Abstract
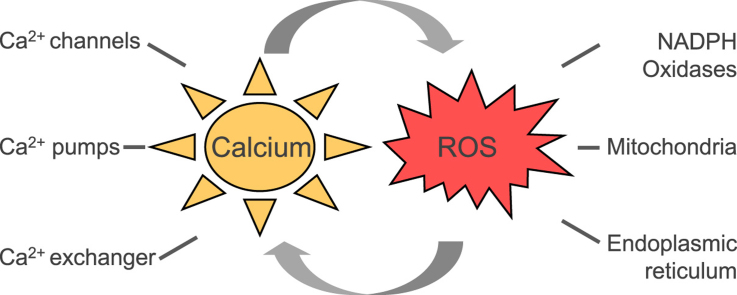
Calcium is an important second messenger involved in intra- and extracellular signaling cascades and plays an essential role in cell life and death decisions. The Ca2+ signaling network works in many different ways to regulate cellular processes that function over a wide dynamic range due to the action of buffers, pumps and exchangers on the plasma membrane as well as in internal stores. Calcium signaling pathways interact with other cellular signaling systems such as reactive oxygen species (ROS). Although initially considered to be potentially detrimental byproducts of aerobic metabolism, it is now clear that ROS generated in sub-toxic levels by different intracellular systems act as signaling molecules involved in various cellular processes including growth and cell death. Increasing evidence suggests a mutual interplay between calcium and ROS signaling systems which seems to have important implications for fine tuning cellular signaling networks. However, dysfunction in either of the systems might affect the other system thus potentiating harmful effects which might contribute to the pathogenesis of various disorders.
Keywords: Calcium, Reactive oxygen species, Mitochondria, NADPH oxidases, Endoplasmic reticulum, Channels
Graphical abstract
Highlights
-
•
Calcium and ROS act as signaling molecules inside the cell and their pathways can interact.
-
•
The mutual interplay of calcium and ROS is required for the fine tuning of signaling.
-
•
Failure in the interplay results in dysfunction and pathologies.
1. Introduction
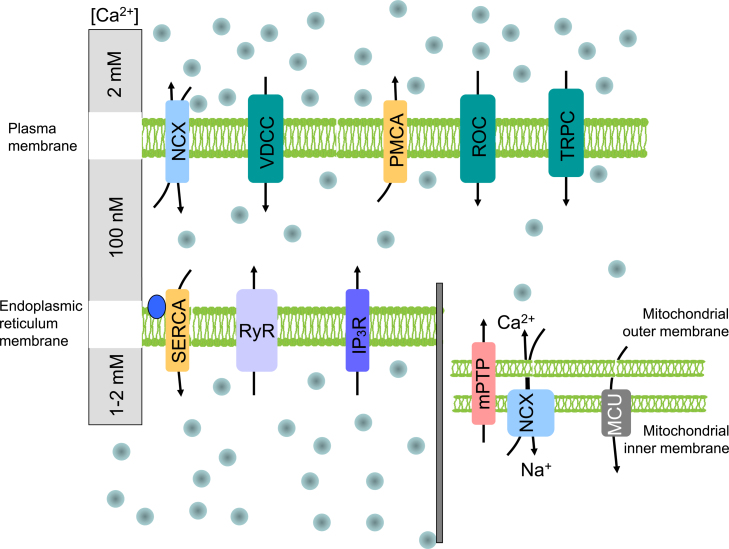
Calcium (Ca2+) as an important second messenger regulates a variety of cellular functions, including contraction, secretion, metabolism, gene expression, cell survival and cell death [14]. Calcium ions enter the cell mostly through transmembrane proteins, called calcium channels. Calcium flow through the calcium channels (either voltage-dependent, or receptor-operated) does not require energy, in contrast to calcium pumps (ATP-ases) that transport calcium from the cells and are ATP-dependent. Part of the intracellular calcium moves to the endoplasmic reticulum (ER) through the sarco/endoplasmic reticulum calcium pump (SERCA) and is released from this store by inositol 1,4,5-trisphosphate (IP3) receptors (IP3R) or ryanodine receptors (RyR) (Fig. 1).
Fig. 1.
Membrane transporters of calcium ions localized in the plasma membrane, the endoplasmic reticulum and in mitochondrial membranes. Calcium channels (illustrated by green boxes) transport calcium ions into the cytoplasm upon changes in membrane potential or ligand binding. Calcium pumps (yellow boxes) transport calcium from the cytoplasm or into the endoplasmic reticulum (ER) and are energy-dependent. Sodium–calcium exchangers belong to antiporters transporting calcium ions against sodium ions. Two receptors that are localized in the ER release calcium from the ER store. In mitochondria, calcium transport is realized through the mitochondrial permeability transition pore, mitochondrial uniporter and sodium calcium exchanger. Abbreviations: NCX – sodium-calcium exchanger; VDCC – voltage-dependent calcium channel; PMCA – plasma membrane calcium ATPase; ROC – receptor operated channels; TRPC – transient receptor potential channel; SERCA – sarco/endoplasmic reticulum calcium ATPase; RyR – ryanodine receptor; IP3R – inositol 1,4,5-trisphosphate receptor; mPTP – mitochondrial permeability transition pore; MCU – mitochondrial uniporter.
In addition, specialized calcium transport systems are also localized in other cellular organelles such as mitochondria, nucleus and Golgi apparatus. Mitochondrial calcium uptake is electrogenic, driven by the large voltage present across the inner mitochondrial membrane developed by the proton pumping by the respiratory chain. In the mitochondria, Ca2+ homeostasis plays a crucial role in cellular physiology and pathophysiology. Mitochondrial Ca2+ uptake controls the rate of energy production, shapes the amplitude and spatio-temporal patterns of intracellular Ca2+ signals, and is instrumental to cell death [110,63,65].
Calcium communicates with a number of other systems and pathways; among them also with reactive oxygen species (ROS), such as superoxide anion (O2•−), hydrogen peroxide (H2O2) and hydroxyl radicals (HO•). ROS at moderate levels represent significant signaling molecules, which are widely involved in physiological processes through oxidizing proteins, lipids and polynucleotides [47,75]. ROS can be generated by various sources: they can be produced as by-products of mitochondrial respiratory chain activity, but also by several extramitochondrial enzymes such as NADPH oxidases, xanthine oxidase, uncoupled nitric oxide synthase, myeloperoxidase, cytochrome P450, cyclooxygenase, and lipoxygenase [127,75]. Interestingly, many of these systems can be modulated by calcium.
In a stark contrast to ROS signaling, Ca2+ exists in only one biologically relevant form that undergoes neither catabolic degradation nor anabolic synthesis. Its biological information-coding ability derives almost entirely from its binding to and unbinding from target proteins as well as from its charge movement to depolarize membrane potential in the form of Ca2+ currents [166].
Interactions among ROS and calcium signaling can be considered as bidirectional, wherein ROS can regulate cellular calcium signaling, while calcium signaling is essential for ROS production [56]. Thus, increased levels of Ca2+ activate ROS-generating enzymes and formation of free radicals. However, the mutual interplay and communication of ROS and calcium is highly dependent on the cell and tissue types. Up to now, this interplay was studied mainly in the cardiovascular system, where ROS and Ca2+ signals converge at dyads, the structural and functional units of cardiac excitation-contraction coupling [166]. Increasing evidence suggests that this cross-talk plays an essential role in many pathophysiological conditions also in other systems, including neurodegenerative diseases such as Parkinson disease and Alzheimer disease, inflammatory diseases, but also cancer [121,131,32,33]. In this review we will discuss the ROS-calcium cross-talk with specific emphasis on mitochondria, the important extramitochondrial ROS source NADPH oxidase, and their interaction with the endoplasmic reticulum.
2. Calcium regulation of ROS formation
2.1. ROS formation
ROS are derived from molecular oxygen by electron transfer reactions resulting in the formation of superoxide anion radical (O2•−), and subsequently hydrogen peroxide (H2O2), either spontaneously, or by the action of superoxide dismutases (SOD). In the presence of iron, superoxide and H2O2 can lead to the formation of highly reactive hydroxyl radicals, which can damage cellular proteins, RNA, DNA and lipids. Interaction of ROS with nitric oxide or fatty acids can lead to the formation of peroxynitrite or peroxyl radicals, respectively, that are also highly reactive. In the presence of chloride, peroxidases can catalyze the generation of hypochlorous acid (HOCl) and singlet oxygen (1O2) from H2O2.
