain activation images reveal that HC and SZ participants recruit the same network. The yellow voxels illustrate regions showing activation overlap in the two groups.
Table 3.
Whole-brain analyses in the combined cohort (FWE, p < .05).
| Region | Volume (mm3) |
Max T | Coordinates |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| A. Immediate reward anticipation versus null | |||||
| Lingual/cuneus | 7352 |
7.51 | 12 | −88 | 2 |
| 6.52 | 12 | −92 | −4 | ||
| 5.88 | 12 | −94 | −22 | ||
| R. putamen/caudate head | 912 | 6.56 | 10 | 8 | −4 |
| L. IPL | 608 | 6.41 | −42 | −36 | 42 |
| L. M/IFG | 480 | 6.13 | −50 | 2 | 40 |
| R. M/IFG | 152 | 5.53 | 40 | −6 | 50 |
| Midbrain | 120 | 5.52 | −8 | −28 | −14 |
| 5.30 | −2 | −34 | −12 | ||
| B. Reward gain versus no monetary gain | |||||
| L. putamen/caudate head | 3952 | 8.71 | −20 | 10 | −8 |
| R. putamen/caudate head | 2664 | 8.01 | 20 | 12 | −6 |
| mPFC/ACC | 1336 | 6.62 | −8 | 36 | −8 |
| 5.56 | −6 | 38 | 10 | ||
| L. IPL | 1512 | 6.60 | −36 | −40 | 38 |
| R. IPL | 360 | 6.14 | 46 | −32 | 40 |
| C. Immediate punishment anticipation versus null | |||||
| Lingual/cuneus | 144 | 5.62 | −20 | −82 | −10 |
| D. Punishment loss versus no monetary loss | |||||
| − | − | − | − | − | − |
We used our whole-brain voxel-wise one-sample t-test in our combined cohort for subsequent region-of-interest (ROI) analyses that tested whether there were quantitative differences in the level of activation between the two groups during reward and punishment processing. The ROIs were centered on the peaks of the whole-brain analyses in our combined sample of HC and SZ participants for each of the contrasts, in order to minimize any bias from selecting clusters that may have been activated predominantly in one group versus the other (Poldrack, 2007). Additionally, in order to minimize multiple comparisons, we only defined ROIs based on regions that showed activation overlap between the two groups. Since the ROIs were selected based on a data-driven functional whole-brain analyses approach from the combined cohort without any knowledge of the results, there is no potential for bias. We calculated mean beta values within a 5 mm radius spherical volume around each of the centroids for each contrast for each participant in the 2 groups (HC and SZ), in which the coordinates of the centroids are shown in Table 4. The mean beta values extracted for each of the contrasts for each ROI were then entered into one-way ANOVAs in SPSS to compare between-group quantitative differences in signal for each contrast. We used Pearson's correlations to examine brain–behavior associations for each group by comparing mean beta signal within the ROIs with our behavioral/clinical variables of interest. Behavioral variables of interest included: TEPS-anticipatory scores, TEPS consummatory scores, BAS-Drive, PANSS and UPSA scores (see Supplementary Table 2). Whole-brain regression analyses were also conducted in order to study whether any of the behavioral/clinical variables predicted neural activation, thus providing additional confirmatory analyses to the brain–behavior ROI correlation findings. Finally, Fisher r-to-z transformations were conducted to assess the significance of group differences between the two correlation coefficients.
Table 4.
Regions-of-interest (mean beta, SD) identified in the whole-brain analyses in the combined cohort that showed voxel-wise activation overlap in the two groups.
| Region | Beta-value (SD) | Coordinates |
p value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| A. Immediate reward anticipation versus Null | |||||
| L. putamen | 0.35 (.60) | −18 | 10 | −6 | 0.20 |
| R. putamen | 0.42 (.52) | 10 | 8 | −4 | 0.14 |
| VS/nucleus accumbens | 0.35 (.58) | −8 | −4 | 6 | 0.046a |
| L. IPL | 0.31 (.40) | −42 | −36 | 42 | 0.66 |
| R. IPL | 0.28 (.42) | 49 | −33 | 42 | 0.19 |
| mPFC | 0.35 (.51) | 6 | 42 | 10 | 0.28 |
| Mid-cingulate | 0.37 (.52) | 8 | 10 | 46 | 0.46 |
| Occipital lobe | 0.67 (.73) | −12 | −88 | 2 | 0.42 |
| B. Reward gain versus no monetary gain | |||||
| L. putamen | 1.40 (1.24) | −20 | 12 | 4 | 0.61 |
| R. putamen | 1.24 (1.28) | 20 | 12 | −3 | 0.69 |
| L. IPL | 0.82 (.96) | −36 | −40 | 38 | 0.62 |
| C. immediate punishment anticipation versus Null | |||||
| L. occipital Lobe | 0.37 (.54) | −15 | −80 | −7 | 0.64 |
| D. Punishment Loss versus no monetary Loss | |||||
| mSFG | 0.96 (1.68) | −2 | 52 | 20 | 0.04a |
Between-group one-way ANOVAs showing significant differences in mean beta signal (p < .05).
