Abstract
Photodynamic therapy (PDT) against cancer has gained attention due to the successful outcome in some cancers, particularly those on the skin. However, there have been limitations to PDT applications in deep cancers and, occasionally, PDT treatment resulted in tumor recurrence. A better understanding of the underlying molecular mechanisms of PDT-induced cytotoxicity and cytoprotection should facilitate the development of better approaches to inhibit the cytoprotective effects and also augment PDT-mediated cytotoxicity. PDT treatment results in the induction of iNOS/NO in both the tumor and the microenvironment. The role of NO in cytotoxicity and cytoprotection was examined. The findings revealed that NO mediates its effects by interfering with a dysregulated pro-survival/anti-apoptotic NF-κB/Snail/YY1/RKIP loop which is often expressed in cancer cells. The cytoprotective effect of PDT-induced NO was the result of low levels of NO that activates the pro-survival/anti-apoptotic NF-κB, Snail, and YY1 and inhibits the anti-survival/pro-apoptotic and metastasis suppressor RKIP. In contrast, PDT-induced high levels of NO result in the inhibition of NF-kB, Snail, and YY1 and the induction of RKIP, all of which result in significant anti-tumor cytotoxicity. The direct role of PDT-induced NO effects was corroborated by the use of the NO inhibitor, l-NAME, which reversed the PDT-mediated cytotoxic and cytoprotective effects. In addition, the combination of the NO donor, DETANONOate, and PDT potentiated the PDT-mediated cytotoxic effects. These findings revealed a new mechanism of PDT-induced NO effects and suggested the potential therapeutic application of the combination of NO donors/iNOS inducers and PDT in the treatment of various cancers. In addition, the study suggested that the combination of PDT with subtoxic cytotoxic drugs will result in significant synergy since NO has been shown to be a significant chemo-immunosensitizing agent to apoptosis.
Abbreviations: ABC, ATP-binding cassette; ABCG2, ATP-binding cassette sub-family G member 2; AIF, apoptosis inducing factor; ALA, aminolevulinic acid; BCC, basal cell carcinoma; BCG, Bacillus Calmette-Guerin; CG, cholangiocarcinoma; CTL, cytotoxic T-lymphocyte; DR4/DR5, TRAIL death receptors; EGF, epithelial growth factor; EMT, epithelial mesenchymal transition; FASL, fas ligand; FDA, food and drug administration; 5-FU, 5-fluorouracil; GI, gastrointestinal; GSNO, S-nitrosoglutathione; HBD, hematoporphyrine-derivative; iNOS, inducible nitric oxide synthase; L-NAME, l-NG-Nitroarginine methyl ester; MAL, methylaminolevulinate; MDR, multidrug resistance; mPEG, monomethoxy-polyethylene glycol; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer; 3O2, molecular singlet oxygen; 1O2, singlet oxygen; PARP, poly ADP ribose polymerase; Pba, pheophorbide a; PDT, photodynamic therapy; PS, photosensitizer; RIPT-1, receptor activity protein I; RKIP, Raf kinase inhibitor protein; ROS, reactive oxygen species; Ru (NO)(NO)(ONO)(pc), nitrosyl-phtalocyanin ruthenium complex; SCC, squamous cell carcinoma; SNAP, S-nitroso-N-acetylpenicillamine; SOD, superoxide dismutase; TNF-α, tumor necrosis factor alpha; TRAIL, TNF-related apoptosis-inducing ligand; TNF-R1/R2, tumor necrosis factor receptor 1/receptor 2; UV, ultraviolet; YY1, Yin Yang 1
Keywords: Nitric oxide, Photodynamic therapy, Tumor response, Resistance, Molecular pathways.
Graphical abstract
Highlights
-
•
PDT-mediated cytotoxic and cytoprotective effects depend also by the induction of NO from tumor.
-
•
The PDT-induced NO modulates the dysregulated NF-kB/Snail/RKIP loop.
-
•
The direct role of NO induction by PDT was corroborated by the use of the NO inhibitor, l-NAME.
-
•
The combination of an NO donor and PDT resulted in a increased cytotoxic effect, in vitro and in vivo.
-
•
Novel potential therapeutic applications are proposed for the use of PDT combined with NO donors.
1. Introduction
Photodynamic therapy (PDT) is a therapeutic modality for certain diseases including cancer. PDT consists primarily of a photosensitizer (PS) and followed by light irradiation of a predetermined wavelength [1]. However, oxygen is an essential mediator of PDT [1,2]. The PDT-generated reactive oxygen species (ROS) and singlet oxygen (1O2) cause damage to the tumor tissues and cells by inducing necrosis and apoptosis. Optimally, the selective effect of PDT is through the localization of the photosensitizer in the desired region and the precise delivery of the light source to the treated areas. The PDT activity has its own limitations, for example, its effect on metastatic cancer lesions.
1.1. The photosensitizer (PS)
Most of the photosensitizers (PSs) used in cancer therapy belong to the protoporphyrin family and are based on a tetrapyrrole structure. An ideal sensitizer must have an absorption peak between 600 and 800 nm (red to deep red). High wavelengths greater than 800 nm produce a limited source of photons since they are poor in exciting oxygen to its singlet state and, thus, reduce reactive oxygen species that are required for cytotoxic effects. The mechanism of tumor localization of PS has been investigated revealing the role of the leaky blood vasculature in cancers and the absence of drainage by the lymphatic system leading to retention [3]. Also, some PSs bind to low density lipoproteins and bind cancers overexpressing LDL receptors and, thus, are more directed on tumors [4]. Other reports also demonstrated the use of PSs covalently linked to binding agents directed at cancer bearing receptors on the tumor cell surface [5]. Such coupling agents include antibody molecules, antibody fragments, peptides, proteins, EGF, etc.
1.2. Light sources for radiation
Red and infrared radiation penetrate into tissues more deep and only in the range of 600 to 800 nm to generate singlet oxygen for toxicity [6]. The choice of light source is dependent on the PS used and is based on the PS absorption, the disease and its size. The fluence rate affects significantly the PDT response [7]. Both lasers and incandescent light sources have been used for PDT and result in similar effects [8]. More detailed analyses of light sources have been reviewed elsewhere [9–14].
1.3. Photochemistry
The light exposure on the PS undergoes a shift from the ground (singlet) state to an excited singlet state. The latter undergoes crossing to an excited triplet state and this can result in the formation of radicals (ROS) (Type I reactions) or transfer the energy to molecular singlet oxygen (3O2) to form singlet oxygen (1O2) (Type II reactions). Singlet oxygen is the predominant cytotoxic molecule in PDT [9].
2. Dual cytotoxic and cytoprotective roles of PDT
2.1. PDT-mediated cytotoxicity
Various PSs target different organelles and subcellular compartments and mediate cytotoxic effects, which will vary based on the targeting – and the sensitivity of the tumor cells to cytotoxic damage [13,15]. Three major types of cell death by PDT have been reported, namely, (1) apoptosis, (2) necrosis and (3) autophagy. Apoptosis is the major cell death mechanism induced by PDT [9,14].
