Abstract
Late-onset epilepsy (LOE), with onset after 50 years of age, is often attributed to underlying occult cerebrovascular disease. LOE is associated with a three-fold increase in subsequent stroke risk, therefore it is important to improve our understanding of pathophysiology. In this exploratory study, we aimed to determine whether established structural magnetic resonance imaging markers and novel physiological imaging markers of occult cerebrovascular disease were more common in patients with LOE than age-matched controls.
Sixteen patients with LOE (mean age ± SD: 67.6 ± 6.5 years) and 15 age-matched control subjects (mean age: 65.1 ± 3.9 years) underwent a 3 T MRI scan protocol. T1-weighted images and T2-weighted fluid attenuated inversion recovery (FLAIR) images were used to determine cortical grey matter volume and white matter hyperintensity (WMH) volume respectively, whilst multiple delay time arterial spin labelling (ASL) images were collected at rest and during a hypercapnic challenge. Cerebral blood flow (CBF) and arterial arrival time (AAT) were calculated from ASL data under both normocapnic and hypercapnic conditions. Cerebrovascular reactivity was also calculated for both CBF and AAT relative to the change in end-tidal CO2.
Patients with LOE were found to have significantly lower cortical volume than control subjects (33.8 ± 3.8% of intracranial volume vs. 38.0 ± 5.5%, p = 0.02) and significantly higher WMH volume (1339 ± 1408 mm3 vs. 514 ± 481 mm3, p = 0.047). Baseline whole brain AAT was found to be significantly prolonged in patients with LOE in comparison to control subjects (1539 ± 129 ms vs. 1363 ± 167 ms, p = 0.005). Voxel-based analysis showed the significant prolongation of AAT to be predominantly distributed in the frontal and temporal lobes. Voxel-based morphometry showed the lower cortical volume to be localised primarily to temporal lobes. No significant differences in CBF or cerebrovascular reactivity were found between the two groups. Baseline whole brain AAT and cortical volume differences persisted upon further analysis to take account of differences in smoking history between patients and control subjects.
These findings suggest that occult cerebrovascular disease is relevant to the pathophysiology of LOE.
Keywords: Late-onset epilepsy, Cerebrovascular disease, Seizures, Arterial spin labelling, Cerebral blood flow, Voxel-based morphometry
Abbreviations: AAT, arterial arrival time; ASL, arterial spin labelling; CBF, cerebral blood flow; CT, computerised tomography; CVD, cerebrovascular disease; CVR, cerebrovascular reactivity; EEG, electroencephalogram; ETCO2, end-tidal CO2; FLAIR, fluid attenuated inversion recovery image; FWHM, full width half maximum; GM, grey matter; ICV, intracranial volume; LOE, late-onset epilepsy; MoCA, Montreal cognitive assessment; MRI, magnetic resonance imaging; oCVD, occult cerebrovascular disease; SVD, small vessel disease; VBA, voxel-based analysis; WMH, white matter hyperintensity; VBM, voxel-based morphometry.
Highlights
-
•
LOE patients were found to have increased WMHs and reduced GM volume on MRI imaging in comparison to HC.
-
•
Baseline arterial arrival time was significantly longer in LOE patients than HC.
-
•
Baseline cerebral blood flow did not differ between LOE patients and HC.
-
•
Cerebrovascular reactivity did not differ between LOE patients and HC.
1. Introduction
A third of all diagnoses of epilepsy are made in people over 60 (Tallis et al., 1991). Late-onset epilepsy (LOE) is increasingly common, and is often attributed to cerebrovascular disease (CVD). The relationship between CVD and LOE is easily recognised where there is a history of stroke, particularly cortical. However, the relationship between LOE and otherwise occult CVD (oCVD) (whether cortical or subcortical) is currently less well established yet causality is often invoked. Limited available evidence from clinical studies suggests, firstly, a relationship between vascular risk factors and the risk of LOE (Ng et al., 1993; Li X. et al., 1997), apart from the relationship that exists through clinically overt stroke; secondly, an excess of clinically unsuspected, radiological CVD, particularly cortical infarction but apparently also excess small vessel disease (SVD) changes, (Roberts et al., 1988; Maxwell et al., 2013) and thirdly, an excess (almost threefold) risk of stroke in patients with LOE (Cleary et al., 2004; Chang et al., 2014; Wannamaker et al., 2015). Recent studies have also found this increased stroke risk to be not limited to those aged above 50 years but present in patients diagnosed with epilepsy as young as 20 years of age (Chang et al., 2014; Wannamaker et al., 2015).
Potential mechanisms of epileptogenesis in otherwise oCVD may include disruption of neurovascular unit integrity; disordered cerebral metabolism and perfusion; blood–brain barrier dysfunction; and inflammation (Shinton et al., 1987; Gibson et al., 2011).
