Abstract
Resistance management for Bt-transgenic crops relies in part on the production of sufficient numbers of susceptible insects in non-toxic refuges. Simulation models suggested that source-sink dynamics could interact with the structure of refuges to impact the production of insects in these areas. We tested the hypothesis that altering isolation between refuges and transgenic cotton by manipulating the width of refuges embedded within cotton fields would alter the density of Heliothis virescens and Helicoverpa zea eggs oviposited in refuges. Three categories of refuge widths were tested over two years: they included narrow (16–24m wide), medium (32-48m wide) and wide (80–96m wide) refuges. Isolation between the two habitats increased as refuge width increased. In 1996, eggs of H. virescens from H. zea were not distinguished, but a significant increase in the density of eggs and a significant decrease in relative yield (refuge yield compared to the yield from immediately surrounding Bt-cotton) was found as refuge width increased. In 1997, eggs from H. virescens were analyzed separately from H. zea using an ELISA test. The density of H. virescens eggs increased with increasing refuge width, and there was a significant decline in density of H. virescens eggs with increasing distance from the refuge. In contrast, there was no impact of refuge width on the density of H. zea eggs, nor was the slope of a regression of egg density and distance from the refuge significantly different from zero. We suggest that these differences reflect differences in the biology of the two insects.
Keywords: Resistance management, Bacillus thuringiensis, Heliothis virescens, Helicoverpa zea
Introduction
Genetically modified plant-pesticidal crops with constitutive expression of insecticidal protein (such as the Cry proteins derived from the bacterium Bacillus thuringiensis) require some form of resistance management program to delay the rapid evolution of resistance in targeted insects to expressed toxins. Because Bt-toxins have substantial sub-lethal effects that could hasten resistance, resistance management for these toxins has focused on a high dose-refuge strategy. This strategy relies on plants expressing a sufficiently high dose of the toxin to kill >95% of susceptible and resistant heterozygous insects, while refuges of non-transformed crops allow large numbers of susceptible insects to survive (Gould 1988, Van Rie 1991, Roush 1994). This strategy can delay the evolution of resistance to the crop, if, among other factors, the refuges produce a sufficient number of susceptible insects. The 1998 scientific advisory panel to the Environmental Protection Agency of the U.S.A. suggested that a refuge should produce 500 susceptible insects for each insect expected to survive in the transgenic crop (SAP-EPA 1998). The emphasis has primarily been on size of the refuge (proportion of overall habitat) needed to meet this or equivalent goals, but one alternative is to increase the efficiency of a refuge by producing more susceptible insects per unit area. This could be accomplished by the use of alternative hosts such as pigeon pea and velvetleaf (Craig 1998).
Caprio (2001) suggested that by altering the spatial structure of refuges one could impact the degree of isolation between refuges and transgenic fields and affect the impact of source-sink dynamics. Source-sink dynamics describe ecosystems in which some populations have net emigration and in which there is net population growth (sources) and other populations (sinks) where there is net immigration and in which deaths exceed births (Pulliam 1988). Utilizing computer simulations, Caprio (2001) modeled varying degrees of isolation between refuges and transgenic fields and found two different trends that resulted in an optimal degree of refuge isolation. When refuges were fine-grained (insects experience both habitats almost at random), with little isolation between refuges and transgenic fields, susceptible females emerging in the refuges laid many of their eggs in the most common habitat, transgenic fields, where few offspring would be expected to survive. The transgenic fields became sink habitats for susceptible moths, and fewer susceptible moths were therefore produced in the refuges and the rate of resistance evolution was increased. At the other extreme, if isolation (decreased dispersal between the two habitats) between refuges and transgenic fields was too great, large numbers of moths were produced in refuges, but few of these moths moved into transgenic fields, increasing the likelihood that resistant moths that survived in this toxic habitat would mate with each other. Between these two extremes was an optimal amount of isolation that would delay resistance the longest. Caprio (2001) suggested that isolation between refuges and transgenic fields could be manipulated by altering the width of refuges embedded within the transgenic fields. Small 1 row wide (ca. 1m) refuges represented the minimum amount of isolation, while refuges placed in separate fields would be representative of more isolated refuges. In each case the total proportion of habitat devoted to refuges would remain identical. There would have to be many more narrow width refuges with less transgenic crop between each in the former case (e.g. one embedded refuge for each 20 rows, versus one refuge for every 1200 rows when refuges were 60 rows wide).