2.2. PDT-mediated cytoprotection
Many cancer cells are not sensitive to PDT-mediated cytotoxicity. Tumor cells develop various mechanisms to protect them from cell death-induced by PDT and many other cytotoxic agents. For instance, certain cancer cells have high levels of antioxidants [16]. Others have overexpression of detoxifying enzymes for ROS [17] and may have protective genes induced by PDT and/or overexpress several anti-apoptotic gene products [18–20]. A more detailed analysis on the mechanisms discussed above would be reported below.
3. Clinical applications of PDT in a variety of human cancers
Historically, Dougherty et al. [21] reported the first clinical study of the application of PDT in patients with a variety of malignant diseases. They treated the patients with PDT with a hematoporphyrine-derivative (HBD). They achieved complete and partial responses in 111 out of 113 treated cancer patients. These initial successful findings of the application of PDT in cancer was followed by hundreds of clinical trials [9,22,23]. PDT was most effective on the surface of lesions due to the limited penetration of the light source deep into the tissues; the range of tumor destruction did not overall exceed one centimeter. Briefly, a few examples of the therapeutic applications of PDT in various cancers are presented.
Còrdoba et al. [24] and Nestor et al. [25] reviewed the response of PDT treatment in premalignant and malignant skin tumors. Noteworthy, PDT was approved in the USA, Canada and Europe for its use in actinic keratosis and also in the European Union and Canada for basal cell carcinoma (BCC). In actinic keratosis, randomized controlled trials reported complete response rates (82–100%) for PDT with aminolevulinic acid (ALA-PDT) or methylaminolevulinate (MAL-PDT) as compared to 67 to 100% for cryotherapy and 74–94% for the application of 5-FU cream at 12 and 24 months [26,27]. In BCC, PDT was superior to cryosurgery or surgery for a selected subset of patients. Also, PDT actinic is a superior cosmetic outcome compared to surgery [28,29]. The use of MAL-PDT was found to be a safe and effective treatment for BCC in patients with Gorlin's syndrome and its efficacy is correlated to the thickness of the region [30]. PDT was also found to have chemo-preventive activity in patients with the Gorlin's syndrome [31].
PDT has been employed in the treatment of head and neck cancer, successfully [32]. Of interest, the study evaluated PDT treatment of patients with advanced diseases and not responding to tumor treatments. They applied Foscan-mediated PDT in 128 patients with a single session of PDT. There was a remarkable response in tumor destruction and complete local tumor clearance [33]. These findings suggest that PDT may be an alternative treatment for patients with early head and neck tumors.
Tumors of the digestive system have been grouped into PDT of the esophagus [34] and tumors beyond the esophagus. The U.S. FDA approved photofrin-mediated PDT for patients with Barret's esophagus and high grade dysplasia who did not undergo surgery [34]. PDT has been applied to other GI digestival tumors under the stomach [35,36], cholangiocarcinoma (CG) [37], with a therapeutic response on unresectable pancreatic cancers [38], and on colon or rectal cancers [39,40].
Intraperitoneal (ovarian, gastrointestinal, sarcoma) have been treated with PDT [41]. There was a suggestion that the median survivals of two years for ovarian cancer and one year for gastrointestinal cancer have been beneficial by PDT compared to controls.
Several reports have shown that the results of PDT treatment of prostate cancer. These studies established the potential use of PDT in prostate cancer and toxicity was considered as a determining factor [42–44].
Superficial bladder cancer is a good target for PDT. Long-term desirable responses of 20–60 of patients who were treated and many of those patients had recurrent disease following BCG treatment [45,46]. While PDT treatment for bladder cancer has been approved in the EU and Canada, it is not yet approved by the U.S. FDA.
In non-small cell lung cancer, the results of PDT treatment are encouraging [47,48]. In patients with malignant pleural mesothelioma, a randomized phase III study compared PDT with surgery and the findings demonstrated the benefit of PDT over surgery [49].
Promising clinical findings of PDT in brain tumors were reported [50,51]. However, more phase III clinical trials are needed to place PDT as superior to other therapeutics in certain cancers. Also a number of applications of laser technologies for accurate dosimetry are needed.
PDT has also been used in the treatment of mycosis fungoides, an indolent subtype of cutaneous T-cell lymphoma. It was reported that consecutive PDT treatments are adjunct for treatments of mycosis fungoides with good cosmetic results [52].
4. Molecular mechanisms of PDT-mediated cytotoxicity and resistance
4.1. PDT-mediated cytotoxicity
It was stated that active PDT-mediated cytotoxicity resulted from apoptosis, necrosis and autophagy. Apoptosis is a mechanism of programmed cell death that is activated by external death ligands (type I) or intracellular effects on the mitochondria (type II) via chemotherapeutic drugs, antibodies, toxins, DNA damaging agents, etc. For example, type I apoptosis is activated from the binding of death ligands (CTL, NK), FasL, TNF-α, and TRAIL to corresponding receptors Fas, TNF-R1/R2, DR4/DR5, respectively. Sensitive cells are induced to apoptosis by activation of caspase-8 and the effector caspase-3 leading to activation downstream of PARP and DNA fragmentation. Type II apoptosis results from altering the mitochondria permeability membrane and inducing the release of cytochrome c and smac/DIABLO, which lead to the activation of caspase-9. Subsequently, there is activation of caspases 7 and 8 and caspase 3, a merging point of type I and type II, and leading downstream to apoptosis. In addition to cytochrome c and smac/DIABLO, AIF is also released and activates apoptosis by a caspase-independent mechanism. Most PDT induce type II apoptosis [14]. PDT also photooxidizes lysosomes leading to the rupture and release of cathepsins which induce Bid cleavage and permeabilization of the mitochondrial outer membrane [53]. Cell death induced by PDT by necrosis has been observed and the underlying mechanism is not really clear [54,55]; although it has been reported that the activation of the receptor activity protein I (RIPT-1), excessive ROS production, lysosomal damage, and calcium are involved [55,56].
PDT also induces autophagy, a lysosomal pathway involved in the degradation and recycling of intracellular proteins and organelles. Autophagy can be induced by oxidative stress [57,58].
4.2. Resistance to PDT-mediated cytotoxicity
Tumor cells utilize several mechanisms to be resistant to the cytotoxic effect of PDT [59,60]. Briefly below, we discuss the most pertinent of those mechanisms, and several of those have been revealed through the utilization of PDT-resistant tumor cell lines. The mitochondrion plays an important role and any perturbation of the content enzymes in cancer cells may result in PDT resistance since PSs mediate their activity in the mitochondria [61,62].
Tumors resistant to several chemotherapeutic drugs exhibit the MDR phenotype [63–65]. The findings of the role of the MDR (Pgp) and the resistance to PDT are controversial depending on the cell lines, the kind of the PS used, and how the PS interacts with the MDR proteins. Another ABC transporter capable of inducing drug resistance in breast cancer cells was termed ABCG2 [66,67]. There is a correlation between the expression of ABCG2 and the resistance to PDT as a function of the PS structure [68]. Overall, high Pgp and ABCG2 expressions potentiate resistance to PDT and inhibitors of those transporters may reverse resistance [69].
