Abstract
Objective(s):
The colorectal cancer stem cells (CSCs) with the CD133+ phenotype are a rare fraction of cancer cells with the ability of self-renewal, unlimited proliferation and resistance to treatment. Quercetin has anticancer effects with the advantage of exhibiting low side effects. Therefore, we evaluated the anticancer effects of quercetin and doxorubicin (Dox) in HT29 cancer cells and its isolated CD133+ CSCs.
Materials and Methods:
The CSCs from HT29 cells were isolated using CD133 antibody conjugated to magnetic beads by MACS. Anticancer effects of quercetin and Dox alone and in combination on HT29 cells and CSCs were evaluated using MTT cytotoxicity assay and flow cytometry analysis of cell cycle distribution and apoptosis induction.
Results:
The CD133+ CSCs comprised about 10% of HT29 cells. Quercetin and Dox alone and in combination inhibited cell proliferation and induced apoptosis in HT29 cells and to a lesser extent in CSCs. Quercetin enhanced cytotoxicity and apoptosis induction of Dox at low concentration in both cell populations. Quercetin and Dox and their combination induced G2/M arrest in the HT29 cells and to a lesser extent in CSCs.
Conclusion:
The CSCs were a minor population with a significantly high level of drug resistance within the HT29 cancer cells. Quercetin alone exhibited significant cytotoxic effects on HT29 cells and also increased cytoxicity of Dox in combination therapy. Altogether, our data showed that adding quercetin to Dox chemotherapy is an effective strategy for treatment of both CSCs and bulk tumor cells.
Keywords: Apoptosis, Cancer stem cells, Cell cycle, Doxorubicin, Drug resistance, Quercetin
Introduction
Colorectal cancer is the third most prevalent cancer and the second cause of malignancy-related death due to therapy resistance (1, 2). According to the Iranian Annual National Cancer Registration Report, colorectal cancer is the fifth prevalent cancer in Iranian men and the third in Iranian women (3). Despite increasing knowledge of tumor biology, the efficacy of treatments for colon cancer has not been remarkably improved. Accumulating evidence confirms that cancer stem cells (CSCs) may play an essential role in the relapse, progression and metastasis of colon cancer (4). Based on the CSC theory, solid tumors are preserved exclusively by a minor fraction of cancer cells with stem cell properties (5). The CSC model suggests that malignancies emanate from a rare fraction of cancer cells that have the ability for initiating, progression and sustaining tumor growth (2). It has been well hypothesized that CSCs are not only the origin of tumor formation but are capable of tumor progression, resistance to therapy, metastasis and tumor recurrence (6-8).
Stem cells can be described by two main properties: the ability to self-renew and the potential to produce all of the differentiated cells of the tissue of origin (multipotency) (9). The CSCs show several hallmarks of normal stem cells. Unlike the extremely regulated differentiation and self-renewal of normal stem cells, it has been proposed that CSCs show abnormal differentiation and uncontrolled self-renewal. In addition, CSCs can metastasize to distant tissues and are more resistant to apoptotic stimuli and conventional chemotherapeutic agents (10, 11). CSCs are identified by expressing specific surface markers in different cancer cells. In the colorectal cancer cells, these markers include CD133, CD44, THY1, epithelial cell adhesion molecule (EpCAM), ATP-binding cassette B5 (ABCB5), and aldehyde dehydrogenase 1 (ALDH1) (10, 12). The cell surface marker CD133 is a well accepted colon CSC marker (13). It has been demonstrated that the CD133+ cells show remarkably higher tumorigenicity in NOD/SCID mice compared to CD133− cells (14). Some studies demonstrated that CD133+ cancer cells are responsible for tumor initiation, resistance to anticancer chemotherapy and have stem-cell-like properties, such as differentiation to other cells and ability to form colonies (15).
Recently, flavonoids received high attention for their potential chemotherapeutic and chemo-preventative properties. The natural product quercetin (3,5,7,30,40-pentahydroxyflavone) is a flavonoid found abundantly in different plants including seeds, fruits, vegetables, nuts, tea, and olive oil, and hence it is extensively present in human diet (16). Quercetin has antioxidative, anti-allergic, anti-inflammatory, anti-viral, and vasodilating properties, and it has been suggested that it is a potential anti-cancer agent with the advantage of low side effects (17). It has been also proposed that quercetin can induce cytotoxic effects by antioxidative activity, inhibition of cell proliferation and induction of apoptosis in various human cancers such as HL60 leukemia cells, liver, lung, colon, and breast cancer cell lines (18). Quercetin cytotoxic effects can be described by different molecular mechanisms including cell cycle arrest at G2/M or G0/G1 phases and induction of caspase-mediated apoptosis, down-regulation of oncogenes, up-regulation of cell cycle control proteins, modification of cellular signal transduction pathways, which affect the anti-apoptotic and pro-apoptotic genes and cell survival or cell proliferation (AKt and MAPKs) processes in several cancer cell models (19-21). Quercetin induces apoptosis through DNA fragmentation, pro-apoptotic Bax up-regulation, anti-apoptotic Bcl-2 post-translational modification, caspase-9 and caspase-3 activation (22, 23).
