Abstract
Objective(s):
Cutaneous Leishmaniasis (CL) is a parasitic disease caused by various species of the flagellated protozoan, Leishmania. Regardless of the numerous studies, there are still serious challenges in the treatment of CL. This study aimed at evaluating the influence of a low dose ultraviolet B (UVB) radiation along with silver nanoparticles (AgNPs) on a mouse model of CL induced by Leishmania major.
Materials and Methods:
L. major promastigotes (MRHO/IR/75/ER) were extracted from infected mice spleens. Two months after subcutaneous injection of 2×106 promastigotes into the footpad of BALB/c mice, when the lesions were developed, the animals were divided into 4 groups including one control group and three study groups: AgNPs, UVB and UVB plus AgNPs. Spleen parasite burden was assessed on day 40 after the first treatment. The data were analyzed by Instat, Elida and SPSS 16 software programs.
Results:
The results showed the highest pronounced inhibitory effect in the group receiving AgNPs plus UVB. In addition, a significant difference was obtained between the group receiving AgNPs alone and the one with combinational therapy. The findings on parasite burden showed a significant difference between the control group and other treatment groups.
Conclusion:
It could be suggested that UVB in the presence of AgNPs, by inhibiting the spread of CL lesions and reducing the rate of visceral progression of the disease, provides a serious anti-leishmanial effect.
Keywords: Cutaneous leishmaniasis, Leishmania major, Nanosilver, Parasite Burden, Phototherapy, Ultraviolet B
Introduction
Cutaneous leishmaniasis (CL) is a parasitic disease caused by the flagellate protozoans of the Leishmania species, and transmitted through the bites of female sandflies from the Phlebotomus and Lutzomyia genera. According to the World Health Organization, more than 350 million people worldwide live in areas where there is the possibility of active disease transmission. Moreover, it is estimated that approximately 14 million people in Africa, Asia, Europe, and America are directly affected by this disease (1). In most regions of the world, meglumine antimoniate (Glucantime) and sodium stibogluconate (Pentostam) are considered the first choice treatment drugs; which are also associated with severe complications such as cardiac, liver, kidney, and blood disturbances when administered intramuscularly (1, 2). Yet, according to several reports in the recent years, the effectiveness of these drugs has significantly reduced (3).
Silver salts are widely used as antimicrobial agents because of their strong anti-bacterial and antiseptic properties (4, 5). In this regard, some researchers have suggested very small silver particles, in the nanometer range, to be used in the treatment of infections due to their high level of germicidal capacity (6). Other studies have reported certain anti-leishmanial effects for silver and gold nanoparticles (7, 8). It is worth noting that due to the toxicity of AgNPs to the liver, brain and spleen, as well as ovarian follicle reduction (9); their precise administration in low doses is highly recommended.
The advantages and disadvantages of ultraviolet (UV) radiation are well known. Vitamin D synthesis is one of its beneficial effects. The harmful effects include sunburn, premature aging of the skin and an increased risk of skin cancer, the latter being the most important health concern in UV ray application. Moreover, proteomic studies have shown such changes to occur in long-term exposure to UV (10).
UVB radiation is currently used to treat skin diseases such as psoriasis and vitiligo (11, 12). The effect of UVB radiation on animal immune systems has been studied via exposing them to UVB before infection by various Leishmania species. However, UVB has not been used in the treatment of leishmaniasis in vivo so far (13-15). There are also the opposite hypotheses, but it seems this effect is dose dependent. Limited data suggest that mice and humans may be immunologically sensitive to similar levels of UVB, which cause equivalent structural damage to skin in both species: selective damage to Langerhans cells (<150 J.m-2), and virtual depletion of ATPase surface markers of these cells, along with damage to other epidermal cells (800 J. m -2) (16).
Here, we studied the efficacy of combination therapy of UVB in the presence of AgNPs on an animal model of CL caused by L. major, based on the lesions’ improvement course and spleen parasitic load.
Materials and Methods
AgNPs preparation
0.001 to 0.01 M silver nitrate solution was used to produce AgNPs; the solution was reduced in a vessel containing two platinum electrodes. Particle size depended on the electric current and voltage fed into the system and was determined after reducing the transparent silver nitrate solution and its conversion into a brown colloid system (17).