3. Results
3.1. Behavioral findings
3.1.1. MID task accuracy
A one-way ANOVA revealed a group difference in overall accuracy (F = 4.05, df = 1,55, p < .05). The effect size (Cohen's d) of the overall accuracy difference between HC and SZ subjects was 0.59. A 2 × 2 repeated-measures ANOVA revealed a main effect of Win/No-Lose accuracy (F = 4.98, df = 1,55, p = .03) and a main effect of group (F = 36.51, df = 1,55, p < .001), but no group × Win/No-Lose accuracy interaction (F = 1.21, df = 1,55, p = .28). Subsequent between-group one-way ANOVAs revealed that HC participants had better accuracy on both Win (F = 38.91, df = 1,55, p < .001) and Lose conditions (F = 29.76, df = 1,55, p < .001) when compared to SZ participants (Fig. 2). However, interestingly, within-group repeated-measures ANOVAs indicated that both HC and SZ groups revealed the same accuracy pattern with higher accuracy scores on the Win versus Lose condition (HC:F = 4.79, df = 1,19, p = .041; SZ: F = 5.37, df = 1,36, p = .026).
3.1.2. MID task Reaction Time (RT)
A one-way ANOVA revealed a group difference in overall RT (F = 10.39, df = 1,55, p < .002). A 2 × 3 repeated-measures ANOVA revealed a main effect of condition (F = 20.88, df = 2,54, p < .001), and a main effect of group (F = 10.39, df = 2,54, p < .002) but no group × condition interaction (F = 1.83, df = 2,54, p = .17). Subsequent between-group one-way ANOVAs revealed that SZ participants were slower than HC participants across all conditions (Win: F = 11.18, df = 1,55, p = .001; Lose: F = 8.53, df = 1,55, p = .005; Null: F = 9.28, df = 1,55, p = .004). Within-group repeated-measures ANOVAs indicated that both HC and SZ groups revealed the same RT pattern, with both groups being slowest in the Null condition when compared to the Win condition (HC: F = 20.77, df = 1,19, p = .001; SZ: F = 13.05, df = 1,36, p = .001), and when the Null was compared to the Lose condition (HC: F = 5.95, df = 1,19, p = .025; SZ: F = 5.84, df = 1,36, p = .02). Both groups were also fastest on the Win condition when compared to the Lose condition (HC: F = 13.37, df = 1,19, p = .002; SZ: F = 10.04, df = 1,36, p = .003).
3.2. fMRI findings
3.2.1. Immediate reward anticipation versus null
As confirmed by our whole-brain conjunction analyses, SZ patients revealed a similar qualitative pattern of cortical–subcortical activation (extending from frontal to striatal regions) to that of HC participants (Fig. 3). However, as predicted, our ROI analyses indicated quantitative between-group activation differences within the ventral striatum (VS) in SZ participants (Table 4). Specifically, one-way ANOVAs revealed that SZ participants showed hypoactivation in only one region, the ventral striatum (VS), when compared to HC participants (F = 4.18, df = 1,53, p = .046).
3.2.2. Association of fMRI findings during immediate reward anticipation with behavioral and clinical measures
3.2.2.1. Self-ratings of reward responsivity and arousal
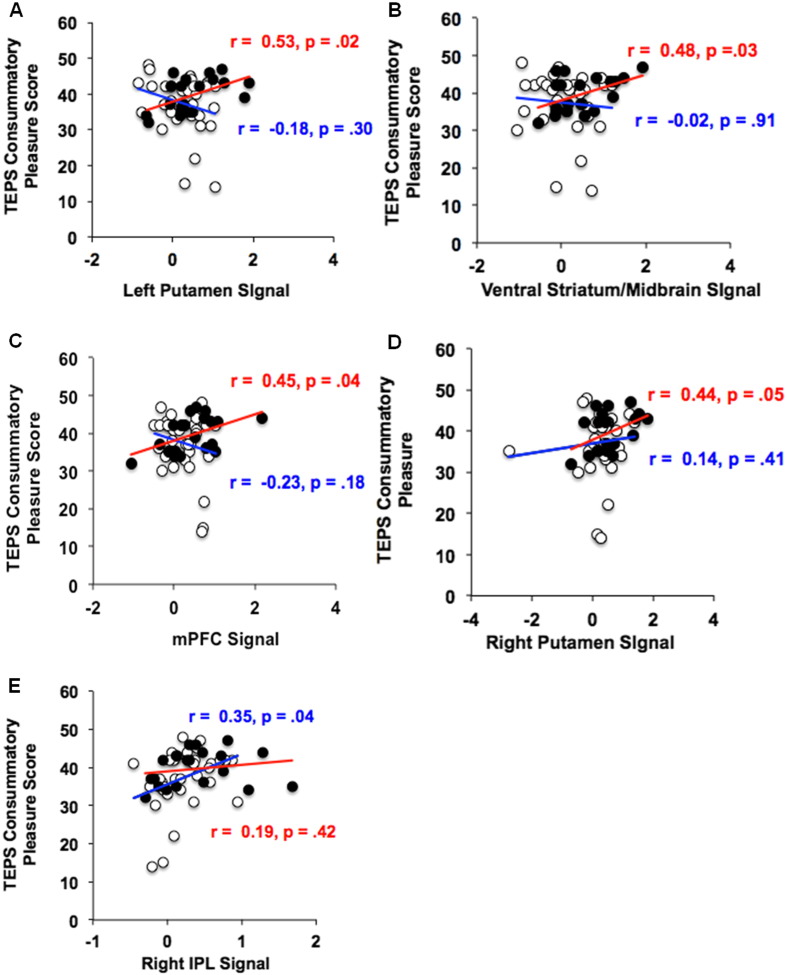
