Abstract
Purpose
The present study reports a case of encephalitis due to herpes simplex virus-1 (HSV-1), following surgical manipulation of the site of a primary infection.
Methods
Herpes simplex virus-1 infection was confirmed by CSF PCR and DNA sequencing.
Results
The patient was an 11-year-old girl who required temporal lobe surgery for epilepsy. She had meningoencephalitis due to HSV at the age of 20 months, and she was treated with acyclovir. Three years later, the patient developed uncontrolled seizures that became more frequent and changed in character at 11 years of age. On the 12th postoperative day, she developed fever and seizures, and she was diagnosed with HSV-1 by positive CSF PCR. She was treated with acyclovir (30 mg/kg/day for 21 days). In this report, we describe the patient and review the relevant literature.
Conclusion
The authors stress the potential risk of reactivation of HSV encephalitis after intracranial surgery. Herpes simplex virus encephalitis must be considered in neurosurgical patients who develop postoperative seizures and fever.
Keywords: Herpes simplex, Encephalitis, Reactivation, Neurosurgery, Epilepsy
1. Introduction
Herpes simplex virus (HSV) is the cause of the most common form of nonepidemic viral encephalitis, with an incidence of 2 or 3 cases per million per year and no seasonal variation [1]. Herpes viruses are characterized by their ability to infect cells and destroy them or remain inactive without causing cell damage. Herpes simplex virus encephalitis after neurosurgery has been described previously [2–15], including three cases of HSV reactivation following neurosurgery to treat uncontrolled epilepsy in patients with a previous history of HSV encephalitis [2,3,9]. We report a case of HSV-1 encephalitis, confirmed by CSF PCR and DNA sequencing, following surgical manipulation of the site of a primary HSV-1 infection.
2. Case report
2.1. History and examination
An 11-year-old right-handed Caucasian female patient was admitted with fever and confusion after surgery for epilepsy. Her medical history was significant for an episode of HSV encephalitis at twenty months of age, which was diagnosed by positive CSF PCR for HSV-1 (Table 1) and resulted in developmental delay and epilepsy. No additional medical and surgical history was relevant. The electroencephalogram (EEG) at twenty months showed dysfunction of the right frontotemporal region. Magnetic resonance imaging (MRI) revealed a signal change in the right temporal, insular, and meningeal arteries, with some bleeding and a lesion in the left hippocampus. She was treated with intravenous acyclovir (30 mg/kg/day for 21 days) and carbamazepine on discharge from the hospital. The seizures were controlled for 3 years, but in 2007, she started having complex partial seizures with secondary generalization.
Table 1.
Cerebrospinal fluid (lumbar puncture) cellular, biochemistry and molecular biology diagnosis.
| CSF | 8 months of age | 12th POD | 16th POD |
|---|---|---|---|
| RBC/mm3 | – | 5 | 20 |
| WBC/mm3 | ↑ | 265 | 128 |
| Lymphocytes % | > 50% | 99 | 97 |
| Monocytes % | – | 1 | 3 |
| Glucose, mg/dL | – | 55 | 59 |
| CSF/blood glucose | – | 0.4 | – |
| Total protein, mg/dL | – | 83 | 51 |
| Microbiological studiesa | Negative | Negative | Negative |
| PCR HSVb | Positive | Positive | Positive |
| PCR enterovirus | ND | Negative | Negative |
ND — not done.
POD — postoperative day.
Gram smear, bacterial and fungus culture.
End-point PCR.
At 11 years of age, her seizures increased in frequency, and the pattern changed, with oculocephalic reflex followed by generalized tonic–clonic seizures and clonic seizures of the eyelids. Despite management with multiple antiepileptic medications, uncontrolled seizures continued with daily frequency. Interictal EEG showed right temporal spikes. Ictal EEG showed generalized complex partial seizures without lateralization. Preoperative MRI revealed a lesion in the right temporal lobe: a small area of encephalomalacia on the medial anterior pole of the right temporal lobe, involving the temporal amygdala, head of the hippocampus, parahippocampal gyrus, and part of the right insular cortex, suggesting residual findings after a previous ischemic insult.
2.2. Surgery
The patient underwent selective right temporal lobe surgery for epilepsy: 3.5 cm of the anterior temporal lobe was resected, and amygdalohippocampectomy was performed, with no complications during surgery. Anatomopathological study of surgical specimens showed nonspecific changes, segmental neuronal loss within the hippocampal subfields, granule cell dispersion, and the presence of diffuse gliosis associated with pyramidal cell loss, consistent with mesial sclerosis. Immunohistochemical reactions with monoclonal anti-HSV-1 antibody (Dako) were negative (Fig. 1).
Fig. 1.