The aim of this study was to obtain exploratory evidence to support the proposed association between LOE and oCVD, i.e. in subjects without clinical stroke, using structural and physiological magnetic resonance imaging (MRI) measures. Accepted structural MRI markers of SVD include white matter hyperintensities (WMHs) and reduced grey matter (GM) volume (Debette and Markus, 2010; Gouw et al., 2011; Wardlaw et al., 2013). WMHs have themselves been shown to be an independent risk factor for future stroke (hazard ratio 3.3, 95% CI: 2.6–4.4) (Debette and Markus, 2010). However, measurements of cerebrovascular function such as cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) may provide more sensitive and direct measures of CVD than these structural markers (Glodzik et al., 2011). Arterial spin labelling (ASL) is an MRI technique which can be used to assess the physiological effects of CVD by enabling both the measurement of CBF and arterial arrival time (AAT) — the time it takes for endogenously labelled blood to reach the tissue (MacIntosh et al., 2010). CVD is also known to impact CVR (ASL can also probe CVR by measuring the CBF and AAT response to a hypercapnic stimulus), the ability of blood vessels to vasodilate in response to a stimulus such as hypercapnia (Terberg et al., 2000; Hajjar et al., 2010). These structural and physiological measures have not previously been investigated in LOE. We therefore sought to undertake an exploratory study to determine whether there are significant differences between patients with LOE and controls in (1) WMH and cortical GM volume, and (2) CBF, AAT and CVR.
2. Methods
2.1. Participants
Patients with a diagnosis of LOE (onset over 50 years of age) were recruited from neurology out-patient clinics in Lancashire between October 2011 and March 2012. Patients with a history of stroke or transient ischaemic attack, radiological evidence of cortical/subcortical infarct >1.5 cm or other structural cause of epilepsy, focal neurological signs, known aetiology for epilepsy, cognitive dysfunction sufficient to interfere with daily activities or other significant medical condition likely to complicate assessment or limit participation in the study, were excluded. Age matched controls without epilepsy were recruited from the same region (i.e. Lancashire) who otherwise met the same eligibility criteria as patients. The two groups were matched for age, gender and cognition. A Montreal Cognitive Assessment (MoCA, http://www.MoCAtest.org) was undertaken by all participants, with permission for use of the test.
This study received relevant regulatory approvals, including ethics (Cheshire Research Ethics Committee), research governance and local university approvals. Written informed consent was obtained from all participants.
Existing clinical reports for all patients regarding computerised tomography (CT)/MRI/electroencephalogram (EEG) were scrutinised. All participants underwent an MRI scan on a 3 T Philips Achieva system using an 8 channel head coil at Salford Royal Hospital.
2.2. Imaging protocol
A high resolution T1-weighted structural image was acquired using a 3D gradient echo sequence with parameters: flip angle 8°, TR 8.4 ms, TE 3.9 ms, FOV 240 × 192 mm in-plane, 162 contiguous axial slices of 1 mm thickness. The images were reconstructed to a voxel size of 1 × 1 × 1 mm.
A T2-weighted fluid-attenuated inversion recovery (FLAIR) image was acquired with the following parameters: TR 11 s, TI 2.8 s, TE 120 ms, FOV 230 × 182 mm (images were reconstructed to give voxel size of 0.45 mm in plane), 30 axial slices of 4 mm thickness with 1 mm gap covering the whole brain.
A Look-Locker ASL sequence (Gunther et al., 2001) was used with STAR labelling (Edelman et al., 1994) and 4 delay times of 800, 1400, 2000, and 2600 ms after labelling, TR: 3500 ms; TE 22 ms; flip angle 40°; 3.5 × 3.5 × 6 mm voxels with a 1 mm gap between slices; 15 slices covering the cerebrum but not the cerebellum with bipolar gradients added to de-phase fast flowing spins and so act as a ‘vascular crusher’ in order to remove large vessel signal. The labelling slab was 15 cm with a 10 mm gap between the labelling and imaging regions. 112 pairs of labelled and control images (at each delay time) were collected, with scan duration approximately 13 min. To allow quantification of CBF an additional scan was acquired with the same scan parameters as above except for: TR = 10 s and 15 read-out times (800–9200 ms), in order to estimate the equilibrium magnetisation of the brain.
An additional echo planar image was collected with the same slice positioning and the same voxel dimensions but with TE = 35 ms to give typical functional MRI contrast for registration and normalisation purposes.
During the ASL acquisition a hypercapnic challenge was administered as described previously (Al-Bachari et al., 2014). In brief, after 5 min of breathing room air (from which the baseline perfusion images were extracted) there followed 6 min of hypercapnia, administered using a non-rebreathing circuit. CO2 flow-rate was altered to ensure all participants reached an increased end tidal level approximately 1% above their baseline. End-tidal CO2 (ETCO2), O2, pulse rate and oxygen saturation were continuously monitored using Powerlab (ADI Instruments, Colorado Springs, USA).
