Abstract
Objective(s):
Silver nanoparticles (Ag-NPs) are used widely in bedding, water purification, tooth paste and toys. These nanoparticles can enter into the body and move into the hippocampus. The aim of this study was to investigate the neurotoxicity of silver nanoparticles in the adult rat hippocampus.
Materials and Methods:
12 male Wistar rats were randomly divided into two experimental and control groups (6 rats in each group). Animals in the experimental group received Ag-NPs (30 mg/kg) orally (gavage) for 28 consecutive days. Control group in the same period was treated with distilled water via gavage. At the end of experiment, animals were deeply anesthetized, sacrificed, and their brains were collected from each group. Finally the brain sections were stained using toluidine blue and TUNEL. Then to compare the groups, dark neurons (DNs) and apoptotic neurons were counted by morphometric method.
Results:
Results showed that the numbers of DNs and apoptotic cells in the CA1, CA2, CA3, and dentate gyrus (DG) of hippocampus significantly increased in the Ag-NPs group in comparison to the control group (P<0.05).
Conclusion:
Exposure to Ag-NPs can induce dark neuron and apoptotic cells in the hippocampus.
Keywords: Apoptosis, Dark neuron, Hippocampus, Neurotoxicity, Silver nanoparticles
Introduction
Silver nanoparticles (Ag-NPs) have been widely used in health industries such as water purification, tooth paste, nursing bottles and other environmental applications (1). These nanoparticles are also used for the treatment of wounds and burns, and as a contraceptive (2). Despite the increasing use of Ag-NPs, there is little information about their possible toxic effects on the nervous system.
Because of their small size, nanomaterials can easily pass into the cells and affect cellular function (3, 4). Moreover, there have been some reports indicating that nanoparticles move into the brain by traversing the blood-brain barrier (BBB) (1, 4). So, nanoparticles have potential toxic effects on human health since they can pass through biological membranes (5). A recent study reported that 28 days of oral exposure to Ag-NPs produced changes in blood chemistry and caused hepatotoxicity (6). Rahman et al suggested that Ag-NPs have neurotoxic effects by generating oxidative stress (1). These studies also showed that after exposure of Ag-NPs, these nanoparticles accumulate in various organs including liver, kidney, testis and brain (6).
It is known that the biological half-life of silver in central nervous system (CNS) is longer than that in other organs (5). This suggests that there may be some pathological risks to the brain. Moreover, it has been shown that Ag-NPs can induce the production of reactive oxygen species (ROS) in the hippocampus (1). On the other hand, the brain has a high metabolic activity, and therefore it is highly vulnerable to oxidative stress (2). Many investigators have shown that ROS can induce apoptosis in many organs (7).
The hippocampus is one of the most important parts of the brain that plays a role in learning and memory (4, 8, 9). It was shown that hippocampal lesions disrupt memory and learning (6, 10).
Moreover, it is reported that the rats’ memory was obstructed after exposure to Ag-NPs (9). Thus, Ag-NPs exposure could possibly affect this important region of the brain and induce cytological changes such as dark neuron and apoptosis.
Apoptosis is a form of cell death that is responsible for maintaining balance in the number of cells in a tissue. This type of cell death that occurs in
normal tissue can also occur due to pathological problems. Apoptosis, which is characterized by morphological characteristics including cell shrinkage, cytoplasmic condensation and chromatin segregation, can be easily detected using TUNEL staining (11). Dark neuron (DN) is a type of cell degeneration (12, 13) that in histological studies is recognized by hyperbasophilia and hyper-electron density properties. These morphological changes are observed in stroke and some metal neurotoxicity (14). DNs can be detected by toluidine blue staining (15). We hypothesized that hippocampal ROS accumulation and memory impairment caused by Ag-NPs would be associated with hippocampal apoptosis and DNs formation. In this regard, in this study, the neurotoxic effect of Ag-NPs exposure on the rat hippocampus was investigated by toluidine blue (for DNs) and TUNEL staining (for apoptosis).
Materials and Methods
Animals and treatment
Male Wistar rats aged 3 to 4 months and weighting 250±20 g were purchased from the Animal Center of Mashhad University of Medical Sciences. The rats were housed under controlled temperature (20±2 °C) and lighting (12 hr light: 12 hr dark photoperiod) conditions and permitted free access to food and water.
Chemicals and preparation
Ag-NPs used in this study were purchased from Sigma-Aldrich, USA (Cat. No. 484059). It is a kind of nano powder with particle size of <100 nm, purity 99% and density 10.49 g/cm3. Ag-NPs suspended in distilled water and were administered by gavage to the rats. Daily administration of the nanoparticles was performed using fresh water.
