Abstract
Objective(s):
Based on the previous reports, silymarin can suppress nitric oxide, prostaglandin E2 (PGE2), leukotrienes, cytokines production, and neutrophils infiltration. Regarding the fact that inflammation plays an important role in neuropathic and formalin-induced pain, it was assumed that silymarin could reduce pain. The present study investigates the analgesic effects of silymarin in chemical nociception and a model of neuropathic pain.
Materials and Methods:
Chemical nociception was produced by injection of 20 µl of formalin (0.5% formaldehyde in saline) into the plantar region of the right hind paw. A sciatic-nerve ligated mouse was applied as the model of neuropathic pain and the antinociceptive response of silymarin was examined 14 days after unilateral nerve-ligation using the hot plate test.
Results:
The intraperitoneal administration of silymarin (25, 50, and, 100 mg/kg) 2 hr prior to the intraplantar formalin injection suppressed the nociceptive response during the late phase of the formalin test significantly, but it was not in a dose-dependent manner. Different doses of silymarin 14 days after unilateral sciatic nerve ligation in hot plate test did not induce obvious antinociception.
Conclusion:
Results of the present study indicated that repeated administration of silymarin prevents the formalin-induced nociceptive behavior. However, it is not effective in the treatment of sciatic neuropathic pain.
Keywords: Silymarin, Formalin test, Sciatic nerve ligation, Inflammatory pain
Introduction
Pain and hyperalgesia, produced by the tissue damages or infections, are common features of the inflammatory process. Inflammation stimulates peripheral nerve fibers and changes local blood flow and vascular permeability (1). In addition, immune cells, activated during the inflammation, release pro-algesic mediators like tumor necrosis factor-α (TNF-α), interleukins (IL-6, IL-8, IL-1β), protons, nerve growth factor, and prostaglandins which induce inflammatory and neuropathic pain (2, 3). Due to the adverse effects of available synthetic medications in the long term treatment of painful conditions and inflammation, many studies have tested different plant extracts and their active compounds for antinociceptive and anti-inflammatory activities (4, 5).
Silymarin is the active complex extract of seeds and fruits of the milk thistle (Silybum marianum) and contains the flavonolignans silybin, isosilybin, silydianin, and silychristin (6). Silymarin possesses many pharmacological effects including antioxidative, antifibrotic, anti-inflammatory, and immunomodulating activities (7). According to different studies, silymarin produces no toxic effects when used in pharmacological doses (7, 8). Because of these beneficial properties, silymarin is clinically used in treatment of hepatitis, chronic alcoholic liver disease, viral cirrhosis, ischemic injury, and radiation toxicity (9). Silymarin is a free radical scavenger that affects various steps in arachidonic acid cascade via cyclooxygenase and lipoxygenase pathways (10). Besides, silymarin modulates immune system through inhibition of neutrophil immigration and mast cell immobilization (11). It also inhibits TNF-α -induced production of reactive oxygen intermediates and lipid peroxidation, and modulates T-cell function (12, 13). So, the purpose of this study was to investigate the effects of intraperitoneal administration of silymarin on neuropathic and formalin-induced pain in mice.
Materials and Methods
Drugs
Silymarin purchased from Sigma-Aldrich, Germany and was suspended in 0.5% carboxymethyl cellulose solution. Imipramine obtained from Sobhan Pharma Group, Iran and diclofenac sodium from Caspian Tamin Pharmaceutical Co., were dissolved in 0.9% saline. All treatments were injected in a volume of 0.1 ml/10 g intraperitoneally (IP).
Animals
Adult Razi male Albino mice, weighing 25–30 g, were provided by Animal House, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran. Mice were housed in standard plastic cages under 12 hours light/dark cycle, 22±2 °C and 40-50% humidity conditions in the colony room. Animals had free access to food and water before and during the study. All the experiments were performed according to Mashhad University of Medical Sciences, Ethical Committee Acts (900545).
Formalin test
Drug treatment
The experimental procedures used for formalin test are summarized in Table 1.
Table 1.
Experimental groups for the formalin test in mice
| Negative control group | Positive control group | Silymarin (25, 50, 100 mg/kg) (group 4, 5, 6) | |||
|---|---|---|---|---|---|
| Experiment A | 0.5% CMC* solution | Dic** 15 mg/kg |  |
||
| Experiment B | 0.5% CMC solution | Dic 15 mg/kg |  |
||
| Experiment C | 0.5% CMC solution | Dic 15 mg/kg |  |
||
Experiment A
Animals in 2 groups received one IP injection of 0.5% carboxymethyl cellulose solution (negative control group) or diclofenac sodium (15 mg/kg as a positive control group) 60 min before the test. Animals in 3 groups injected with different doses of silymarin (25, 50, and 100 mg/kg) and pain responses were measured after 120 min.