Superoxide is not freely diffusible, but can cross membranes via ion channels. Extracellular superoxide has been shown to enter the cell via the anion blocker sensitive chloride channel-3 [69], while mitochondrial outer membrane´s voltage-dependent anion channels can direct superoxide flux from mitochondria to the cytosol [64]. On the other hand, hydrogen peroxide, which is not a radical, is diffusible over membranes and therefore has been frequently considered to act as a second messenger. Efficient transmembrane diffusion of hydrogen peroxide can be directed by aquaporins, which probably fine tune hydrogen peroxide levels in the cytoplasm, intracellular organelles, and the extracellular space [16]. High ROS levels in the cell can be achieved endogenously (e.g. in several cardiac pathologies), or exogenously (e.g. by administration of some types of chemotherapeutics). There is increasing evidence that in addition to the detrimental effects of high ROS levels exceeding the cellular antioxidant capacity, the cell is able to generate ROS in lower amounts that act as important signaling molecules controlling cell proliferation and cell death, cellular migration, vascular tone, and other cellular functions [75,99].
2.2. Calcium and mitochondrial ROS generation
Although the role of mitochondrial ROS is not completely understood, it is proposed that mitochondrial dysfunction causing excessive ROS production may be a prominent feature of several diseases [2]. Newer evidence suggests that mitochondrial ROS can also act as signaling molecules to activate pro-growth responses [139].
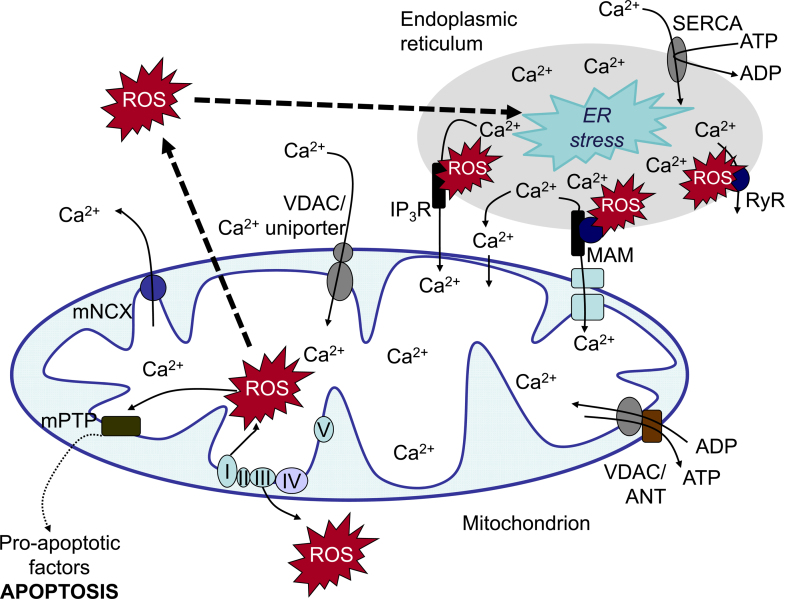
Generation of ROS by mitochondria has been considered for a long time to be only a byproduct of oxidative metabolism in the course of ATP production [151,164]. However, clear evidence exists that mitochondrial ROS might also have a function in signaling within mitochondria or between mitochondria and other organelles [130,34]. Under normal conditions, up to 1% of the electrons flowing to molecular oxygen through the electron transport chain may be diverted to form superoxide. Superoxide can be generated at different sites within the mitochondria [22]. Among them, the ubiquinone-binding sites in complex I (site IQ) and complex III (site IIIQo) of the respiratory chain, glycerol 3-phosphate dehydrogenase, the flavin in complex I (site IF), the electron transferring flavoprotein:Q oxidoreductase (ETFQOR) of fatty acid beta-oxidation, and pyruvate and 2-oxoglutarate dehydrogenases have the highest capacity to generate superoxide. Interestingly, only site IIIQo (on complex III) and glycerol 3-phosphate dehydrogenase can release superoxide into the intermembrane space suggesting that these sites are of a high importance of mitochondrial ROS release into the cytosol ([102], Fig. 2).
Fig. 2.
Calcium and ROS crosstalk between endoplasmic reticulum and mitochondria. The endoplasmic reticulum (ER) is a major site of calcium storage. Calcium from ER cisternae is flowing mainly through calcium release channels as inositol 1,4,5-trisphosphate receptors (IP3R) and ryanodine receptors (RyR). These channels are accumulated in mitochondrial associated membranes (MAMs), which associate with the mitochondrial outer membrane. Calcium ions from the cytoplasm enter the mitochondria through voltage dependent anion channels (VDAC) or calcium uniporter. High levels of calcium stimulate respiratory chain activity leading to higher amounts of reactive oxygen species (ROS). ROS can further target ER-based calcium channels leading to increased release of calcium and further increased ROS levels. Increased ROS and calcium load can open the mitochondrial permeability transition pore (mPTP) resulting in the release of pro-apoptotic factors. Abbreviations: SERCA – sarco/endoplasmic reticulum Ca2+ ATPase; RyR – ryanodine receptors; IP3R – IP3 receptor; VDAC – voltage-dependent anion channel; ANT – adenine-nucleotide transporter; mPTP – mitochondrial permeability transition pore; mNCX – mitochondrial sodium/calcium exchanger.
The metabolic state of the cell has an important impact on ROS production capacity of mitochondria. The chemical nature of the substrates fueling the respiratory chain, the amplitude of the membrane potential in mitochondria (ΔΨm), the pH of the matrix, and the oxygen tension in the surroundings are important factors controlling ROS production in mitochondria [5].
Since Ca2+ primarily promotes ATP synthesis by stimulating enzymes of the Krebs cycle and oxidative phosphorylation in the mitochondria, it has been suggested that the increased metabolic rate would consume more oxygen resulting in increased respiratory chain electron leakage and ROS levels [26]. Indeed, mitochondrial ROS generation correlated with metabolic rate [136]. Under normal conditions, Ca2+ diminished ROS from both complexes I and III while it enhanced ROS generation when these complexes were inhibited by pharmacological agents. One explanation has been that Ca2+ induces a three-dimensional conformation change of the respiratory chain complexes which leads to mitochondrial ROS generation [26].
There is further evidence that the metabolic state of the mitochondria determines the effects of calcium on mitochondrial ROS levels. When the membrane potential is high (no ATP synthesis), Ca2+ uptake results in decreased ROS generation. When the membrane potential is set to a depolarized range (ATP synthesis), ROS generation is stimulated, or not influenced by Ca2+, depending on the amount of the Ca2+ load [1]. When mitochondria are overloaded with Ca2+, ROS production might increase independently of the metabolic state of mitochondria [94].
Mitochondrial permeability transition pore (mPTP) is a voltage and Ca2+-dependent, cyclosporin A sensitive, high conductance channel, whose prolonged opening leads to a brisk increase in the permeability of the inner mitochondrial membrane to solutes with molecular mass up to 1500 Da [13]. As a consequence, a bioenergetic catastrophe occurs: equilibration of the proton gradient causes mitochondrial depolarization, followed by respiratory inhibition and generation of ROS, massive release of matrix Ca2+, and swelling of mitochondria which leads to breaches in the outer mitochondrial membrane that induce the release of intermembrane proteins. Thus, mPTP opening prompts the demise of the cell, and its (dys)regulation turned out to be a crucial step in the pathogenesis of a variety of diverse diseases, encompassing ischemia-reperfusion damage, lysosomal storage diseases, liver damage, many acute and chronic disorders of the central nervous system and cancer (for review see [119]).
2.3. Calcium and NADPH oxidases
The family of NADPH oxidases (NOXes) has been considered unique in that their sole function is to generate superoxide or hydrogen peroxide, respectively, and that they are responsive to receptor stimulation [115,9]. Up to date, this family comprises 7 members, which differ in their catalytic subunits as well as in the requirement of regulatory proteins. The initially identified NADPH oxidase contains the NOX2 core unit, which builds together with the p22phox subunit the cytochrome b558. It is also known as the “respiratory burst” enzyme of neutrophils and is a part of the innate immune response. Upon binding of particles, bacteria, fungi or soluble inflammatory mediators to specific receptors on the neutrophil cell surface, NOX2 is activated and mediates release of large amounts of ROS [108]. This activation is regulated by cytosolic subunits p47phox, p67phox, p40phox and the Rac GTPase, which need to be phosphorylated by calcium activated protein kinase C (PKC) in order to translocate to the plasma membrane and join the NOX2/p22phox complex [30].
The majority of neutrophil-activating receptors induce extracellular calcium entry as an early signaling response to activate effector functions, including phagocytosis, degranulation, and chemotaxis [108]. These membrane receptors induce generation of inositol 1,4,5-trisphosphate (IP3) which activates IP3Rs and Ca2+ release from the intracellular stores which is important for phagocytosis [137]. Depleted stores are reloaded by the sarco/endoplasmic reticulum Ca2+ ATPase SERCA, whereby calcium influx into the cell is enhanced through store-operated calcium channels [23]. This Ca2+ influx is also required for neutrophil ROS generation by stimulating Ca2+-dependent recruitment of S100A8/A9 proteins which act as Ca2+ sensors and can interact with flavocytochrome b558 and p67phox to promote ROS generation [25]. Moreover, Hv1 voltage-gated proton channels have been shown to extrude the protons and compensate the charge generated by NADPH oxidases, thereby enhancing the driving force for extracellular Ca2+ entry and sustaining NADPH oxidase activity [46].