Experimental procedure
12 male Wistar rats were randomly divided into two groups (6 rats in each group). Animals in the experimental group received Ag-NPs (30 mg/kg) orally (gavage) for 28 consecutive days (6). Control group in the same period was treated by distilled water via gavage. Experimental procedures were carried out according to Mashhad University of Medical Sciences, Ethical Committee Acts.
Tissue sampling
After 28 days, all animals were sacrificed after being anesthetized by chloroform. The brains were excised carefully and were washed with normal saline. The collected brains from each group were fixed in 10% neutral buffered formalin for 5 days.
Toluidine blue staining
After fixation, the specimens were dehydrated with an ascending ethanol series, cleared with xylene and embedded in paraffin. The brain samples were processed for embedding in paraffin using routine protocols. The brain tissue blocks were cut into 5 μm coronal serial sections (6 sections in each animal at 150 μm intervals) and stained with toluidine blue (16).
Apoptotic cell death staining
The in situ apoptotic cell death identification was carried out by the TUNEL technique. Apoptotic nuclei were identified by the presence of dark brown staining (17). Coronal serial sections (5 μm thick) were cut at the level of the dorsal hippocampus (3–4 mm posterior from bregma) using microtome, and 6 sections from each animal at 150 μm intervals were collected. Then the sections were deparaffinized with xylene, rehydrated through descending concentrations of ethanol and rinsed in phosphate buffered saline (PBS) twice for 10 min at room temperature. Proteinase-K digestion was applied as a pre-treatment for 20 min. The sections were rinsed in PBS subsequently. Then the TUNEL reaction was carried out by the in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany). The kit was used in accordance our previous study protocol (18).
Measurements
The photography was performed from different regions of the hippocampus using the microscope (Olympus BX51, Japan). Then on the computer monitor, the number of DNs and apoptotic cells were counted under 40 × objectives and by using rectangular grids placed randomly on the investigated areas. Morphometrical methods were used to count DNs and apoptotic cells per unit area in CA1, CA2, CA3 and dentate gyrus (DG) of the hippocampus. Finally, the mean of DN and apoptotic positive cells number per unit area (NA) was calculated in different regions of hippocampus using the following formula (19).
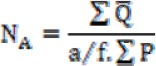
In this formula, “∑Q” is the sum of counted particles appeared in sections, “a/f” is the area associated with each frame, and “∑P” is the sum of frame associate points hitting the reference (18).
Data and statistical analysis
Statistical analysis was performed using the SPSS 11.5 software for windows. The results obtained from the chemically-treated group was compared to the control group. The values were compared using the independent T-test. The P-value less than 0.05 was considered to be statistically significant.
Results
Number of DNs in the hippocampus
The numbers of DNs per unit area (NA) of the CA1, CA2, CA3 and DG subdivisions of the hippocampus were counted. Then the mean number of DNs in the hippocampus of experimental group compared with the control group. A few DNs were found in different regions of hippocampus in control group. In comparison to control group, the mean of DNs number per unit area in CA1, CA2, CA3 and DG of hippocampus was significantly increased in Ag-NPs group (P<0.05 for CA1 and P<0.01 for other areas) (Figure 1, 2).
Figure 1.