2.3. Statistical analysis
Demographic data were compared between groups using un-paired t-tests or chi-squared tests. Smoking was measured by pack years and alcohol in units per week. Cortical GM volume and lateral ventricular volume were measured from the T1-weighted images on an individual basis using stereological techniques as implemented in the ‘Easymeasure’ software (Garcia-Finana et al., 2003). Intracranial volume (ICV) was also measured in order to normalise the measures (i.e. make them independent of head size). Isotropic grid sizes of 15, 10 and 5 mm were used for ICV, cortex and ventricles respectively. WMH volume was measured from the T2-weighted FLAIR image using the same stereological technique with a 3 mm in-plane grid-size performed on every slice (i.e. at 5 mm intervals). This choice of grid sizes ensured that the coefficient of error of the volume estimates were always below 5% (Garcia-Finana et al., 2003). Differences between groups were tested using student t-tests.
ASL data were analysed using in-house MATLAB (Mathworks, MA, USA) routines using a single blood compartment model (Parkes et al., 2002), adapted for LL readout as described previously (Al-Bachari et al., 2014). Baseline CBF and AAT maps were calculated using the first 5 min of ASL data, during breathing of air. CVR maps were calculated using subtraction images of CBF and AAT between periods of air (5 min) and hypercapnia (last 5 min, omitting the first minute of hypercapnia to allow equilibrium to be reached) and dividing these by the value of change in end-tidal CO2 (ΔETCO2) on an individual basis.
Whole brain values for CBF, AAT, CVRCBF (%ΔCBF/ΔETCO2) and CVRAAT (ΔAAT/ΔETCO2) were calculated using a simple threshold mask based on the ASL calibration image on an individual basis. Differences between groups were tested using student t-tests. Linear regression was used to assess whether there were any significant relationships between the MRI measurements which showed significant group differences.
Voxel-based analysis (VBA) was also performed using the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) to compare CBF, AAT and CVR maps between groups. Image pre-processing in SPM included (1) motion correction, (2) registration and normalisation of the EPI image to the EPI template within SPM, and application of this procedure to the perfusion maps, and (3) smoothing of the normalised images using a 12 mm full-width-half-maximum (FWHM) kernel. Voxel-based morphometry (VBM) was performed on the T1-weighted images in SPM8 following standard procedures (http://www.fil.ion.ucl.ac.uk/~john/misc/VBMclass10.pdf) including modulation to allow comparisons of volumes, proportional scaling to individual intracranial volume and smoothing using an 8 mm FWHM kernel. Voxel-wise comparisons of grey matter volume, CBF, AAT, CVRCBF, and CVRAAT between groups were carried out using a two-sample t-test (unequal variances). Regions were considered significant at a p value of <0.001 uncorrected, with a cluster size of 100 voxels (at the re-sampled voxel size of 2 mm isotropic), which we feel provides an appropriate balance between type I and type II errors in view of the exploratory nature of the study (Lieberman and Cunningham, 2009).
3. Results
3.1. Participants
Eighteen patients with LOE and seventeen controls were recruited. Two patients and two controls withdrew due to intolerance of the set-up of the gas apparatus in the scanner. Sixteen patients underwent the full scanning protocol, but for three only the T1-weighted and FLAIR images were useable due to their ΔETCO2 gas response being outside the expected limits (4–12 mm Hg).
Fifteen controls underwent the full protocol but for one, only the T1-weighted image was useable due to technical problems at the scanner.
Baseline demographics for the two groups are shown in Table 1. The two groups were well matched for age, gender, co-morbidities, cognition and cardiovascular risk factors other than smoking.
Table 1.
Baseline demographics, expressed as mean ± SD.
| Controls n = 15 |
LOE patients n = 16 |
p | |
|---|---|---|---|
| Age (years) | 65.1 ± 3.9 | 67.6 ± 6.5 | 0.5 |
| Gender (% male) | 60 | 56 | 0.6 |
| Number of CVD risk factors | 1 ± 1.1 | 1.9 ± 1.1 | 0.08 |
| Smoking history (pack years) | 2.4 ± 4.5 | 10.4 ± 10.6 | 0.01 |
| Alcohol intake (units per week) | 9.3 ± 9.5 | 5.3 ± 7.0 | 0.1 |
| Number of co-morbidities | 0.7 ± 1.1 | 1.4 ± 1.3 | 0.3 |
| MoCA score | 28.3 ± 2.7 | 27.3 ± 2.0 | 0.4 |
All patients presented with symptoms suggestive of focal seizures with or without secondary generalisation as evaluated by a consultant neurologist. Additional patient characteristics, including seizure semiology, type, EEG findings, routine clinical brain imaging findings and response to antiepileptic drug treatment, are available in Supplementary Table 1. They all underwent routine clinical investigations including CT and/or MRI brain scans alongside baseline scalp EEG. Two patients also underwent sleep-deprived EEG.
Of the 15 patients for whom baseline clinical imaging results were available, 9 had CT and/or MRI scans reported as normal; 6 had SVD documented in either (or both) their CT or (and) MRI report(s).
Scalp EEG reports were available for 13 patients. EEG was normal in 6; 1 showed non-specific changes; 6 showed changes in keeping with epilepsy of temporal lobe origin, with focal sharp waves and spikes being reported either unilaterally or bilaterally in the temporal lobe(s). Two patients whose baseline EEG was reported as normal underwent a sleep-deprived EEG. Both sleep-deprived EEGs reported focal sharp waves in the anterior temporal regions in keeping with an epileptic focus in the temporal lobe.