The use of relatively narrow (16-100 row wide) refuges embedded into transgenic cotton, comprising 5% of the area, is a compromise between sprayed and unsprayed refuges. Sprayed refuges in the U.S.A. are mandated by the US-EPA to be larger than unsprayed refuges, but may be treated with a non-Bt based foliar product. Sprayed refuges have the potential disadvantage that use of highly efficacious sprays could make these refuges ineffective. Fields with sprayed refuges also utilize two technologies simultaneously, the conventional technology applied to the refuges and the transgenic technology (a high dose transgenic crop produces too few individuals to act as a refuge for the conventionally treated refuges). In essence, the conventional technology is being utilized to protect the transgenic technology. Spatially separate unsprayed refuges are ideal because they are refuges not only for transgenic crops, but also for areas treated with conventional insecticides. Unfortunately, these refuges are easy to manage in a manner different than the surrounding Bt-crop (reduced fertilizer input or irrigation, different planting dates, etc.) and have therefore become a target for minimizing compliance to resistance management guidelines. The embedded refuge concept attempts to deploy refuges within the transgenic crop in small enough units so that growers are unlikely to manage these refuges differently than the surrounding Bt-crop. This has the disadvantage, relative to true unsprayed refuges, that the embedded refuges are sprayed whenever the Bt-crop is sprayed, but attempts to minimize the likelihood of non-compliance with resistance management guidelines.
The Caprio (2001) model predicted, that in systems where pests persist for multiple generations in fields, that as refuges increase in width (but overall proportion remains the same), the density of the population in the refuges should increase. The null hypothesis tested here is that refuge population density is independent of refuge width (assuming that proportion of the total habitat devoted to refuges remains constant) for the two major insect pests the tobacco budworm, Heliothis virescens (Fabricius), and the cotton bollworm, Helicoverpa zea (Boddie) of the Mid-South cotton agroecosystem. We also hypothesize that if insects are emerging primarily from local refuges a decline in egg density should be seen as one moves away from the refuge into the Bt-crop. Alternatively, if insects are moving into fields from outside sources, no relationship should exist between refuges and insect egg density within this crop.
Materials and methods
In each of two years, 1996 and 1997, 3 replicates were used, each replicate consisting of a farm with three different embedded refuge widths, including narrow (16-24 rows wide), medium (32-48 rows wide) and wide (80-96 rows wide) refuges in a randomized design. Each refuge was surrounded with the appropriate amount of Bollgard transgenic cotton expressing the cry 1Ac gene isolated from B. thuringiensis so that the overall proportion of refuge cotton was 4% of the cotton habitat. Because of the large size needed for each replicate (>140 ha), grower fields were used, and actual refuge widths depended on grower planter width. Cotton fields in Mississippi generally remain furrowed throughout the year so that rows are clearly visible to growers prior to planting. This allowed growers to plant their Bt-cotton and then fill in the non-Bt refuges. This particular crop management practice simplified the use of embedded refuges, an advantage that may not be available in many other crops.
At each farm the three refuge widths for a randomized complete block design with were replicated with farms as blocks. Each farm was sampled once per week and these counts were pooled to produce seasonal totals. Four sites were sampled at each refuge, the refuge and three distances away from the refuge. The refuge itself was sampled by randomly selecting plants from two randomly selected rows that were in the central third of the refuge. The other three samples sites at each refuge were in the transgenic cotton at distances of, in 1996, 20, 40 and 60 meters from the refuge edge, while in 1997 the distances were 40, 80 and 120 meters. At each site, 40 randomly selected plants in one row were sampled for the presence of eggs, for a total of 160 plants per refuge. In 1997 eggs were collected and identified to species (budworm or bollworm) using an ELISA test (Zeng et al. 1999). Because this test was not available in 1996, and the eggs are morphologically indistinguishable, no attempt was made to separate budworm from bollworm larvae in that year.