Similar to the NOX2 containing enzyme in neutrophils, NOX1 activity in keratinocytes has been described to be dependent on calcium in response to UVA light [153]. NOX1 activity requires the recruitment of cytosolic activators similar to NOX2, suggesting that calcium might also act in resembling way. Moreover, it has been recently shown that NOX1 can directly be phosphorylated by the calcium activated PKCβ1 suggesting that calcium may via this way enhance NOX1 activity [138].
Apart from these more indirect ways of calcium-dependent NOX activation, the NOX5 as well as the DUOX1 and DUOX2 containing enzymes have been shown to be calcium-binding proteins, which require calcium for ROS generation. NOX5 contains an N-terminal regulatory domain (called NOX5-EF) with four EF-hands. When Ca2+ binds to this domain, hydrophobic residues can interact with the C-terminal catalytic domain and activate the enzyme [7]. Besides of EF-hands, NOX5 can bind calcium-activated calmodulin to the C-terminal domain, leading to a conformational change and increased N-terminal enzymatic activity. Furthermore calcium-activated calcium/calmodulin-dependent kinase II (CAMKII) can positively regulate NOX5 activity via the phosphorylation of Ser475 [111]. Calcium-dependent NOX5 activity has been found to contribute to vascular proliferation and vessel formation [10], to proliferation in different cancer cell lines [3] and also might play a role in kidney disease [76] and in coronary artery disease [61].
Two other family members, dual oxidase 1 (DUOX1) and 2 (DUOX2) have been originally identified in the mammalian thyroid gland. DUOX1 is also highly expressed in airway epithelial cells and DUOX2 in the salivary glands and gastrointestinal tract. Dual oxidases contain an EF-hand calcium-binding cytosolic region similar to that in NOX5 and an N-terminal, extracellular domain with considerable sequence identity with mammalian peroxidases. DUOX enzymes are activated by calcium and release hydrogen peroxide rather than superoxide. In the thyroid, hydrogen peroxide produced by DUOX2 is utilized by thyroperoxidase as an electron acceptor to generate protein-bound iodothyronines (T3 and T4) [109,27,88]. Recently, it was shown that epidermal wounding induces a calcium flash which activates hydrogen peroxide production via DUOX1 and subsequently the recruitment of immune cells to migrate to the wound [122]. Similarly, calcium flashes have been shown to trigger DUOX-dependent hydrogen peroxide in zebrafish after mechanical injury, resulting in leukocyte recruitment [107]. Genetic studies in Drosophila have demonstrated that DUOX can generate microbicidal ROS in the gut epithelia [91].
Recent studies suggested a cross-talk between NADPH oxidases and mitochondrial ROS generation. For example, NOX2 was shown to stimulate mitochondrial ROS production by activating reverse electron transfer in angiotensin-II induced hypertension, while mitochondrial superoxide induced by activation of mitochondrial ATP-sensitive K+ channels has been demonstrated to stimulate NOX2, contributing to the development of endothelial oxidative stress and hypertension [106,43]. Although the exact mechanisms of this cross-talk are not clear yet, these findings might explain some discrepancies found in the literature regarding the sources of ROS. Since both ROS generating systems are sensitive to calcium, they show the importance of the calcium-ROS cross-talk under (patho)physiological conditions.
3. Regulation of calcium homeostasis by reactive oxygen species
The reciprocal interaction between Ca2+ modulated ROS production and ROS modulated Ca2+ signaling underlies the concept of ROS and Ca2+ crosstalk. Thus, in addition to calcium regulating ROS generation, redox state and ROS have been shown to modulate the activity of a variety of Ca2+ channels, pumps and exchangers.
3.1. ROS modulation of plasma membrane Ca2+ channels
Several calcium transporters are localized in the plasma membrane (Fig. 1) and can be regulated by ROS.
Voltage-dependent Ca2+ channels (VDCC) have been described to be redox sensitive due to cysteine residues in the pore forming α1-subunit [101,78]. Activated or inhibited redox status can affect activity, expression, open-time probability, as well as trafficking [149,19]. For example, in guinea pig ventricular myocytes, exogenous ROS suppressed L-type Ca2+ current [54]. Similarly, application of sulfhydryl oxidants inhibited the activity of rabbit smooth muscle L-type Ca2+ channels expressed in chinese hamster ovarian (CHO) cells. Also, free sulfhydryl groups of L-type Ca2+ channels were responsible for ROS induced alterations of the gating process [87].
On the contrary, ROS were shown to stimulate Ca2+ entry through L-type and T-type voltage-gated channels in vascular smooth muscle cells [144]. Application of hydrogen peroxide also increased the current in cells expressing human cardiac L-type α1-subunits in a voltage-dependent manner [78]. Similarly, ROS derived from NOX1 NADPH oxidase have been shown to be involved in Ca2+ mobilization in smooth muscle cells, in part through regulation of Ca2+ influx by L-type calcium channels in response to thrombin [168].
Disunity in the redox regulation of L-type calcium channels might be due to the extensive phosphorylation of this channel by different kinases, which are activated by ROS and might at least partially counterbalance the inhibitory effects of direct ROS oxidation of this channel [126]. However, differences in source, species, amount and timing of ROS might also contribute to variable ROS effects on L-type calcium channels.
Receptor-induced Ca2+ signals are crucial to the function of all cells and involve both the release of Ca2+ from its stores and the entry of Ca2+ through plasma membrane channels. Two major types of channel proteins appear to be involved in receptor-induced Ca2+ entry signals; members of the family of transient receptor potential (TRP) channels and the store-operated Ca2+ channels (SOC) mediated by the widely expressed Orai channel proteins [155].
Members of the TRP superfamily are nonselective cation channels, which carry predominantly Ca2+ ions. Similar to VDCC, TRP channels are a part of the superfamily of six transmembrane spanning cation channels, but they lack the voltage sensitivity. According to their mechanism of activation and presence of regulatory domains in the N- and C-termini, subtypes are classified as the classical or canonical TRP (TRPC1–TRPC7), vanilloid-receptor-related TRP (TRPV1–TRPV4), and melastatin-related TRP (TRPM1–TRPM8) channels [42].
Members of all three subfamilies have been associated with redox regulation and oxidative stress [146]. TRPM2 channel was the first identified ROS-sensitive TRP channel. Activation by hydrogen peroxide was suggested to be mediated by ADP-ribose (ADPR) and cyclic ADPR which interact within a binding cleft in the C-terminal NUDT9-H domain of TRPM2 [113,66]. This pathway has been related to cell death [66], to insulin secretion in pancreatic β-cells [152] and to chemokine production in monocytes [162].
In the endothelium, oxidant mediated disruption of cholesterol-rich lipid rafts was able to activate TRPC3 and TRPC4 channels [116] suggesting the involvement of this pathway in atherosclerosis [154]. Endothelial NOX2-generated ROS triggered Ca2+ influx via TRPC6 in response to pulmonary ischemia-reoxygenation [159]. Furthermore, NOX4 has been shown to enhance TRPC1 and 6 expression resulting in enhanced proliferation in response to BMP4 in pulmonary artery smooth muscle cells suggesting an involvement of this pathway in pulmonary hypertension [80].
While reducing agents such as dithiotreitol have also been shown to activate TRPC5 by cleaving a disulfide bridge in the predicted extracellular loop adjacent to the ion selectivity filter [161], TRPV1 channels were activated by oxidizing agents thereby sensitizing TRPV1 to pH alterations which seems to play a role in heat or pain sensation [142].
Oxidants have also been shown to modulate store-operated Ca2+ entry (SOCE) which is regulated by translocation of the ER Ca2+ sensors STIM1 and STIM2 (stromal interaction molecule 1/2) to the plasma membrane where they bind and activate primarily the Ca2+-permeable Orai channels to initiate calcium entry and store refilling [133,20]. While hydrogen peroxide induced S-glutathionylation of STIM 1 at cysteine 56, resulting in clustering of full length STIM1 and activation of SOCE [60], STIM1 oligomerization and SOCE have been shown to be negatively modulated by the ER oxidoreductase ERp57 [70]. Orai has, similar to TRPC, been suggested to be redox-sensitive. A reactive cysteine in Orai1 may serve as a detection system primarily for changes in the extracellular oxidative environment [19]. While STIM1 and Orai1 have been shown to contribute to ROS generation by NOX2 [24], NOX2 has also been shown to drive Ca2+ signaling via STIM1 and SOCE thereby promoting vascular barrier dysfunction and sepsis-induced acute lung injury [53].
3.2. ROS regulation of intracellular calcium channels
Major calcium release channels from sarcoplasmic/endoplasmic reticulum (SR/ER) are ryanodine receptors (RyR) in excitable cells and inositol 1,4,5-trisphosphate receptors (IP3R) in non-excitable cells (Figs. 1 and 2).