SZ patients exhibited marginally reduced levels of future representations of anticipatory pleasure (i.e., TEPS anticipatory pleasure) compared to healthy participants (p = .08), although they exhibited similar arousal levels when viewing the Win cue, as well as similar levels of consummatory pleasure (i.e., TEPS consummatory pleasure) as HC participants (all p values > .20). However, interestingly, we found distinct differences between the HC and SZ groups in terms of their neural signal association with the TEPS consummatory pleasure scale. Specifically, the HC group revealed neural activity in four regions during reward anticipation that predicted higher self-ratings of TEPS consummatory pleasure; this association was found in: left putamen (r = .53, df = 18, p = .02), VS (r = .48, df = 18, p = .03), mPFC (r = .45, df = 18, p = .04), and right putamen (r = .44, df = 18, p = .05). In contrast, the SZ group revealed signal increase in only one region, the right IPL, that predicted higher self-ratings in TEPS consummatory pleasure (r = .35, df = 33, p = .04) (Fig. 4). Signal in right IPL was also associated with higher self-ratings on BAS-Drive (r = .39, df = 33, p = .02) while signal in the left IPL predicted better real-world functional capacity, as measured by the UPSA (r = .34, df = 33, p = .05). Finally, whole-brain regression analyses provide additional confirmatory evidence that TEPS anticipatory pleasure, TEPS consummatory pleasure and BAS-Drive predicted R. IPL signal during immediate reward anticipation in schizophrenia patients (see Supplementary Fig. 1). Although, Fisher r-to-z transformations did not reveal any significant between-group difference in the strength of the association between R. IPL and TEPS consummatory pleasure (z = .58, p = .28), we did find a significant between-group difference in the relation between TEPS consummatory pleasure and ventral striatum (z = 1.91, p = .03), left putamen (z = 2.57, p = .005), as well as mPFC (z = 2.57, p = .005). Together, these findings suggest that SZ and HC participants show significantly different associations between frontal–striatal activation during anticipation of immediate rewards, and self-ratings of real-world reward responsivity.
Fig. 4.
ROI analyses during immediate reward anticipation: correlations with trait hedonic pleasure. HC = black circles, red correlation values; SZ = white circles, blue correlation values.
3.2.3. Clinical and neuropsychological measures
Overall, symptom ratings were low in this clinically stable group of SZ participants (average rating slightly over 2, mild). However, we did find a positive correlation between positive symptom severity with mean beta signal in mPFC (r = .33, df = 33, p = .05) and mid-cingulate (r = .37, df = 33, p = .03) during immediate reward anticipation. This positive relationship between mid-cingulate signal with positive symptoms was also confirmed by our whole-brain voxel-wise regression analyses in which positive symptom severity predicted activation in the mid-cingulate region during reward anticipation (see Supplementary Fig. 2). There was no association between mean beta signal in any of the ROIs during reward anticipation with either problem-solving or global cognition (all p values > 0.10).