At the end of the season, 3 replicates of 10 row feet from the center of the refuge as well as 10 row feet of the surrounding Bt-cotton (8 rows from the refuge-Bt cotton interface) were harvested by hand for yield estimation. To reduce variance due to local field effects, the ratio of yield of refuge cotton compared to yield of surrounding Bt-cotton was analyzed. Bt-cotton was utilized as a standard (adjusting for local environmental effects such as soil, other pests and pathogens because the two yields were from cottons grown in a similar environment) against which the yield of the refuges could be compared.
Statistical analysis
Analysis of variance was performed on the mean egg density found in each refuge on each farm, with farms and refuge widths as categories. Means were separated with Fisher's LSD procedure. Homogeneity of variance between treatments was tested using a Levene's test (Wilkinson et al. 1996). Linear regression was used to determine the change in average egg density with distance. A slope of the regression line significantly different from zero was considered evidence of an effect of distance on egg density and an indication that eggs were being oviposited by adults originating in the refuges (if the slope was negative).
Results
1996 study
A significant effect of refuge size on combined H. virescens and H. zea egg density was observed over the 1996 sample periods (F=5.49; df=2,30; p=0.026). Wider refuges had higher relative densities of eggs than narrow refuges (Fig. 1). No decline in egg density was recorded with increasing distance from the refuge for the distances sampled in this year (F=0.102; df=1,34; p=0.75; r2=0.003; Fig. 2). There was a significant decline in the relative yield of the refuge cotton compared with the surrounding Bt-cotton as the refuge width increased (F=8.67; df=2,56; p<0.001). The yield in the narrowest refuges was 81% of the yield of surrounding Bt-cotton, while the largest refuges yielded only 71% (Fig. 3).
Figure 1.
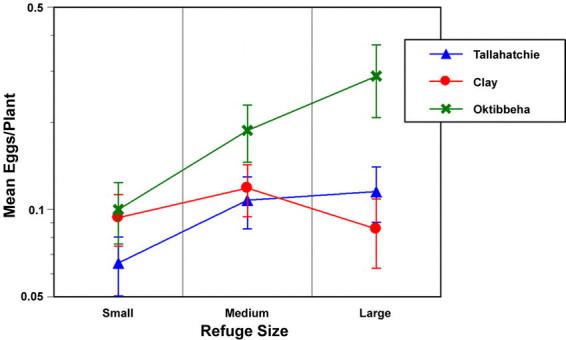
The change in mean (±SEM) egg density as a function of embedded refuge width for three farms in 1996. The small refuges were 16–24 m wide, the medium width refuges were 32–48 m wide, and the wide refuges were 80–96 m wide.
Figure 2.
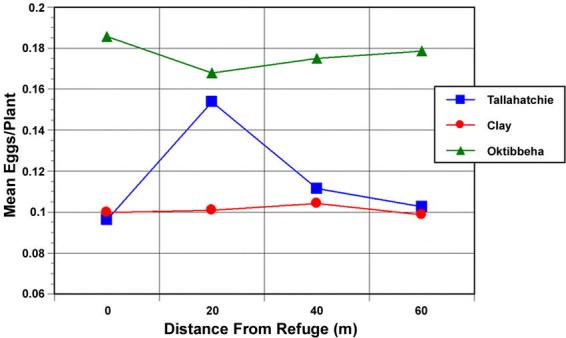
The change in mean (±SEM) heliothine egg density as a function of distance from the refuge edge for three farms in 1996.
Figure 3.
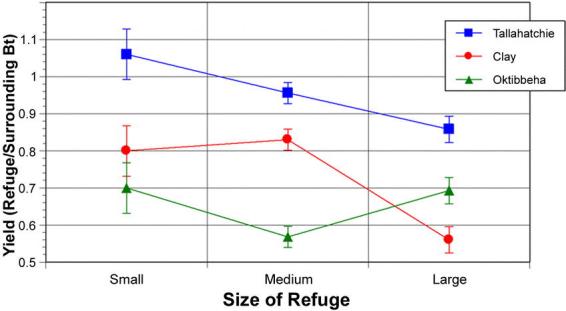
The relationship of relative yield (yield in the refuge relative to immediately surrounding Bt-cotton) to embedded refuge width for three farms in 1996. The small refuges were 16–24 m wide, the medium width refuges were 32–48 m wide, and the wide refuges were 80–96 m wide.