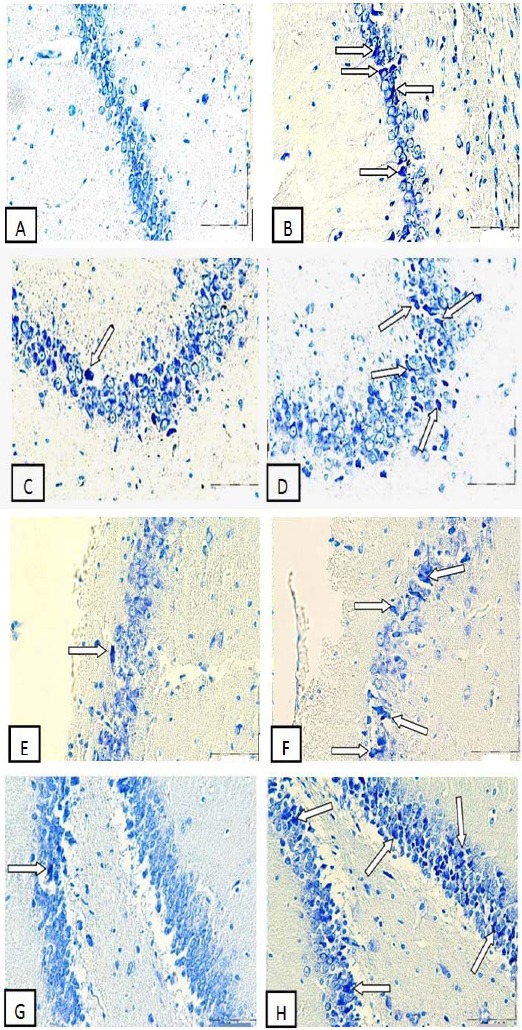
Photomicrographs of the adult rat hippocampus, Exposure to Ag-NPs increased formation of DNs in the hippocampus. (A) CA1 Control group, (B) CA1 Ag-NPs group, (C) CA2 Control group, (D) CA2 Ag-NPs group, (E) CA3 Control group, (F) CA3 Ag-NPs group, (G) DG Control group, (H) DG Ag-NPs group. Arrow heads point to representative DNs. Scale bars, 150 µm
Figure 2.
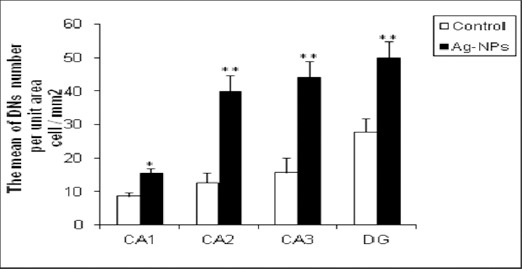
Ag-NPs exposure for 28 days induces DNs production in the adult rat hippocampus. Bars marked with an asterisk or double asterisks mean that it is significantly different from the control at the 5% or 1% confidence level, respectively. The data are presented as the mean ± SEM
Number of TUNEL positive cells in the hippocampus
The numbers of TUNEL positive cells per unit area (NA) of the CA1, CA2, CA3 and DG subdivisions of the hippocampus were counted. In comparison to control group, the mean of TUNEL positive cells number per unit area in CA1, CA2, CA3 and DG of hippocampus was increased significantly in Ag-NPs group (P<0.01), (Figures 3 and 4).
Figure 3.
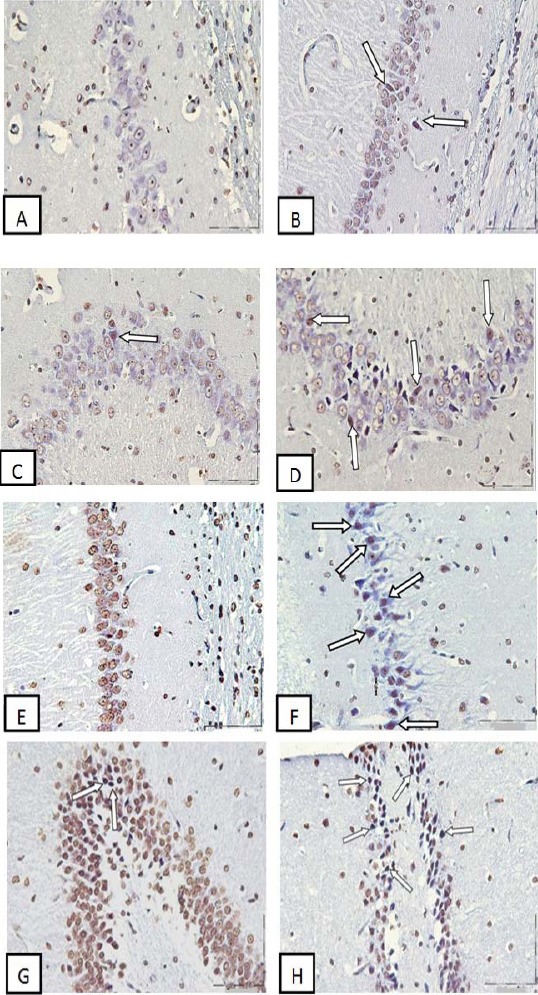
Photomicrographs of the adult rat hippocampus, Exposure to Ag-NPs increased apoptotic cells in the hippocampus. (A) CA1 Control group, (B) CA1 Ag-NPs group, (C) CA2 Control group, (D) CA2 Ag-NPs group, (E) CA3 Control group, (F) CA3 Ag-NPs group, (G) DG Control group, (H) DG Ag-NPs group, Arrow heads point to representative TUNEL positive cells with dark brown nucleus. Scale bars, 150 µm
Figure 4.