Experiment B
Animals in 2 groups received one IP injection of 0.5% carboxymethyl cellulose solution (negative control group) or diclofenac sodium (positive control group), 60 min before the test. In group 3, 4, and 5 mice received three IP injections of different doses of silymarin (25, 50, and 100 mg/kg). Two injections were on one day before the test (morning and evening) and the third one was on the day of the test. Pain responses were measured 120 min after the last injection of silymarin.
Experiment C
Animals in 2 groups received one IP injection of 0.5% carboxymethyl cellulose solution (negative control group) or diclofenac sodium (positive control group), 60 min before the test. In group 3, 4, and 5 mice received 5 IP injections of different doses of silymarin (25, 50, and 100 mg/kg). Two injections were on 2 days before the test (morning and evening), the next two injections were on one day before the test (morning and evening) and the fifth dose was on the day of the test (14). Pain responses were measured 120 min after the last injection of silymarin.
Test procedure
The antinociceptive effect of silymarin was evaluated using formalin test described by Dubuisson and Dennis (15) with some modification. In this method, 20 µl of formalin (0.5% formaldehyde in saline) was injected into plantar region of right hind paw. Each animal was placed in a transparent plastic cage and the time spent licking and/or biting the injected hind paw during 5 min periods over a 40 min observation time was recorded. The formalin test consists of two distinct periods; an early phase is due to a direct effect on nociceptors lasting the first 10 min (neurogenic pain) and a late phase is due to a direct effect of inflammatory mediators lasting from 30 to 40 min (inflammatory pain) after the injection of formalin (16, 17).
Model of neuropathic pain
Surgical procedure for nerve ligation
Animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg). Unilateral peripheral neuropathy was produced on the right hind limb, according to the method of Seltzer et al (18) with some modification (19). The animal’s right sciatic nerve was exposed, a 2-3 mm long nerve segment was then dissected and the nerve was ligated with a copper wire. Only one ligature with fine metal wire was made around the dissected nerve. In sham-operated animal the nerve was exposed, but not ligated.
Analgesic measurement
Fourteen days after nerve ligation, based on the result of formalin test, animals received 3 IP injections of different doses of silymarin (25, 50, and 100 mg/kg) intraperitoneally. Two injections were one day before the test (morning and evening) and the third was on the day of the test. Pain sensitivity was assessed 120 min after the last injection of silymarin using hot plate as described by Eddy and Liembach (20), with some modification. Briefly, the animal was placed on a circular surface (diameter 19 cm) maintained at 55±0.2 °C, and surrounded by a plexiglass wall (12 cm high). The apparatus (Eghbal Lab, IRAN) was equipped with a timer and a thermocouple to maintain a constant temperature. Licking the forepaws, lifting hind paws, and jumping from the surface were used as the end points for determination of the response latencies (19). Failure to respond by 45 seconds resulted in the termination of the test (cut-off). Animals in negative and positive control groups received IP injection of a similar volume of 0.5% carboxymethyl cellulose (vehicle) solution and imipramine (10 mg/kg) one hour before the test, respectively.
Statistical analysis
Data were analyzed using GraphPad InStat version 3.00 (GraphPad Software, San Diego, California, USA) with One-way Analysis of Variance (ANOVA) followed by Tukey post-hoc test and plotted in GraphPad Prism version 3.00 (GraphPad Software, San Diego California USA). All data presented as mean±Standard deviation (SD). P-values less than 0.05 were considered to be statistically significant.
Results
Effects of silymarin on the nociceptive responses of mice in formalin test
Injection of 0.5% formalin into the hind paw of the mice caused significant early nociceptive response (licking and/or biting) and lasted for about 10 min (phase I). The second phase of the formalin test started 20-30 min after the formalin administration and lasted for about 10 min (Figure 1).
Figure 1.