RyR are present in skeletal (RyR1 isoform) and cardiac (RyR2 isoform) muscle cells, where they evoke muscle contractions, but also in the brain (RyR3). These large proteins form tetramers in the SR and ER membranes and contain many cysteins, thus making them good targets for redox regulation [100]. Shifts in the ratios of cellular redox buffers, such as the GSH/GSSG redox pair and the NADH/NAD+ redox pair, can modulate channel function [71]. ROS can directly modulate RyR activity by oxidizing redox-sensing thiol groups [167,85]. Oxidation of RyR thiols enhances channel activity, augments SR Ca2+ leak [147] and increases Ca2+ spark frequency [163]. In skeletal muscle, modification of the redox sensitive Cys3635 by S-nitrosylation caused RyR1 activation and protection from calmodulin binding-mediated inhibition at high Ca2+ concentrations [6].
RyRs were shown to be regulated by NADPH oxidases [167,48,72]. Specifically, SR based NOX4 co-immunoprecipitated with RyR1, thereby locally modulating its Ca2+ release activity [140]. In Langendorff perfused rat hearts mild oxidative stress enhanced the Ca2+ response of RyR2 channels and this process was mediated by NOX2 [44]. In cardiomyocytes, stretch induced NOX2 dependent ROS production in the sarcolemmal and t-tubule membranes which sensitized nearby RyR2 in the SR to trigger a burst of Ca2+ sparks, resulting in the induction of arrhythmogenic Ca2+ waves [117]. NOX2 dependent ROS generation activated RyR1 by S-glutathionylation in skeletal muscle cells [72] leading to insulin-dependent GLUT4 translocation [35]. In the mouse model for Duchenne muscular dystrophy, elevated ROS signaling through divergent Ca2+ derived from the SR cooperated to cardiomyopathy [117]. In addition, mitochondria-derived ROS have recently been connected to the oxidation of RyRs [21,74].
Post-translational modifications of RyRs by ROS that destabilize interdomain interactions within RyRs [104] have been implicated in alterations of Ca2+ homeostasis in conditions accompanied by oxidative stress, such as heart failure or myocardial infarction [11,147,62]. Age-associated increase in the rate of ROS production by mitochondria leads to a thiol-oxidation of RyRs, which results in hyperactivity of RyRs and thereby shortened refractoriness of Ca2+ release in cardiomyocytes from the ageing heart [59]. Furthermore, the arrhythmogenic effect of cardiac glycosides has been linked to the generation of mitochondrial ROS and oxidation of RyRs [74].
IP3Rs are the primary Ca2+ release channel in the endoplasmic reticulum in nonexcitable cells and constitute a minor proportion of SR Ca2+ release channels in cardiac cells (for review see [86]). ER Ca2+ release via IP3R is initiated by binding of the signaling molecule inositol 1,4,5-trisphosphate (IP3). Three different IP3R isoforms are expressed in different amounts in various cells, and the different isoforms are capable of forming homo- and heterotetramers [81].
Various exogenously added oxidants, e.g. thimerosal [83], t-butylhydroperoxide [17], and diamide [95] can stimulate IP3R-mediated Ca2+ release. In the case of thimerosal, the proposed mechanism involved an increased sensitivity of IP3R to IP3 by modifying cysteine residues and thereby stabilizing an active conformation of the receptor [83] resulting in Ca2+ oscillations at the ambient concentration of IP3 present in unstimulated cells. Although sensitization to IP3 may be a general mechanism applicable to other oxidants, it has also been suggested that they may alter the Ca2+ sensitivity of the receptor. Diamide promoted Ca2+ release from IP3-sensitive internal Ca2+ stores and elevated basal Ca2+ levels due to diamide-induced S-glutathionylation of the IP3R and the plasmalemmal Ca2+-ATPase Ca2+ pump, respectively, in endothelial cells [95]. Sensitization of IP3R to IP3 through modulation of thiol groups has also been observed in response to ROS derived from xanthine oxidase [8]. Moreover, S-glutathionylation of IP3R1 was increased in endothelial cells challenged with hydrogen peroxide [96]. It has been shown that insulin induced activation of NOX increased IP3R activity and Ca2+ release in skeletal muscle [49]. Furthermore, the ER-resident oxidoreductase, ERp44 inhibited Ca2+ release by IP3R [73].
3.3. ROS modulation of Ca2+ATPases
The sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) is importantly involved in refilling calcium stores in the ER/SR (Fig. 2). Three SERCA isoforms are expressed differentially in various tissues. SERCA1 occurs predominantly in fast skeletal muscle, SERCA2a is expressed in slow skeletal and cardiac muscle. The SERCA2b isoform is found mostly in smooth muscle cells, while non-muscle cells express SERCA3.
The redox state of specific cysteine residues of SERCA is important for enzymatic function, so that modifications of different SERCA cysteine residues may result in both inhibition and activation of the protein [132]. Thiol oxidizing compounds and ROS inhibit SERCA Ca2+ pumping activity, while reducing agents including dithiothreitol and GSH stimulate SERCA [167]. For its proper function, SERCA is dependent on ATP hydrolysis. ROS have been indicated to prevent ATP binding to SERCA, thus uncoupling ATP hydrolysis from Ca2+ pumping [132,36]. Cys674 appears to play a prominent role in the redox control of SERCA activity: VEGF induction of NOX2 and NOX4 increased SERCA activity by reversible S-glutathionylation resulting in enhanced endothelial migration [50]. In contrast, irreversible oxidative sulfonation of this residue by exposure to high glucose or in the senescent heart was associated with decreased SERCA activity [118,148].
The plasma membrane Ca2+ATPase (PMCA) is a much slower pump compared to SERCA. PMCA belongs to the major calcium extrusion systems in a variety of cells and has been found to be very sensitive to oxidative stress [165]. In neurodegenerative diseases such as Alzheimer disease or Parkinson disease oxidative modification of one or more cysteines has been indicated to disrupt the structural coupling between ATP binding and hydrolysis and to reduce PMCA activity [165]. Oxidative modification and subsequent degradation of the PMCA protein has been found in models of global ischemia-reperfusion injury and seizures [15].
4. Mitochondrial ROS and calcium
Mitochondrial Ca2+ homeostasis plays an important role in cellular physiology and pathophysiology. Balanced calcium uptake by the mitochondria is essential: at appropriate levels, it can stimulate important metabolic processes, such as activation of mitochondrial dehydrogenases, but higher mitochondrial calcium can be detrimental for a cell, by initiating cell death pathways, such as apoptosis and necrosis [110,63,65]. The outer mitochondrial membrane is highly permeable to Ca2+, primarily through the non-specific voltage-dependent anion channel, which is also used to expulse superoxide from the mitochondria, whereas the Ca2+ permeability of the inner membrane is orders of magnitude lower, thus rendering the inner mitochondrial membrane rate-limiting for Ca2+ influx into the mitochondrial matrix [51].
Mitochondrial Ca2+ uptake is electrogenic, driven by the large voltage present across the inner mitochondrial membrane (ΔΨm) developed by proton pumping by the respiratory chain [12]. Patch clamp electrophysiology of isolated mitoplasts (mitochondria with the outer membrane removed) demonstrated that Ca2+ influx was mediated by a highly Ca2+ selective ion channel [84]. Recently, a mitochondrial calcium uniporter (MCU) was identified as an ion-conducting pore of the uniporter [31,41]. The driving force for calcium uptake by the MCU was found to be the steep negative membrane potential established by the respiratory chain [124]. The MCU has two transmembrane domains, and is localized to the inner mitochondrial membrane. Recently, the mitochondrial matrix was identified as the site of both the N- and C-termini. MCU appears to oligomerize within the mitochondrial inner membrane as part of a larger molecular weight complex, consistent with the presence of MICU1 and MICU2, its major regulators (for review see [67]).
In cardiomyocytes, mitochondria-derived ROS have been found necessary to maintain spontaneous RyR2-mediated Ca2+ spark activity while excessive mitochondrial ROS production exerted a bidirectional regulation of Ca2+ spark activity in a dose- and time-dependent fashion supporting a mitochondrial control of SR Ca2+ release [163].
Various pro-oxidant agents are able to induce mPTP opening as a key effect to promote cellular death [119]. Oxidants increase intracellular Ca2+ release from the endoplasmic reticulum and inhibit Ca2+ extrusion from the plasma membrane [29]. The increase in Ca2+ results in transient opening of the mPTP to protect cells against cytosolic Ca2+ overload as has been observed during ischemia. However, in the reperfusion period ROS can lead to a sustained mPTP opening resulting in cell death[169]. Tumor cells can desensitize the mPTP to Ca2+ [169] and ROS thereby increasing their resistance to death [119]. Therefore, the mPTP could be a target for anticancer chemotherapeutics.