DNA damage may be induced by PDT [70]. PDT induces activation of early response genes [61] with the activation of cell survival pathways. Hence, the hyper activation of survival pathway may result in resistance of PDT-mediated cytotoxicity. In addition, tumor cells overexpress anti-apoptotic gene products that play a role in the resistance of PDT-mediated cell death.
PDT is also antagonized by antioxidant defense mechanisms including the glutathione system, superoxide dismutase (SOD), catalase, and lipoamine dehydrogenases [17,71]. The increase of heat shock proteins may also be involved in PDT resistance [72]. Modification of the extra cellular matrix in tumor cells affects PDT cell toxicity [59].
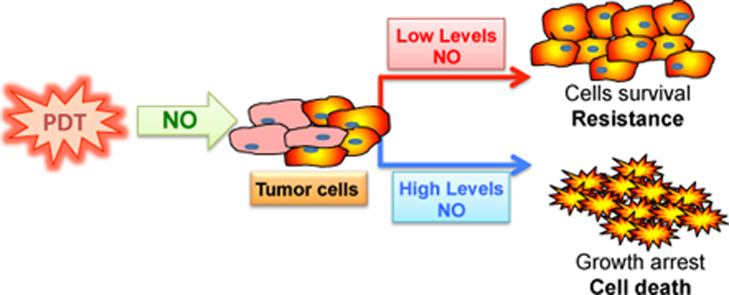
The role of NO in resistance has been controversial and depending on the level of NO. Low levels of NO mediate chemo and radio-resistance whereas high levels of NO mediate cytotoxicity and sensitize tumor cells to chemo-immunotherapy (see chapter Bonavida and Garbon in this volume). The subset of NO in PDT will be discussed below separately.
5. Dual roles of NO-mediated anti-tumor effects
The induction of NO in tumor cells may result in some protective effects by mediating cell proliferation, survival, and resistance. For example, reports by Sikora [73] reported that the inhibition of inducible nitric oxide synthase (iNOS) repressed the growth of human melanoma in vivo and synergized with cisplatin. Noteworthy, Eyler et al. [74] reported that glioblastoma stem cells expressed higher levels iNOS than normal stem cells and the iNOS inhibition reduced cell proliferation in vitro. In vivo, in a mouse xenograft model, an iNOS inhibitor slowed tumor progression and prolonged survival. Such studies and others demonstrated that many cancer cells utilized low levels of iNOS/NO to reduce apoptosis, to stimulate cell proliferation and to induce invasion and metastases. NO at low concentration can act as antioxidant [75] and also S-nitrolsylate proteins that activate pro-survival pathways [76].
The dual roles of NO have been the subject of many studies in both NO-mediated cytoprotective and cytotoxic effects. NO at high concentration, in the µM range, is cytotoxic due to its conversion to oxidized intermediates that damage the DNA, exerts lipid peroxidation on the membrane and inhibits certain proteins by S-nitrosylation [77–80]. NO dual contrasting roles in PDT have been reviewed elsewhere [81–84].
6. NO donors and PDT
Recently, several groups have begun to synthesize NO donors to promote PDT-mediated anti-tumor cytotoxicity. For instance, Carneiro et al. [85] reported the synthesis and activity of a nitrosyl-phtalocyanin ruthenium complex [Ru (NO) (NO) (ONO) (pc)] and studied its effect on a murine melanoma cell line, B16F10, in the presence or absence of light irradiation. Their findings demonstrated that the complex was more effective in inhibiting B16F10 cell growth than the free [Ru (pc)] demonstrating the importance of NO release. Also, the encapsulation of the complex into liposomes was >25% more effective than the non-capsulated complex. The phototoxicity of the complex on B16F10 cells was primarily due to apoptosis.
Giles et al. [86] designed 2 photolabile NO-releasing prodrugs, tert-butyl-S-nitrosothiol and tert-dodecaire-S-nitrosothiol. These prodrugs have better kinetics of NO release than available nitrosothiols and are stable in vitro in the absence of radiation. Experimentally, irradiation increased the cytotoxic activity of these prodrugs and the authors suggested their therapeutic potential. In subsequent studies, the same group designed a superior NO donor than the conventional GSNO SNAP [87]. Tert-dodecaire-S-nitrosothiol released high NO than GSNO or SNAP, and exhibited a photodynamic response. The authors concluded that this compound is the most effective known S-nitrosothiol for PDT application. Rapozzi et al. [88] reported the superior activity of a new complex DR2 constituted of a PS (Pheophorbide a, Pba) connected to a non-steroidal antiandrogen molecule able to release cytotoxic NO under the exclusive control of light in prostate cancer cells.
Reported studies implicated the role of the high level of NO in its interference with the dysregulation of NF-κB/Snail/YY1/RKIP in cancer cells [89]. This loop provided the tool to examine its role and implication in PDT on one hand and its regulation in NO-mediated PDT treatment resulting in either the cytoprotective tumor recurrence or antitumor cytotoxicity. Below briefly, we present our findings that have been recently published [90,91].
6.1. NO-mediated PDT-induced cytotoxicity
It is well established that treatment with PDT results in the induction of NO through the activity of the PS and light as a result of the induction of iNOS [81,82]. The induction of NO by PDT is the result of both the activity of iNOS expression by both the tumor cells and the tumor microenvironment [83,84]. Whether the NO-mediated induction by PDT plays a major role in PDT-mediated cytotoxicity was investigated. This hypothesis was examined in vitro in a tumor cell model using the amelanotic murine cell line, B78-H1 [90].
In this model, Pba was used as the PS. Treatment of tumor cells with Pba induced iNOS expression and the level of iNOS are a function of the concentration of Pba used. In addition, treatment with Pba inhibited tumor cell viability as assessed by a reduction of metabolic activity of the treated cells. The direct role of NO-induction by PDT was corroborated by the use of l-NAME, an inhibitor of NO, and such a treatment reversed the cytotoxic activity.
We then examined the effect of Pba on the expression of the loop gene products. Treatment of B78-H1 cells with Pba resulted in the inhibition of NF-kappa B and Snail expressions while upregulating the expression of RKIP. In addition, treatment with Pba induced the activation of caspases 3 and 7 above control levels and suggested that the cytotoxic mechanism involved apoptosis. The in vivo findings of Pba-induced inhibition of cell proliferation and induced cytotoxicity were corroborated in a murine bearing B78-H1 tumor cells whereby the administration of mPEG Pba resulted in significant inhibition of tumor growth in vivo [90].