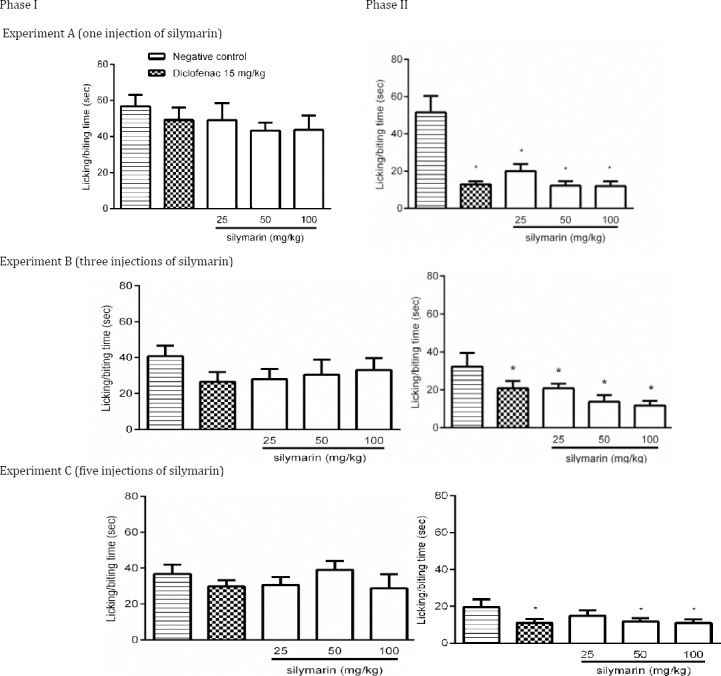
Effect of silymarin on formalin-induced nociceptive behavior during phase I and phase II. Mice were treated with one (A), three (B) or five (C) injections of silymarin at various doses intraperitoneally. Twenty µl of formalin (0.5% formaldehyde in saline) was subcutaneously injected into the plantar region of the right hind paw 120 min after the last silymarin administration. Time of licking and/or biting the injected paw was measured during the period of 0–10 min (1st phase) and 20-40 min (2nd phase). All groups were compared to negative control group (0.5% carboxymethyl cellulose solution) according to ANOVA followed by Tukey post-hoc test. Data are shown as mean±SD. The number of animals in each group was 6 (Positive control: diclofenac)
Phase I
In 3 experiments, after interplanetary injection of formalin, neither diclofenac nor different doses of silymarin produced antinociceptive response as compared with control group (Figure 1A, B, C, phase I).
Phase II
Intraperitoneal administration of different doses of silymarin in three experiments inhibited paw licking completely similar to 15 mg/kg diclofenac in the second phase of formalin test (P<0.05) (Figure 1A, B, C, phase II). However, the difference among various doses of silymarin in three experiments was not significant. In experiment C, 25 mg/kg of silymarin failed to produce antinociceptive response (Figure 1C, phase II).
Effects of silymarin on the nociceptive responses of intact or nerve-ligated mice in the hot plate test
The hyperalgesic response was significantly induced 14 days after nerve ligation. In hot plate test, no difference in latency period was found at three doses of silymarin in nerve-ligated mice compared to the negative control group. The sensitivity of the mice in sham-operated control group and those received imipramine were significantly reduced (P<0.05) (Figure 2).
Figure 2.
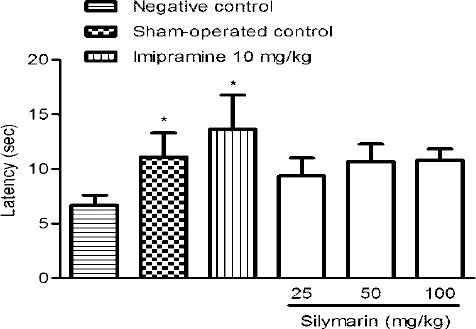
Antinociceptive effect of silymarin in intact and nerve-ligated mice using hot-plate test. Mice received 3 injections of different doses of silymarin intraperitoneally. Two injections were on one day before the test (morning and evening) and one was on the day of the test. All groups were compared to negative control group (0.5% carboxymethyl cellulose solution) according to ANOVA followed by Tukey post-hoc test. Data are shown as mean±SD. The number of animals in each group was 6. *, P<0.05. (Positive control: imipramine)
Discussion
In the present study, silymarin showed antinociceptive effects in phase II of the formaldehyde-induced nociception model. Despite that, silymarin was ineffective against neuropathic pain induced by constriction of the sciatic nerve (nerve-ligation). Many studies have reported that silymarin has antioxidant, anti-inflammatory, antifibrotic, antiproliferative, antiviral, and immunomodulating properties (7). In the model of carrageenan-induced paw edema in rats, silymarin inhibited leukocyte accumulation and significantly reduced the number of neutrophils migrated into the inflamed site (21). Additionally, silymarin showed inhibitory effects on IL-1β and PGE2 production in macrophages and blocked mRNA expression of IL-1β and cyclooxygenase-2 in LPS-stimulated RAW 264.7 cells (12). It was reported that silymarin suppressed the induction of TNF-α in dialysis patients with chronic inflammation and modulated the immune system by inhibition of neutrophil immigration and mast cell immobilization (11). In this study, formalin test, which is a valid, reliable, sensitive, and simple behavioral biphasic model of nociception (16), was used to analyze the mechanism of action of silymarin. The first phase