Interestingly, quercetin can act as an efflux pump inhibitor. It has been demonstrated that quercetin can increase the bioavailability of drugs by competitively inhibiting BCRP, P-gp, MRP1, and the metabolizing enzyme CYP3A4 (18). Quercetin can reduce BCRP and P-gp expression and their activity in a dose-dependent manner (17). Thus, quercetin co-administration with conventional chemotherapeutic agents may exert better anticancer effects at nontoxic concentrations of quercetin (18). Furthermore, doxorubicin is one of the most frequently used drugs in chemotherapy regimens for treatment of a variety of human cancers. It has been proposed that co-administration of quercetin with doxorubicin may affect activity and toxicity of doxorubicin in cancer therapy (17).
Therefore, considering the importance of CSCs and necessity to target this fraction of tumor cells, the main aim of this study was to evaluate the effects of quercetin alone or in combination with doxorubicin on cellular proliferation, cell cycle distribution and apoptosis induction of colorectal cancer HT29 cells and its isolated CD133+ CSCs.
Materials and Methods
Materials
RPMI 1640 and FBS were purchased from Biosera (UK). Pen-strep and trypsin- EDTA were purchased from Gibco (UK). MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide), Propidium Iodide (PI), Annexin V-FITC and quercetin were purchased from Sigma (Germany). DAPI (4, 6-diamidine-2-phenylindole) and Nonidet P40 were purchased from Roche (Germany). Doxorubicin (Dox) was purchased from Ebewe Pharma (Austria). CD133 isolation kit, LD separator column, anti-CD133-PE antibody were purchased from Miltenyi Biotech GmbH (Germany).
Cells and cell culture
The HT29 cell line (purchased from NCBI of Iran Pasteur Institute), were grown in RPMI-1640 media supplemented with penicillin (100 U/ml) and streptomycin (100 µg/ml), 10% heat-inactivated fetal bovine serum (FBS) at 95% air and 5% CO2 in a humidified 37° C incubator.
Drug preparation
Quercetin was initially solubilized in dimethyl-sulphoxide (DMSO) at a concentration of 10 mM, stored at 4°C and protected from the light. Different concentrations of quercetin were then freshly prepared in complete culture medium before use and added to the cells in different experiments. In all experiments, DMSO concentration never exceeded 1%, which has no effect on HT29 cells. Dox (20 mg/10 ml) was diluted in complete culture medium freshly before use and added to the cells at different concentrations.
Magnetic cell sorting and flow cytometry
The CSCs were isolated by magnetic bead sorting using the magnetic cell sorting system (MACS). Cells were centrifuged at 300 g for 10 min, and pellet was resuspended in 0.35 ml PBS with 0.5% BSA and 2 mM EDTA. Cells were then incubated with a monoclonal CD133 antibody conjugated to MicroBeads (Miltenyi Biotech) for 30 min at 4 °C. The CD133+ cells were then enriched using a QuadroMACS magnet and LS columns (Miltenyi Biotech). All MACS procedures were performed according to the manufacturer’s instructions. The purity of isolated CD133+ cells was evaluated by flow cytometry with specific anti-CD133-PE antibody.
Cytotoxicity assay
Proliferation of HT29 cells and isolated CD133+ CSCs under various treatment conditions was evaluated by using the colorimetric MTT assay. Briefly, 5000 cells per well were seeded in 96-well plates. After 48 hr, culture media was removed and the cells were treated with 100, 250, 500, 750, and 1000 nM of Dox and 25, 50, 75, and 100 µM of quercetin alone and in combination at different time points (72 and 96 hr). Then MTT solution (4 mg/ml in phosphate-buffered saline (PBS)) was added to each well and incubated for 3 hr at 37 °C and 5% CO2. The insoluble formazan crystals were dissolved by adding DMSO to each well, and the absorbance of each well was read at 540 nm using a microplate reader (Sunrise, Tecan, Switzerland). The results were presented as a percentage to the control RPMI. The growth inhibition rate was calculated using the following equation: inhibition rate (%)= (ODexp/ODcon)×100 in which ODexp and ODcon represent the optical densities of treated and untreated (RPMI) control cells, respectively. Drug concentration that inhibited cell proliferation to 50% (IC50) in comparison to the control RPMI was determined using curve fitting method from at least three independent experiments in a quadruplicate format for each treatment.