1997 study
Significantly higher H. virescens egg densities were found in larger refuges (F= 3.78; df = 2,36; p = 0.032, Fig. 4). In contrast, H. zea egg densities did not vary with refuge size (F= 1.80; df = 2,36; p = 0.179, Fig. 4). Egg densities for H. virescens decreased with increasing distance from the refuges (density = 35.1 − 0.166 * distance; r2 = 0.295; p = 0.013, Fig. 5), while a regression of egg density on distance from the refuge for H. zea was not significant (density = 29.6 − 0.075 * distance; r2 = 0.084; p = 0.219, Fig. 5). Yield data were not collected in 1997.
Figure 4.
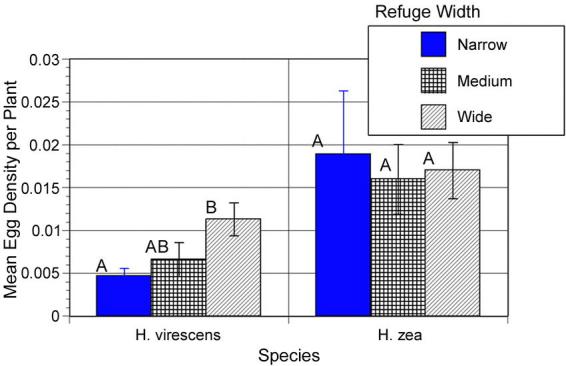
The mean density (± SEM) of H. virescens and H. zea eggs in embedded refuges of three different widths in 1997. The narrow refuges were 16–24 m wide, the medium width refuges were 32–48 m wide, and the wide refuges were 80–96 m wide.
Figure 5.
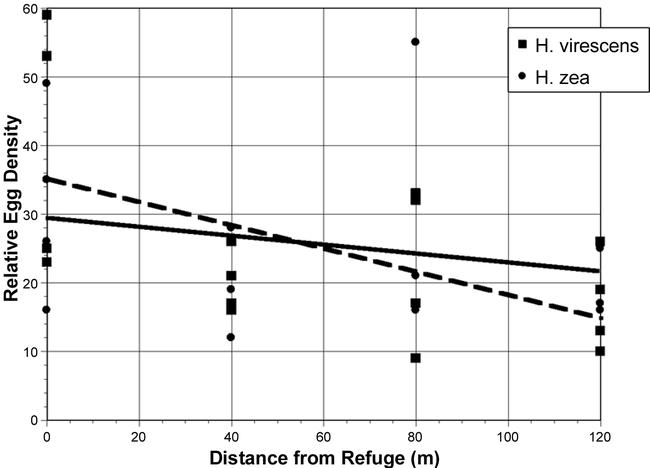
The relationship of egg density to distance from the edge of an embedded refuge for H. virescens and H. zea in 1997. The slope of the H. virescens regression was significantly different from 0, while the slope of the H. zea regression was not.
Discussion
These data support the source-sink hypothesis proposed by Caprio (2001). As predicted by modeling, we found significantly higher egg densities in wider refuges for tobacco budworm, at least for the combined (1996) and the 1997 H. virescens data. Increased isolation between the refuges and Bt-cotton allowed more of the reproductive effort of females emerging in refuges to be expended in the refuges, thus increasing the population of moths in the refuges. Theoretical considerations (Caprio 2001) suggest that this increase in population density would more than compensate for the increased isolation of the refuges. While individual moths emerging in the refuges would have a lower probability of mating with a resistant individual in the Bt-crop, the overall probability that a resistant individual mating with an insect emerging out of the refuge would increase because of the greater refuge population size.
The fine-scale structure of egg density within refuges was not investigated. Larval densities and yield loss are expected to differ within the refuge in relation to distance from the transgenic crop (Alstad and Andow 1995). However, the sampled eggs reflect adult dispersal patterns rather than larval movement patterns and interactions with crop types. Adult H. virescens do not oviposit preferentially on either host type (Parker 1997). Given the distance over which we had to measure outside of the refuges to detect declines in oviposition, we believe it unlikely that egg density varied significantly with refuges and feel that the densities measured reflect mean densities for the entire refuge.