Promastigotes preparation
1. Infected mice with L. major (MRHO/IR/75/ER), which causes visceral disease, were sacrificed. Splenectomy was performed and mice spleen amastigotes were cultured on Novy-MacNeal-Nicolle (NNN) medium containing Agar (4 mg/100 ml) and defibrinated rabbit blood (10%). After releasing the amastigotes from the spleen cells and transformation from amastigotes to promastigote-like forms, the growth of promastigo-tes was continued in RPMI 1640 culture medium (HIMED IA; AT 028) containing 100 μg/ml penicillin, 100 μg/ml streptomycin supplemented with 10% fetal calf serum (FCS), purchased from the GIBCO Company in a 28 °C incubator for two weeks. During several passages the parasite suspension was precipitated in a refrigerated centrifuge for 10 min at 4500 rpm, and the supernatant was removed. Thus, the parasites achieved a fixed growth phase (stationary-phase) after 12 days, and were ready for CL injection and induction (2, 18). It should be noted that the promastigotes have shown more intense infectivity at the stationary phase in comparison with the logarithmic phase. In this stage the number of metacyclic promastigotes is more than the other forms. It should be noted that repeated cultivations of promastigotes reduces their infectivity property. The parasites at the stationary phase, with 3 or 4 consecutive counts were quantified; an unchanged or decreased count of parasites was the criterion for confirming the stationary phase. At each passage, parasite sampling was performed and the growth of parasites was examined (19). It is mentioned that in this study the third passage of promastigotes was used to inoculate the lesions.
Leishmaniasis animal models
Six to eight week mice were purchased from the Pasteur Institute, Karaj branch. The mice were maintained at 22–25 °C under conditions of 12 hr darkness and 12 hr light. The third or fourth passage of promastigotes in the stationary phase of growth was used to infect the mice. After proliferation and harvesting, the parasites were washed with PBS, and a parasite suspension was prepared at a concentration of 40 × 106 L. major parasites per mL. 50 μl of the solution containing live promastigotes (2×106 parasites) was subcutaneously injected in the right hind paw of each mouse. After 20 days, the lesions began to appear. Following a random sampling of the animals and confirmation of CL by a pathologist, the 40 animals were randomly divided into four treatment groups. All experiments involving animals have been reviewed and confirmed by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Phototherapy conditions
Three Lamps with 70 cm length and 20 W powers were placed concentrically in the interior walls of a cylindrical tube. To determine the output wavelength, range of the UV lamps, an AvaSpec-2048TEC spectrometer equipped with a cooled CCD at the wavelengths of 200–1100 nm was utilized. Using a Leybold radiometric probe, UV power density was measured in the region where the lesion would be placed and irradiated. Intensity of UV radiation emitted by the lamp was determined as 135 μW/cm2.
To provide the required dermatologic conditions, the suberythemogenic dose, i.e. 70% of minimal erythema dose (MED) was administered to each mouse in the first session. However, for practical reasons, phototesting was often not performed (20). Based on the dermatologist’s experience, irradiation was started by 0.03 J/cm2 UVB (21) but due to severe erythema, the second irradiation dose was decreased to 0.02 J/cm2. Irradiation was performed using 0.05 J/cm2 in the third and fourth subsequent sessions. The treatment period was calculated according to the following equation:
Treatment time (sec) = Dose (mJ/cm2)/Intensity (mW/cm2)
The time required for delivering the desired dose was 222 sec for 30 mJ/cm2, 148 sec for 20 mJ/cm2 and 370 sec for 50 mJ/cm2.
Experimental groups
The mice infected with L. major were randomly divided into four groups, each consisting of 10 mice as follows:
Group 1 with no treatment, was the control group; Group 2, received AgNPs at a dose of 2 mg/kg under anesthesia (22); the effect of phototherapy alone was studied in Group 3 and the effect of AgNPs in combination with UVB was assessed in Group 4. 4 treatment sessions were given to the lesions on days zero, 4, 8, and 12. The administrative doses of UVB irradiation and/or AgNPs were similar in the treatment groups, if they were required.
The administrative dose of AgNPs in each session was 2 mg/kg. UVB irradiations were performed as 0.03, 0.02, 0.05, and 0.05 J/cm2 on days 0, 4, 8, and 12, respectively.