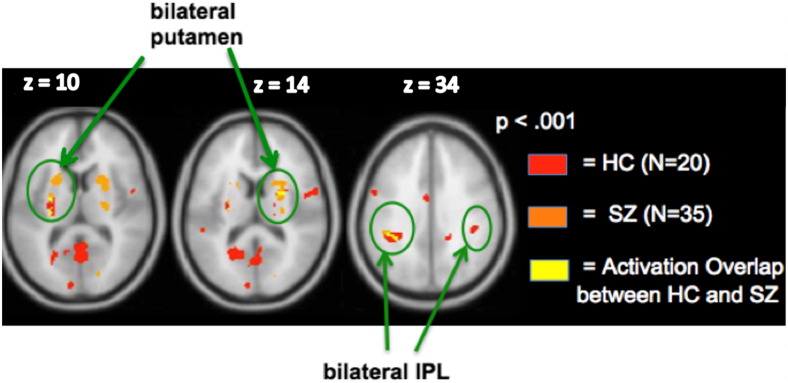
3.2.4. Reward gain versus no monetary gain
Whole-brain conjunction analyses revealed that the two groups activated the same network, with activation overlap observed within bilateral putamen and L.IPL (Fig. 5). Additionally, further ROI analyses revealed that signal in none of the three ROIs correlated with any clinical/neuropsychological measures (all ps > .10).
Fig. 5.
Conjunction analyses: Reward gain versus no monetary gain. Bilateral putamen and L. IPL regions show activation overlap in the two groups.
3.2.5. Immediate punishment anticipation versus null
Whole-brain conjunction analyses revealed similar qualitative activation patterns between HC and SZ participants during anticipation of immediate punishments versus the Null condition. Specifically, schizophrenia patients recruited the same regions that healthy participants activated (i.e., mid-cingulate, bilateral putamen and occipital regions), although functional overlap was only observed within the left occipital region (Fig. 6). Further ROI analyses indicated that the left occipital region did not correlate with any clinical/neuropsychological measures (all ps > .20).
Fig. 6.
Conjunction analyses: immediate punishment anticipation versus Null. Only one region, left occipital cortex, shows activation overlap in the two groups.
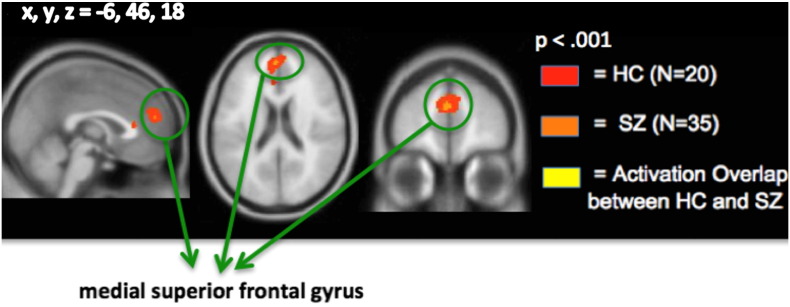
3.2.6. Punishment loss versus no monetary loss
Whole-brain 1-sample t-tests in the combined cohort revealed that only one region, the medial superior frontal gyrus (extending to the anterior cingulate cortex, mSFG/ACC), showed increased activation during the outcome phase when participants were notified that they had lost money compared to no monetary loss (Fig. 7). Interestingly, ROI analyses indicated that the mSFG/ACC was also the only region in which between-group quantitative differences in signal were found. Specifically, SZ participants revealed significantly reduced activation during monetary loss versus no loss when compared to HC participants (F = 4.69, df = 1,53 p = .035) (Table 4).
Fig. 7.
Conjunction analyses: punishment Loss versus no monetary Loss. Only one region, medial superior frontal gyrus, shows activation overlap in the two groups.
4. Discussion
We conducted an fMRI study investigating neural activation patterns in schizophrenia during immediate prediction and outcome of rewards and punishments on the MID task, and investigated how neural signal related to measures of real-world reward sensitivity, responsivity, and functional outcome. We found that:
-
1)
SZ patients showed impaired accuracy on both Win (reward) and Lose (punishment) conditions when compared to HC participants. Although accuracy was titrated for each participant at roughly 68% based on the previous run, we still observed group differences in accuracy. These findings suggest that HC participants performed better than SZ participants with each subsequent run. Additionally, although SZ participants were slower than HC participants on each condition, they showed the same accuracy and RT patterns as the HC group in that both groups had higher accuracy scores on the Win compared to Lose conditions, and both groups also had the slowest RT on the Null condition and fastest RT on the Win condition. Further, one-way between-group ANOVAs did not yield any brain regions in which SZ patients showed increased activation to neutral stimuli when compared to HC participants, confirming that the current findings cannot be explained by aberrant salience to neutral stimuli compared to motivationally salient information (Esslinger et al., 2012).
-
2)
During immediate reward anticipation, both HC and SZ participants showed increased activation in several regions, including: VS, bilateral putamen, bilateral IPL, mPFC, mid-cingulate and occipital areas. However, there were distinct differences between groups in brain–behavior correlations with self-reports of reward responsivity. HC participants showed a significant relationship between reward-mediating regions (i.e., VS, mPFC, bilateral putamen) and self-ratings of TEPS consummatory pleasure, whereas SZ participants showed a significant association between right IPL and self-ratings of TEPS consummatory pleasure.