The ability to discriminate and observe the differences between H. zea and H. virescens using the ELISA in the 1997 study revealed important differences in the biology of these two insects. Populations of H. virescens are primarily restricted to cotton for four generations during the summer, with smaller populations on some wild hosts (Stadelbacher et al. 1986). One would then expect that fields are initially infested with moths moving in from springtime wild hosts, but then a substantial proportion of subsequent populations originate in cotton fields. This behavior increases the potential effects of the source-sink dynamics, and is reflected in the significant effects of refuge size and a significant decline in egg density as distance from the refuges increased. H. zea, on the other hand, infests primarily corn during the first two generations of the summer, and only after corn ceases to be a suitable habitat in mid-July do bollworm moths begin to move into cotton and possibly other alternative hosts (Neunzig 1969). Lopez et al. (1995) also found that H. zea is more mobile than H. virescens during mid-summer months in Texas. This biological pattern limits the effects of source-sink dynamics because many of the eggs found in a cotton field will have come from moths dispersing into cotton from other fields or habitats rather than immediately adjacent refuges. This is consistent with the lack of an effect of refuge size for this species, and with the lack of a significant decline in egg density with distance from the refuges.
These data, combined with the results from Caprio (2001), suggest that source-sink dynamics can be important for some species in agricultural systems. The studies suggest that a suitable improvement in the strategy to delay the evolution of resistance to Bt-cotton would be to deploy embedded refuges, strips of non-Bt cotton planted within Bt-cotton. It is essential that such strips be wide enough to generate sufficient isolation between the refuges and Bt-cotton. As a rough guide, the mean proportion of eggs oviposited in refuges by females emerging in the refuges should be the inverse of the mean rate of increase in population size per generation. If the mean rate of increase per generation is 5 fold, the embedded refuges should be wide enough so that at least 20% of the eggs oviposited by females emerging in refuges would be laid in refuges. This would ensure that the population size in the refuges would approximately remain constant over the course of the season. Further empirical tests would be required to validate such strategies prior to deployment. The results presented in this paper suggest that refuges of 24–48 m wide are probably not wide enough to generate this isolation for H. virescens, and embedded refuges should be at least 50m wide.
Deployment of embedded refuges has a number of potential advantages and disadvantages. A disadvantage of conventional unsprayed refuges, which can be physically separated from Bt-fields, is that such refuges may be subject to illicit insecticidal sprays. Embedded refuges could reduce such proscribed spraying by reducing the likelihood of micro-management of pest populations in such refuges, essentially hiding the refuge within the crop at large. However, for pests such as H. zea that have significant survivorship in Bt-fields, a mechanism must exist to allow embedded refuges to be sprayed when the surrounding Bt-fields are sprayed. In these cases, spraying of refuges should occur only when Bt-fields are treated for the targeted pests. In the absence of synergy between the Bt-crop and the applied insecticide, one would expect equal mortality of all genotypes related to Bt-resistance in both Bt-fields and refuges, and the over-spraying should not impact the rate of resistance evolution (though in comparison, an unsprayed refuge, in which the refuge population was not exposed to the insecticide, would actually benefit from applications applied to the Bt-cotton). Embedded refuges, while maintaining some isolation between refuges and Bt-fields, would also help limit the maximal distance between refuges and Bt-plants. Further, in contrast to sprayed refuges, embedded refuges would acts as refuges for all pesticides used for lepidopteran control in cotton (except when Bt-fields are over-sprayed). Because Bt-fields are expected to produce very low numbers of H. virescens, they will not act as refuges for conventional sprayed fields, and one would expect that resistance to conventional insecticides will continue to evolve at a rate almost independent of the presence of plants expressing Bt-toxins.
Sprayed refuges use conventional insecticides to preserve susceptibility to Bt-toxins and utilize two generations of toxins (Bt transgenic plants and conventional foliar insecticides) simultaneously. Unsprayed refuges and, to a lesser degree, embedded refuges, represent a more balanced approach in which transgenics and conventional insecticides each would be capable of managing resistance in the absence of the other. Finally, embedded refuges should provide some advantage to a grower by limiting population growth in refuges and limiting yield loss compared to larger refuges. The optimal size for a refuge will be a compromise between grower desires (narrow refuges and larger yields) and long term resistance management (wider refuges and increased yield loss). Inevitably, however, production of susceptible insects for the management of resistance requires some yield loss.