The above findings demonstrated clearly that treatment of B78-H1 tumor cells with PDT resulted in cytotoxicity via inhibition of the constitutively activated NF-kappa B pathway, responsible for cell proliferation and viability, and downstream inhibition of its target gene product, Snail, and resulting in the derepression of the metastasis suppressor gene product RKIP. In addition, the induction of RKIP potentiated the inhibition of NF-kappa B activity as reported [92]. PDT-mediated effects are the result, in part, of the induction of NO. NO has been reported to inhibit the NF-κB activity via the S nitrosylation of p50 and p65 [93]. Thus, based on these findings, we have postulated that NO-mediated PDT cytotoxic activity may be enhanced by the combined treatment of PDT and an NO donor. Accordingly, we have used the NO donor, DETANONOate, which was reported to have a significant effect in interfering with the loop in cancer cells and resulting in the inhibition of cell survival and sensitization to chemotherapeutic drugs [89]. Therefore, the combination of PDT and DETANONOate was examined and the findings revealed, in contrast to single agent treatment alone, that the combination resulted in significant potentiation of (a) inhibition of metabolic activity (b) inhibition of NF-κB activity (c) inhibition of Snail and (d) upregulation of RKIP. In addition, in vivo studies in mice revealed that the combination of Pba and DETANONOate resulted in significant inhibition of tumor growth compared to single treatment alone and significant prolongation of survival in mice [90].
Overall, the above findings demonstrated clearly that the NO-induced by PDT plays a pivotal role in PDT-induced cytoxicity and that the addition of exogenous NO potentiated the cytotoxic activity against the tumor cells. A schematic diagram representing PDT-induced NO-mediated cytotoxity is shown in Fig. 1.
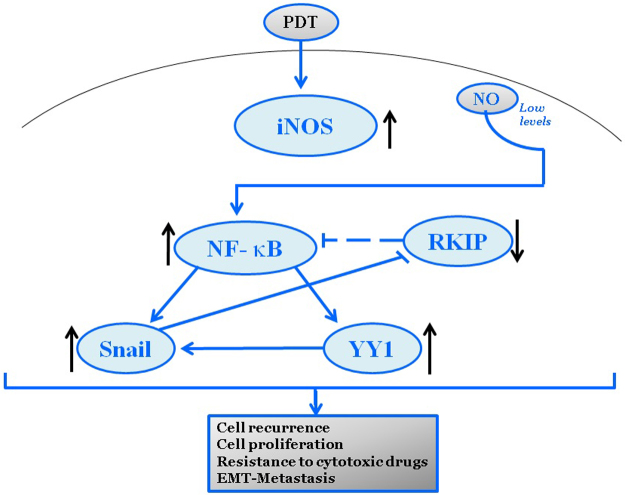
Fig. 1.
Cytoprotective role of PDT-induced NO.
6.2. NO-mediated PDT inhibition of cytotoxicity and epithelial mesenchymal transition
The protective role of NO-induced by PDT in cytoprotection has been the subject of many reports and recently reviewed by Girotti [82]. We have reported that the level of NO-induced by PDT has contrasting effects on the NF-κB/Snail/YY1/RKIP loop i.e. low level of NO activates NF-κB, YY1, and Snail and inhibits RKIP whereas high level inhibits NF-κB, Snail, YY1, and induces RKIP [90,91,94].
It was also reported that the activity of the above loop not only regulated cell survival and viability, but also regulates the epithelial to mesenchymal transition (EMT) [95]. Thus, based on the findings that a suboptimal PDT-treatment resulted in a transient inhibition of tumor cell growth followed by recurrence, we examined the role of PDT-induced NO in a model of tumor cell recurrence as well as its role in EMT [91]. The tumor model used consisted of the human PC3 prostate cancer cell line. A suboptimal concentration of Pba was used, which had no effect on PC3 cells, and treatment was repeated 4 or 8 times and the properties of the treated tumor cells were examined at these time intervals. There was significant enhancement of cell proliferation and with a mild and constant production of iNOS in the cells. Noteworthy, there was significant activation of NF-κB and YY1 [91] expressions following 8 treatments along with inhibition of RKIP. Also, there was significant activation of the AKT pathway, which regulates NF-κB activity [91]. In addition, the modulation of the loop by PDT treatments resulted in the induction of the EMT phenotype of PC3 cells as determined by inhibition of E-cadherin and the induction of vimentin. The direct role of NO on both the effects on the loop and EMT by repeated PDT treatments was corroborated by the use of the NO-inhibitor, l-NAME, that resulted in the reversal of the observed effects.
The above findings demonstrated a mechanism by which the cytoprotective effect of PDT is mediated by low levels of NO-induction resulting in cell proliferation and EMT [91]. A schematic diagram representing these effects is shown in Fig. 2.
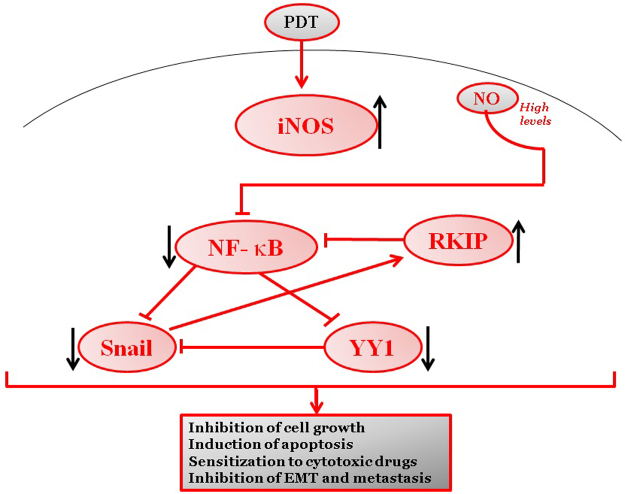
Fig. 2.
Cytoyoxic role of PDT-induced NO.
We hypothesized that low levels of PDT-induced NO may activate the loop and result in cell proliferation and tumor recurrence. However, high levels of induced NO will inhibit the loop and result in the inhibition of cell proliferation, reduced cell viability, and induction of cell apoptosis.
7. Concluding remarks and future directions
7.1. Concluding Remarks
It is clear that the induction of NO by PDT plays an essential role in its cytotoxic anti-tumor activity provided the amount of NO released is optimal for mediating cytotoxicity. Since the amount of NO-induced by PDT varies and is dependent on the tumor tissue, the PS used, the light source, one may consider various means to overcome such limitations. The combined treatment of NO donors and PDT resulted in a significant synergistic cytotoxic activity using tumor model systems described here. Clearly, the overall activity mediated by PDT-induced NO or the addition of exogenous NO resulted, in part, in the interference of a dysregulated loop (the pro-survival/anti-apoptotic/NF-κB/Snail/YY1/RKIP) found to be present in many cancers. This dysregulated loop has been shown to be central for tumor cell survival, proliferation, resistance, invasion, angiogenesis, EMT and metastasis. Thus, the combined treatment of PDT and NO donors results in pleiotropic activities that not only induce cytotoxicity against a tumor, but also, prevent invasion and metastasis as well as reverse resistance. Several NO donors have been used and others are being developed for their anti-tumor mediated activities used alone or in combination with sensitizing agents and drugs. Clearly, the therapeutic implication for the use of the combination of NO donors and PDT in cancer patients warrants clinical trials to determine toxicity and efficacy. At present, there have been a few ongoing clinical trials with NO donors as single agents, but clearly, these would be followed with clinical trials for the combination treatments with PDT.