-
3)
During punishment outcome, SZ patients revealed hypoactivation in the mSFG when compared with HC participants.
Our whole-brain analyses indicate that as subjects anticipated immediate monetary rewards, several regions revealed increased activation, including VS, bilateral putamen, bilateral IPL, mPFC and mid-cingulate, in our combined cohort of HC and SZ participants. Further, whole-brain conjunction analyses revealed all regions that showed activated voxels that were common to both HC and SZ groups, suggesting similar whole-brain qualitative patterns of neural activity in the 2 groups. Interestingly, whole-brain regression analyses revealed that anticipatory representations of future reward (assayed with TEPS anticipatory pleasure scale) as well as consummatory pleasure ratings (assayed with the TEPS consummatory pleasure scale) predicted signal in only one region, the right IPL, in SZ patients during immediate reward anticipation (i.e., during the Win cue). It has also been previously shown that remembered consummatory pleasure is a critical predictor of motivation in schizophrenia (Trémeau et al., 2010), which seem to be supported with the findings in our current neuroimaging study.
Our ROI analyses indicated that in SZ patients, compared with HC participants, the VS showed reduced activation during immediate reward anticipation, and the medial SFG showed reduced activation during punishment outcome. The medial SFG/ACC region has been implicated in conflict monitoring and error detection (Alain et al., 2002; Garavan et al., 2003; Gehring and Knight, 2000). Thus, it may be possible that HC participants recruit the mSFG to a greater extent during error-monitoring, which, in turn, likely contributes to better subsequent performance during punishment trials and, consequently, may help them perform better at avoiding monetary loss in the future, when compared to SZ participants.
Given past research showing that striatal activation is associated with reward anticipation and that SZ patients reveal lower striatum signal during anticipation of immediate upcoming rewards (Juckel et al., 2006), the present findings corroborate prior research confirming that although SZ patients do show similar whole-brain neural activation patterns to HC participants, they do exhibit reduced striatal activation during anticipation of immediate rewards. Our findings also revealed that during immediate reward anticipation, SZ patients showed a positive association between mPFC and mid-cingulate signal with positive symptom severity. While some prior studies have shown an inverse relationship between reward-related activation with positive symptoms (Schlagenhauf et al., 2009), other studies have shown positive associations between frontal and striatal signals with positive symptom severity (Rotarska-Jagiela et al., 2010; Wotruba et al., 2014). For example, Rotarska-Jagiela et al. (2010) has shown that frontal–temporal connectivity positively correlated with positive symptom severity in schizophrenia, which suggests that psychopathology may be associated with intrinsic frontal aberrations. One theory that may explain this positive correlation between frontal–temporal regions with positive symptoms (such as hallucinations) is that there is impaired connectivity between frontal areas (involved in anticipatory processing for an upcoming action) and temporal areas (involved in auditory perception) in which hallucinations are thought to result from misinterpreted speech intentions (Rotarska-Jagiela et al., 2010). In other words, frontal regions (such as the mid-cingulate) may be activated during prediction errors between anticipatory speech intentions and outcome. Similarly, in our study, one speculation is that the mid-cingulate may also be activated during prediction errors between immediate reward anticipation and outcome, in which mid-cingulate signal correlates with positive symptom severity. In our clinically stable sample of patients, since most of our patients were on atypical antipsychotic medications, we do not believe that these findings are due to effects of dopaminergic-blocking typical versus atypical medications (Juckel et al., 2006; Kirsch et al., 2007). Furthermore, we did not find any regions in the brain where CPZ medication dosage predicted reward anticipatory/outcome activation in our whole-brain regression analyses. Rather, our findings do confirm that aberrations in recruitment of the frontal–striatal circuitry during immediate reward anticipation are inherent to the illness even in our low-symptom clinically stable sample of schizophrenia patients.
The present paper also extends prior research in that SZ and HC participants showed different responses in their brain–behavior associations with self-ratings of reward responsivity (i.e., TEPS consummatory pleasure ratings). HC participants revealed associations in bilateral putamen, mPFC and VS with hedonic consummatory pleasure ratings while SZ participants revealed correlations with consummatory pleasure ratings within the right IPL. It must also be noted that we did not find significant activation group differences within the IPL or a significant between-group difference in the strength of the association between R. IPL and reward responsivity. However, we did find a significant between-group difference in the strength of the relation between reward responsivity, and left putamen, VS and mPFC. We think that we did not observe a significant between-group difference in R. IPL because the slopes are in the same direction for both groups (unlike the slopes within mPFC, VS and left putamen in which we do find a significant between group difference). These findings hint at the possibility that both HC and SZ groups may benefit from enhanced IPL signal during immediate reward anticipation (although the association with reward responsivity seems to be stronger in the SZ than that in the HC group). Together, these findings suggest that SZ and HC participants show significantly distinct associations between frontal–striatal activation during anticipation of reward, and self-ratings of real-world reward responsivity.