3.2. Structural measures of oCVD
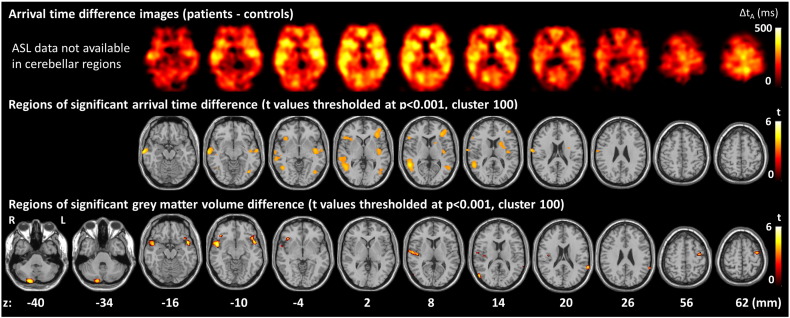
A comparison of structural measures of oCVD between groups is shown in Table 2. Patients were found to have both significantly lower cortical GM volume (33.8 ± 3.8% of ICV vs 38.0 ± 5.5%, p = 0.02) and significantly higher WMH volume (1340 ± 1408 mm3 vs 514 ± 481 mm3, p = 0.047) than controls. No difference in ventricular volume between patients and controls was found. VBM demonstrated focal regions of lower grey matter volume in patients compared to controls, mainly in temporal lobes (Fig. 1 bottom row and Table 3). There was one region of increased grey matter volume in patients compared to controls in the right superior frontal gyrus (164 voxels, peak t = 4.4, peak p < 0.0001, peak MNI coordinates 39 29 50).
Table 2.
Structural measures of oCVD, expressed as mean ± SD.
| Controls n = 15 |
Patients n = 16 |
p | |
|---|---|---|---|
| Ventricular volume (% of ICV) | 1.9 ± 0.8 | 2.3 ± 1.1 | 0.3 |
| Cortical GM volume (% of ICV) |
38.0 ± 5.5 | 33.8 ± 3.8 | 0.02 |
| WMH volume (mm3) |
514 ± 481 | 1340 ± 1408 | 0.047 |
Fig. 1.
Regions of prolonged AAT (top and middle rows) and lower grey matter volume (bottom row) in patients compared with controls.
Table 3.
Regions of lower grey matter volume in patients compared to controls.
| Region | Cluster size (n voxels) | Cluster p (FWE-corrected) | Peak t value |
Peak p value uncorrected | Peak MNI coordinates |
|---|---|---|---|---|---|
| R mid temporal gyrus BA21 | 648 | 0.04 | 5.5 | <0.0001 | 53 5 −11 |
| R cerebellum posterior lobe | 627 | 0.05 | 4.9 | <0.0001 | 20 −86 −39 |
| R mid temporal gyrus BA39 | 119 | 0.9 | 4.9 | <0.0001 | 56 −72 12 |
|
L sup temporal gyrus BA38 L inf frontal gyrus BA47 L inf frontal gyrus BA47 |
541 | 0.08 | 4.8 4.3 3.9 |
<0.0001 <0.0001 .0003 |
−47 9 −15 −41 21 −12 −48 30 −12 |
| R inf frontal gyrus BA47 | 111 | 0.9 | 4.8 | <0.0001 | 44 27 −8 |
| L sup temporal gyrus BA22 L supramarginal gyrus BA40 |
176 | 0.7 | 4.5 3.7 |
<0.0001 0.0005 |
−66 −45 20 −65 −48 27 |
| R cerebellum posterior lobe | 139 | 0.8 | 4.4 | <0.0001 | 35 −66 −49 |
| R sup temporal gyrus BA22 R sup temporal gyrus BA13 R sup temporal gyrus BA22 |
314 | 0.3 | 4.2 4.0 4.0 |
0.0001 0.0002 0.0002 |
59 −11 9 45 −17 9 68 −8 8 |
| L precentral gyrus BA6 | 211 | 0.6 | 4.2 | 0.0001 | −39 −5 62 |
Thresholded at p < 0.001, cluster size 100. BA = Brodmann area.
The top row shows AAT difference images (patients − controls) and the middle row shows regions where these differences are significant. The bottom row shows regions of significantly lower grey matter volume in patients compared to controls. t-Statistic maps are thresholded at p < 0.001 with cluster size 100. The z-coordinate in MNI space is given.
3.3. Baseline global and regional CBF and AAT
Whole brain baseline CBF was found not to be significantly different between the two groups (Table 4). VBA also failed to show any significant regional differences in baseline CBF between groups.
Table 4.
