Abstract
Objective(s):
Valproic acid (VPA), a drug used in the treatment of neurological disorders, has been shown to have cytotoxic effects on cancer cells through different mechanisms. Telomerase, a ribonucleoprotein reverse transcriptase, is responsible for elongation of the telomere and is activated in cancers. A relation between telomerase activity and resistance to apoptosis has been established. This study focused on probable effects of VPA on MCF-7 cancer cells. In particular, we investigated VPA effects on viability, apoptosis and telomerase activity.
Materials and Methods:
Cytotoxicity effects of VPA on MCF-7 cells were determined by neutral red uptake assay. Cells were treated with different concentrations of VPA (0-32 mM) and telomerase activity and Bax and Bcl-2 protein levels were determined using TRAP assay (PCR-ELISA) method and ELISA method, respectively.
Results:
The cytotoxic effects of different concentration of VPA on MCF-7 cells were observed as a reduction in cell viability and telomerase activity and altered expression of Bcl-2 family protein levels. The results also showed that there is a significant correlation between reduction of telomerase activity and increase in Bax/Bcl-2 ratio (P=0.001).
Conclusion:
Our study demonstrated that cell viability of MCF-7 cells was decreased after treatment with VPA, probably through a reduction of telomerase activity and an increase in Bax/bcl-2 ratio. Therefore, it could be concluded that VPA is a potent anti-cancer agent for breast cancer cells through inhibition of telomerase activity and induction of apoptosis.
Keywords: Bax, Bcl-2, MCF-7, Telomerase, Valproic acid
Introduction
Breast cancer is the most common malignancy and the leading cause of death in women, worldwide. It is gradually increasing and brings economic burden to patients (1).
Valproic acid (VPA, 2- pentatonic propyl acid), a drug used in the treatment of neurological disorders, has anti-cancer effects through inhibition of histone deacetylase enzymes (HDACi) (2). VPA inhibits both class I and class II of histon deacetylase enzymes via hyperacetylation of histon H3 and H4. HDACi, through modifying chromatin structure and making dynamic changes in the nucleosomal packaging of DNA, play an important role in regulation of gene expression (3). Studies have shown that inhibitors of histone deacetylase exert their effects via inhibition of cell growth, induction of differentiation and stimulation of apoptosis (3). The exact mechanisms via which HDACi reduce tumor growth are still unclear, but a factor that is suppressed by these inhibitors is the telomerase enzyme (4).
Telomerase enzyme is activated in more than 90% of cancers including breast cancer whereas it has negative or very weak activity in the most somatic cells (5). Telomerase, a cellular reverse transcriptase, protects the ends of chromosomes (telomeres). Telomeres are specialized structures at the ends of eukaryotic chromosomes that normally shorten during each cell division and eventually cause aging and cell death. The loss of telomere length is inhibited by telomerase in cancer cells (6). Telomerase is a ribonucleoprotein complex with a catalytic subunit (hTERT) and an RNA component (hTR) as a template to add telomeric repeats (TTAGGG) to the 3’ ends of chromosomes (7). Studies show that the level of hTERT expression in cells was associated with the degree of telomerase activity, whereas hTR gene expression in cells is independent of the activity of this enzyme (8). Scientists seek for anti-cancer agents that specifically target cancer cells and have fewer side effects on normal cells in this regard, telomerase now and over the last decade has
been considered by many studies (9). Also, targeting the process of apoptosis, the programmed cell death, is an appropriate strategy for prevention and treatment of cancer. Due to the fact that many cancer cells can inhibit apoptosis in various ways, inducing programmed cell death is an important mechanism for cancer treatment (10). Many studies have shown that inhibition of telomerase can affect cell viability by inducing apoptosis without having long periods of treatment (11).
Thus, according to studies on telomerase activity in cancer cells and its effect on the proliferation and growth of cancer cells, this study examined the effects of VPA on telomerase activity and two factors affecting apoptosis: (1) Bax and (2) Bcl-2, in MCF-7 breast cancer cell line.
Materials and Methods
Cell culture and VPA treatment
Human breast adenocarcinoma Cell line (MCF-7) (Pasteur Institute, Tehran, Iran) was maintained at 37 °C in a humidified condition of 95% air and 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin(Gibco, Germany).
VPA (Sigma, Germnay) was dissolved in PBS to prepare a stock solution of 100 mM and stored at -20 °C.