The sensitivity of the mPTP to both, ROS and Ca2+ overload, suggests the presence of an amplification loop that is generated by the impairment of either Ca2+ or ROS signaling that triggers activation of the mPTP and outer mitochondria membrane permeabilization (Fig. 2). This amplification loop could be strengthened by means of ROS-induced ROS release, or Ca2+-induced Ca2+ release, that then propagate throughout the mitochondrial population [4].
Excess generation of mitochondrial ROS and cytosolic calcium accumulation plays a major role in the initiation of programmed cell death during acute myocardial infarction. During ischemia, calcium handling between the sarcoplasmic reticulum and myofilament is disrupted and calcium is diverted to the mitochondria causing swelling. Reperfusion, while essential for survival, reactivates energy transduction and contractility and causes the release of ROS and additional ionic imbalance. During acute ischemia–reperfusion, the principal death pathways are programmed necrosis and apoptosis through the intrinsic pathway, initiated by the opening of the mPTP and outer mitochondrial membrane permeabilization, respectively [157]. An emerging body of evidence indicates that the generation of ROS by mitochondria plays a critical role in damaging cellular components and initiating cell death under ischemia-reperfusion conditions [167,82].
Dysfunctional mitochondrial ROS and calcium have been also implicated in the pathogenesis of neurodegenerative diseases. The role of ROS in the regulation of mitochondrial remodeling was studied in primary astrocytes. Changes in mitochondrial morphology induced by calcium occur predominantly through ROS-mediated remodeling [39]. Compared to somatic regions, dendritic regions exhibited a smaller degree of mitochondrial Ca2+ uptake, lower fold-induction of NADH and larger reduction in ATP levels. Collectively, these data reveal that dendritic regions of primary neurons are vulnerable to greater energetic and redox fluctuations than the cell body, which may contribute to disease-associated dendritic damage [68].
Dysruption of mitochondrial ROS and calcium homeostasis has been also related to TNF-alpha function indicating an important role in inflammatory diseases such as osteoarthritis or even sepsis [18,32,38]. Moreover, inflammatory responses evoked by dysfunctional mitochondria have also been found at different steps of cancer development [58]. There is increasing evidence that mitochondrial alterations are intricately involved in tumor metabolism resulting in increased ROS generation and abnormal calcium handling with consequences for tumor growth, survival and metastasis [125]. In fact, mitochondria have been now identified as an interesting target for tumor therapy [158].
5. Endoplasmic reticulum stress, calcium and ROS
The endoplasmic reticulum (ER) is a huge organelle, which forms a membranous network inside the cell and serves for proper assembly and folding of nascent proteins. In order to accomplish these functions, the ER lumen possesses a unique environment with molecular chaperones, folding enzymes, high concentrations of ATP and calcium and, at the same time, offering an oxidizing environment for intra- and intermolecular disulfide bond formation. Perturbations in ER function trigger a process named “ER stress”, a tightly orchestrated collection of intracellular signal transduction reactions designed to restore protein homeostasis in the so-called unfolded protein response (UPR) [128,57]. If the ER stress response is exaggerated, pro-apoptotic cellular cascades are activated.
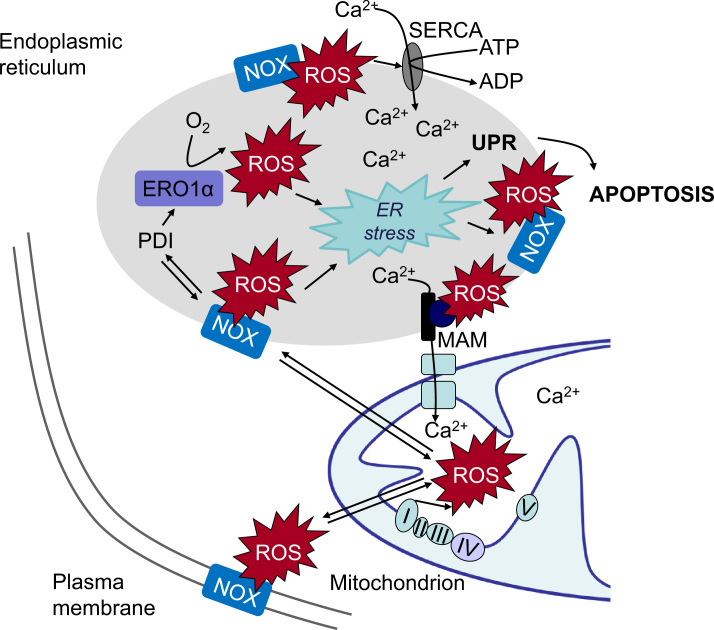
Increases in ROS and rapid decreases in Ca2+ concentrations in the ER lumen belong to common features of cellular ER stress and UPR activation (Fig. 3). With respect to early signals leading to ER stress, calcium is an optimal candidate for rapid and extensive changes of diverse pathways. The ER is the main intracellular calcium store containing roughly 2 mM total Ca2+, corresponding to a free Ca2+ concentration of about 500 μM, a concentration which is much higher than the free Ca2+ concentration in the cytosol [134]. Thus, the ER can act as a major buffering system that functions as a sink for Ca2+ storage. Loss of calcium ions during ER stress is mediated through activation/expression of calcium handling proteins localized in the ER and through calcium release in the cytosol via IP3R or RyR [40]. Both, IP3R and RyR are sensitive to ROS, as well as to Ca2+. Thus ROS- as well as calcium-induced calcium release can set up propagated calcium waves [32].
Fig. 3.
ROS and calcium crosstalk can induce ER stress. In the course of oxidative protein folding in the endoplasmic reticulum (ER), reactive oxygen species (ROS) are generated during electron transfer between protein disulfide isomerase (PDI) and endoplasmic reticulum oxidoreductin-1 (ERO1α). PDI can also associate with ROS-generating NADPH oxidases (NOX), further increasing ROS levels in the ER. NOX-derived ROS can modulate SERCA activity thus increasing ER calcium levels, contribute to ER stress and activate the unfolded protein response (UPR). ROS increase calcium release to the mitochondria which activates ROS generation in the mitochondrial respiratory chain. Mitochondrial ROS can affect NOX thus further increasing ROS and calcium load in the ER resulting in a vicious circle ultimately leading to apoptosis. Abbreviations: SERCA – sarco/endoplasmic reticulum Ca2+ ATPase; MAM – mitochondria-associated ER membranes; NOX NADPH oxidase; PDI – protein disulfate isomerase; ERO1α – endoplasmic reticulum oxidoreductin-1.
Approximately 25% of ROS generated in the cell are derived from the ER [150] mainly required for oxidative protein folding and the formation of disulfide bonds among cysteines. During protein overload, ROS are generated in the ER as a part of an oxidative folding process during electron transfer between protein disulfide isomerase (PDI) and endoplasmic reticulum oxidoreductin-1 (ERO1α) [97]. Electrons are transferred from substrate's thiol groups through PDI and ERO1α to molecular oxygen and produce hydrogen peroxide as a by-product (Fig. 3). PDI directly accepts electrons, resulting in the oxidation of cysteine residues and the formation of disulfide bonds. ERO1α then uses a flavin-dependent reaction to transfer electrons from PDI to molecular oxygen, thereby oxidizing PDI. Specific and limited PDI oxidation by ERO1α is essential to avoid ER hyperoxidation. Under normal physiological conditions, the ER forms an oxidizing environment and Ca2+ stores are filled, allowing proper function of the various chaperones [57]. During ER stress ERO1α is upregulated in a CHOP-dependent manner, leading to ER hyperoxidation. Since conformation of the third luminal loop of the IP3R depends on the oxidation state, hyperoxidation could disrupt the interaction between ERp44 and IP3R, causing IP3R hypersensitivity, increased calcium release and induction of apoptosis [92].
The ER redox environment is further characterized by low levels of reduced glutathione (GSH). GSH is consumed during reduction of unstable and improperly formed disulfide bonds resulting in a GSH/GSSH ratio of 1:1 to 3:1 in the ER, compared to the cellular ratios which vary from 30:1 to 100:1 [32].
In addition, the NOX2, NOX4 and NOX5 NADPH oxidases have been found localized in the ER where they are processed and activated [10,114,89]. PDI has been shown to be able to interact in particular with NOX1 and NOX2 suggesting an involvement of NADPH oxidases in ER stress related ROS generation [79]. In support, NOX2 has been related to ER stress induced apoptosis and renal dysfunction [93], and NOX4 was found to mediate the UPR in response to ER stress, resulting in autophagy [160]. NOX4 has also been suggested to participate in ER stress responses and to possibly contribute to the hyperoxidative ER environment that triggers upstream UPR signals [129] (Fig. 3).