Physiological measures of oCVD, expressed as mean ± SD.
| Controls n = 14 |
Patients n = 13 |
p | |
|---|---|---|---|
| Baseline CBF (mL/min/100 mL) |
37.5 ± 9.0 | 42.2 ± 26.8 | 0.5 |
| Baseline AAT (ms) | 1363 ± 167 | 1539 ± 129 | 0.005 |
| CVRCBF (%Δ/Δmm Hg) | 2.1 ± 2.5 | 1.4 ± 3.8 | 0.6 |
| CVRAAT (Δms/Δmm Hg) | −21.0 ± 6.3 | −18.1 ± 14.2 | 0.5 |
| ΔETCO2 (mm Hg) | 7.9 ± 3.8 | 9.3 ± 3.5 | 0.3 |
Whole brain baseline AAT was found to be significantly longer in patients than controls (1539 ± 129 ms vs. 1363 ± 167 ms, p = 0.005) (Table 4). VBA demonstrated widespread regions of increased baseline AAT in patients by comparison with controls (Fig. 1 top row), with regions of significant increase distributed mainly in the temporal and frontal lobes (Fig. 1 middle row and Table 5).
Table 5.
Regions of prolonged AAT in patients compared to controls.
| Region | Cluster size (n voxels) | Cluster p (FWE-corrected) | Peak t value |
Peak p value uncorrected | Peak MNI coordinates |
|---|---|---|---|---|---|
| R mid temporal gyrus BA 21 | 352 | 0.4 | 4.8 | <0.0001 | 70 −12 −14 |
| R sup temporal gyrus BA 39 R mid temporal gyrus BA 37 R mid temporal gyrus BA 21 | 1156 | 0.08 | 4.6 4.1 3.9 |
<0.0001 0.0002 0.0003 |
54 −48 8 48 −56 0 64 −32 −2 |
| L mid frontal gyrus BA10 L mid frontal gyrus BA 46 | 510 | 0.3 | 4.1 3.8 |
0.0002 0.0004 |
−34 36 6 −44 48 4 |
| R post central gyrus, BA 43 | 139 | 0.7 | 4.1 | 0.0002 | 72 −8 20 |
| L mid temporal gyrus BA 21 L insula, BA13 L insula, BA13 | 571 | 0.3 | 4.0 4.0 3.8 |
0.0002 0.0002 0.0004 |
−62 −10 −8 −42 −10 −4 −38 −14 12 |
| L fusiform gyrus, BA19 L sup temporal gyrus, BA 39 |
272 | 0.5 | 4.0 3.7 |
0.0002 0.0005 |
−40 −68 −8 −54 −58 8 |
| R inf frontal gyrus, BA 47 R inf frontal gyrus, BA 45 | 254 | 0.5 | 3.8 3.8 |
0.0004 0.0005 |
38 24 −2 52 26 4 |
| L caudate L putamen |
184 | 0.6 | 3.8 3.7 |
0.0004 0.0005 |
−18 10 12 −26 0 16 |
Thresholded at p < 0.001, cluster size 100. BA = Brodmann area.
3.4. Global and regional CVRCBF and CVRAAT
Whole-brain CVRCBF or CVRAAT were not significantly different between patients and controls (Table 4). VBA of CVRCBF did not show any significant regional differences between the two groups, but CVRAAT showed one region in the right inferior frontal gyrus where CVR response was lower in patients by comparison with controls (105 voxels, peak t = 5.1, peak p = 0.00001, peak MNI coordinates 8 −36 18).
There was no significant difference in ETCO2 following induction of hypercapnia between the groups (Table 4).
Linear regression between all pairs of measurements showing significant group differences (cortical volume, WMH volume and AAT) showed that cortical volume was significantly correlated with WMH volume (r = −0.43, p = 0.02, n = 31) and with AAT (r = −0.38, p = 0.05, n = 27) but WMH volume and AAT were not related (r = 0.28, p = 0.2, n = 27). The correlations were largely driven by group differences, but the relationship between cortical volume and WMH volume remained significant within the control group.
3.5. Additional analysis accounting for differences in smoking history
Given the difference in smoking history between the two groups, we repeated the analysis with the 4 heaviest smokers from the patient group removed (rendering the difference in smoking between the groups as non-significant: LOE 6.9 ± 8.5 pack years vs. controls 2.4 ± 4.5 pack years, p = 0.2). AAT remained significantly longer in the patient group compared to controls (1547 ± 128 ms vs. 1363 ± 167 ms, p = 0.007), and cortical volume significantly lower (33.9 ± 3.4% of ICV vs. 38.0 ± 5.5%, p = 0.03). However, the difference in white matter lesion volume was no longer significant (888 ± 731 mm3 vs. 514 ± 481 mm3, p = 0.2).
4. Discussion
All patients had been diagnosed with LOE associated with focal seizures with or without secondary generalisation, and had no history otherwise of clinical CVD. The seizure types in this study population are in keeping with previous studies of seizures in patients aged 50–85 years (Paradowski and Zagrajek, 2005).