Determination of cell growth and viability
The sensitivity of MCF-7 cells to VPA was evaluated in vitro using neutral red assay. It is based on the ability of viable cells to incorporate the supravital dye neutral red in the lysosomes. For this assay, 5×104 cells/ml were seeded in triplicate wells of a 24-well microtiter plate and cultured overnight prior to treatment. Cells were exposed to various concentrations of VPA (0-32 mM) for 24, 48, 72 hr. Untreated samples were considered as control cells. The extent of viability was determined by the neutral red assay. The absorbance of the samples was measured using a spectrophotometer at a wavelength of 450 nm. Cell viability was calculated as (mean OD of treated cells/mean OD of control or untreated cells) × 100. Lethal concentrations (LC50) of VPA were obtained from concentration-response curves. One million (1×106) cells were grown after determination of LC50 values in 25 cm2 flasks. After 24 hr of incubation, cells were treated with various concentrations of VPA (0, 2, 8 and 16 mM) for 24, 48 and 72 hr and this treatment was repeated for three times; Then, cells were trypsinized and washed with phosphate-buffered saline (PBS). Next, the viable cells were scored with a hemocytometer based on the exclusion of trypan blue.
Measurement of telomerase activity
Based on the protocol of Kim et al, quantitative measurement of telomerase activity was conducted using polymerase chain reaction-based telomeric repeat amplification protocol (TRAP Assay) (Telo TAGGG telomerase PCR ELISAPLUS; Roche, Germany) (12).
The TRAP assay is a two-step process in which the telomerase-mediated elongation products are subsequently amplified by PCR to allow highly sensitive detection of telomerase activity. According to the protocol, 5 µg of total protein of each sample as measured by the Bradford method (Bio-Rad Protein Assay, USA), was added to the reaction mixture. The PCR reaction for 30 cycles (25 °C for 20 min, 94 °C for 5 min, 94 °C for 30 sec, 50 °C for 30 sec, 72 °C for 90 sec, 72 °C for 10 min) was performed using Thermal cycler. Products of PCR (2.5 μl) were used for the ELISA reaction and the absorbance of each sample was read at 450 nm using ELISA reader device.
Measurement of Bax and Bcl-2 levels
Bax and Bcl-2 protein levels were measured using ELISA kits (Biospes, China), according to the manufacturer protocol as duplicate. Briefly, treated and untreated cells were lysed according to the ELISA kit instruction. Bax and Bcl-2 protein from the cell lysate specifically bound to the primary antibody and were detected by Horseradish peroxidase (HRP)-conjugated secondary antibody. Then, Bax and Bcl-2 protein levels were determined by measuring the absorbance at 450 nm using a microplate reader.
Statistical analysis
The normality of data distribution was measured using Kolmogorov -Smirnov test. All data were analyzed by One-way Analysis of variance (ANOVA), followed by post-hoc Tukey’s test. The relationship between telomerase activity and the ratio of Bax/Bcl-2 was measured by Pearson correlation test. A P-value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 16.
Results
Cytotoxic effects of VPA on MCF-7 cells
To determine the effects of VPA on MCF-7, cells were exposed to various concentrations of VPA (0-32 mM) for 24, 48 and 72 hr. Assessment of cell viability by neutral red test showed that with increasing dose and treatment time, an increase in cytotoxic effects was observed and the concentrations of 8, 16 and 32 mM had the greatest effects compared with control (P<0.05). Also, LC50 value for VPA in MCF-7 cells after 48 and 72 hr were about 12 and 7 mM, respectively (Figure 1).
Figure 1.
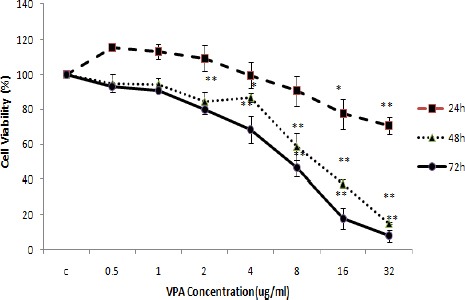
Valproic acid (VPA) cytotoxic effects (means±SD) on MCF-7 breast cancer cells. One-way ANOVA followed by post-hoc Tucky’s test was used to compare the mean Cell Viability (%) among different concentrations of VPA at 24, 48 and 72 hr (*P<0.05 and **P<0.005 compared to the control for 24, 48 and 72 hr, respectively)
After determination of LC50 values, cells were treated with selected concentrations of VPA (2, 8 and 16 mM).