Consistent with prior studies, our findings indicate that SZ patients did not differ from HC participants in the level of their consummatory pleasure ratings (Gard et al., 2007) but only in terms of the brain regions that predicted consummatory pleasure. Furthermore, SZ patients did not differ from HC participants in their positive affect and arousal ratings to the rewarding cue. These findings suggest that patients' experience of positive affect and arousal in response to immediate rewarding stimuli is intact, in agreement with previous literature (Cohen and Minor, 2010; Llerena et al., 2012), but point towards distinct differences between reward-related anticipatory neural activity and the reported subjective experience of pleasure. Specifically, while healthy participants recruited regions known to mediate reward-anticipatory activity such as the basal ganglia (bilateral putamen) and VS (Barch and Dowd, 2010; Knutson et al., 2001; Nielsen et al., 2012) that predicted trait hedonic pleasure, SZ patients revealed right IPL activation — a region that is not considered central to reward processing (Singh-Curry and Husain, 2009).
During anticipation of immediate rewards, the right IPL was also correlated with overall motivational drive (i.e., BAS-Drive) to move towards a desired goal in SZ participants, while the left IPL predicted better functional capacity (as measured by the UPSA). Together, these findings indicate that the signal in the IPL (rather than reward-mediating regions) predicts enhanced functional capacity in individuals with schizophrenia. Finally, our findings suggest that activating impaired frontal circuits during the reward anticipatory phase may not be beneficial for clinical symptoms in our sample of chronically-ill albeit clinically stable sample of SZ participants, but point towards recruitment of more intact systems within the IPL that are likely to predict enhanced motivation and functional capacity.
Although the IPL is involved in multiple cognitive and emotional processes, it is particularly important for attention during saliency processing and consequently, facilitates goal-directed activities. Salient information activates both the right and left IPL; however, the right IPL is thought to be more involved in directing attention to salient relevant information, while the left IPL is activated particularly during suppression of irrelevant information (Mevorach et al., 2006). Thus, both right and left IPL support attention to salient information that is needed for goal-directed functional capacity. This is consistent with findings from other studies that indicate that the parietal cortex is modulated when participants engage in increased attentional effort across a broad range of cognitive tasks (Kanwisher and Wojciulik, 2000; Shuman and Kanwisher, 2004). Furthermore, damage to the IPL has been reliably associated with hemi-spatial attentional neglect (Mesulam, 1999), in which patients fail to attend to information in their left visual field, indicating that IPL is necessary for attention processes. Finally, Small et al. (2005) have specifically shown that IPL is modulated by changes in attentional cues that mediate motivational salience on their monetary incentive task, and IPL signal is enhanced when motivationally salient information (i.e., rewarding cues) is combined with transcranial magnetic stimulation (TMS) on the MID task (Stanford et al., 2013).
Together, these findings suggest that in SZ participants, enhanced IPL activity during immediate reward anticipation and its association with BAS-Drive supports enhanced attention to incoming salient information, which then increases patients' motivation to move towards a desired goal, thus, contributing to enhanced functional capacity (as shown by IPL signal association with the UPSA scale). Indeed, Gard et al. (2009) also conducted path analyses to find that motivation plays a mediating role between neurocognition and functional outcome. These findings corroborate prior research, suggesting that the IPL may have a functional attention-mediating compensatory role during reward motivation in psychiatric disorders such as schizophrenia (Stanford et al., 2013).
5. Conclusions
The motivation of getting rewards or avoiding punishments reinforces goal-directed behavior. The present findings indicate that SZ patients showed reduced accuracy on reward and punishment trials when compared to HC participants. However, both HC and SZ participants showed a similar accuracy and RT pattern, being most accurate and fastest on the Win condition and slowest on the Null condition. Therefore, we do not believe that the current findings can be explained by SZ participants showing reduced willingness to expend effort in order to achieve monetary rewards. The results point to common whole-brain qualitative activation patterns during immediate reward and punishment anticipation and outcome in the two groups, as well as quantitative between-group differences (with SZ patients showing hypoactivation in ventral striatum) during immediate reward anticipation. Finally, whereas HC participants activated reward-related circuits in VS, bilateral putamen and mPFC during immediate reward anticipation, which predicted hedonic pleasure, SZ participants activated regions such as the IPL (mediating attention rather than reward mechanisms) that predicted hedonic pleasure and functional capacity. Together, these findings suggest that the study and treatment of motivation deficits in patients with schizophrenia may require a more detailed investigation of the role of the IPL as a circuit modulator during immediate reward anticipation that may contribute to real-world functioning capacity.