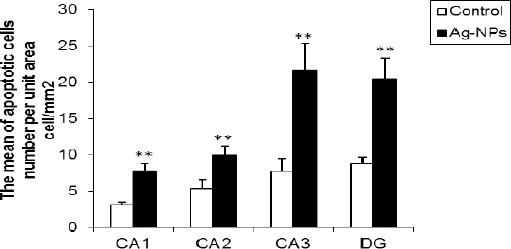
The influence of Ag-NPs exposure on the adult rat hippocampal cells; these nanoparticles induce apoptosis in the hippocampus. Bars marked with double asterisks mean that it is significantly different from the control at the 1% confidence level. The data are presented as the mean ± SEM
Discussion
The aim of this study was to assess the effect of Ag-NPs on DNs formation and apoptosis induction in the rat hippocampus. Our data indicate that administration of Ag-NPs to adult rats can induce apoptosis and DNs formation in the hippocampus. Ag-NPs are widely used in everyday life. Our body can receive Ag-NPs via three routes including inhalation, dermal contact, and ingestion. Among the above mentioned ways, oral ingestion may be important in using consumer products such as toothpaste, nursing nipples and toys (1, 2). Because of this, we have used orally administration for Ag-NPs in the present study.
Previous studies have shown that a daily intake of silver nanoparticles could lead to the accumulation of these particles in different organs (6, 18). Nanomaterials are able to enter in to cells in different ways including passive diffusion, receptor-mediated endocytosis and clarthrin mediated endocytosis (17).
Tang et al examined the distribution and toxicity of Ag-NPs in rats following subcutaneous injection. They found that these nanoparticles were translocated to the circulation and finally distributed to the brain and some other target organs including kidney, liver and lung. Because Ag-NPs can induce blood–brain barrier destruction, it can easily enter to the brain (4). These nanoparticles can also stimulate ROS generation and activate oxidative stress in the brain. On the other hand, hippocampal cells are highly sensitive to oxidative stress following exposure to nanoparticles (20). It can cause damage to cellular components such as membranes and proteins (21). Moreover, oxidative stress can induce apoptosis in the cells. So, induction of apoptosis leads to decrease of hippocampal neurons. In fact, one possible mechanism for Ag-NPs cytotoxicity is free radical formation, particularly ROS generation (22, 23). Moreover, inflammatory responses were significantly induced by oral administration of Ag-NPs for 28 days. It was shown that production of cytokines including IL-1, IL-6, IL-4, IL-10, IL-12, and TGF-β are increased after silver nanoparticles exposure (24). So, another mechanism of damage by these nanoparticles is the induction of inflammatory reactions.
DN formation is a type of cell death that is neither apoptosis nor necrosis (25). DNs formations have been reported in ischemia, epilepsy and hypoglycemia (26). In all of these pathological conditions, excitatory neurotransmitters such as glutamate are increased. On the other hand, the excitatory effects of Ag-NPs on hippocampal neurons are similar with glutamate, which plays a key role in the pathological process of brain disorders (27). Thus in the present study, apoptosis induction and DNs formation in the hippocampus after Ag-NPs treatment may occur due to oxidative stress, inflammation and glutamate increasing.
Results of a recent study indicated that the rats’ spatial memory was obstructed after Ag-NPs exposure (9). It may be due to apoptosis and induction of DNs in the hippocampus. It has been proved that hippocampal lesions are associated with impairments in learning and memory (4, 6), and in our study, it is shown that Ag-NPs increased DNs and apoptotic cells in the hippocampus.
Conclusion
Based on the results of this study, it is suggested that Ag-NPs have neurotoxic effect and induce apoptosis and DNs formation in hippocampus.