The effect of VPA on telomerase activity
Telomerase activity was investigated at the concentrations of 0, 2, 8 and 16 mM at 24, 48 and 72 hr (Figure 2). Telomerase activity was reduced by increasing concentrations of VPA. Maximum reduction of telomerase activity was obtained at 16 mM VPA at 48 hr. Thetelomerase activity was not significantly different between 48 and 72 hr, while there was significant differences between 24 and 48 hr as well as 24 and 72 hr at concentrations 0,8 and 16 mM.
Figure 2.
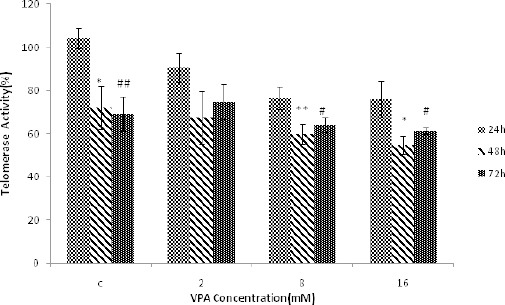
The effect of different concentrations of Valproic acid (VPA) (means ± SD) at 24, 48 and 72 hr on telomerase activity. The level of telomerase activity significantly decreased at 48 hr (*P<0.05 and **P<0.005) and 72 hr (#P<0.05 and ##P<0.005) compared to 24 hr treatment. Telomerase activity at 48 hr was not significantly different from that of 72 hr treatment
The effect of VPA on the expression of Bax and Bcl-2 and Bax / Bcl-2 ratio
Bax, Bcl-2 and Bax / Bcl-2 were measured following 24, 48 and 72 hr treatment with different concentra- tions of VPA (0, 2, 8, 16 mM). Bax protein levels were augmented by increasing concentrations of VPA, and maximum protein concentration was obtained at 16 mM after 48 hr of treatment (Figure 3). Bcl-2 protein levels decreased with increasing concentrations of VPA and the lowest value was obtained at 16 mM following 72 hr treatment (Figure 4). Bax / Bcl-2 Ratio was also increased with increasing concentrations of valproic acid and showed the highest value at 16 mM in 48 hr (Figure 5).
Figure 3.
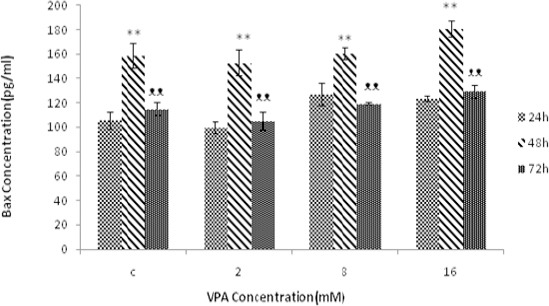
The effect of different concentrations of VPA (means ± SD) at 24, 48 and 72 hr on the amount of Bax protein. The amount of Bax protein increased significantly in 48 hr (*P-value<0.05 and **P-value<0.005) compared to 24 hr. The amount of Bax protein is decreased in 72 hr (ᴥP-value<0.05 and ᴥᴥP-value<0.005) compared to 48 hr. The Bax protein level was not significant between 24 hr and 72 hr
Figure 4.
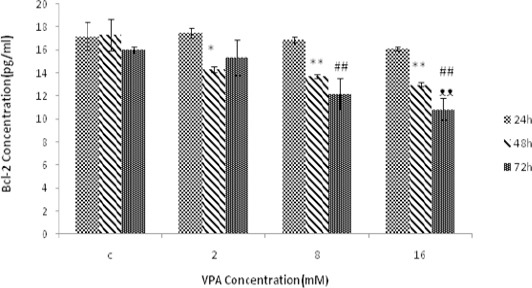
The effect of different concentrations of Valproic acid (VPA) (means ± SD) treatment for 24, 48 and72 hr on Bcl-2 expression. The amount of Bcl-2 protein significantly decreased after 48 hr (*P<0.05 and **P<0.005) and 72 hr (#P<0.05 and ##P<0.005) compared to 24 hr. The amount of Bcl-2 protein is also decreased after 72 hr (ᴥP<0.05 and ᴥᴥP<0.005) compared to 48 hr
Figure 5.