Although the ER and mitochondria play distinct cellular roles, these organelles also form physical interactions with each other at sites defined as mitochondria-associated ER membranes (MAMs), which are essential for calcium, lipid and metabolite exchange [105]. A number of molecular entities have been described to support the physical interaction between the ER and mitochondria. VDAC was shown to be linked to the IP3R in the ER through the molecular chaperone glucose-regulated protein 75 (GRP-75). Functional interaction between the channels was demonstrated to enhance Ca2+ accumulation in mitochondria [143]. Since VDAC are also able to transport superoxide anions, and the IP3R has been shown to be redox-sensitive, a regulatory role of ROS and the ER-mitochondria cross-talk via calcium might be envisaged. ROS that are produced in the mitochondria and in the ER/SR can exert local control of the Ca2+ transport by the SR (Fig. 2). In yeast, dysfunctional mitochondria have been shown to promote the loss of redox homeostasis and ROS accumulation in the ER by the NADPH oxidase Ynop1 [103,90].
During Ca2+ overload, calcium influx increases in mitochondria and ER, thereby causing changes in mitochondrial pH and ROS production accompanied by altered mitochondrial membrane potential and opening of the mPTP with subsequent release of cytochrome c, cardiolipin peroxidation, and activation of several calcium-dependent proteins and kinases [135]. Thus, calcium-induced ROS increase and ROS-mediated calcium vulnerability create a self-amplifying loop [112]. However, severe ER stress induces mitochondrial Ca2+ overload, ROS accumulation, and ATP depletion and thus activates mitochondria-dependent apoptosis ([120], Fig. 2). Thus, mutual local interactions between Ca2+ and ROS signaling are likely to occur and control various functions at the SR/ER–mitochondria associations [45].
Increasing evidence suggests that disruption of the calcium-ROS balance at the SR/ER-mitochondria interface might have implications for various disorders. Motor neuron death due to dysregulated ER and mitochondrial ROS and calcium balance is found as an underlying cause in amyotrophic lateral sclerosis [145]. A role for calcium and ROS dysfunction has also been described in other neurodegenerative diseases such as Parkinson’s disease and Alzheimer's disease [28].
Dysfunctional calcium load and oxidative stress via mitochondria and ER has been associated with myocardial infarction and other ischemic diseases. Consequently, cardiomyocytes develop hypertrophy, fibrosis, apoptosis, inflammation and structural cardiac remodeling eventually leading to cardiomyopathy and even heart failure [32]. In the aging heart, increased mitochondrial ROS result in thiol-oxidation of RyR channel hyperactivity and shortened refractoriness of Ca2+ release in cardiomyocytes. This mechanism probably plays an important role in the increased incidence of arrhythmias and sudden death in the ageing population [37].
Dysfunctional ROS and calcium signaling has also been described in diabetes [123] and other metabolic diseases as well as in various inflammatory diseases [32].
There is also increasing evidence that signaling cascades at the ER mitochondrial interface might be involved in tumor progression [98]: for example, the tumor suppressor promyelocytic leukemia protein (PML) is specifically enriched at the ER and MAMs and complexes with IP3R resulting in increased cell survival through ER-mitochondrial Ca2+ and ROS signaling [55]. On the other hand, these pathways may also be used for anticancer approaches. Recently it was shown that hyperthermia induced tumor cell apoptosis via the ROS, ER stress, mitochondria, and caspase pathways [77], and several chemotherapeutic approaches seem to disrupt calcium-ROS signaling in the ER and mitochondria [141,156,52].
Thus, further understanding of the molecular mechanisms underlying these interconnecting pathways which have been associated with numerous diseases, may lead to the discovery of novel therapeutic strategies.
6. Conclusion
Without doubt, ROS and calcium are mutually interconnected. Calcium can increase production of the ROS. On the other hand, ROS can significantly affect calcium influx into the cell and intracellular calcium stores. Improved understanding of the mechanism that fine tune the levels of ROS and calcium within the different cellular organelles could result in novel therapeutic strategies for various diseases affected by dysfunction of the calcium ROS balance.
Acknowledgment
The authors of this review were in part supported by the European Cooperation in Science and Technology (COST Action BM1203/EU‐ROS), DZHK (German Centre for Cardiovascular Research), German Research Foundation (DFG-GO709/4-5), German Federal Ministry of Education and Research (Acidox, Epiros), Vedecká Grantová Agentúra MŠVVaŠ SR a SAV 2/0074 and CEMAN.
Contributor Information
Agnes Görlach, Email: goerlach@dhm.mhn.de.
Olga Krizanova, Email: olga.krizanova@savba.sk.
References
- 1.Adam-Vizi A., Starkov A.A. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimers Dis. 2010;20(Suppl. 20):S413–S426. doi: 10.3233/JAD-2010-100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antony S., Wu Y., Hewitt S.M., Anver M.R., Butcher D., Jiang G., Meitzler J.L., Liu H., Juhasz A., Lu J., Roy K.K., Doroshow J.H. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic. Biol. Med. 2013;65:497–508. doi: 10.1016/j.freeradbiomed.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aon M.A., Cortassa S., Marbán E., O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 5.Aon M.A., Cortassa S., O’Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochim. Biophys. Acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aracena-Parks P., Goonasekera S.A., Gilman C.P., Dirksen R.T., Hidalgo C., Hamilton S.L. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J. Biol. Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- 7.Banfi B., Tirone F., Durussel I., Knisz J., Moskwa P., Molnar G.Z., Krause K.H., Cox J.A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 8.Bánsághi S., Golenár T., Madesh M., Csordás G., Ramachandra Rao S., Sharma K., Yule D.I., Joseph S.K., Hajnóczky G. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem. 2014;289:8170–8181. doi: 10.1074/jbc.M113.504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 10.BelAiba R.S., Djordjevic T., Petry A., Diemer K., Bonello S., Banfi B., Hess J., Pogrebniak A., Bickel C., Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic. Biol. Med. 2007;42:446–459. doi: 10.1016/j.freeradbiomed.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 11.Belevych A.E., Terentyev D., Terentyeva R., Ho H.T., Gyorke I., Bonilla I.M., Carnes C.A., Billman G.E., Gyorke S. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ. Res. 2012;110:569–677. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 13.Bernardi P., Krauskopf A., Basso E., Petronilli V., Blachly-Dyson E., Di Lisa F., Forte M.A. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 14.Berridge M.J. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- 15.Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bienert G.P., Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta. 2014;1840:1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Bird G.S., Burgess G.M., Putney J.W., Jr Sulfhydryl reagents and cAMP-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. J. Biol. Chem. 1993;268:17917–17923. [PubMed] [Google Scholar]
- 18.Blanco F.J., Rego I., Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011;7:161–169. doi: 10.1038/nrrheum.2010.213. [DOI] [PubMed] [Google Scholar]
- 19.Bogeski I., Kummerow C., Al-Ansary D., Schwarz E.C., Koehler R., Kozai D., Takahashi N., Peinelt C., Griesemer D., Bozem M., Mori Y., Hoth M., Niemeyer B.A. Differential redox regulation of ORAI ion channels: a mechanism to tune cellular calcium signaling. Sci. Signal. 2010;3:ra24. doi: 10.1126/scisignal.2000672. [DOI] [PubMed] [Google Scholar]
- 20.Bogeski I., Kilch T., Niemeyer B.A. ROS and SOCE: recent advances and controversies in the regulation of STIM and Orai. J. Physiol. 2012;590:4193–4200. doi: 10.1113/jphysiol.2012.230565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bovo E., Lipsius S.L., Zima A.V. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J. Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bréchard S., Tschirhart E.J. Regulation of superoxide production in neutrophils: role of calcium influx. J. Leukoc. Biol. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bréchard S., Melchior C., Plançon S., Schenten V., Tschirhart E.J. Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44:492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Bréchard S., Plançon S., Tschirhart E.J. New insights into the regulation of neutrophil NADPH oxidase activity in the phagosome: a focus on the role of lipid and Ca(2+) signaling. Antioxid. Redox Signal. 2013;18:661–676. doi: 10.1089/ars.2012.4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brookes PS1 Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 27.Caillou B., Dupuy C., Lacroix L., Nocera M., Talbot M., Ohayon R., Deme D., Bidart J.M., Schlumberger M., Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J. Clin. Endocrinol. Metab. 2001;86:3351–3358. doi: 10.1210/jcem.86.7.7646. [DOI] [PubMed] [Google Scholar]
- 28.Cali T., Ottolini D., Brini M. Calcium and endoplasmic reticulum–mitochondria tethering in neurodegeneration. DNA Cell Biol. 2013;32:140–146. doi: 10.1089/dna.2013.2011. [DOI] [PubMed] [Google Scholar]