The judgment parameters for evaluating treatment efficacy
The effects of different treatments were compared on the basis of both changes in the lesion and spleen parasite burden. The sizes of lesions were recorded by a digital caliper twice a week and normalized to the change the day before treatment. In order to determine the number of viable parasites in the spleen, the mice were sacrificed on day 40 after the first treatment, and their spleen tissue was removed. Spleen parasite burden was determined in the following manner (23). A two-phase system consisting of a liquid phase containing RPMI-1640, 10% FCS, 100 μg/ml penicillin and 100 μg/ml streptomycin on a solid substrate of blood Agar was prepared. Solid agar medium containing 10% sterile defibrinated rabbit blood was prepared under sterile conditions. To assess the mice’s parasite burden, the spleens of the animals were removed after spinal block under sterile conditions and placed into 6-well plates containing complete RPMI-1640 medium. After homogenization of the spleen tissue, the content of every well was raised to 200 microliters with complete RPMI-1640 medium. Afterwards, ten-fold serial dilutions were prepared in 7 steps from each homogenized spleen, and each sample was divided into four replicates. The prepared plates were kept for 10 days at 28 °C. Finally, the positive (with parasite) and negative (without parasite) results were recorded in different dilutions on the basis of the microscopic observations.
Statistical analysis
The Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA) for Windows® version 12.0 was used to analyze the data. Data were analyzed for significant differences (P<0.05) and normality of the data was assessed using the Kolmogorov-Smirnov test. As the obtained data did not follow a normal distribution, Mann-Whitney and Kruskal Wallis tests were used. The results of spleen parasite burden were reported as the number of parasites per ml and were analyzed using ANOVA and Tukey tests.
Results
Characterization of silver nanoparticles
Nanoparticle size distribution was determined using a particle size analyzer (Malvern Instruments, Southborough, MA, USA). The peak particle size distribution was recorded at 9.67 nm (Figure 1).
Figure 1.
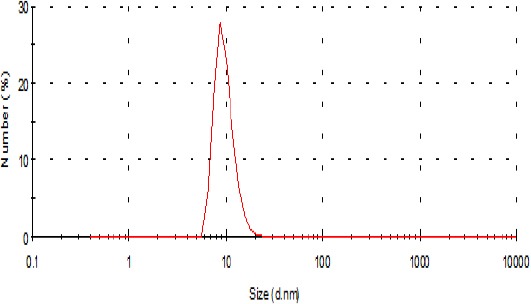
Size distribution of silver nanoparticles
Also, we utilized transmission electron microscopy (CM 120, Philips, Germany) operating at an acceleration voltage of 120 kV to examine the morphology of AgNPs (Figure 2).
Figure 2.
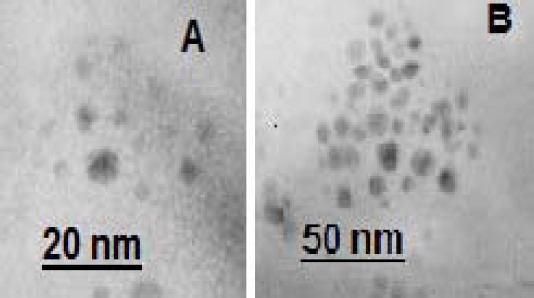
Two transmission electron microscope images of the silver nanoparticles at different magnifications
Phototherapy by UVB lamps
The lesions were irradiated by UV lamps (Philips ultraviolet BTL 20 w/12 rs) manufactured by Philips of the Netherlands with maximum wavelength output at 310 and 437 nm (Figure 3).
Figure 3.
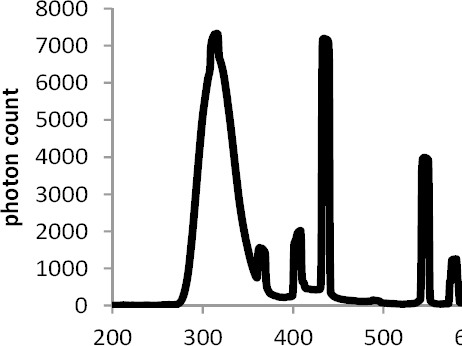
Emission spectrum recorded from a UVB lamp (Philips ultraviolet BTL 20 w/12 rs)
The effects of UVB radiation alone and in the presence of AgNPs on the right footpad lesion in BALB/c mice were studied. The relative area of the lesion was calculated as the lesion area at each day divided by the lesion area at day zero (before the first treatment). At the end of the 12 day treatment period the lesion areas, in the control group (Group 1) and the group receiving AgNPs (Group 2) were increased nearly 1.5 times. At day 22, in comparison with day 12, it showed no significant variation in Group 2, but in the control group the lesions’ relative area was significantly increased to 2.7 times (Figure 4).