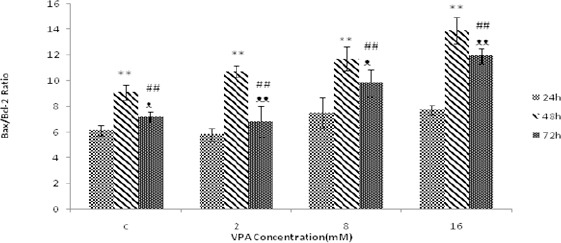
The effect of different concentrations of Valproic acid (VPA) (means ± SD) treatment for 24, 48 and 72 hr on Bax/Bcl-2 ratio. Bax/Bcl-2 ratio increased significantly after 48 hr (*P<0.05 and **P<0.005) and 72 hr (#P-value<0.05 and ##P<0.005) compared to 24 hr. Bax/Bcl-2 ratio is also increased after 72 hr (ᴥP<0.05 and ᴥᴥP<0.005) compared to 48 hr
Figure 6.
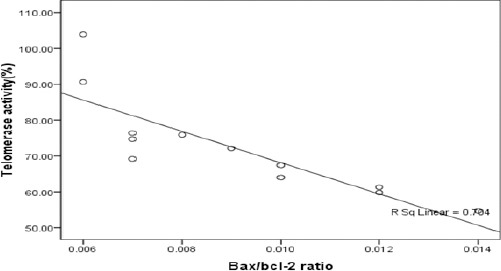
Correlation between telomerase activity and Bax/Bcl-2 ratio (P=0.001)
The relationship between telomerase activity and Bax/Bcl-2 ratio
The results also reported that there was a significant correlation between reduction in telomerase activity and increasing in Bax/Bcl-2 ratio (P=0.001).
Discussion
In this study, we demonstrated the cytotoxic effects of VPA on MCF-7 cells, including reduction in cell viability, telomerase activity and altered expression of Bcl-2 family protein levels.
This study showed that VPA has cytotoxic effects on MCF-7 breast cancer cell lines in a dose and time-dependent manner and causes a significant decrease in cell survival. These results are consistent with previous studies showing that treatment with VPA resulted in a significant reduction in the rate of cell proliferation (2, 13). VPA is considered as an inhibitor of histone deacetylase with strong anti-proliferative activity in various cancer cells through multiple mechanisms including cell cycle arrest, differentiation and apoptosis (14).
The present study also described that with increasing concentrations of VPA, telomerase activity was declined and maximum reduction of telomerase activity in MCF-7 cells was obtained after 48 hr of treatment. Consistent with these results, numerous studies have shown that some inhibitors of histone deacetylase reduced telomerase activity through suppression of hTERT mRNA levels (15-17). In fact, HDACi-induced hyperacetylation facilitates binding of some suppressor to hTERT gene control region, resulting in inhibition of telomerase activity (4, 18).
In our study, it was also found that VPA resulted in an increase in Bax and reduction of Bcl-2 protein levels in MCF-7 cells. In addition, Bax/Bcl-2 ratio increased dose-dependently and the maximum value was observed at 16 mM after 48 hr treatment. Several investigations showed that Bax/Bcl-2 ratio could be considered as an apoptotic index and the balance between Bax and Bcl-2 expression affects the activation of mitochondrial apoptosis pathways (19); Thus, an increase in Bax/Bcl-2 ratio which was observed in this study may partly reflect the activation of mitochondrial apoptosis pathway in MCF-7 cells after treatment with VPA. In accordance with our results, previous studies showed that HDACi can induce apoptosis through repression of apoptotic and regulation of anti-apoptotic genes (20).
Although following VPA treatment a significant correlation was observed between reduction of telomerase activity and increase in Bax/Bcl-2 ratio, but it is still not clear whether there is any association between changes in Bcl-2 family proteins and telomerase activity induced by HDACi; However, some reports showed that inhibition of hTERT and telomerase activity may facilitate the induction of mitochondrial apoptosis (21).
Conclusion
Our results showed that VPA causes a decrease in cell viability and induces apoptosis by reducing telomerase activity and increasing Bax/Bcl-2 ratio. Our study illustrated that loss of telomerase activity may be a useful biomarker to evaluate the antitumor activity of VPA in breast cancer cells but still further studies are needed. The significant correlation that was observed between decreased telomerase activity and increased Bax/Bcl-2 ratio may provide a new perspective about the molecular mechanisms involved in cancer cells.