Cell cycle distribution analysis
DAPI staining was used to determine the distribution of cells in different phases of cell cycle by flow cytometry analysis. Briefly, cells were seeded into 6-well plates at a density of 2.5×105 cells/well. The cells were treated with IC50 of quercetin and Dox alone and in combination. Cells were collected by trypsinization and stained with DAPI solution at 4 °C for 30 min in dark to analyze cell cycle distribution based on DNA content of cells by Partec flow cytometer using UV light. Data analysis was performed using FloMax software.
Apoptosis analysis
Apoptosis induction under different treatment conditions was determined using Annexin V-FITC and PI dual staining and flow cytometry analysis. Briefly, cells were seeded into 6-well plates at a density of 2.5×105 cells/well. The cells were treated with IC50 of quercetin and Dox alone and in combination. Cells were collected by trypsinization and stained with Annexin V-FITC and PI for 15 min at 4°C in dark. Finally, stained cells were analyzed for percentage of apoptotic cells by Partec flow cytometer and FloMax software.
Statistical analysis
All experiments were repeated at least three times and data were presented as mean±SE. Data sets were examined by one-way analysis of variance (ANOVA) followed by Tukey post hoc test. Significant difference between treatments in comparison to control RPMI was denoted by # for P<0.01 and *, for P<0.001.
Results
Isolation of CD133+ cancer stem cells by magnetic cell sorting
HT29 cancer stem cells were identified and isolated based on the expression of surface marker CD133. Flow cytometry analysis showed presence of about 10% CSC population that highly expressed CD133 in the HT29 cancer cells (Figure 1A). After isolation of CD133+ CSCs by MACS, the purity of isolated CSCs was determined using specific antibody against CD133 by flow cytometry, which showed 94% purity (Figure 1B).
Figure 1.
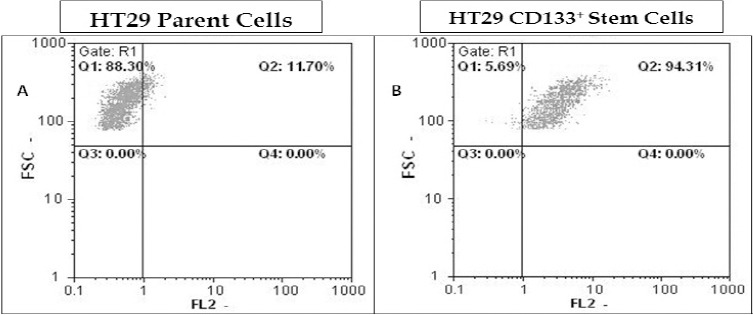
Isolation of CD133+ cancer stem cells from HT29 cancer cells by magnetic cell sorting system. The CD133+ CSCs of HT29 cancer cells were isolated by CD133-magnetic beads using the Miltenyi’s MACS. The parental HT29 cancer cells (A) and its isolated CD133+ CSCs (B) were subjected to immunostaining with anti-CD133-PE antibody and analyzed by flow cytometry to determine the percentage of CD133+ cells within each population of HT29 cells
Cytotoxicity of different treatments on HT29 cancer cells
The effects of different treatments on cell proliferation in HT29 cell line was determined by MTT cytotoxicity assay. Cell proliferation was remarkably decreased following treatment with Dox or quercetin in a concentration- and time-dependent manner in HT29 cell line (Figure 2). The IC50 for Dox and quercetin were determined to be about 750 nM and 75 µM after 72 hr treatment of HT29 cells, respectively.
Figure 2.
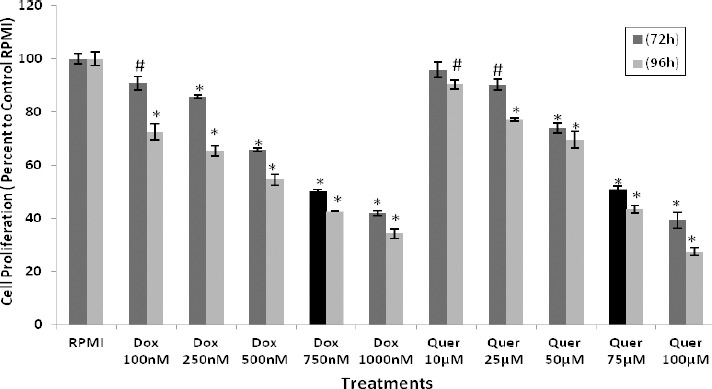
Cytotoxic effects of quercetin and doxorubicin on HT29 cancer cells. The HT29 cancer cells were treated with different concentrations of Dox and Quer to determine cell proliferation using MTT assay. The results were expressed as mean±SE of three independent experiments in quadruplicate layout for each concentration. # denotes P<0.01 and *, P<0.001 for significant difference between treatments in comparison to control RPMI. Dox: Doxorubicin; Quer: Quercetin
Effect of quercetin on cytotoxicity of doxorubicin in HT29 cancer cells
The effect of quercetin (Quer) on cytotoxicity of Dox in HT29 cancer cells was determined using MTT assay. Co-treatment of HT29 cells with Quer and Dox resulted in significant sensitization of these cells to Dox chemotherapy (Figure 3). Quer at low concentration significantly reduced the IC50 of Dox from 750 nM to 250 nM in HT29 cancer cells. This demonstrates that Quer enhances the efficacy of Dox treatment and in turn reduces side effects of Dox, which are usually seen at higher concentrations of Dox in normal cells. comparison to parental HT29 cells. The IC50 of Dox and Quer alone that were determined on parental HT29 cancer cells showed 18% and 23% reduction in cell proliferation of CD133+ CSCs, respectively. Importantly, combination of Quer and Dox at low concentration was more effective in inhibiting the CSCs proliferation (28%) in comparison to Quer and Dox alone.