- 29.Camello-Almaraz C., Gomez-Pinilla P.J., Pozo M.J., Camello P.J. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2006;291:1082–1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 30.Cathcart M.K. Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages. Arterioscler. Thromb. Vasc. Biol. 2004;24:23–28. doi: 10.1161/01.ATV.0000097769.47306.12. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhuri D., Sancak Y., Mootha V.K., Clapham D.E. MCU encodes the pore conducting mitochondrial calcium currents. Elife. 2013;2:e00704. doi: 10.7554/eLife.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhari N., Talwar P., Parimisetty A., Lefebvre d’Hellencourt C., Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell Neurosci. 2014;8:213. doi: 10.3389/fncel.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinopoulos C., Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 34.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cochemé H.M., Murphy M.P. Mitochondrial redox signalling at a glance. J. Cell Sci. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 35.Contreras-Ferrat A., Lavandero S., Jaimovich E., Klip A. Calcium signaling in insulin action on striated muscle. Cell Calcium. 2014;56:390–396. doi: 10.1016/j.ceca.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Cook N.L., Viola H.M., Sharov V.S., Hool L.C., Schöneich C., Davies M.J. Myeloperoxidase-derived oxidants inhibit sarco/endoplasmic reticulum Ca2+-ATPase activity and perturb Ca2+ homeostasis in human coronary artery endothelial cells. Free Radic. Biol. Med. 2012;52:951–961. doi: 10.1016/j.freeradbiomed.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper L.L., Li W., Lu Y., Centracchio J., Terentyeva R., Koren G., Terentyev D. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J. Physiol. 2013;591:5895–5911. doi: 10.1113/jphysiol.2013.260521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dada L.A., Sznajder J.I. Mitochondrial Ca²+ and ROS take center stage to orchestrate TNF-α-mediated inflammatory responses. J. Clin. Invest. 2011;121:1683–1685. doi: 10.1172/JCI57748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deheshi S., Dabiri B., Fan S., Tsang M., Rintoul G.L. Changes in mitochondrial morphology induced by calcium or rotenone in primary astrocytes occur predominantly through ros-mediated remodeling. J. Neurochem. 2015;133:684–699. doi: 10.1111/jnc.13090. [DOI] [PubMed] [Google Scholar]
- 40.Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 41.De Stefani D., Patron M., Rizzuto R. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta. 2015 doi: 10.1016/j.bbamcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietrich A., Chubanov V., Kalwa H., Rost B.R., Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells .Pharmacol. Ther. 2006;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Dikalov S.I., Nazarewicz R.R., Bikineyeva A., Hilenski L., Lassègue B., Griendling K.K., Harrison D.G., Dikalova A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2012;20:281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donoso P., Finkelstein J.P., Montecinos L., Said M., Sanchez G., Vittone L., Bull R. Stimulation of NOX2 in isolated hearts reversibly sensitizes RyR2 channels to activation by cytoplasmic calcium. J. Mol. Cell. Cardiol. 2014;68:38–46. doi: 10.1016/j.yjmcc.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Eisner V., Csordás G., Hajnóczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle-pivotal roles in Ca2+ and reactive oxygen species signaling. J. Cell Sci. 2013;15:2965–2978. doi: 10.1242/jcs.093609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El Chemaly A., Okochi Y., Sasaki M., Arnaudeau S., Okamura Y., Demaurex N. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J. Exp. Med. 2010;207:129–139. doi: 10.1084/jem.20091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ermak G., Davies K.J. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 48.Espinosa A., Leiva A., Peña M., Müller M., Debandi A., Hidalgo C., Carrasco M.A., Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J. Cell Physiol. 2006;209:379–388. doi: 10.1002/jcp.20745. [DOI] [PubMed] [Google Scholar]
- 49.Espinosa A., García A., Härtel S., Hidalgo C., Jaimovich E. NADPH oxidase and hydrogen peroxide mediate insulin-induced calcium increase in skeletal muscle cells. J. Biol. Chem. 2009;284:2568–2575. doi: 10.1074/jbc.M804249200. [DOI] [PubMed] [Google Scholar]
- 50.Evangelista A.M., Thompson M.D., Bolotina V.M., Tong X., Cohen R.A. Nox4- and Nox2-dependent oxidant production is required for VEGF-induced SERCA cysteine-674 S-glutathiolation and endothelial cell migration. Free Radic. Biol. Med. 2012;53:2327–2334. doi: 10.1016/j.freeradbiomed.2012.10.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foskett J.K., Philipson B. The mitochondrial Ca(2+) uniporter complex. J. Mol. Cell Cardiol. 2015;78:3–8. doi: 10.1016/j.yjmcc.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gajate C., Mollinedo F. Lipid rafts, endoplasmic reticulum and mitochondria in the antitumor action of the alkylphospholipid analog edelfosine. Anticancer Agents Med. Chem. 2014;14 doi: 10.2174/1871520614666140309222259. 509-2. [DOI] [PubMed] [Google Scholar]
- 53.Gandhirajan R.K., Meng S., Chandramoorthy H.C., Mallilankaraman K., Mancarella S., Gao H., Razmpour R., Yang X.F., Houser S.R., Chen J., Koch W.J., Wang H., Soboloff J., Gill D.L., Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Invest. 2013;123:887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gill J.S., McKenna W.J., Camm A.J. Free radicals irreversibly decrease Ca2+ currents in isolated guinea-pig ventricular myocytes. Eur. J. Pharmacol. 1995;292:337–340. doi: 10.1016/0926-6917(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 55.Giorgi C., Ito K., Lin H.K., Santangelo C., Wieckowski M.R., Lebiedzinska M., Bononi A., Bonora M., Duszynski J., Bernardi R., Rizzuto R., Tacchetti C., Pinton P., Pandolfi P.P. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330:1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordeeva A.V., Zvyagilskaya R.A., Labas Y.A. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry (Mosc) 2003;68:1077–1080. doi: 10.1023/a:1026398310003. [DOI] [PubMed] [Google Scholar]
- 57.Görlach A., Klappa P., Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 58.Grek C.L., Tew K.D. Redox metabolism and malignancy. Curr. Opin. Pharmacol. 2010;10:362–368. doi: 10.1016/j.coph.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grivennikova V.G., Kareyeva A.V., Vinogradov A.D. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim. Biophys. Acta. 2010;1797:939–944. doi: 10.1016/j.bbabio.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grupe M., Myers G., Penner R., Fleig A. Activation of store-operated ICRAC by hydrogen peroxide. Cell Calcium. 2010;48:1–9. doi: 10.1016/j.ceca.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guzik T.J., Chen W., Gongora M.C., Guzik B., Lob H.E., Mangalat D., Hoch N., Dikalov S., Rudzinski P., Kapelak B., Sadowski J., Harrison D.G. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gyorke S., Carnes C. Dysregulated sarcoplasmic reticulum calcium release: potential pharmacological target in cardiac disease. Pharmacol. Ther. 2008;119:340–354. doi: 10.1016/j.pharmthera.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hajnóczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 64.Han D., Antunes F., Canali R., Rettori D., Cadenas E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003;278:5557–5563. doi: 10.1074/jbc.M210269200. [DOI] [PubMed] [Google Scholar]
- 65.Hansford R.G. Physiological role of mitochondrial Ca2+ transport. J. Bioenerg. Biomembr. 1994;26:495–508. doi: 10.1007/BF00762734. [DOI] [PubMed] [Google Scholar]
- 66.Hara Y., Wakamori M., Ishii M., Maeno E., Nishida M., Yoshida T., Yamada H., Shimizu S., Mori E., Kudoh J., Shimizu N., Kurose H., Okada Y., Imoto K., Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol. Cell. 2002;9:163–17310. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 67.Harrington J.L., Murphy E. The mitochondrial calcium uniporter: mice can live and die without it. J. Mol. Cell Cardiol. 2015;78:46–53. doi: 10.1016/j.yjmcc.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hasel P., McKay S., Qiu J., Hardingham G.E. Selective dendritic susceptibility to bioenergetic, excitotoxic and redox perturbations in cortical neurons. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbamcr.2014.12.021. 10.1016/j.bbamcr.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawkins B.J., Madesh M., Kirkpatrick C.J., Fisher A.B. Superoxide flux in endothelial cells via the chloride channel-3 mediates intracellular signaling. Mol. Biol. Cell. 2007;18:2002–2012. doi: 10.1091/mbc.E06-09-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawkins B.J., Irrinki K.M., Mallilankaraman K., Lien Y.C., Wang Y., Bhanumathy C.D., Subbiah R., Ritchie M.F., Soboloff J., Baba Y., Kurosaki T., Joseph S.K., Gill D.L., Madesh M. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J. Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hidalgo C., Donoso P., Carrasco M.A. The ryanodine receptors Ca2+ release channels: cellular redox sensors? IUBMB Life. 2005;57:315–322. doi: 10.1080/15216540500092328. [DOI] [PubMed] [Google Scholar]
- 72.Hidalgo C., Sanchez G., Barrientos G., Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J. Biol. Chem. 2006;281:26473–26482. doi: 10.1074/jbc.M600451200. [DOI] [PubMed] [Google Scholar]