Figure 4.
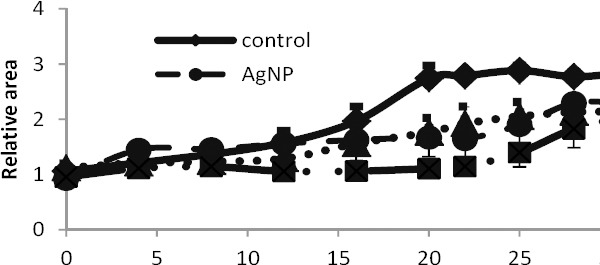
Variations of the relative surface area of the lesions in mice in different groups during consecutive days; the data has been presented as mean±SEM. The number of mice in each group was 10
In the group receiving the UVB alone (Group 3), no increase in lesion size was observed until the final treatment session (day 12); While on day 22 the relative lesion area, in the group receiving UVB (Group 3) was nearly doubled.
At the end of the designed treatments and 10 days later (days 12 and 22, respectively), variations of the relative lesion area, in the group receiving both AgNPs and UVB (Group 4) were not significant.
The relative lesion area, in all treatment groups was enlarged during the days 22 to 32. The least increase was recorded in the group 4 in comparison with the control group.
It is worth noting that in all treatment and control groups, the lesion area showed no significant increase within the first 10 days during treatments.
In a general comparison we can say, there are significant differences between all treatment groups compared to the control group (P<0.001). No significant difference in the lesions’ size was observed between the treatment groups receiving AgNPs or UVB rays (Groups 2 and 3). The lesion extent in the group receiving combinational treatment (Group4) showed significant differences in comparison with Groups 2 and 3 (P <0.029).
As shown in Table 1, the number of parasites in the spleen, in the control group and the receiving groups of AgNPs and UVB with AgNPs showed significant differences (P<0.001), whereas no meaningful difference was observed between the groups of UVB radiation alone, or AgNPs alone in comparison with combinational treatment.
Table 1.
The number of Leishmania major parasites in the spleen of BALB/c mice in different groups; 40 days after the first treatment. Assume 10 mice in each group
| Experimental groups | Number of parasites in 1 ml spleen cells (mean±SEM) |
|---|---|
| control | 26,600,000+2,500,000 |
| AgNPs | 1.73+ 1.69 |
| UVB | 12,060+ 11,780 |
| UVB+AgNPs | 46+ 7.9 |
AgNps: silver nanoparticles; UVB: ultraviolet B; UVB+ AgNps: ultraviolet B+ silver nanoparticles
We also had some qualitative observations. As shown in Figure 5, the scaling of the lesions began in the third and fourth treatment sessions in the groups treated with UVB radiation alone and UVB radiation in combination with AgNPs, which could be a sign of healing.
Figure 5.
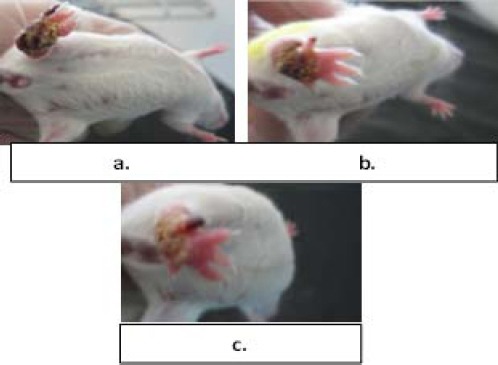
Three images taken from the ulcers on the mice’s footpad belonged to various treatment groups: a. treated by AgNPs, b. irradiated by UVB, c. UVB irradiated with AgNPs
These observations were associated with the pale pink discoloration caused by UVB which returned to the initial state after 48 hr, whereas in the control group the mice lost the leg from day 12.
Discussion
The key point of this study is the application of UVB radiation with a lower dose than MED (20), which is safer in human models and also has a lower radiation period (24). The use of AgNPs, besides providing sites for targeting and increasing cellular uptake, reduces the required dose of AgNPs as well as the side effects of silver toxicity. In addition, the likelihood of implementing the topical treatment should not be ignored.
Torabi et al studied the effect of gold nanoparticles in treating CL induced by L. major at two doses in 2011. Their findings showed a significant decrease in the number of lesion’s amastigotes in comparison with the control group at two concentrations of gold nanoparticles. But no significant difference was recorded between the animal groups receiving the two doses (8).