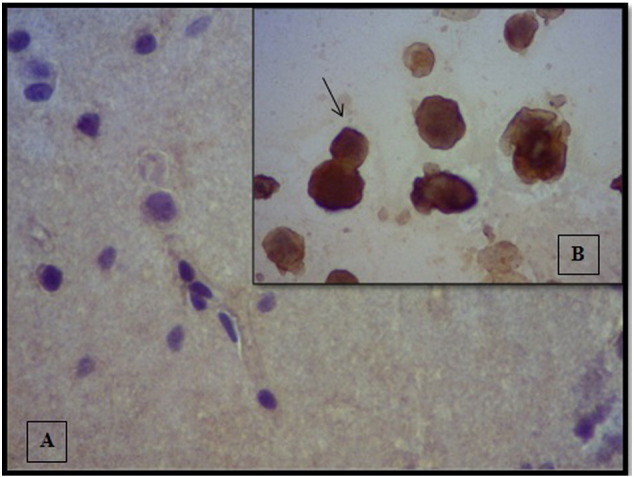
The immunohistochemical reactions with monoclonal antibody anti-HSV- 1. A. Reactions in the surgical specimens were negative. B. Positive control: reaction of monoclonal antibody with HSV-positive Vero cell culture. The arrow indicates a positive reaction. Magnification: 400×.
2.3. Postoperative course
No epileptiform activity was detected in EEG recordings after surgery. She was discharged from the hospital on the 4th postoperative day (POD) in good general condition, with no fever or neurological symptoms. The surgical wound was clean and dry.
On the 12th POD, the patient developed fever (39–40 °C) and severe headache, vomiting, and had deteriorating level of consciousness and worsening of general condition. Neurological examination results were as follows: sleepiness although obeying commands, Glasgow coma scale score of 14/15 (eye opening: 4, verbal response: 4, and motor response: 6), pupils bilaterally isochoric and reactive to light, neck stiffness, and spontaneous respiration. An infectious workup, including lumbar puncture (Table 1) and blood and urine cultures, identified no bacteria or fungus. Acyclovir was administered intravenously (15 mg/kg/dose) for 21 days. Magnetic resonance imaging (MRI) showed sequelae with hyperintense signal, cavitations, volume loss, and disruption of brain parenchyma (Fig. 2). Cerebrospinal fluid (CSF) analyzed by end-point PCR was positive for HSV and negative for enterovirus (Table 1). Herpes simplex virus-1 was identified using sequencing of DNA polymerase UL30. The patient was discharged from the hospital with no clinical or EEG evidence of seizures and was on the following antiepileptic drugs: divalproex sodium (13.5 mg/kg/day; TID), carbamazepine (15 mg/kg/day; TID), and clobazam (0.25 mg/kg/day; once daily).
Fig. 2.
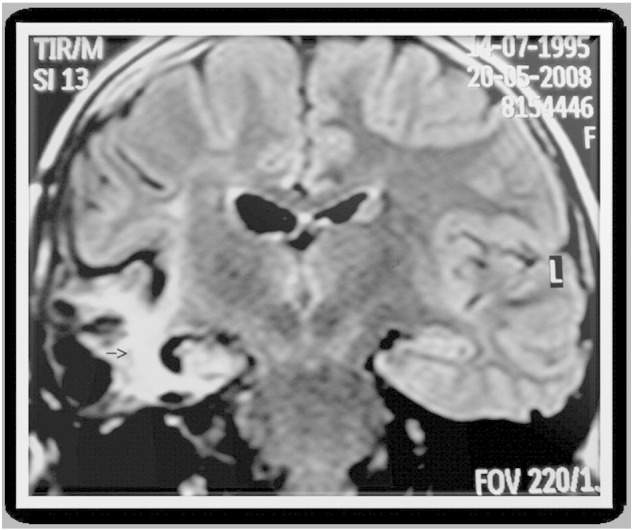
MRI image, coronal, FLAIR (fluid acquisition inversion recovery) sequence. TR: 11,000 ms; TE: 140 ms; FOV: 220 mm2. Showing sequelae: area of hyperintense signal, with cavitations, volumetric loss, and disruption of brain parenchyma.
2.4. Follow-up
The patient was monitored in the epilepsy outpatient clinic and maintained on anticonvulsant therapy: divalproex sodium (13.5 mg/kg/day; TID) and carbamazepine (15 mg/kg/day; TID).
3. Discussion
The present study reports a patient with a recurrence of HSV-1 encephalitis, following temporal lobe surgery for epilepsy.