Patient and control groups were well matched for all baseline demographics other than for smoking, with a significantly higher pack year history among patients than controls. Cardiovascular risk factors such as dyslipidaemia (Li et al., 1997) and hypertension (Ng et al., 1993) have already been reported as independent risk factors for LOE. Smoking is a known risk factor for oCVD (Van Dijk et al., 2008), and the difference in smoking history between the two groups is unsurprising. We recognise this as a potential limitation, however re-analysis with the 4 heaviest smokers removed from the patient group (rendering the difference in smoking between the groups as non-significant) showed similar results. Only 6 of the patients' clinical imaging scan reports documented the presence of otherwise oCVD. This is perhaps unsurprising given that features of oCVD, for instance, may not be reported by some radiologists, who simply deem these findings (perhaps particularly those of SVD) to be in keeping with ageing.
Patients were found to have significantly lower cortical GM volume and a significantly greater WMH volume than controls. These findings are in-keeping with those of previous radiological studies (Shorvon et al., 1984; Roberts et al., 1988; Maxwell et al., 2013). Brain atrophy in the context of SVD on imaging has been defined as a lower brain volume unrelated to a specific macroscopic focal injury such as trauma or infarction (Wardlaw et al., 2013). In the present study, ventricular volume did not differ between groups, excluding significant central atrophy (which is associated with increased ventricular size and basal ganglia atrophy). We found significantly lower cortical GM volume, accompanied by, and significantly correlated with, significantly greater WMH volume, suggesting that the cortical atrophy in patients with LOE is related to SVD.
VBM showed that the atrophy was most significant in temporal and frontal regions (Fig. 1 bottom row and Table 3). This is intriguing as it may relate to the seizure origin, and is in keeping with studies of TLE (Keller and Roberts, 2008). Diffuse cerebral atrophy is also a common finding in LOE, with one study showing it is mainly of cortical origin (Regesta and Tangenelli, 1992). This same study found no correlation between grade of atrophy and seizure frequency, suggesting that atrophy is not a consequence of seizures.
A recent retrospective study found epilepsy associated with leukoaraiosis to mainly affect the temporal lobe (Gasparini et al., 2015). However, the authors commented on the issue of whether epileptogenesis arises from cortical vascular lesions such as cortical microinfarcts or microhaemorrhage — for which conventional clinical MRI has inadequate sensitivity, or from subcortical injury due to disruption of subcortical–cortical connections, being unresolved.
Baseline CBF, when analysed globally or regionally, was not found to differ between the two groups. Baseline AAT values were significantly longer in patients than controls, on both global and regional analyses.
Given the hypothesis concerning the relevance of oCVD to LOE, it might be expected that baseline CBF, as well as AAT, would differ between patients with LOE and controls. Potentially, chronic CVD, even without other clinical manifestations, might result in compensatory mechanisms in the brain to maintain a constant CBF by means of chronic arteriolar vasodilatation and/or recruitment of secondary collateral vessels (Farkas and Luiten, 2001; Derdeyn et al., 2002). Our use of ‘vascular crushers’ in the ASL sequence removes signal from larger vessels and ‘weights’ the AAT measurements towards the microvasculature from which we believe this prolongation of AAT in LOE may arise.
Preservation of CBF in patients with LOE has been reported previously in a positron emission tomography study, with reduction in CBF noted only among patients with both LOE and radiological WMHs (De Reuck et al., 1996). Other studies investigating CBF in TLE have reported a marked reduction in CBF in the affected temporal lobe on both inter-ictal positron emission tomography and ASL maps (Pense et al., 2010; Boscolo Galazzo et al., 2015). Future studies addressing CBF in the different sub-types of LOE are required to clarify this discrepancy.
VBA found patients with LOE to have significantly longer baseline AAT times in widespread brain regions but with the greatest prolongation within the temporal and frontal lobes. Clearly, it is tempting to speculate that temporal and/or frontal lobe AAT prolongation may have pathophysiological relevance in patients with LOE of temporal and/or frontal lobe origin, but further study would obviously be needed to confirm this observation, its distribution, and to define any potential epileptogenic mechanisms.
There were no significant differences in whole brain CVRCBF or CVRAAT between the two groups and only a small frontal region of lower CVRAAT in the patient group, suggesting that CVR is largely preserved in patients with LOE. This is unexpected in view of the observed baseline AAT prolongation. If prolonged AAT is indicative of chronic arteriolar vasodilatation then one might expect impaired CVRCBF and CVRAAT due to reduced capacity for cerebral vasodilatation of the arterioles under hypercapnic conditions. Preserved CVR despite baseline AAT prolongation in this study might be attributable to the relatively small sample size, or may indicate that prolonged baseline AAT may be due to recruitment of secondary collaterals rather than chronic arteriolar vasodilatation.
There are a number of other MRI markers of CVD that could have been included in this study, such as microbleeds and lacunes (Wardlaw et al., 2013), but our imaging protocol could not be extended. Given the lack of sensitivity of CVR to LOE, future studies may wish to consider the inclusion of alternative imaging techniques such as susceptibility weighted imaging and diffusion weighted imaging.