Figure 3.
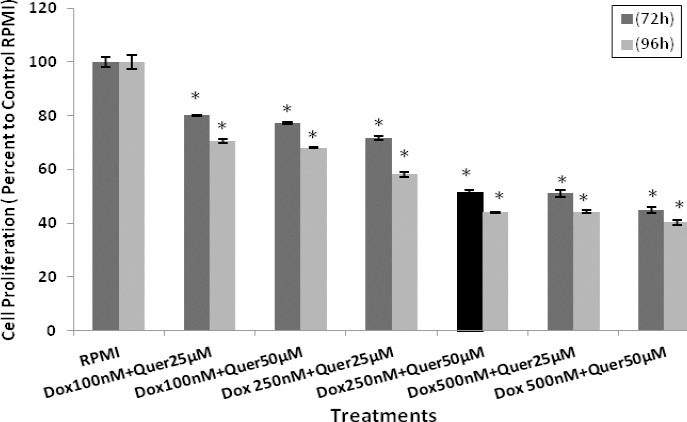
Effects of quercetin on doxorubicin cytotoxicity in HT29 cancer cells. The HT29 cells were co-treated with different concentrations of Quer + Dox to determine cell proliferation using MTT assay. The results were expressed as mean±SE of three independent experiments in quadruplicate layout for each concentration. *denotes P<0.001 for significant difference between treatments in comparison to control RPMI. Dox: Doxorubicin; Quer: Quercetin
Figure 4.
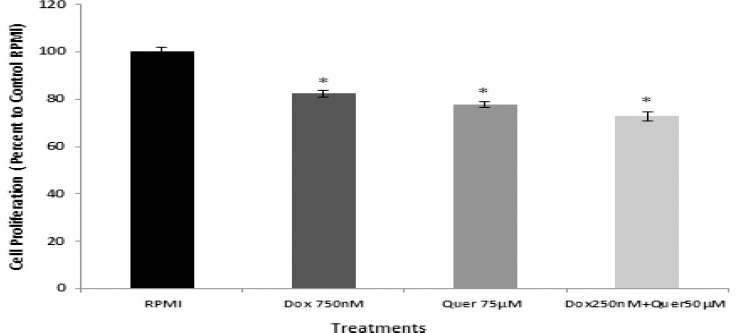
Cytotoxic effects of quercetin and doxorubicin on isolated CD133+ cancer stem cells. The isolated CD133+ cancer stem cells were treated with IC50 of Dox and Quer alone and in combination for 72 hr to determine cell proliferation using MTT assay. The results were expressed as mean±SE of three independent experiments in quadruplicate layout for each concentration. *denotes P<0.001 for significant difference between treatments in comparison to control RPMI. Dox: Doxorubicin; Quer: Quercetin
Effects of treatments on cell cycle distribution of HT29 cells and its CD133+ CSCs
In order to understand whether the growth inhibitory effect of Quer and Dox was due to cell cycle arrest, we evaluated the effects of treatments on cell cycle distribution using DAPI staining and flow cytometry analysis. In HT29 cell line, 55.42% of control (RPMI) treated cells were in G0/G1, 23.55% in S and 21% in G2/M phases of cell cycle (Figure 5A). Flow cytometry analysis revealed that Dox treatment at IC50 induced accumulation of HT29 cells in G2/M phase (60.7%) compared to the control cells (21%). Quer treatment at IC50 also induced G2/M arrest (60%) in HT29 cells almost the same as Dox treatment. Importantly, similar level of G2/M arrest (64%) was observed in HT29 cells treated with combination of Dox and Quer at much lower concentration than their IC50 (Figure 5A). Importantly, the pattern of cell cycle distribution of the isolated CD133+ CSCs showed significantly higher percentage of cells at G0/G1 (79%) in comparison to parental HT29 cells (55%) in control (RPMI) condition (Figure 5A and B). This further indicated that CSCs were more in quiescent phase and therefore, less responsive to Dox treatment at IC50, which resulted in significantly less accumulation of CSCs in G2/M phase (33.79%) in comparison to parental HT29 cells (60.7%). Similar results for G2/M arrest (35.96%) were observed following Quer treatment at IC50 or combination of Dox and Quer (40.48%) at much lower concentration than their IC50 (Figure 5B).