7.2. Future directions
Several future directions for investigations are being currently contemplated, for example, analysis of the effect of the combination of NO donors and PDT on cytotoxicity on cancer stem cells. Also, several reports have demonstrated the significant chemo and immuno-sensitizing activities of NO donors. Thus, analysis of the combination of NO donors and suboptimal PDT in resistance as chemoimmunosensitizing agents are warranted for investigation. There are also reports on the analysis of the superiority of NO-drug conjugates in comparison with single agents. Novel synthesized PDT-NO complexes have been synthesized and are currently being investigated. Preliminary findings demonstrated that a selected PDT-NO complex is more cytotoxic than single agents alone or combination [88]. In addition, the application of nanoparticles coated with the PS-NO complex will be examined for their superior activity. The findings demonstrating PDT-induced NO on the dysregulated NF-κB/Snail/YY1//RKIP loop suggested that inhibitors of NF-κB/Snail/YY1 or inducers of RKIP may be useful if used in combination with PDT for anti-tumor cell activity, and such studies are currently being explored.
This schematic diagram represents treatments with suboptimal PDT result in the low induction of iNOS and resulting in low levels of NO. Under these conditions, NO induces the expressions and activities osf NF-κB and downstream its target gene products Snail and YY1. The overexpression of SNAIL represses the transcription of RKIP. Thus, under the conditions of low levels of NO, the modified dysregulated loop results in the overexpression of NF-κB, YY1, and Snail and the inhibition of RKIP expression. Hence, the tumor cells undergo recurrence, cell proliferation, resistance to cytotoxic drugs, expression of the EMT phenotype, and metastasis.
This schematic diagram represents optimal PDT treatments which result in the high induction of iNOs expression and high levels of NO. High levels of NO inhibit NF-κB, Snail, and YY1 activities and expressions and resulting in the derepression and overexpression of RKIP. In addition, the overexpression of RKIP, in turn, potentiates the inhibition of NF-κB and its target genes Snail and YY1. These manifestations by high levels of NO result in inhibition of tumor cell growth, induction of apoptosis in sensitive cells, sensitization to cytotoxic drugs, and inhibition of EMT and metastasis.
Acknowledgments
We acknowledge the assistance of the Jonsson Comprehensive Cancer Center at UCLA (BB) and the assistance of Kathy Nguyen, Leah Moyal, and Ailina Heng Lao in the preparation of this manuscript.
Contributor Information
Valentina Rapozzi, Email: valentina.rapozzi@uniud.it.
Emilia Della Pietra, Email: emydellapietra@yahoo.com.
Benjamin Bonavida, Email: bbonavida@mednet.ucla.edu.
References
- 1.Dougherty T.J., Gomer C.J., Henderson B.W., Jori G., Kessel D., Korbelik M., Moan J., Peng Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolmans D., Fukumura D., Jain R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 3.Iyer A.K., Greish K., Seki T., Okazaki S., Fang F., Takeshita K., Maeda H. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J. Drug Target. 2007;15:496–506. doi: 10.1080/10611860701498252. [DOI] [PubMed] [Google Scholar]
- 4.Kessel D. The role of low-density lipoproteins in the biodistribution of photosensitizing agents. J. Photochem. Photobiol. B. 1992;14:261–266. doi: 10.1016/1011-1344(92)85103-2. [DOI] [PubMed] [Google Scholar]
- 5.Sibani S.A., McCarron P.A., Woolfson A.D., Donnelly R.F. Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opin. Drug Deliv. 2008;5:1241–1254. doi: 10.1517/17425240802444673. [DOI] [PubMed] [Google Scholar]
- 6.Juzeniene A., Nielsen K.P., Moan J. Biophysical aspects of photodynamic therapy. J. Environ. Pathol. Toxicoly Oncol. 2006;25:7–28. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 7.Henderson B.W., Busch T.M., Snyder J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006;38:489–493. doi: 10.1002/lsm.20327. [DOI] [PubMed] [Google Scholar]
- 8.Brancaleon L., Moseley H. Laser and non-laser light sources for photodynamic therapy. Lasers Med. Sci. 2002;17:173–186. doi: 10.1007/s101030200027. [DOI] [PubMed] [Google Scholar]
- 9.Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Hahn S.M., Hamblin M.R., Juzeniene A., Kessel D., Korbelik M., Moan J., Mroz P., Nowis D., Piette J., Wilson B.C., Golab J. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson B.C., Patterson M.S. The physics, biophysics and technology of photodynamic therapy. Phys. Med. Biol. 2008;53:R61–R109. doi: 10.1088/0031-9155/53/9/R01. [DOI] [PubMed] [Google Scholar]
- 11.Plaetzer K., Krammer B., Berlanda J., Berr F., Kiesslich T. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med. Sci. 2009;24:259–268. doi: 10.1007/s10103-008-0539-1. [DOI] [PubMed] [Google Scholar]
- 12.Juzeniene A., Juzenas P., Ma L.W., Iani V., Moan J. Effectiveness of different light sources for 5-aminolevulinic acid photodynamic therapy. Lasers Med. Sci. 2004;19:139–149. doi: 10.1007/s10103-004-0314-x. [DOI] [PubMed] [Google Scholar]
- 13.Allison R.R., Sibata C.H. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagn. Photodyn. Ther. 2010;7:61–75. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Buytaert E., Dewaele M., Agostinis P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta. 2007;1776:86–107. doi: 10.1016/j.bbcan.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Castano A.P., Demidova T.N. HamblinMR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sattler U.G., Mueller-Klieser W. The anti-oxidant capacity of tumour glycolysis. Int. J. Radiat. Biol. 2009;85:963–971. doi: 10.3109/09553000903258889. [DOI] [PubMed] [Google Scholar]