Acknowledgments
This work was supported by the National Institutes of Health grants R01MH82818-01A2, T32MH89920-5 and K01MH105615. We thank Daniel H. Mathalon, Todd Thompson, Michael M. Merzenich, Judith Ford, Patrick Slattery, Aditi Shastri, Coleman Garrett, Hong Yin, Benjamin Brandrett, Lisa Howard and Abby Rowlands for their assistance and input on this project.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2015.08.001.
Appendix A. Supplementary data
Supplementary material.
References
- Abi-Dargham A. Probing cortical dopamine function in schizophrenia: what can D1 receptors tell us? World Psychiatry. 2003;2(3):166–171. 16946930 [PMC free article] [PubMed] [Google Scholar]
- Alain C., McNeely H.E., He Y., Christensen B.K., West R. Neurophysiological evidence of error-monitoring deficits in patients with schizophrenia. Cereb. Cortex. 2002;12(8) doi: 10.1093/cercor/12.8.840. 12122032 [DOI] [PubMed] [Google Scholar]
- Barch D.M., Dowd E.C. Goal representations and motivational drive in schizophrenia: the role of prefrontal–striatal interactions. Schizophr. Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. 20566491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie C.R., Reichenberg A., Patterson T.L., Heaton R.K., Harvey P.D. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am. J. Psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. 16513862 [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol. 1994;67(2):319–333. [Google Scholar]
- Cohen A.S., Minor K.S. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. 18562345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esslinger C., Englisch S., Inta D., Rausch F., Schirmbeck F., Mier D., Kirsch P., Meyer-Lindenberg A., Zink M. Ventral striatal activation during attribution of stimulus saliency and reward anticipation is correlated in unmedicated first episode schizophrenia patients. Schizophr. Res. 2012;140(1–3):114–121. doi: 10.1016/j.schres.2012.06.025. 22784688 [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. patient edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders, research version. [Google Scholar]
- Foussias G., Remington G. Negative symptoms in schizophrenia: avolition and Occam's razor. Schizophr. Bull. 2010;36(2):359–369. doi: 10.1093/schbul/sbn094. 18644851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Kaufman J., Stein E.A. A midline dissociation between error-processing and response–conflict monitoring. NeuroImage. 2003;20(2):1132–1139. doi: 10.1016/S1053-8119(03)00334-3. 14568482 [DOI] [PubMed] [Google Scholar]
- Gard D.E., Fisher M., Garrett C., Genevsky A., Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophr. Res. 2009;115(1):74–81. doi: 10.1016/j.schres.2009.08.015. 19783407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard D.E., Kring A.M., Gard M.G., Horan W.P., Green M.F. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. 17490858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring W.J., Knight R.T. Prefrontal–cingulate interactions in action monitoring. Nat. Neurosci. 2000;3(5):516–520. doi: 10.1038/74899. 10769394 [DOI] [PubMed] [Google Scholar]
- Gold J.M., Waltz J.A., Prentice K.J., Morris S.E., Heerey E.A. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr. Bull. 2008;34(5):835–847. doi: 10.1093/schbul/sbn068. 18591195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey E.A., Matveeva T.M., Gold J.M. Imagining the future: degraded representations of future rewards and events in schizophrenia. J. Abnorm. Psychol. 2011;120(2):483–489. doi: 10.1037/a0021810. 21171727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbener E.S., Song W., Khine T.T., Sweeney J.A. What aspects of emotional functioning are impaired in schizophrenia? Schizophr. Res. 2008;98(1–3):239–246. doi: 10.1016/j.schres.2007.06.025. 17689054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G., Schlagenhauf F., Koslowski M., Wüstenberg T., Villringer A., Knutson B., Wrase J., Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kanwisher N., Wojciulik E. Visual attention: insights from brain imaging. Nat. Rev. Neurosci. 2000;1(2):91–100. doi: 10.1038/35039043. 11252779 [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Kirsch P., Ronshausen S., Mier D., Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–198. doi: 10.1055/s-2007-984463. 17874350 [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. 11459880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Bennett S.M., Adams C.M., Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. NeuroImage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. 12595181 [DOI] [PubMed] [Google Scholar]
- Knutson B., Rick S., Wimmer G.E., Prelec D., Loewenstein G. Neural predictors of purchases. Neuron. 2007;53(1):147–156. doi: 10.1016/j.neuron.2006.11.010. 17196537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. fMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. 10875899 [DOI] [PubMed] [Google Scholar]