An advantage of the simultaneous application of AgNPs and UVB radiation in treating cutaneous lesions is that AgNPs can enforce antiparasitic effects (through ROS), and the effect of UV radiation on AgNPs gradually releases Ag+ ions, which give rise to silver-cysteine?? complexes by affecting the cysteine groups in the structure of enzymes and proteins. The application of UV photons on these complexes generates monosulfide radicals, resulting in the eventual death of the organism (15).
The advantage of AgNPs over colloidal solutions of silver is that the latter has plenty of silver ions, which are diluted by blood in the wounded site and uselessly distributed in all body organs. In contrast, AgNPs can remain in the wound site for a longer period of time, gradually releasing 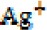 ions and inducing toxicity to the parasites. In the study by Darroudi et al, it was shown that the size of nanoparticles decreases by applying UV radiation on AgNPs (25), enhancing its toxic effects. The production mechanism of silver ions during UV radiation is presented in the following equations:
ions and inducing toxicity to the parasites. In the study by Darroudi et al, it was shown that the size of nanoparticles decreases by applying UV radiation on AgNPs (25), enhancing its toxic effects. The production mechanism of silver ions during UV radiation is presented in the following equations:
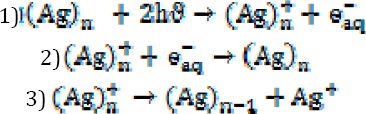
Application of UV photons on 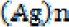 nanoparticles releases an aqueous electron
nanoparticles releases an aqueous electron 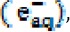 , and an ion is generated from
, and an ion is generated from 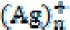 silver nanoparticles. This ion may combine with an aqueous electron
silver nanoparticles. This ion may combine with an aqueous electron 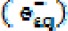 and revert to its original status or may convert to an
and revert to its original status or may convert to an 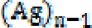 nanoparticle and produce an
nanoparticle and produce an 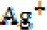 ion (5).
ion (5).
Evidence suggests that UV radiation shows both beneficial and harmful effects, depending on the organism type, the UV region (UVA, UVB, UVC) and light dose (intensity × time duration) (26).
In the present study on an animal model for the treatment of CL (L. major), the beneficial effects of UV light at wavelengths of 280 to 320 nm (UVB), at a cumulative dose of 150 mJ/cm2 was confirmed. In a study by N Khaskhely in 2002, low-dose UVB was applied to mice before infection by L. amazonensis. In this study, the theory that UVB irradiation invariably leads to suppression of the immune system was rejected, and low dose UVB was reported to be useful for L. amazonenis infections (15).
In the study by Allahverdiyev et al in 2011, the anti-leishmanial effect of nanosilver in the presence of UVB was evaluated on promastigotes and amastigotes of L. tropica. The results indicated enhanced anti-leishmanial effects by nanosilver, so that after UVB irradiation on L. tropica promastigotes and amastigotes, the proliferation and metabolic activity of the parasite was reduced 1.5 to 3 times and 2 to 6.5 times, respectively (14).
The findings of this research indicate that synergism of UVB radiation with a cumulative dose of 150 mJ/cm2 with AgNPs at a concentration of 2 mg/kg (in each treatment round) is capable of inhibiting the extension of the lesion and controlling disease visceralization in groups receiving AgNPs, UVB radiation or the combined treatment. The following points should be mentioned with respect to the possible mechanisms of action of AgNPs and UVB radiation:
AgNPs with a small size, vast surface area and the capability to attach to sulfur and phosphorus groups have favorable anti-leishmanial effects, providing a high capacity for ROS production, to which the Leishmania parasite seems to be vulnerable.
Once again the hypothesis of immune suppression by UV radiation is questioned, and UVB radiation at low doses is reported to be useful for L. major infections.
Toxic[s19] effects of AgNPs increases when irradiated by UV photons via releasing silver ions. On the other hand, in addition to UVB, part of the light output of the lamp used in our study, was in the visible region of the light spectrum. Therefore, we suggest that the treatment effects of narrow band UVB and blue light in the presence of AgNPs be studied on CL lesions in animal models to further distinguish the effect of every part of the light spectrum.