Figure 5.
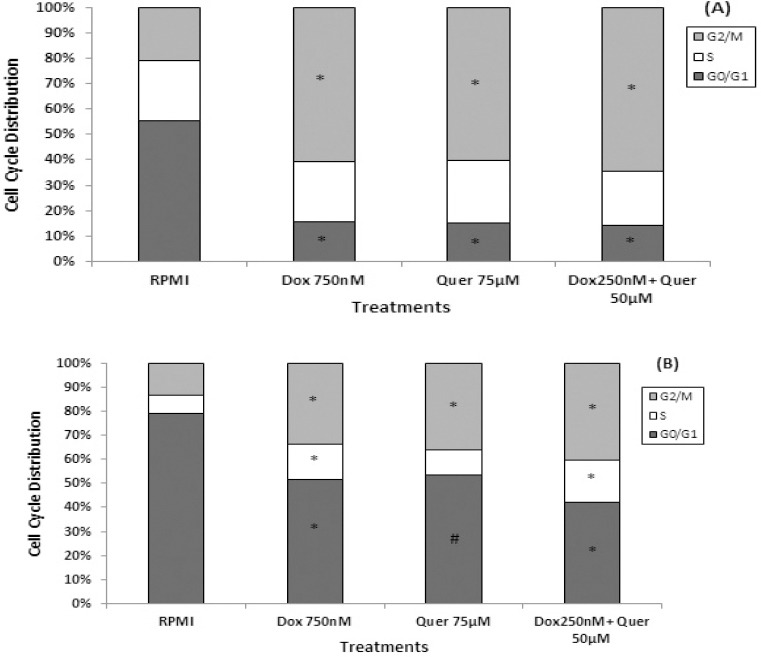
Cell cycle alteration in HT29 cell line and its isolated CD133+ cancer stem cells. The HT29 cancer cells (A) and its isolated CD133+ cancer stem cells (B) were treated with Dox and Quer alone and in combination for 72 hr to determine cell cycle distribution pattern using DAPI staining by flow cytometry analysis. Data are presented as the mean±SE of three independent experiments. # denotes P<0.01 and *, P<0.001 for significant difference between treatments in comparison to control RPMI. Dox: Doxorubicin; Quer: Quercetin
Effects of treatments on apoptosis induction in HT29 cells and its CD133+ CSCs
In order to study the induction of apoptosis, HT29 cells and its isolated CD133+ CSCs were treated with IC50 of Dox and Quer alone and in combination. Then cells were double-stained with AnnexinV-FITC and PI and analyzed by flow cytometry. Flow cytometry analysis data were processed with FloMax software to determine the percentage of necrotic (Q1: AnnexinV-FITC-/PI+), late apoptotic (Q2: AnnexinV-FITC+/PI+), viable (Q3: AnnexinV-FITC-/PI-), and early apoptotic (Q4: AnnexinV-FITC+/PI-) cells. The sums of percentages of early (Q4) and late (Q2) apoptotic cells in HT29 cells treated with IC50 of Dox (44.98%) and Quer (49.7%) alone and in combination (56.77%) were significantly greater than that of control cells (0.26%) (Figure 6A).
Figure 6.