- Kring A.M., Moran E.K. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. 18579556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llerena K., Strauss G.P., Cohen A.S. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr. Res. 2012;142(1–3):65–70. doi: 10.1016/j.schres.2012.09.005. 23040736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354(1387):1325–1346. doi: 10.1098/rstb.1999.0482. 10466154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach C., Humphreys G.W., Shalev L. Opposite biases in salience-based selection for the left and right posterior parietal cortex. Nat. Neurosci. 2006;9(6):740–742. doi: 10.1038/nn1709. 16699505 [DOI] [PubMed] [Google Scholar]
- Murray G.K., Corlett P.R., Clark L., Pessiglione M., Blackwell A.D., Honey G., Jones P.B., Bullmore E.T., Robbins T.W., Fletcher P.C. Substantia nigra/ventral tegmental reward prediction error disruption in psychosis. Mol. Psychiatry. 2008;13(3):267–276. doi: 10.1038/sj.mp.4002058. 17684497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.Ø., Rostrup E., Wulff S., Bak N., Lublin H., Kapur S., Glenthøj B. Alterations of the brain reward system in antipsychotic naïve schizophrenia patients. Biol. Psychiatry. 2012;71(10):898–905. doi: 10.1016/j.biopsych.2012.02.007. 22418013 [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Patterson T.L., Goldman S., McKibbin C.L., Hughs T., Jeste D.V. UCSD performance-based skills assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr. Bull. 2001;27(2):235–245. doi: 10.1093/oxfordjournals.schbul.a006870. 11354591 [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. Region of interest analysis for fMRI. Soc. Cogn. Affect. Neurosci. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. 18985121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska-Jagiela A., van de Ven V., Oertel-Knöchel V., Uhlhaas P.J., Vogeley K., Linden D.E.J. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. 20097544 [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F., Juckel G., Koslowski M., Kahnt T., Knutson B., Dembler T., Kienast T., Gallinat J., Wrase J., Heinz A. Reward system activation in schizophrenic patients switched from neuroleptics to olanzapine. Psychopharmacology (Berl.) 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. 18097655 [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F., Sterzer P., Schmack K., Ballmaier M., Rapp M., Wrase J., Juckel G., Gallinat J., Heinz A. Reward feedback alterations in unmedicated schizophrenia patients: relevance for delusions. Biol. Psychiatry. 2009;65(12):1032–1039. doi: 10.1016/j.biopsych.2008.12.016. 19195646 [DOI] [PubMed] [Google Scholar]
- Schlosser D.A., Fisher M., Gard D., Fulford D., Loewy R.L., Vinogradov S. Motivational deficits in individuals at-risk for psychosis and across the course of schizophrenia. Schizophr. Res. 2014;158(1–3):52–57. doi: 10.1016/j.schres.2014.06.024. 25008792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman M., Kanwisher N. Numerical magnitude in the human parietal lobe; tests of representational generality and domain specificity. Neuron. 2004;44(3):557–569. doi: 10.1016/j.neuron.2004.10.008. 15504334 [DOI] [PubMed] [Google Scholar]
- Simon J.J., Biller A., Walther S., Roesch-Ely D., Stippich C., Weisbrod M., Kaiser S. Neural correlates of reward processing in schizophrenia — relationship to apathy and depression. Schizophr. Res. 2010;118(1–3):154–161. doi: 10.1016/j.schres.2009.11.007. 20005675 [DOI] [PubMed] [Google Scholar]
- Singh-Curry V., Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47(6):1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. 19138694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.M., Gitelman D., Simmons K., Bloise S.M., Parrish T., Mesulam M.M. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb. Cortex. 2005;15(12) doi: 10.1093/cercor/bhi063. 15746002 [DOI] [PubMed] [Google Scholar]
- Stanford A.D., Luber B., Unger L., Cycowicz Y.M., Malaspina D., Lisanby S.H. Single pulse TMS differentially modulates reward behavior. Neuropsychologia. 2013;51(14):3041–3047. doi: 10.1016/j.neuropsychologia.2013.09.016. 24041669 [DOI] [PubMed] [Google Scholar]
- Strauss G.P., Morra L.F., Sullivan S.K., Gold J.M. The role of low cognitive effort and negative symptoms in neuropsychological impairment in schizophrenia. Neuropsychology. 2015;29(2):282–291. doi: 10.1037/neu0000113. 25000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G.P., Wilbur R.C., Warren K.R., August S.M., Gold J.M. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187(1–2):36–41. doi: 10.1016/j.psychres.2011.01.012. 21295860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trémeau F., Antonius D., Cacioppo J.T., Ziwich R., Butler P., Malaspina D., Javitt D.C. Anticipated, on-line and remembered positive experience in schizophrenia. Schizophr. Res. 2010;122(1–3):199–205. doi: 10.1016/j.schres.2009.10.019. 19906511 [DOI] [PubMed] [Google Scholar]
- Wotruba D., Heekeren K., Michels L., Buechler R., Simon J.J., Theodoridou A., Kollias S., Rössler W., Kaiser S. Symptom dimensions are associated with reward processing in unmedicated persons at risk for psychosis. Front. Behav. Neurosci. 2014;8:382. doi: 10.3389/fnbeh.2014.00382. 25477792 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.