- 17.Golab J., Nowis D., Skrzycki M., Czeczot H., Baranczyk-Kuzma A., Wilczynski G.M., Makowski M., Mroz P., Kozar K., Kaminski R., Jalili A., Kopec’ M., Grzela T., Jakobisiak M. Antitumor effects of photodynamic therapy are potentiated by 2-methoxyestradiol – A superoxide dismutase inhibitor. J. Biol. Chem. 2003;278:407–414. doi: 10.1074/jbc.M209125200. [DOI] [PubMed] [Google Scholar]
- 18.Hanlon J.G., Adams K., Rainbow A.J., Gupta R.S., Singh G. Induction of Hsp60 by photofrin-mediated photodynamic therapy. J. Photochem. Photobiol. B. 2001;64:55–61. doi: 10.1016/s1011-1344(01)00189-0. [DOI] [PubMed] [Google Scholar]
- 19.Nonaka M., Ikeda H., Inokuchi T. Inhibitory effect of heat shock protein 70 on apoptosis induced by photodynamic therapy in vitro. Photochem. Photobiol. 2004;79:94–98. [PubMed] [Google Scholar]
- 20.Oleinick N.L., Morris R.L., Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem. Photobiol. Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 21.Dougherty T.J., Kaufman J.E., Goldfarb A., Weishaupt K.R., Boyle D., Mittleman A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628–2635. [PubMed] [Google Scholar]
- 22.Gao F., Bai Y., Ma S.-R., Liu F., Li Z.S. Systematic review: photodynamic therapy for unresectable cholangiocarcinoma. J Hepatbiliary-Pancreat. Sci. 2010;17:125–131. doi: 10.1007/s00534-009-0109-3. [DOI] [PubMed] [Google Scholar]
- 23.Fayter D., Corbett M., Heirs M., Fox D., Eastwood A. A systematic review of photodynamic therapy in the treatment of pre-cancerous skin conditions, Barrett’s oesophagus and cancers of the biliary tract, brain, head and neck, lung, oesophagus and skin. Health Technol. Assess. 2010;14:1–288. doi: 10.3310/hta14370. [DOI] [PubMed] [Google Scholar]
- 24.Córdoba F., Braathen L.R., Weissenberger J., Vallan C., Kato M., Nakashima I., Weis J., von Felbert V. 5-Aminolaevulinic acid photodynamic therapy in a transgenic mouse model of skin melanoma. Exp. Dermatol. 2005;14:429–437. doi: 10.1111/j.0906-6705.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- 25.Nestor M.S., Gold M.H., Kauvar A.N., Taub A.F., Geronemus R.G., Ritvo E.C., Goldman M.P., Gilbert D.J., Richey D.F., Alster T.S., Anderson R.R., Bank D.E., Carruthers A., Carruthers J., Goldberg D.J., Hanke C.W., Lowe N.J., Pariser D.M., Rigel D.S., Robins P., Spencer J.M., Zelickson B.D. The use of photodynamic therapy in dermatology: results of a consensus conference. J. Drugs Dermatol. 2006;5:140–154. [PubMed] [Google Scholar]
- 26.Morton C.A. Methyl aminolevulinate: atinic keratoses and Bowen’s disease. Dermatol. Clin. 2007;25:81–87. doi: 10.1016/j.det.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Salim A., Leman J.A., McColl J.H., Chapman R., Morton C.A. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br. J. Dermatol. 2003;148:539–543. doi: 10.1046/j.1365-2133.2003.05033.x. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes L.E., de Rie M.A., Leifsdottir R., Yu R.C., Bachmann I., Goulden V., Wong G.A., Richard M.A., Anstey A., Wolf P. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch. Dermatol. 2007;143:1131–1136. doi: 10.1001/archderm.143.9.1131. [DOI] [PubMed] [Google Scholar]
- 29.Berroeta L., Clark C., Dawe R.S., Ibbotson S.H., Fleming C.J. A randomized study of minimal curettage followed by topical photodynamic therapy compared with surgical excision for low-risk nodular basal cell carcinoma. Br. J. Dermatol. 2007;157:401–403. doi: 10.1111/j.1365-2133.2007.07996.x. [DOI] [PubMed] [Google Scholar]
- 30.Basset-Seguin N., Bissonnette R., Girard C., Haedersdal M., Lear J.T., Paul C., Piaserico S. Consensus recommendations for the treatment of basal cell carcinomas in Gorlin syndrome with topical methylaminolaevulinate-photodynamic therapy. J. Eur. Acad. Dermatol. Venereol. 2014;28:626–632. doi: 10.1111/jdv.12150. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe C.M., Green W.H., Cognetta A.B., Jr, Hatfield H.K.A. possible chemopreventive role for photodynamic therapy in Gorlin syndrome: a report of basal cell carcinoma reduction and review of literature. Australas. J. Dermatol. 2013;54:64–68. doi: 10.1111/j.1440-0960.2012.00922.x. [DOI] [PubMed] [Google Scholar]
- 32.Jerjes W., Upile T., Akram S., Hopper C. The Surgical palliation of advanced head and neck cancer using photodynamic therapy. Clin. Oncol. 2010;22:785–791. doi: 10.1016/j.clon.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 33.D’Cruz A.K., Robinson M.H., Biel M.A. mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients. HeadNeck. 2004;26:232–240. doi: 10.1002/hed.10372. [DOI] [PubMed] [Google Scholar]
- 34.Wolfsen H.C. Carpe luz-seize the light: endoprevention of esophageal adenocarcinoma when using photodynamic therapy with porfimer sodium. Gastrointest. Endosc. 2005;62:499–503. doi: 10.1016/j.gie.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura H., Yanai H., Nishikawa J., Okamoto T., Hirano A., Higaki M., Omori K., Yoshida T., Okita K. Experience with photodynamic therapy (endoscopic laser therapy) for the treatment of early gastric cancer. Hepatogastroenterology. 2001;48:1599–1603. [PubMed] [Google Scholar]
- 36.Yanai H., Kuroiwa Y., Shimizu N., Matsubara Y., Okamoto T., Hirano A., Nakamura Y., Okita K., Sekine T. The pilot experience of immunotherapy-combined photodynamic therapy for advanced gastric cancer in elderly patients. Int. J. Gastrointest. Cancer. 2002;32:139–142. doi: 10.1385/IJGC:32:2-3:139. [DOI] [PubMed] [Google Scholar]