5. Conclusions
We have found WMH volume to be significantly increased and cortical GM volume to be significantly reduced in patients with LOE, by comparison with age-matched controls. We also found significant prolongation of baseline AAT, with VBA demonstrating this prolongation to be predominantly within frontal and temporal lobes. We found no significant differences in CVR measures. These findings lend further support to the relevance of oCVD, without other clinical manifestations in LOE.
Given the small sample size, this work should be viewed as an exploratory study. Further study is required to confirm and extend these observations and to better define potential pathophysiological mechanisms of epileptogenesis, including discrimination of cortical/subcortical anatomical substrates, as well as potential opportunities for intervention.
The following are the supplementary data related to this article.
Table demonstrating seizure type, results from baseline investigations and response to anti-epileptic medication for all patients with LOE who participated in the study.
Conflicts of interest
None.
Acknowledgements
We would like to sincerely thank all the participants for their kind participation in the study. In addition, the radiographers at Salford Royal for their support and expertise and Mr Matthew Wright for his support and input whilst performing the scans. We'd also like to thank the University of Manchester's MRI facility for their financial contribution towards the scanning costs.
References
- Al-Bachari S., Parkes L.M., Vidyasagar R. Arterial spin labelling reveals prolonged arterial arrival time in idiopathic Parkinson's disease. Neuroimage Clin. 2014;6:1–8. doi: 10.1016/j.nicl.2014.07.014. 25379411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscolo Galazzo I., Storti S.F., Del Felice A., Pizzini F.B., Arcaro C. Patient-specific detection of cerebral blood flow alterations as assessed by arterial spin labeling in drug-resistant epileptic patients. PLOS One. 2015;10(5):e0123975. doi: 10.1371/journal.pone.0123975. 25946055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.S., Liao C.H., Lin C.C., Lane H.Y., Sung F.C., Kao C.H. Patients with epilepsy are at an increased risk of subsequent stroke: a population-based cohort study. Seizure. 2014;23(5):377–381. doi: 10.1016/j.seizure.2014.02.007. 24630806 [DOI] [PubMed] [Google Scholar]
- Cleary P., Shorvon S., Tallis R. Late-onset seizures as a predictor of subsequent stroke. Lancet. 2004;363(9416) doi: 10.1016/S0140-6736(04)15946-1. 15081649 [DOI] [PubMed] [Google Scholar]
- Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. 20660506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdeyn C.P., Videen T.O., Yundt K.D. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamics impairment revisited. Brain. 2002;125(3):595–607. doi: 10.1093/brain/awf047. 11872616 [DOI] [PubMed] [Google Scholar]
- Edelman R.R., Siewert B., Darby D.G. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192(2):513–520. doi: 10.1148/radiology.192.2.8029425. 8029425 [DOI] [PubMed] [Google Scholar]
- Farkas E., Luiten P.G. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog. Neurobiol. 2001;64(6):575–611. doi: 10.1016/s0301-0082(00)00068-x. 11311463 [DOI] [PubMed] [Google Scholar]
- García-Fiñana M., Cruz-Orive L.M., Mackay C.E., Pakkenberg B., Roberts N. Comparison of MR imaging against physical sectioning to estimate the volume of human cerebral compartments. Neuroimage. 2003;18(2):505–516. doi: 10.1016/s1053-8119(02)00021-6. 12595203 [DOI] [PubMed] [Google Scholar]
- Gasparini S., Ferlazzo E., Beghi E. Epilepsy associated with leukoaraiosis mainly affects temporal lobe: a casual or causal relationship? Epilepsy Res. 2015;109:1–8. doi: 10.1016/j.eplepsyres.2014.10.012. 25524836 [DOI] [PubMed] [Google Scholar]
- Gibson L.M., Allan S.M., Parkes L.M., Emsley H.C. Occult cerebrovascular disease and late-onset epilepsy: could loss of neurovascular unit integrity by a viable model? Cardiovasc. Psychiatry Neurol. 2011;2011:130406. doi: 10.1155/2011/130406. 21461380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik L., Rusinek H., Brys M. Framington cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J. Cereb. Blood Flow Metab. 2011;31(2):671–679. doi: 10.1038/jcbfm.2010.145. 20842159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw A.A., Seewann A., van der Flier W.M. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry. 2011;82(2):126–135. doi: 10.1136/jnnp.2009.204685. 20935330 [DOI] [PubMed] [Google Scholar]
- Günther M., Bock M., Schad L.R. Arterial spin labeling in combination with a look-locker sampling strategy: inflow turbo-sampling EPI-FAIR (ITS-FAIR) Magn. Reson. Med. 2001;46(5):974–984. doi: 10.1002/mrm.1284. 11675650 [DOI] [PubMed] [Google Scholar]