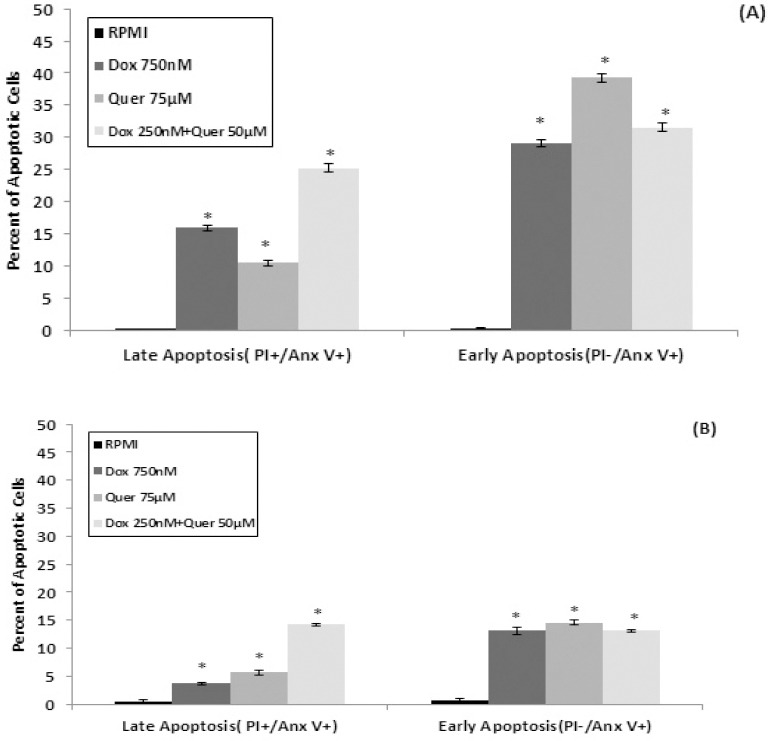
Apoptosis induction in HT29 cell line and its isolated CD133+ cancer stem cells. The HT29 cancer cells (A) and its isolated CD133+ cancer stem cells (B) were treated with Dox and Quer alone and in combination for 72 hr to evaluate induction of apoptosis using Annexin V-FITC/PI double-staining by flow cytometry analysis. The percentage of early and late apoptotic cells were presented for each treatment group. Data are presented as the mean±SE of three independent experiments. *denotes P<0.001 for significant difference between treatments in comparison to control RPMI. Dox: Doxorubicin; Quer: Quercetin
In the isolated CD133+ CSCs, Dox (16.79%) and Quer (20.25%) alone and in combination (27.4%) induced apoptosis but at significantly lower percentages than that observed in the parent HT29 cells (Figure 6B). Apoptosis data also confirmed that the CSCs are more resistant to the effects of treatments than the parent HT29 cells.
Discussion
Colorectal cancer is the second most common cause of cancer related deaths, demonstrating that current therapies cannot eradicate some of cancer cells effectively (24). The colorectal cancer mortality rate is high due to the tendency for early metastasis and high resistance to chemotherapy and radiation (12). Evidence indicates that CSCs are capable of self-renewal and multipotent differentiation and play an important role in colorectal cancer initiation, progression, metastasis, chemoresistance, and relapse despite of their small quantity (8). CSCs possess inherent resistance to anti-proliferative agents, a specification which is the main clinical challenge for complete eradication of cancer cells (11). CSCs are recognized by expression of specific surface markers such as the CD133 in the colorectal cancers (10, 12). Data of present study showed that about 10% of HT29 cancer cells are CD133+ CSCs. Currently available anticancer drugs are mostly effective against non-stem cancer cells and therefore, cancer research centers around the world have focused their studies on introducing natural and synthetic compounds to target and eradicate CSCs.
Many studies have confirmed the presence of cytotoxic compounds in different plants that show potential anticancer effects in various cancer cells (25-27). Quer is one of the important flavonoids that exert anti-proliferative effects on cancer cells mediated by various mechanisms of action including cell cycle arrest and apoptosis induction, alterations in gene expression of oncogenes, tumor suppressor genes and apoptosis related genes such as Bcl-2 and caspases (28).
In this report, effects of Quer and Dox on proliferation, cell cycle distribution and apoptosis induction were evaluated in human colorectal cancer HT29 cells and its isolated CD133+ CSCs. On the other hand, Dox is an effective and commonly used anthracycline chemotherapeutic agent in different human cancers. The major anticancer activities of Dox are DNA intercalation, topoisomerase II inhibition and free radical formation resulting in cell death or growth arrest (29). Results of this study indicate that Quer and Dox can decrease proliferation of HT29 cancer cells in a concentration- and time-dependent manner. Importantly, adding low concentration of Quer to 1/3 of IC50 of Dox was able to show similar or higher anticancer effects than IC50 of either compound alone. This indicated that Quer can increase efficacy of Dox chemotherapy at much lower concentration that in turn also reduces the side effects associated with Dox chemotherapy on normal cells at higher doses. Results of MTT assay showed that the IC50 of Dox and Quer alone had significantly lower cytotoxic effects on the isolated CD133+ CSCs in comparison to the parental HT29 cancer cells. This data further supports the previous findings such as the study conducted by Eramo et al (5), showing CSCs are more resistant to chemotherapeutic agents. Importantly, combination treatment of Dox and Quer at much lower concentration than their IC50 was able to exhibit antiproliferative effects similar to IC50 of each treatment alone. This data also indicated that Quer can enhance anticancer effects of Dox at lower concentration, which in turn decreases the side effects associated with Dox on normal cells.