Acknowledgment
The results described in this paper were from a MSc student thesis and research protocol (89375), which was supported financially by the Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Rahman M, Wang J, Patterson T, Saini U, Robinson B, Newport G, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187:15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Oberdörster E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile largemouth bass. Environ Health Perspect. 2004;112:1058. doi: 10.1289/ehp.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang J, Xiong L, Wang Sh, Xiong L, Wang J, Li J, et al. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Appl Surf Sci. 2008;255:502–504. [Google Scholar]
- 5.Liu Z, Ren G, Zhang T, Yang Z. Action potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal CA1 neurons by silver nanoparticles. Toxicology. 2009;264:179–184. doi: 10.1016/j.tox.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 7.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 8.Yang WM, Shim KJ, Choi MJ, Park SY, Choi BJ, Chang MS, et al. Novel effects of Nelumbo nucifera rhizome extract on memory and neurogenesis in the dentate gyrus of the rat hippocampus. Neurosci Lett. 2008;443:104–107. doi: 10.1016/j.neulet.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Guan W, Ren G, Yang Zh. The possible mechanism of silver nanoparticle impact on hippocampal synaptic plasticity and spatial cognition in rats. Toxicol Lett. 2012;209:227–231. doi: 10.1016/j.toxlet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Cui K, Luo X, Xu K, Ven Murthy MR. Role of oxidative stress in neurodegeneration: recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuro sychopharmacol Biol Psychiatry. 2004;28:771–799. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Hara A, Niwa M, Iwai T, Nakashima M, Bunai Y, Uematsu T, et al. Neuronal apoptosis studied by a sequential TUNEL technique: a method for tract-tracing. Brain Res Protoc. 1999;4:140–146. doi: 10.1016/s1385-299x(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 12.Zsombok A, Tóth Z, Gallyas F. Basophilia, acidophilia and argyrophilia of “dark” (compacted) neurons during their formation, recovery or death in an otherwise undamaged environment. J Neurosci Methods. 2005;142:145–152. doi: 10.1016/j.jneumeth.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Kherani ZS, Auer RN. Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol. 2008;116:47–452. doi: 10.1007/s00401-008-0386-y. [DOI] [PubMed] [Google Scholar]
- 15.Jafarian M, Rahimi S, Behnam F, Hosseini M, Haghir H, Sadeghzadeh B, et al. The effect of repetitive spreading depression on neuronal damage in juvenile rat brain. Neuroscienc. e2010;169:388–394. doi: 10.1016/j.neuroscience.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Sadeghi A, Ebrahimzadeh-bideskan AR*, Alipour F, Fazel AR, Haghir H. The effect of ascorbic acid & garlic administration on lead-induced neural damage in rat offspring’s hippocampus. Iran J Basic Med Sci. 2013;16:156–164. [PMC free article] [PubMed] [Google Scholar]
- 17.Singh N, Manshian B, Jenkins G, Griffiths SM, Williams P, Maffeis T, et al. The DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Lale Ataei M, Ebrahimzadeh-bideskan AR. The effects of nano-silver and garlic administration during pregnancy on neuron apoptosis in rat offspring hippocampus. Iran J BasicMed Sci. 2014;17:411–418. [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadipour A, Fazel AR, Haghir H, Motejaded F, Rafatpanah H, Zabihi H, et al. Maternal exposure to titanium dioxidenanoparticles during pregnancy;impaired memory and decreased hippocampal cell proliferation in rat offspring. Environ Toxicol Pharmacol. 2014;37:617–625. doi: 10.1016/j.etap.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Liu ZW, Allaker RP, Reip P, Oxford J, Ahmad, et al. A review of nanoparticle functionality and toxicity on the central nervous system. J R Soc Interfac. 2010;7:411–422. doi: 10.1098/rsif.2010.0158.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beal MF. Mitochondria, free radicals, and neurodegeneration. Curr Opin Neurobiol. 1996;6:661–666. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 22.Hsin Y, Chen C, Huang S, Shih T, Lai P, Chueh P. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Choi O, Hu Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sic Thechnol. 2008;42:4583–4588. doi: 10.1021/es703238h. [DOI] [PubMed] [Google Scholar]
- 24.Eun-Jung P, Eunjoo B, Jongheop Y, Younghun K, Kyunghee Ch, Sang HL, et al. Repeated dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Gallyas F, Kiglics V, Baracskay P, Juhász G, Czurkó A. The mode of death of epilepsy-induced “dark” neurons is neither necrosis nor apoptosis: an electron-microscopic study. Brain Res. 2008;1239:207–215. doi: 10.1016/j.brainres.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 26.Catarzi D, Colotta V, Varana F. Competitive AMPA receptor antagonists. Med Res. 2007;27:239–278. doi: 10.1002/med.20084. [DOI] [PubMed] [Google Scholar]
- 27.Liu Zh, Zhang T, Ren G, Yang Zh. Nano-Ag inhibiting action potential independent glutamatergic synaptic transmission but increasing excitability in rat CA1 pyramidal neurons. Nanotoxicology. 2012;6:414–423. doi: 10.3109/17435390.2011.583996. [DOI] [PubMed] [Google Scholar]