Limitations of this study
To determine the treatment’s efficacy, there were the other more validated procedures, i.e. Stauber’s method and quantitative Real-time PCR. In Stauber’s method, parasitological diagnosis in tissue smears (spleen and liver) is performed on the microscopic slides after fixation and staining by Giemsa are examined under optical microscopy to identify Leishmania amastigote forms, and the results are expressed as LDU index values. These values correspond to the number of amastigotes per 1000 nucleated cells multiplied by the organ weight. The LDU index has the disadvantage of low sensitivity for detecting amastigotes by optical microscopy. Moreover, reproducibility in detecting amastigotes requires a trained technician. Finally, a further disadvantage is that the technique relies on the dispersion of the parasites in the imprints stained with Giemsa, which are distributed in the sample in a nonuniform way. Also, a long period after infection (6 and 9 months) is needed prior to being able to visualize and quantify the amastigotes by optical microscopy in the spleen and liver. The most valuable approach is quantitative Real-time PCR (27), but it is a relatively expensive method and we lacked the funds to execute it.
Conclusion
The presence of AgNPs in low concentration (which is not toxic for the liver and spleen) beside UVB irradiation in a low cumulative dosage is quite effective in treating the L. major induced skin lesions, both by preventing the visceral course of the disease and reducing the parasite load in the spleen.
Acknowledgment
The authors would also like to thank Ms Soudmand for her help in the preparation of animals and Dr Ali Badiee who kindly cooperated in this research. This study was financially supported by Research Deputy of Mashhad University of Medical Sciences, Mashhad, Iran. The results reported in this paper are a part of an MSc student thesis.
References
- 1.Igbineweka O, Aghedo F, Idusuyi O, Hussain N. Evaluating the efficacy of topical silver nitrate and intramuscular antimonial drugs in the treatment of cutaneous leishmaniasis in sokoto, Nigeria. Afr J Clin Exp Microbiol. 2012;13:90–97. [Google Scholar]
- 2.Sazgarnia A, Zabolinejad N, Layegh P, Rajabi O, Berenji F, Javidi Z, et al. Antileishmanial activity of liposomal clarithromycin against Leishmania major Promastigotes. Iran J Basic Med Sci. 2012;15:1210–1214. [PMC free article] [PubMed] [Google Scholar]
- 3.Sazgarnia A, Taheri AR, Soudmand S, Parizi AJ, Rajabi O, Darbandi MS. Antiparasitic effects of gold nanoparticles with microwave radiation on promastigots and amastigotes of Leishmania major. Int J Hyperthermia. 2013;29:79–86. doi: 10.3109/02656736.2012.758875. [DOI] [PubMed] [Google Scholar]
- 4.Kvítek L, Panacek A, Prucek R, Soukupova J, Vanickova M, Kolar M, et al., editors. IOP Publishing; 2011. Antibacterial activity and toxicity of silver–nanosilver versus ionic silver. [Google Scholar]
- 5.Darroudi M, Ahmad MB, Shameli K, Abdullah AH, Ibrahim NA. Synthesis and characterization of UV-irradiated silver/montmorillonite nanocomposites. Solid State Sci. 2009;11:1621–1624. [Google Scholar]
- 6.Kheybari S, Samadi N, Hosseini SV, Fazeli A, Fazeli MR. Synthesis and antimicrobial effects of silver nanoparticles produced by chemical reduction method. DARU. 2010;18:168–172. [PMC free article] [PubMed] [Google Scholar]
- 7.Khosravi A, Sharifi I, Barati M, Zarean M, Hakimi-Parizi M. Anti-leishmanial effect of nanosilver solutions on Leishmania tropica promastigotes by in-vitro assay. ZJRMS. 2011;13:8–12. [Google Scholar]
- 8.Torabi T, Mohebali M, Shahverdi AR, Rezayat SM, Edrissian GH, Esmaeili J, et al. Nanogold for the treatment of zoonotic cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER):An animal trial with methanol extract of Eucalyptus camaldulensis. JPHS. 2011;1:15–18. [Google Scholar]
- 9.Ghorbanzadeh V, Moshtaghian SJ, Habibian S, Ebadi AG. Influence of nano-silver on graffian follicles via intraperitoneal injection in rats. Middle East J Sci Res. 2011;8:228–230. [Google Scholar]