It is currently well accepted that most conventional chemotherapeutic agents target rapidly dividing tumor cells and therefore, have minor effects on the slow dividing and quiescent CSCs (30). Furthermore, the cell cycle arrest followed by apoptosis induction in tumor cells after treatment with chemotherapeutic agents is the main efficient strategy to prevent the uncontrolled cell proliferation of cancer cells. Results of cell cycle analysis by flow cytometry in our studies further support that a high percentage of CSCs are in the G0/G1 phase as observed in the isolated CD133+ CSCs of the HT29 colorectal cancer cells under control (RPMI) culture conditions. In the present study we examined the effects of Quer and Dox alone or in combination on cell cycle pattern of HT29 cancer cells and its isolated CD133+ CSCs. Consistent with the previous findings (31), in this study HT29 cancer cells were mostly arrested in G2/M phase when treated with Quer that was similar to the effects of Dox treatment in these cells. It has been reported that Quer induces G2/M phase accumulation due to enhanced level of the cyclin B and decreased level of the cyclin E, cyclin D, E2F1, and E2F2 (31). In addition, Dox and Quer alone or in combination induced G2/M arrest in the isolated CD133+ CSCs but to a lesser extent than observed in the parental HT29 cancer cells.
Furthermore, CSCs have been proposed to be resistant to death-inducing signals by different mechanisms including being relatively quiescent (30), slow cycling (9), showing high expression of drug efflux pumps such as breast cancer resistance protein (BCRP) (32), showing high DNA-repair capacity (9), and high expression of anti-apoptotic proteins such as Bcl2. In this study, flow cytometry analysis revealed that Dox and Quer alone induced apoptosis significantly more in the parental HT29 cancer cells than in the isolated CD133+ CSCs. The resistance of CSCs to apoptosis can be explained by different mechanisms, one of the important mechanisms being dysregulation of balances between anti- and pro-apoptotic Bcl2 genes (33-34). Furthermore, it has been shown that activation of Wnt/β-catenin signaling pathway in CSCs can inhibit apoptosis (33). It is important to note that the results of our study can be clinically relevant: adding Quer to low concentration of Dox (1/3 of IC50) can induce apoptosis to similar extent as IC50 of each compound alone in both parental HT29 cancer cells and its isolated CD133+ CSCs.
Conclusion
Findings of this study further support the previously reported data that despite CSCs being quantitatively a minor population within the majority of bulk of tumor cells, using compounds to target these resistant cells is very essential for a successful cancer therapy. Furthermore, accumulation of CSCs at G0/G1 phase, a quiescent and slow cycling phenotype, which is observed in the isolated CD133+ CSCs of HT29 colorectal cancer cells, partly explains the resistance of CSCs to chemotherapy that mostly targets rapidly dividing cells. Quer can enhance the efficacy of low concentration of Dox chemotherapy in inhibiting cell proliferation, inducing cell cycle arrest and apoptosis in HT29 parental and more importantly in its CD133+ CSCs. This effect of Quer is clinically very important because of reducing life threatening side effects associated with Dox chemotherapy at high concentration on normal cells. Results of this and other similar studies will advance our knowledge about characteristics of CSCs and natural or synthetic compounds to target and eradicate both differentiated cancer cells as well as multipotent CSCs.
Acknowledgment
The results reported in this article are part of a PhD dissertation that was financially supported by Tehran University of Medical Sciences, Tehran, Iran.