of the test starts a few minutes (0-10 min) after intraplantar injection of formalin and is due to direct stimulation of nociceptors, especially C fibers which results in neurogenic pain (22). So drugs that act primarily on central nervous system, such as opioids, can block the pain in this phase (22, 23). The second phase or inflammatory phase starts 20-30 min after formalin injection and inflammatory mediators such as prostaglandins, bradykinin, histamine, sympathomimetic amines, TNF-α, and ILs are released. The second phase is sensitive to peripherally-acting drugs such as Nonsteroidal Anti-inflammatory Drugs (NSAIDs), and corticosteroids (22, 23). During the formalin test, pretreatment with silymarin significantly reduced the pain in phase II and its effect was not dose-dependent. It seems that pain relieving effect of silymarin is attributed to inhibition of peripherally acting mediators like IL-1β, PGE2, and TNF-α (1-3).
Neuropathic pain, caused by damages to the peripheral and central nerves, comprises a complex combination of negative symptoms such as dysesthesia and paresthesia (24). NSAIDs, opiates, tricyclic antidepressants, serotonin and norepinephrine reuptake inhibitors, and anti-convulsants are used to alleviate neuropathic pain but they have limited efficacy and undesirable side effects and neuropathic pain responses poorly to the drug treatment (25). Based on the increasing evidence, both inflammation and immune cells are involved in induction of neuropathic pain. Activation of mast cells and subsequent secretion of various inflammatory mediators (histamine and TNF-α) are results of peripheral nerve damage. Thereafter, these mediators affect and sensitize nociceptors and contribute to the recruitment of neutrophils and macrophages (26-28). In the present study, 14 days after the unilateral sciatic nerve-ligation, hyperalgesia to thermal stimulation was significantly observed. It was indicated that stimulation of β2-adrenergic receptors (29) and indirect anti-TNFα action (30) in peripheral nervous system are necessary for the mechanism by which antidepressants alleviate neuropathic pain. The results of the current study showed that repeated IP injection of silymarin induced an analgesic effect in formalin test but it was not able to attenuate the thermal hyperalgesia following nerve injury. It seems that silymarin has no β2-adrenergic receptors stimulation effects in peripheral nervous system. In addition, changes in the dose and time of silymarin administration may be involved in its antinociceptive action. Moreover, different receptors for neurotransmitters, responsible in the pain transmission, are not the same in these two models. Therefore, various activity profiles of different classes of analgesic drugs may be seen. For example, clonidine and gabapentin tend to have activity in the second phase of the formalin test and low activity in neuropathic pain. Baclofen, tramadol, and amitriptyline show better results in animal models of neuropathic pain. So, correlation between pharmacological activities in these two models needs further validation (31, 32).
Conclusion
The present study suggests that repeated administration of silymarin significantly prevents the formalin-induced nociceptive behavior, but it is ineffective in reducing the thermal threshold after the sciatic nerve ligation. More studies are needed to elucidate the silymarin function on sciatic neuropathic pain.
Conflict interests
The authors declare that they have no competing interests.
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences and Iran National Science Foundation for financial support. The results described in this paper were part of a Pharm D thesis.