- 37.Witzigmann H., Berr F., Ringel U., Caca K., Uhlmann D., Schoppmeyer K., Tannapfel A., Wittekind C., Mossner J., Hauss J., Wiedmann M. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma – Palliative photodynamic therapy plus stenting is comparable to R1/R2 resection. Ann. Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bown S.G., Rogowska A.Z., Whitelaw D.E., Lees W.R., Lovat L.B., Ripley P., Jones L., Wyld P., Gillams A., Hatfield A.W. Photodynamic therapy for cancer of the pancreas. Gut. 2002;50:549–557. doi: 10.1136/gut.50.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura T., Fukui H., Ishii Y., Ejiri K., Ejiri M. Photodynamic therapy with polypectomy for rectal cancer. Gastrointest. Endosc. 2003;57:266–269. doi: 10.1067/mge.2003.57. [DOI] [PubMed] [Google Scholar]
- 40.Abulafi A.M., Dejode M., Allardice J.T., Ansell J., Rogers J., Williams N.S. Adjuvant intraoperative photodynamic therapy in experimental colorectal-cancer. Br. J. Surg. 1995;82:178–181. doi: 10.1002/bjs.1800820212. [DOI] [PubMed] [Google Scholar]
- 41.Wilson J.J., Jones H., Burock M., Smith D., Fraker D.L., Metz J., Glatstein E., Hahn S.M. Patterns of recurrence in patients treated with photodynamic therapy for intraperitoneal carcinomatosis and sarcomatosis. Int. J. Oncol. 2004;24:711–717. [PubMed] [Google Scholar]
- 42.Du K.L., Mick R., Busch T.M., Zhu T.C., Finlay J.C., Yu G., Yodh A.G., Malkowicz S.B., Smith D., Whittington R., Stripp D., Hahn S.M. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg. Med. 2006;38:427–434. doi: 10.1002/lsm.20341. [DOI] [PubMed] [Google Scholar]
- 43.Trachtenberg J., Bogaards A., Weersink R.A., Haider M.A., Evans A., McCluskey S.A., Scherz A., Gertner M.R., Yue C., Appu S., Aprikian A., Savard J., Wilson B.C., Elhilali M. Vascular targeted photodynamic therapy with palladium-bacteriopheophorbide photosensitizer for recurrent prostate cancer following definitive radiation therapy: assessment of safety and treatment response. J. Urol. 2007;178:1974–1979. doi: 10.1016/j.juro.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 44.Trachtenberg J., Weersink R.A., Davidson S.R.H., Haider M.A., Bogaards A., Gertner M.R., Evans A., Scherz A., Savard J., Chin J.L., Wilson B.C., Elhilali M. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 2008;102:556–562. doi: 10.1111/j.1464-410X.2008.07753.x. [DOI] [PubMed] [Google Scholar]
- 45.Berger A.P., Steiner H., Stenzl A., Akkad T., Bartsch G., Holtl L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: a single-center study. Urology. 2003;61:338–341. doi: 10.1016/s0090-4295(02)02123-4. [DOI] [PubMed] [Google Scholar]
- 46.Waidelich R., Beyer W., Knuchel R., Stepp H., Baumgartner R., Schroder J., Hofstetter A., Kriegmair M. Whole bladder photodynamic therapy with 5-aminolevulinic acid using a white light source. Urology. 2003;61:332–337. doi: 10.1016/s0090-4295(02)02164-7. [DOI] [PubMed] [Google Scholar]
- 47.Weinberg B.D., Allison R.R., Sibata C., Parent T., Downie G. Results of combined photodynamic therapy (PDT) and high dose rate brachytherapy (HDR) in treatment of obstructive endobronchial non-small cell lung cancer (NSCLC) Photodiagn. Photodyn. Ther. 2010;7:50–58. doi: 10.1016/j.pdpdt.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Corti L., Toniolo L., Boso C., Colaut F., Fiore D., Muzzio P.C., Koukourakis M.I., Mazzarotto R., Pignataro M., Loreggian L., Sotti G. Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg. Med. 2007;39:394–402. doi: 10.1002/lsm.20513. [DOI] [PubMed] [Google Scholar]
- 49.Pass H.I., Temeck B.K., Kranda K., Thomas G., Russo A., Smith P., Friauf W., Steinberg S.M. Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Ann. Surg. Oncol. 1997;4:628–633. doi: 10.1007/BF02303746. [DOI] [PubMed] [Google Scholar]
- 50.Muller P.J., Wilson B.C. Photodynamic therapy of brain tumors – A work in progress. Lasers Surg. Med. 2006;38:384–389. doi: 10.1002/lsm.20338. [DOI] [PubMed] [Google Scholar]
- 51.Stylli S.S., Kaye A.H., MacGregor L., Howes M., Rajendra P. Photodynamic therapy of high grade glioma-long term survival. J. Clin. Neurosci. 2005;12:389–398. doi: 10.1016/j.jocn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Quereux G., Brocard A., Saint-Jean M., Peuvrel L., Knol A.C., Allix R., Khammari A., Renaut J.J., Dréno B. Photodynamic therapy with methyl-aminolevulinic acid for paucilesional mycosis fungoides: a prospective open study and review of the literature. J. Am. Acad. Dermatol. 2013;69:890–897. doi: 10.1016/j.jaad.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 53.Reiners J.J., Caruso J.A., Mathieu P., Chelladurai B., Yin X.M., Kessel D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves bid cleavage. Cell Death Diff. 2002;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kessel D. Relocalization of cationic porphyrins during photodynamic therapy. Photochem. Photobiol. Sci. 2002;1:837–840. doi: 10.1039/b206046a. [DOI] [PubMed] [Google Scholar]
- 55.Vanlangenakker N., Vanden Berghe T., Krysko D.V., Festjens N., Vandenabeele P. Molecular mechanisms and pathophysiology of necrotic cell death. Curr. Mol. Med. 2008;8:207–220. doi: 10.2174/156652408784221306. [DOI] [PubMed] [Google Scholar]
- 56.Zong W.X., Thompson C.B. Necrotic death as a cell fate. Genes Dev. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 57.Dewaele M., Maes H., Agostinis P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy. 2010;6:838–854. doi: 10.4161/auto.6.7.12113. [DOI] [PubMed] [Google Scholar]
- 58.Reiners J.J., Jr., Agostinis P., Berg K., Oleinick N.L., Kessel D. Assessing autophagy in the context of photodynamic therapy. Autophagy. 2010;6:7–18. doi: 10.4161/auto.6.1.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casas A., Di Venosa G., Hasan T., Batlle A. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011;18:2486–2515. doi: 10.2174/092986711795843272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.B. Bonavida, V. Rapozzi, G. Jori (Eds.) Resistance to Photodynamic Therapy in Cancerof Resistance to Targeted Anti-cancer Therapeutics, vol. 5, Springer, 2015. doi:10.1007/978-3-319-12730-9.