- Hajjar I., Zhao P., Alsop D., Novak V. Hypertension and cerebral vasoreactivity: a continuous arterial spin labeling magnetic resonance imaging study. Hypertension. 2010;56(5):859–864. doi: 10.1161/HYPERTENSIONAHA.110.160002. 20876450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S.S., Roberts N. Voxel based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49(5):741–757. doi: 10.1111/j.1528-1167.2007.01485.x. 18177358 [DOI] [PubMed] [Google Scholar]
- Li X., Breteler M.M., de Bruyne M.C., Meinardi H., Hauser W.A., Hofman A. Vascular determinants of epilepsy: the Rotterdam Study. Epilepsia. 1997;38(11):1216–1220. doi: 10.1111/j.1528-1157.1997.tb01219.x. 9579923 [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. 20035017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntosh B.J., Filippini N., Chappell M.A., Woolrich M.W., Mackay C.E., Jezzard P. Assessment of arterial arrival times derived from multiple inversion time pulsed arterial spin labeling MRI. Magn. Reson. Med. 2010;63(3):641–647. doi: 10.1002/mrm.22256. 20146233 [DOI] [PubMed] [Google Scholar]
- Maxwell H., Hanby M., Parkes L.M., Gibson L.M., Coutinho C., Emsley H.C. Prevalence and subtypes of radiological cerebrovascular disease in late-onset isolated seizures and epilepsy. Clin. Neurol. Neurosurg. 2013;115(5):591–596. doi: 10.1016/j.clineuro.2012.07.009. 22840415 [DOI] [PubMed] [Google Scholar]
- Ng S.K., Hauser W.A., Brust J.C., Susser M. Hypertension and the risk of new-onset unprovoked seizures. Neurology. 1993;43(2):425–428. doi: 10.1212/wnl.43.2.425. 8437714 [DOI] [PubMed] [Google Scholar]
- Paradowski B., Zagrajek M.M. Epilepsy in middle-aged and elderly people: a three-year observation. Epileptic Disord. 2005;7(2):91–95. 15929910 [PubMed] [Google Scholar]
- Parkes L.M., Tofts P.S. Improved accuracy of human cerebral blood perfusion measurements using arterial spin labelling: accounting for capillary water permeability. Magn. Reson. Med. 2002;48(1):27–41. doi: 10.1002/mrm.10180. [DOI] [PubMed] [Google Scholar]
- Pendse N., Wissmeyer M., Altrichter S. Interictal arterial spin-labeling MRI perfusion in intractable epilepsy. J. Neuroradio. 2010;37(1):60–63. doi: 10.1016/j.neurad.2009.05.006. 19674791 [DOI] [PubMed] [Google Scholar]
- Regesta G., Tanganelli P. Late onset epilepsy and diffuse cryptogenous cerebral atrophy. Epilepsia. 1992;33(5):821–825. doi: 10.1111/j.1528-1157.1992.tb02188.x. 1396423 [DOI] [PubMed] [Google Scholar]
- Roberts R.C., Shorvon S.D., Cox T.C., Gilliatt R.W. Clinically unsuspected cerebral infarction revealed by computerised tomography scanning in late onset epilepsy. Epilepsia. 1988;29(2):190–194. doi: 10.1111/j.1528-1157.1988.tb04418.x. 3349970 [DOI] [PubMed] [Google Scholar]
- Shinton R.A., Gill J.S., Zezulka A.V., Beevers D.G. The frequency of epilepsy preceding stroke. Case–control study in 230 patients. Lancet. 1987;1(8523):11–13. doi: 10.1016/s0140-6736(87)90701-x. 2879091 [DOI] [PubMed] [Google Scholar]
- Shorvon S.D., Gilliatt R.W., Cox T.C., Yu Y.L. Evidence of vascular disease from CT scanning in late onset epilepsy. J. Neurol. Neurosurg. Psychiatry. 1984;47(3):225–230. doi: 10.1136/jnnp.47.3.225. 6707668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallis R., Hall G., Craig I., Dean A. How common are epileptic seizures in old age? Age Ageing. 1991;20(6):442–448. doi: 10.1093/ageing/20.6.442. 1776595 [DOI] [PubMed] [Google Scholar]
- Terborg C., Gora F., Weiller C., Röther J. Reduced vasomotor reactivity in cerebral microangiopathy: a study with near-infrared spectroscopy and transcranial Doppler sonography. Stroke. 2000;31(4):924–929. doi: 10.1161/01.str.31.4.924. 10754000 [DOI] [PubMed] [Google Scholar]
- De Reuck J., Decoo D., Boon P. Late-onset epileptic seizures in patients with leukoaraiosis: a positron emission tomographic study. Eur. Neurol. 1996;36(1):20–24. doi: 10.1159/000117194. 8719645 [DOI] [PubMed] [Google Scholar]
- Van Dijk E.J., Prins N.D., Vrooman H.A., Hofman A., Koudstaal P.J., Breteler M.B. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39(10):2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- Wannamaker B.B., Wilson D.A., Malek A.M., Selassie A.W. Stroke after adult-onset epilepsy: a population-based retrospective cohort study. Epilepsy Behav. 2015;43:93–99. doi: 10.1016/j.yebeh.2014.11.028. 25575071 [DOI] [PubMed] [Google Scholar]
- Wardlaw J.M., Smith E.E., Biessels G.J. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. 23867200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table demonstrating seizure type, results from baseline investigations and response to anti-epileptic medication for all patients with LOE who participated in the study.