The data presented here suggest that varying the width of refuges embedded within Bt-cotton can impact the growth rate of H.virescens populations in those refuges. Theoretical work suggests that these changes can significantly impact the rate of resistance evolution to Bt-cotton. Based on these data as well as data on spatial aspects of female reproductive efforts (M.A.C., unpublished data), we suggested that refuges in cotton for H. virescens be between 50 and 96 m wide.
Acknowledgments
We are grateful for the assistance of J. Adamczyk, F. Gould, J. Reed and H. Pitre for reviewing earlier versions of this manuscript. The assistance of Tucker Miller, Wade Worley and Dennis Reginelli was deeply appreciated. This study was supported in part by grant no. 96-35313-3807 USDA-NRI. This is article number J10188 of the Journal Series of the Mississippi Agricultural and Forestry Experiment Station.
References
- Alstad D.N, Andow D.A. Managing the evolution of insect resistance to transgenic plants. Science. 1995;268:1394–1396. doi: 10.1126/science.268.5219.1894. [DOI] [PubMed] [Google Scholar]
- Caprio M.A. Source-sink dynamics between transgenic and nontransgenic habitats and their role in the evolution of resistance. Journal of Economic Entomology. 2001;94:698–705. doi: 10.1603/0022-0493-94.3.698. [DOI] [PubMed] [Google Scholar]
- Craig C.C. 1998 Development of a trap crop for Lygus lineolaris (Heteroptera: Miridae) and a refuge for production of Heliothis virescens (Lepidoptera: Noctuidae) susceptible to cotton expressing insecticidal proteins. M.S. Thesis, Mississippi State University. [Google Scholar]
- Gould F. Genetic engineering, integrated pest management and the evolution of pests. Tree. 1988;3:515–519. doi: 10.1016/0169-5347(88)90131-0. [DOI] [PubMed] [Google Scholar]
- Lopez J.D, Beerwinkle K.R, Witz J.A, and Goodenough J.L. 1995 Spatial and temporal patterns of catches in pheromone traps of Helicoverpa zea Lepidoptera: Noctuidae and Heliothis virescens Lepidoptera: Noctuidae in Central Texas. Southwest Entomologist. Suppl. 18:5–24. [Google Scholar]
- Neunzig H.H. 1969 The biology of the tobacco budworm and corn earworm in North Carolina: with particular reference to tobacco as a host. North Carolina Agricultural Experiment Station Technical Bulletin 196. [Google Scholar]
- Parker C.D. 1997 Effects of mixtures of delta-endotoxin-producing transgenic cotton and ‘Coker 312’ cotton on adult oviposition and larval behavior of Heliothis virscens (Lepidoptera: Noctuidae). M. S. Thesis, Mississippi State University, Mississippi State. [Google Scholar]
- Roush R.T. Managing pests and their resistance to Bacillus thuringiensis: Can transgenic crops be better than sprays? Biocontrol Science and Technology. 1994;4:501–516. [Google Scholar]
- SAP-EPA. 1998 FIFRA Scientific advisory panel, subpanel on Bacillus thuringiensis (Bt) Plant-pesticides and resistance management, February 9 and 10, 1998. Transmittal of the final report. US EPA Docket Number OPP #00231. [Google Scholar]
- Stadelbacher E.A, Graham H.M, Harris V.E, Lopez J.D, Phillips J.R, and Roach S.H. 1986. Heliothis populations and wild host plants in the southern U.S. Southern Cooperative Series Bulletin No. 316; Theory and tactics of Heliothis population management; cultural and biological control. [Google Scholar]
- Van Rie J. Insect control with transgenic plants: resistance proof? Tree. 1991;11:1137–1144. [Google Scholar]
- Wilkinson L, Blank G, and Gruber C. 1996 Desktop data analysis with SYSTAT. Prentice Hall, Upper Saddle River, New Jersey. [Google Scholar]
- Zeng F, Ramaswamy S.B, Luttrell R.G, Reed J, Parker C.D, Stewart S, Harris A, Knighten K, Robbins J, Xia J, Setula C. Comparison of monoclonal antibody and laboratory rearing techniques to identify Heliothentina (Lepidoptera: Noctuidae) eggs from Mississippi cotton fields. Environmental Entomology. 1999;28:275–281. [Google Scholar]