Postoperative infections that occur as complications of neurosurgical procedures are usually bacterial in origin and include meningitis, subdural empyema, and cerebral abscess [16]. Viral encephalitis complicating the early postoperative course is rare, and diagnosis of postoperative HSV encephalitis is difficult because the clinical features can mimic other more common postoperative complications. Review of the literature revealed three cases of reactivation of HSV following epilepsy surgery in patients with a previous history of HSV encephalitis [2,3,9] very similar to the case reported in the present study. Neurosurgery was performed at the site of the previous encephalitis, which raises the possibility that surgical manipulation caused reactivation of the infection. Cases of HSV reactivation without a previous history of HSV encephalitis have also been reported [4–7,10,13]. Herpes simplex virus-1 was identified in the present case, which is common in most reported cases of postoperative HSV encephalitis, with only one case reporting HSV-2 [8]. In some of the abovementioned cases, surgical manipulation occurred far from the previous encephalitis site, which leads us to hypothesize that stress due to surgery or use of corticosteroids could also be related to HSV reactivation [11].
In all reported cases with a previous history of HSV encephalitis, including the case reported here, the elapsed time after HSV encephalitis varied from 14 months to 10 years [2,3]. The appearance of the early signs of encephalitis in the postoperative period usually occurs in the first two weeks (mean ± SD, 10 ± 7; median, 9; IQR, 5–16.5; range: 1–21 days). The immunohistochemical reactions with monoclonal anti-HSV-1 antibody in the surgical specimens were negative, indicating later reactivation of the infection following the surgical manipulation.
The reasons for HSV recurrence are poorly understood. Risk factors include stress, trauma, bacterial infections, exposure to ultraviolet radiation, and menstruation [17]. In the case reported here, the right temporal lobe was the site previously affected by HSV, and surgical manipulation could have been the main trigger, although stress due to surgery or use of corticosteroids might also be implicated.
Latency is a characteristic of the herpes virus family. Herpes simplex virus establishes a latent infection within sensitive neurons that innervate the site of the primary infection [18]. Transcription of the viral genome depends on a specific cellular protein of octameric structure known as Oct-1 that is present in most human cells. However, some neurons express the Oct-2 protein, which restrains the transcription of specific purinic and pyrimidinic bases, preventing the reactivation of latent strains of HSV. The predominance of the Oct-2 protein depends on neuronal growth factor (NGF). Herpes simplex virus recurrence after tissue injury can be caused by a decrease in the level of NGF in neurons expressing Oct-2, allowing Oct-1 to prevail and, consequently, the transcription of the HSV genome [18]. These mechanisms can be involved in the reactivation of HSV following epilepsy surgery [1].
Herpes simplex virus encephalitis must be considered in neurosurgery patients with postoperative altered state of consciousness, seizures, and fever. Polymerase Chain Reaction (PCR) is the method of choice for diagnosis of HSV encephalitis. It is positive 24–48 h after the commencement of symptoms and remains positive 2–5 days after treatment. The sensitivity of conventional PCR is 98%, specificity 94%, positive predictive value 95%, and negative predictive value 98% [19].
Acyclovir is the treatment of choice for HSV encephalitis. Its mechanisms of action include competitive inhibition of viral DNA polymerase, incorporation into and subsequent termination of the growing viral DNA chain, and inactivation of DNA polymerase [17]. Adequate prophylactic administration of acyclovir has been proposed by some authors for neurosurgery that involves the site of a previous herpetic lesion [2,20]; however, the following points should be considered: reactivation following neurosurgery is a very rare event, acyclovir only acts on replicating viruses and not latent viruses, and acyclovir crosses the blood–brain barrier and easily reaches therapeutic levels in the brain. Concentrations in the CSF are approximately 50% of plasma values. Plasma protein binding is relatively low (9–33%), and drug interactions involving binding site displacement are not anticipated. Although it is a very safe drug, side effects have been described, including neurological symptoms. Acyclovir resistance is uncommon. It is usually only seen in immunocompromised patients with multiple recurrent herpes lesions and past treatment with acyclovir and results from changes in viral thymidine kinase or DNA polymerase [17]. A primary genetic immunodeficiency can predispose individuals to HSV infection [21,22]. Identifying those at risk could help to select patients with previous HSV encephalitis who would benefit from prophylactic therapy when undergoing neurosurgery.
4. Conclusions
The potential risk of reactivation of HSV encephalitis after intracranial surgery must be considered in neurosurgery patients with postoperative altered state of consciousness, seizures, and fever. The prophylactic use of acyclovir needs further evaluation, and research and consensus in this area are needed to delineate best practice for prophylaxis.