Acknowledgment
This work was supported by a grant (Grant No. 92021002) from Golestan University of Medical Science, Gorgan, Iran.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA-Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Xia Q, Sung J, Chowdhury W, Chen C-l, Höti N, Shabbeer S, et al. Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res. 2006;66:7237–7244. doi: 10.1158/0008-5472.CAN-05-0487. [DOI] [PubMed] [Google Scholar]
- 3.Ververis K, Hiong A, Karagiannis TC, Licciardi PV. Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics. 2013;7:47–60. doi: 10.2147/BTT.S29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman R, Osteso-Ibanez T, Hirst RA, Levesley J, Kilday J-P, Quinn S, et al. Histone deacetylase inhibition attenuates cell growth with associated telomerase inhibition in high-grade childhood brain tumor cells. Mol Cancer Ther. 2010;9:2568–2581. doi: 10.1158/1535-7163.MCT-10-0272. [DOI] [PubMed] [Google Scholar]
- 5.Rao Y, Xiong W, Liu H, Jia C, Zhang H, Cui Z, et al. Inhibition of telomerase activity by dominant-negative hTERT retards the growth of breast cancer cells. Breast Cancer. 2014:1–8. doi: 10.1007/s12282-014-0553-z. [DOI] [PubMed] [Google Scholar]
- 6.Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discovery. 2006;5:577–584. doi: 10.1038/nrd2081. [DOI] [PubMed] [Google Scholar]
- 7.Wai LK. Telomeres, telomerase, and tumorigenesis--a review. Med Gen Med. 2004;6:19. [PMC free article] [PubMed] [Google Scholar]
- 8.Massard C, Zermati Y, Pauleau A, Larochette N, Metivier D, Sabatier L, et al. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene. 2006;25:4505–4514. doi: 10.1038/sj.onc.1209487. [DOI] [PubMed] [Google Scholar]
- 9.Buseman C, Wright W, Shay J. Is telomerase a viable target in cancer? Muta Res. 2012;730:90–97. doi: 10.1016/j.mrfmmm.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woo HJ, Lee SJ, Choi BT, Park Y-M, Choi YH. Induction of apoptosis and inhibition of telomerase activity by trichostatin A, a histone deacetylase inhibitor, in human leukemic U937 cells. Expmolpathol. 2007;82:77–84. doi: 10.1016/j.yexmp.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Rubis B, Holysz H, Gladych M, Toton E, Paszel A, Lisiak N, et al. Telomerase downregulation induces proapoptotic genes expression and initializes breast cancer cells apoptosis followed by DNA fragmentation in a cell type dependent manner. Mol Biol Rep. 2013;40:4995–5004. doi: 10.1007/s11033-013-2600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PdL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 13.Byun S-S, Kim FJ, Khandrika L, Kumar B, Koul S, Wilson S, et al. Differential effects of valproic acid on growth, proliferation and metastasis in HTB5 and HTB9 bladder cancer cell lines. Cancer let. 2009;281:196–202. doi: 10.1016/j.canlet.2009.02.045. [DOI] [PubMed] [Google Scholar]
- 14.Fortunati N, Bertino S, Costantino L, Bosco O, Vercellinatto I, Catalano MG, et al. Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Lett. 2008;259:156–164. doi: 10.1016/j.canlet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Yin S, Feng S, Nie D, Xie S, Ma L, et al. Effect of valproic acid on apoptosis of leukemia HL-60 cells and expression of h-tert gene. Zhongguoshiyanxue ye Xuezazhi. 2010;18:1445–1450. [PubMed] [Google Scholar]
- 16.Khaw AK, Silasudjana M, Banerjee B, Suzuki M, Baskar R, Hande MP. Inhibition of telomerase activity and human telomerase reverse transcriptase gene expression by histone deacetylase inhibitor in human brain cancer cells. Mutat Res. 2007;625:134–144. doi: 10.1016/j.mrfmmm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Wu P, Meng L, Wang H, Zhou J, Xu G, Wang S, et al. Role of hTERT in apoptosis of cervical cancer induced by histone deacetylase inhibitor. Biochem Biophys Res Commun. 2005;335:36–44. doi: 10.1016/j.bbrc.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 18.Meeran SM, Patel SN, Tollefsbol TO. Sulforaphane causes epigenetic repression of hTERT expression in human breast cancer cell lines. PloS One. 2010;5:e11457. doi: 10.1371/journal.pone.0011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 2007;19:488–496. doi: 10.1016/j.coi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujimoto Y, Shimizu S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis. 2007;12:835–840. doi: 10.1007/s10495-006-0525-7. [DOI] [PubMed] [Google Scholar]
- 21.Bokelmann I, Mahlknecht U. Valproic acid sensitizes chronic lymphocytic leukemia cells to apoptosis and restores the balance between pro-and antiapoptotic proteins. Mol Med. 2008;14:20–27. doi: 10.2119/2007-00084.Bokelmann. [DOI] [PMC free article] [PubMed] [Google Scholar]