References
- 1.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Samadder NJ, Curtin K, Wong J, Tuohy TM, Mineau GP, Smith KR, et al. Epidemiology and familial risk of synchronous and metachronous colorectal cancer: a population-based study in Utah. Clin Gastroenterol Hepatol. 2014;12:2078–2084. doi: 10.1016/j.cgh.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Safaee A, Moghimi-Dehkordi B, Pourhoseingholi MA, Vahedi M, Maserat E, Ghiasi S, et al. Risk of colorectal cancer in relatives: a case control study. Indian J Cancer. 2010;47:27–30. doi: 10.4103/0019-509X.58855. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 5.Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- 6.Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl) 2009;87:1097–1104. doi: 10.1007/s00109-009-0518-4. [DOI] [PubMed] [Google Scholar]
- 7.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Yu SC, Ping YF, Yi L, Zhou ZH, Chen JH, Yao XH, et al. Isolation and characterization of cancer stem cells from a human glioblastoma cell line U87. Cancer Lett. 2008;265:124–134. doi: 10.1016/j.canlet.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 10.Fulda S, Pervaiz S. Apoptosis signaling in cancer stem cells. Int J Biochem Cell Biol. 2010;42:31–38. doi: 10.1016/j.biocel.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Sampieri K, Fodde R. Cancer stem cells and metastasis. Semin Cancer Biol. 2012;22:187–193. doi: 10.1016/j.semcancer.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Fan X, Ouyang N, Teng H, Yao H. Isolation and characterization of spheroid cells from the HT29 colon cancer cell line. Int J Colorectal Dis. 2011;26:1279–1285. doi: 10.1007/s00384-011-1248-y. [DOI] [PubMed] [Google Scholar]
- 13.Feng HL, Liu YQ, Yang LJ, Bian XC, Yang ZL, Gu B, et al. Expression of CD133 correlates with differentiation of human colon cancer cells. Cancer Biol Ther. 2010;9:216–223. doi: 10.4161/cbt.9.3.10664. [DOI] [PubMed] [Google Scholar]
- 14.Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, et al. CD133+CD44+population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- 15.Piao LS, Hur W, Kim TK, Hong SW, Kim SW, Choi JE, et al. CD133+liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315:129–137. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Zhang M, Yu L, Zhao Y, He N, Yang X. Antitumor activities of quercetin and quercetin-5’,8-disulfonate in human colon and breast cancer cell lines. Food Chem Toxicol. 2012;50:1589–1599. doi: 10.1016/j.fct.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Du G, Lin H, Yang Y, Zhang S, Wu X, Wang M, et al. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int Immunopharmacol. 2010;10:819–826. doi: 10.1016/j.intimp.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Zhou J, Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sci. 2010;87:333–338. doi: 10.1016/j.lfs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Vidya Priyadarsini R, Senthil Murugan R, Maitreyi S, Ramalingam K, Karunagaran D, Nagini S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur J Pharmacol. 2010;649:84–91. doi: 10.1016/j.ejphar.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res. 2010;33:1181–1191. doi: 10.1007/s12272-010-0808-y. [DOI] [PubMed] [Google Scholar]
- 21.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–325. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Szczepanski M, Lee YJ. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem Pharmacol. 2008;75:2345–2355. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocathechingallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal. 2010;5:14. doi: 10.1186/1750-2187-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeulen L, de Sousae Melo F, Richel DJ, Medema JP. The developing cancer stem-cell model: clinical challenges and opportunities. Lancet Oncol. 2012;13:83–89. doi: 10.1016/S1470-2045(11)70257-1. [DOI] [PubMed] [Google Scholar]
- 25.Momtazi-borojeni AA, Behbahani M, Sadeghi-aliabadi H. Antiproliferative activity and apoptosis induction of crude extract and fractions of avicennia marina. Iran J Basic Med Sci. 2013;16:1203–1208. [PMC free article] [PubMed] [Google Scholar]
- 26.Abdolmohammadi MH, Fouladdel Sh, Shafiee A, Amin Gh, Ghaffari SM, Azizi E. Anticancer effects and cell cycle analysis on human breast cancer T47D cells treated with extracts of Astrodaucus persicus (Boiss.). Drude in comparison to doxorubicin. DARU. 2008;16:112–118. [Google Scholar]
- 27.Kang TB, Liang NC. Studies on the inhibitory effects of quercetin on the growth of HL-60 leukemia cells. Biochem Pharmacol. 1997;54:1013–1018. doi: 10.1016/s0006-2952(97)00260-8. [DOI] [PubMed] [Google Scholar]
- 28.Avila MA, Velasco JA, Cansado J, Notario V. Quercetin mediates the down-regulation of mutant p53 in the human breast cancer cell line MDA-MB468. Cancer Res. 1994;54:2424–2428. [PubMed] [Google Scholar]
- 29.Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 30.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Lee TJ, Kim OH, Kim YH, Lim JH, Kim S, Park JW, et al. Quercetin arrests G2/M phase and induces caspase-dependent cell death in U937 cells. Cancer Lett. 2006;240:234–242. doi: 10.1016/j.canlet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Moorthi C, Kathiresan K. Curcumin- Piperine/Curcumin–Quercetin/Curcumin- Silibinin dual drug-loaded nanoparticulate combination therapy:A novel approach to target and treat multidrug-resistant cancers. J Med Hypotheses and Ideas. 2013;7(1):15–20. [Google Scholar]
- 33.Kruyt FA, Schuringa JJ. Apoptosis and cancer stem cells:Implications for apoptosis targeted therapy. Biochem Pharmacol. 2010;80:423–430. doi: 10.1016/j.bcp.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 34.Shahveisi K, Mousavi SH, Hosseini M, Khajavi Rad A, Jalali SA, Rajaei Z, et al. The role of local renin-angiotensin system on high glucose-induced cell toxicity, apoptosis and reactive oxygen species production in PC12 cells. Iran J Basic Med Sci. 2014;17:613–621. [PMC free article] [PubMed] [Google Scholar]