References
- 1.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 2.Rittner H, Machelska H, Stein C. Leukocytes in the regulation of pain and analgesia. J Leukocyte Biol. 2005;78:1215–1222. doi: 10.1189/jlb.0405223. [DOI] [PubMed] [Google Scholar]
- 3.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Iranshahi M, Askari M, Sahebkar A, Adjipavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU. 2009;17:99–103. [Google Scholar]
- 5.Monsef HR, Ghobadi A, Iranshahi M, Abdollahi M. Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test. J Pharm Pharm Sci. 2004;7:65–69. [PubMed] [Google Scholar]
- 6.Wellington K, Jarvis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–489. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci. 2011;14:308–317. [PMC free article] [PubMed] [Google Scholar]
- 8.Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 9.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gupta OP, Sing S, Bani S, Sharma N, Malhotra S, Gupta BD, et al. Anti-inflammatory and anti-arthritic activities of silymarin acting through inhibition of 5-lipoxygenase. Phytomedicin. 2000;7:21–24. doi: 10.1016/S0944-7113(00)80017-3. [DOI] [PubMed] [Google Scholar]
- 11.Kang JS, Jeon YJ, Park S-K, Yang K-H, Kim HM. Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1βand prostaglandin E2 synthesis by silymarin. Biochem Pharmacol. 2004;67:175–181. doi: 10.1016/j.bcp.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–6809. [PubMed] [Google Scholar]
- 13.Nazemian F, Karimi G, Moatamedi M, Charkazi S, Shamsara J, Mohammadpour AH. Effect of silymarin administration on TNF-alpha serum concentration in peritoneal dialysis patients. Phytother Res. 2010;24:1654–1657. doi: 10.1002/ptr.3175. [DOI] [PubMed] [Google Scholar]
- 14.Taghiabadi E, Imenshahidi M, Abnous Kh, Mosafa F, Sankian M, Memar M, et al. Protective Effect of Silymarin against Acrolein-Induced Cardiotoxicity in Mice. Evid Based Complement Alternat Med 2012. 2012:352091. doi: 10.1155/2012/352091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 16.Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 17.Mota VG, de Carvalho FL, de Morais LCSL, Bhattacharyya J, de Almeida RN, de Alencar JL. Antinociceptive activity of the chloroform fraction of Dioclea virgata (Rich.). Amshoff (Fabaceae) in mice. Biomed Res Int 2011. 2011:342816. doi: 10.1155/2011/342816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seltzer Ze, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 19.Karimi G, Tabrizian K, Rezaee R. Evaluation of the analgesic effect of dextromethorphan and its interaction with nitric oxide on sciatic nerve ligated rats. J Acupunct Meridian Stud. 2010;3:38–42. doi: 10.1016/S2005-2901(10)60006-4. [DOI] [PubMed] [Google Scholar]
- 20.Eddy NB, Leimbach D. Synthetic analgesics II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- 21.Puerta R, Martinez E, Bravo L, Ahumada M. Effect of silymarin on different acute inflammation models and on leukocyte migration. J Pharm Pharmacol. 1996;48:968–970. doi: 10.1111/j.2042-7158.1996.tb06014.x. [DOI] [PubMed] [Google Scholar]
- 22.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira AA, Amaral FA, Duarte IDG, Oliveira PM, Alves RB, Silveira D, et al. Antinociceptive effect from Ipomoea cairica extract. J Ethnopharmacol. 2006;105:148–153. doi: 10.1016/j.jep.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 25.Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123–139. doi: 10.1016/S0304-3959(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 26.Olsson Y. Degranulation of mast cells in peripheral nerve injuries. Acta Neurol Scand. 1967;43:365–374. doi: 10.1111/j.1600-0404.1967.tb05739.x. [DOI] [PubMed] [Google Scholar]
- 27.Perry V, Brown M, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Yalcin I, Choucair-Jaafar N, Benbouzid M, Tessier LH, Muller A, Hein L, et al. beta(2)-adrenoceptors are critical for antidepressant treatment of neuropathic pain. Ann Neurol. 2009;65:218–225. doi: 10.1002/ana.21542. [DOI] [PubMed] [Google Scholar]
- 30.Bohren Y, Tessier LH, Megat S, Petitjean H, Hugel S, Daniel D, et al. Antidepressants suppress neuropathic pain by a peripheral beta2-adrenoceptor mediated anti-TNFalpha mechanism. Neurobiol Dis. 2013;60:39–50. doi: 10.1016/j.nbd.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Vissers KC, Geenen F, Biermans R, Meert TF. Pharmacological correlation between the formalin test and the neuropathic pain behavior in different species with chronic constriction injury. Pharmacol Biochem Behav. 2006;84:479–486. doi: 10.1016/j.pbb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Munro G. Pharmacological assessment of the rat formalin test utilizing the clinically used analgesic drugs gabapentin, lamotrigine, morphine, duloxetine, tramadol and ibuprofen: influence of low and high formalin concentrations. Eur J Pharmacol. 2009;605:95–102. doi: 10.1016/j.ejphar.2009.01.004. [DOI] [PubMed] [Google Scholar]