- 61.ACE Moor. Signaling pathways in cell death and survival after photodynamic therapy. J. Photochem. Photobiol. B. 2000;57:1–13. doi: 10.1016/s1011-1344(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 62.Kessel D., Luo Y., Mathieu P., Reiners J.J., Jr. Determinants of the apoptotic response to lysosomal photodamage. Photochem. Photobiol. 2000;71:196–200. doi: 10.1562/0031-8655(2000)071<0196:dotart>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 63.Gros P., Ben-Neriah Y., Croop J.M., Housman D.E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986;323:728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- 64.Roninson I.B., Chin J.E., Choi K., Gros P., Housman D.E., Fojo A., Shen D.W., Gottesman M.M., Pastan I. Isolation of human MDR-DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cell. Mol. Life Sci. 2008;65:3145–3167. doi: 10.1007/s00018-008-8111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doyle L.A., Yang W.D., Abruzzo L.V., Krogmann T., Gao Y.M., Rishi A.K., Ross D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allikmets R., Schriml L.M., Hutchinson A., Romano-Spica V., Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–5339. [PubMed] [Google Scholar]
- 68.Busch T.M., Hahn S.M. Multidrug resistance in photodynamic therapy. Cancer Biol. Ther. 2005;4:195–196. doi: 10.4161/cbt.4.2.1463. [DOI] [PubMed] [Google Scholar]
- 69.Robey R.W., Steadman K., Polgar O., Bates S.E. ABCG2-mediated transport of photosensitizers: potential impact on photodynamic therapy. Cancer Biol. Ther. 2005;4:187–194. [PubMed] [Google Scholar]
- 70.Fiel R.J., Dattagupta N., Mark E.H., Howard J.C. Induction of DNA damage by porphyrin photosensitizers. Cancer Res. 1981;41:3543–3545. [PubMed] [Google Scholar]
- 71.Oberdanner C.B., Plaetzer K., Kiesslich T., Krammer B. Photodynamic treatment with fractionated light decreases production of reactive oxygen species and cytotoxicity in vitro via regeneration of glutathione. Photochem. Photobiol. 2005;81:609–613. doi: 10.1562/2004-08-23-RN-284. [DOI] [PubMed] [Google Scholar]
- 72.Gomer C.J., Ryter S.W., Ferrario A., Rucker N., Wong S., Fisher A,M.R. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355–2360. [PubMed] [Google Scholar]
- 73.Sikora A.G., Gelbard A., Davies M.A., Sano D., Ekmekcioglu S., Kwon J., Hailemichael Y., Jayaraman P., Myers J.N., Grimm E.A., Overwijk W.W. Targeted inhibition of inducible nitric oxide synthase inhibits growth of human melanoma in vivo and synergizes with chemotherapy. Clin. Cancer Res. 2010;16:1834–1844. doi: 10.1158/1078-0432.CCR-09-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eyler C.E., Wu Q., Yan K., MacSwords J.M., Chandler-Militello D., Misuraca K.L., Justin D., Lathia J.D., Forrester M.T., Lee J., Stamler J.S., Goldman S.A., Bredel M., McLendon R.E., Sloan A.E., Hjelmeland A.B., Rich J.N. Glioma Stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 2011;146:53–66. doi: 10.1016/j.cell.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S., Kirk M., Freeman B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 76.Foster M.W., Hess D.T., Stamler J.S. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol. Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ridnour L.A., Thomas D.D., Donzelli S., Espey M.G., Roberts D.D., Wink D.A., Isenberg J.S. The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 2006;8:1329–1337. doi: 10.1089/ars.2006.8.1329. [DOI] [PubMed] [Google Scholar]
- 78.Thomas D.D., Ridnour L.A., Isenberg J.S., Flores-Santana W., Switzer C.H., Donzelli S., Hussain P., Vecoli C., Paolocci N., Ambs S., Colton C.A., Harris C.C., Roberts D.D., Wink D.A. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wink D.A., Hines H.B., Cheng R.Y., Switzer C.H., Flores-Santana W., Vitek M.P., Ridnour L.A., Colton C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burke A.J., Sullivan F.J., Giles F.J., Glynn S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis. 2013;34:503–512. doi: 10.1093/carcin/bgt034. [DOI] [PubMed] [Google Scholar]
- 81.Gupta S., Ahmad N., Mukhtar H. Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res. 1998;58:1785–1788. [PubMed] [Google Scholar]
- 82.Girotti A.W. Tumor-generated nitric oxide as an antagonist of photodynamic therapy. Photochem. Photobiol. Sci. 2015 doi: 10.1039/c4pp00470a. [DOI] [PubMed] [Google Scholar]
- 83.Reeves K.J., Reed M.W.R., Brown N.J. Is nitric oxide important in photodynamic therapy? J Photochem Photobiol B. 2009;95:141–147. doi: 10.1016/j.jphotobiol.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 84.Korbelik M., Parkins C.S., Shibuya H., Cecic I., Stratford M.R.L., Chaplin D.J. Nitric oxide production by tumour tissue: impact on the response to photodynamic therapy. Br. J. Cancer. 2000;82:1835–1843. doi: 10.1054/bjoc.2000.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carneiro Z.A., de Moraes J.C., Rodrigues F.P., de Lima R.G., Curti C., da Rocha Z.N., Paulo M., Bendhack L.M., Tedesco A.C., Formiga A.L., da Silva R.S. Photocytotoxic activity of a nitrosyl phthalocyanine ruthenium complex—a system capable of producing nitric oxide and singlet oxygen. J. Inorg. Biochem. 2011;105:1035–1043. doi: 10.1016/j.jinorgbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Giles N.M., Kumari S., Gang B.P., Yuen C.W., Billaud E.M., Giles G.I. The molecular design of S-nitrosothiols as photodynamic agents for controlled nitric oxide release. Chem. Biol. Drug Des. 2012;80:471–478. doi: 10.1111/j.1747-0285.2012.01420.x. [DOI] [PubMed] [Google Scholar]
- 87.Kumari S., Sammut I.A., Giles G.I. The design of nitric oxide donor drugs: s-nitrosothiol tDodSNO is a superior photoactivated donor in comparison to GSNO and SNAP. Eur. J. Pharmacol. 2014;737:168–176. doi: 10.1016/j.ejphar.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 88.Rapozzi V., Ragno D., Guerrini A., Ferroni C., Pietra E.D., Cesselli D., Castoria G., Di Donato M., Saracino E., Benfenati V., Varchi G. Androgen receptor targeted conjugate for bimodal photodynamic therapy of prostate cancer in vitro. Bioconjug. Chem. 2015 doi: 10.1021/acs.bioconjchem.5b00261. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 89.Bonavida B., Baritaki S. Dual role of NO donors in the reversal of tumor cell resistance and EMT: downregulation of the NF-kappa B/Snail/YY1/RKIP circuitry. Nitric Oxide. 2011;24:1–7. doi: 10.1016/j.niox.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Rapozzi V., Della Pietra E., Zorzet S., Zacchigna M., Bonavida B., Xodo L.E. Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide. 2013;30:26–35. doi: 10.1016/j.niox.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 91.Della Pietra E., Simonella F., Bonavida B., Xodo L.E., Rapozzi V. Repeated sub-optimal photodynamic treatments with pheophorbide a induce an epithelial mesenchymal transition in prostate cancer cells via nitric oxide. Nitric Oxide. 2015;45:43–53. doi: 10.1016/j.niox.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Yeung K.C., Rose D.W., Dhillon A.S., Yaros D., Gustafsson M., Chatterjee D., McFerran B., Wyche J., Kolch W., Sedivy J.M. Raf kinase inhibitor protein interacts with NF-κB-inducing kinase and TAK1 and inhibits NF-κB activation. Mol. Cell. Biol. 2001;21:7207–7217. doi: 10.1128/MCB.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reynaert N.L., Ckless K., Korn S.H., Vos N., Guala A.S., Wouters E.F., van der Vliet A., Janssen-Heininger Y.M. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rapozzi V., Umezawa K., Xodo L.E. Role of NF-kappaB/Snail/RKIP loop in the response of tumor cells to photodynamic therapy. Lasers Surg. Med. 2011;43:575–585. doi: 10.1002/lsm.21095. [DOI] [PubMed] [Google Scholar]
- 95.Bonavida B., Baritaki S. The novel role of yin yang 1 in the regulation of epithelial to mesenchymal transition in cancer via the dysregulated NF-κB/snail/YY1/RKIP/PTEN circuitry. Crit. Rev. Oncogenes. 2011;16:211–226. doi: 10.1615/critrevoncog.v16.i3-4.50. [DOI] [PubMed] [Google Scholar]