Conflict of interest
The authors have no conflicts of interest to disclose.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Kleinschmidt-Demasters B.K., Gilden D.H. The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol. 2001;11:440–451. doi: 10.1111/j.1750-3639.2001.tb00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgeois M., Vinikoff L., Lellouch-Tubiana A., Sainte-Rose C. Reactivation of herpes virus after surgery for epilepsy in a pediatric patient with mesial temporal sclerosis: case report. Neurosurgery. 1999;44:633–635. doi: 10.1097/00006123-199903000-00106. [DOI] [PubMed] [Google Scholar]
- 3.Gong T., Bingaman W., Danziger-Isakov L., Tuxhorn I., Goldfarb J. Herpes simplex virus reactivation after subtotal hemispherectomy in a pediatric patient. Pediatr Infect Dis J. 2010;29:1148–1150. doi: 10.1097/INF.0b013e3181ecc4b4. [DOI] [PubMed] [Google Scholar]
- 4.Fearnside M.R., Grant J.M.F. Acute necrotizing encephalitis complicating bifrontal craniotomy and pituitary curettage. J Neurosurg. 1972;36:499–502. doi: 10.3171/jns.1972.36.4.0499. [DOI] [PubMed] [Google Scholar]
- 5.Perry J.D., Girkin C.A., Miller N.R., Kerr D.A. Herpes simplex encephalitis and bilateral acute retinal necrosis syndrome after craniotomy. Am J Ophthalmol. 1998;126:456–460. doi: 10.1016/s0002-9394(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 6.Spuler A., Blaszyk H., Parisi J.E., Davis D.H. Herpes simplex encephalitis after brain surgery: case report and review of the literature. J Neurol Neurosurg Psychiatry. 1999;67:239–242. doi: 10.1136/jnnp.67.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochsner F. Contamination d'un gliome par le virus herpetique. Arch Suisses Neurol Neurochir Psychiatr. 1981;129:19–30. [PubMed] [Google Scholar]
- 8.Ihekwaba U.K., Battersby R.D. Type 2 herpes simplex reactivation after craniocervical decompression for hind brain hernia and associated syrinx. Br J Neurosurg. 2009;23:326–328. doi: 10.1080/02688690802425671. [DOI] [PubMed] [Google Scholar]
- 9.Lund M. Herpes simplex virus reactivation and encephalitis after topectomy. J Pediatr Health Care. 2011;25:323–327. doi: 10.1016/j.pedhc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Kwon J.W., Cho B.K., Kim E.C., Wang K.C., Kim S.K. Herpes simplex encephalitis after craniopharyngioma surgery: case report. J Neurosurg Pediatr. 2008;2:355–358. doi: 10.3171/PED.2008.2.11.355. [DOI] [PubMed] [Google Scholar]
- 11.Salomon A., Delanghe F., Jeanjean P. Une méningo-encéphalite herpétique après chirurgie lombaire: à propos d'un cas. Ann Fr Anesth Reanim. 2010;29:732–735. doi: 10.1016/j.annfar.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Ploner M., Turowski B., Wobker G. Herpes encephalitis after meningioma resection. Neurology. 2005;65:1674–1675. doi: 10.1212/01.wnl.0000184491.21252.9b. [DOI] [PubMed] [Google Scholar]
- 13.Filipo R., Attanasio G., De Seta E., Viccaro M. Post-operative Herpes simplex virus encephalitis after surgical resection of acoustic neuroma: a case report. J Laryngol Otol. 2005;119:558–560. doi: 10.1258/0022215054352243. [DOI] [PubMed] [Google Scholar]
- 14.Aldea S., Joly L.M., Roujeau T., Oswald A.M., Devaux B. Postoperative herpes simplex virus encephalitis after neurosurgery: case report and review of the literature. Clin Infect Dis. 2003;36:e96–e99. doi: 10.1086/368090. [DOI] [PubMed] [Google Scholar]
- 15.Hengstman G.J., Gons R.A., Menovsky T., Lunel F.V., van de Vlasakker C.J., de Vries J. Delayed cranial neuropathy after neurosurgery caused by herpes simplex virus reactivation: report of three cases. Surg Neurol. 2005;64:67–69. doi: 10.1016/j.surneu.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 16.McClelland S., Hall W.A. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007;45:55–59. doi: 10.1086/518580. [DOI] [PubMed] [Google Scholar]
- 17.Solomon T., Hart I.J., Beeching N.J. Viral encephalitis: a clinician's guide. Pract Neurol. 2007;7:288–305. doi: 10.1136/jnnp.2007.129098. [DOI] [PubMed] [Google Scholar]
- 18.Lillycrop K.A., Liu Y.Z., Theil T., Möröy T., Latchman D.S. Activation of herpes simplex virus immediate-early promoters by neuronally expressed POU family transcription factors. Biochem J. 1995;307:581–584. doi: 10.1042/bj3070581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakeman F.D., Whitley R.J. Diagnosis of herpes simplex encephalitis: application of polymerase chain reaction to cerebrospinal fluid from brain biopsied patients and correlation with disease. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S., Kameyama T., Nagaya S., Hashizume Y., Yoshida M. Relapsing herpes simplex encephalitis: pathological confirmation of viral reactivation. J Neurol Neurosurg Psychiatry. 2003;74:262–264. doi: 10.1136/jnnp.74.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casrouge A., Zhang S.Y., Eidenschenk C., Jouanguy E., Puel A., Yang K. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006;314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S.Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]