- 10.Pastila R. Effects of ultraviolet radiation on skin cell proteome. Ad Exp Med Biol. 2013;990:121–127. doi: 10.1007/978-94-007-5896-4_9. [DOI] [PubMed] [Google Scholar]
- 11.El-Zawahry BM, Bassiouny DA, Sobhi RM, Abdel-Aziz E, Zaki NS, Habib DF, et al. A comparative study on efficacy of UVA1 vs. narrow-band UVB phototherapy in the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2012;28:84–90. doi: 10.1111/j.1600-0781.2011.00643.x. [DOI] [PubMed] [Google Scholar]
- 12.Evers AW, Kleinpenning MM, Smits T, Boezeman J, van de Kerkhof PC, Kraaimaat FW, et al. Treatment nonadherence and long-term effects of narrowband UV-B therapy in patients with psoriasis. Arch Dermatol. 2010;146:198–199. doi: 10.1001/archdermatol.2009.382. [DOI] [PubMed] [Google Scholar]
- 13.Giannini M. Suppression of pathogenesis in cutaneous leishmaniasis by UV irradiation. Infect Immun. 1986;51:838–843. doi: 10.1128/iai.51.3.838-843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, et al. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int J Nanomed. 2011;6:2705–2714. doi: 10.2147/IJN.S23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaskhely NM, Maruno M, Uezato H, Takamiyagi A, Ramzi ST, Al-Kasem KM, et al. Low-dose UVB contributes to host resistance against Leishmania amazonensis infection in mice through induction of gamma interferon and tumor necrosis factor alpha cytokines. Clin Diagnost Lab Immun. 2002;9:677–686. doi: 10.1128/CDLI.9.3.677-686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannini SH. Effects of ultraviolet B irradiation on cutaneous leishmaniasis. Parasitol Today. 1992;2:44–48. doi: 10.1016/0169-4758(92)90083-e. [DOI] [PubMed] [Google Scholar]
- 17.Yadegari-Dehkordi S, Sadeghi HR, Attaran-Kakhki N, Shokouhi M, Sazgarnia A. Silver nanoparticles increase cytotoxicity induced by intermediate frequency low voltages. Electromagn Biol Med. 2014;5:1–5. doi: 10.3109/15368378.2014.919590. [DOI] [PubMed] [Google Scholar]
- 18.Mohebali M, Rezayat MM, Gilani K, Sarkar S, Akhoundi B, Esmaeili J, et al. Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study. DARU. 2009;4:285–289. [Google Scholar]
- 19.Ehrchen J, Sindrilaru A, Grabbe S, Schlesiger C, Sorg C, Scharffetter-Kochanek K, et al. Senescent BALB/c mice are able to develop resistance to Leishmania major infection. Infect Immun. 2004;72:5106–5114. doi: 10.1128/IAI.72.9.5106-5114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honigsmann HST, Bolognia J, Jorizzo JL, Rapini RP. New York: Mosby; 2012. Ultraviolet Therapy In Dermatology. [Google Scholar]
- 21.Zanolli MD FS, Clark AR, Fleischer AB., Jr . Parthenon Publishing; 2000. Phototherapy Treatment Protocols:For Psoriasis and Other Phototherapy Responsive Dermatoses. [Google Scholar]
- 22.Xue Y, Zhang S, Huang Y, Zhang T, Liu X, Hu Y, et al. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J Appl Toxicol. 2012;32:890–899. doi: 10.1002/jat.2742. [DOI] [PubMed] [Google Scholar]
- 23.Buffet P, Sulahian A, Garin Y, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob Agents Chemother. 1995;39:2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damian DL, Barnetson RS, Halliday GM. Effects of low-dose ultraviolet radiation on in vivo human cutaneous recall responses. Austr J Dermatol. 2001;42:161–167. doi: 10.1046/j.1440-0960.2001.00507.x. [DOI] [PubMed] [Google Scholar]
- 25.Darroudi M, Ahmad MB, Zak AK, Zamiri R, Hakimi M. Fabrication and characterization of gelatin stabilized silver nanoparticles under UV-light. Int J Mol Sci. 2011;12:6346–6356. doi: 10.3390/ijms12096346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hockberger PE. A history of ultraviolet photobiology for humans, animals and microorganisms. Photochem Photobiol. 2002;76:561–579. doi: 10.1562/0031-8655(2002)0760561AHOUPF2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 27.Moreira Nd, Vitoriano-Souza J, Roatt BM, Vieira PM, Ker HG, de Oliveira Cardoso JM, et al. Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: “Leishman Donovan Units” versus real-time PCR. PLoS One. 2012;7:2012. doi: 10.1371/journal.pone.0047907. [DOI] [PMC free article] [PubMed] [Google Scholar]