- 73.Higo T., Hattori M., Nakamura T., Natsume T., Michikawa T., Mikoshiba K. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 74.Ho H.T., Stevens S.C., Terentyeva R., Carnes C.A., Terentyev D., Gyorke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J. Physiol. 2011;589:4697–4708. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 76.Holterman C.E., Thibodeau J.F., Towaij C., Gutsol A., Montezano A.C., Parks R.J., Cooper M.E., Touyz R.M., Kennedy C.R. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J. Am. Soc. Nephrol. 2014;25:784–797. doi: 10.1681/ASN.2013040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hou C.H., Lin F.L., Hou S.M., Liu J.F. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int. J. Mol. Sci. 2014;15:17380–17395. doi: 10.3390/ijms151017380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hudasek K., Brown S.T., Fearon I.M. H2O2 regulates recombinant Ca2+ channel alpha1C subunits but does not mediate their sensitivity to acute hypoxia. Biochem. Biophys. Res. Commun. 2004;318:135–141. doi: 10.1016/j.bbrc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Janiszewski M., Lopes L.R., Carmo A.O., Pedro M.A., Brandes R.P., Santos C.X., Laurindo F.R. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Q., Fu X.1, Tian L., Chen Y., Yang K., Chen X., Zhang J., Lu W., Wang J. NOX4 mediates BMP4-induced upregulation of TRPC1 and 6 protein expressions in distal pulmonary arterial smooth muscle cells. PLoS One. 2014;9:e107135. doi: 10.1371/journal.pone.0107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joseph S.K., Lin C., Pierson S., Thomas A.P., Maranto A.R. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J. Biol. Chem. 1995;270:23310–23316. doi: 10.1074/jbc.270.40.23310. [DOI] [PubMed] [Google Scholar]
- 82.Kalogeris T., Bao Y., Kotthuis R.J. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs preconditioning. Redox Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan S.R. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J. Urol. 2013;189:803–811. doi: 10.1016/j.juro.2012.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 85.Kourie J.I. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- 86.Krizanova O., Ondrias K. The inositol 1,4,5-trisphosphate receptor-transcriptional regulation and modulation by phosphorylation. Gen. Physiol. Biophys. 2003;22:295–311. [PubMed] [Google Scholar]
- 87.Lacampagne A., Duittoz A., Bolaños P., Peineau N., Argibay J.A. Effect of sulfhydryl oxidation on ionic and gating currents associated with L-type calcium channels in isolated guinea-pig ventricular myocytes. Cardiovasc. Res. 1995;30:799–806. [PubMed] [Google Scholar]
- 88.Lacroix L., Nocera M., Mian C., Caillou B., Virion A., Dupuy C., Filetti S., Bidart J.M., Schlumberger M. Expression of nicotinamide adenine dinucleotide phosphate oxidase flavoprotein DUOX genes and proteins in human papillary and follicular thyroid carcinomas. Thyroid. 2001;11:1017–1023. doi: 10.1089/105072501753271699. [DOI] [PubMed] [Google Scholar]
- 89.Laurindo F.R., Araujo T.L., Abrahão T.B. Nox NADPH oxidases and the endoplasmic reticulum. Antioxid. Redox Signal. 2014;20:2755–2775. doi: 10.1089/ars.2013.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leadsham J.E., Sanders G., Giannaki S., Bastow E.L., Hutton R., Naeimi W.R., Breitenbach M., Gourlay C.W. Loss of cytochrome c oxidase promotes RAS-dependent ROS production from the ER resident NADPH oxidase, Yno1p, in yeast. Cell Metab. 2013;18:279–286. doi: 10.1016/j.cmet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Lee K.A., Kim B., Bhin J., Kim do H., You H., Kim E.K., Kim S.H., Ryu J.H., Hwang D., Lee W.J. Bacterial uracil modulates Drosophila DUOX-dependent gut immunity via Hedgehog-induced signaling endosomes. Cell Host Microbe. 2015;17:191–204. doi: 10.1016/j.chom.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 92.Li G., Mongillo M., Chin K.T., Harding H., Ron D., Marks A.R., Tabas I. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li G., Scull C., Ozcan L., Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X., Fang P., Mai J., Choi E.T., Wang H., Yang X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;25:6–19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lock J.T., Sinkins W.G., Schilling W.P. Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H493–H506. doi: 10.1152/ajpheart.01073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lock J.T., Sinkins W.G., Schilling W.P. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J. Physiol. 2012;590:3431–3447. doi: 10.1113/jphysiol.2012.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malhotra J.D., Kaufman R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manié S.N., Lebeau J., Chevet E. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 3. Orchestrating the unfolded protein response in oncogenesis: an update. Am. J. Physiol. Cell Physiol. 2014;307:C901–C907. doi: 10.1152/ajpcell.00292.2014. [DOI] [PubMed] [Google Scholar]
- 99.Martindale J.L., Holbrook N.J. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 100.Meissner G. Regulation of ryanodine receptor ion channels through posttranslational modifications. Curr. Top. Membr. 2010;66:91–113. doi: 10.1016/S1063-5823(10)66005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 102.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murphy M.P. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell Metab. 2013;18:145–146. doi: 10.1016/j.cmet.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Mochizuki M., Yano M., Oda T., Tateishi H., Kobayashi S., Yamamoto T., Ikeda Y., Ohkusa T., Ikemoto N., Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 105.Naon D., Scorrano L. At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim. Biophys. Acta. 2014;1843:2184–2194. doi: 10.1016/j.bbamcr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 106.Nazarewicz R.R., Dikalova A.E., Bikineyeva A., Dikalov S.I. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H1131–H1140. doi: 10.1152/ajpheart.00063.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Niethammer P., Grabher C., Look A.T., Mitchison T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nunes P., Demaurex N., Dinauer M.C. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic. 2013;14:1118–1131. doi: 10.1111/tra.12115. [DOI] [PubMed] [Google Scholar]
- 109.Ohye H., Sugawara M. Dual oxidase, hydrogen peroxide and thyroid diseases. Exp. Biol. Med. (Maywood) 2010;235:424–433. doi: 10.1258/ebm.2009.009241. [DOI] [PubMed] [Google Scholar]
- 110.Orrenius S., Zhivotovsky B., Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 111.Pandey D.1, Gratton J.P., Rafikov R., Black S.M., Fulton D.J. Calcium/calmodulin-dependent kinase II mediates the phosphorylation and activation of NADPH oxidase 5. Mol. Pharmacol. 2011;80:407–415. doi: 10.1124/mol.110.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng T.I., Jou M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. NY Acad. Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 113.Perraud A.L., Takanishi C.L., Shen B., Kang S., Smith M.K., Schmitz C., Knowles H.M., Ferraris D., Li W., Zhang J., Stoddard B.L., Scharenberg A.M. Accumulation of free ADP-ribose from mitochondria mediates oxidative stress-induced gating of TRPM2 cation channels. J. Biol. Chem. 2005;280:6138–6148. doi: 10.1074/jbc.M411446200. [DOI] [PubMed] [Google Scholar]
- 114.Petry A., Djordjevic T., Weitnauer M., Kietzmann T., Hess J., Görlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid. Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 115.Petry A., Weitnauer M., Görlach A. Receptor activation of NADPH oxidases. Antioxid. Redox Signal. 2010;13:467–487. doi: 10.1089/ars.2009.3026. [DOI] [PubMed] [Google Scholar]
- 116.Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M.X., Romanin C., Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 117.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 118.Qin F., Siwik D.A., Lancel S., Zhang J., Kuster G.M., Luptak I., Wang L., Tong X., Kang Y.J., Cohen R.A., Colucci W.S. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J. Am. Heart Assoc. 2013 doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rasola A., Bernardi P. Reprint of “The mitochondrial permeability transition pore and its adaptive responses in tumor cells”. Cell Calcium. 2015;56:437–445. doi: 10.1016/j.ceca.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raturi A., Simmen T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM) Biochim. Biophys. Acta. 2013;1833:213–224. doi: 10.1016/j.bbamcr.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 121.Raturi A., Ortiz-Sandoval C., Simmen T. Redox dependence of endoplasmic reticulum (ER) Ca2+ signaling. Histol. Histopathol. 2014;29:543–552. doi: 10.14670/HH-29.10.543. [DOI] [PubMed] [Google Scholar]
- 122.Razzell W., Evans I.R., Martin P., Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr. Biol. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rieusset J. Mitochondria and endoplasmic reticulum: mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int. J. Biochem. Cell Biol. 2011;43:1257–1262. doi: 10.1016/j.biocel.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 124.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 125.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sag C.M., Wagner S., Maier L.S. Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes. Free Radic. Biol. Med. 2013;63:338–349. doi: 10.1016/j.freeradbiomed.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 127.Sauer H., Wartenberg M., Hescheler j. Reactive oxygen species as intracellular messenger during cell growth and differentiaton. Cell Physiol. Biochem. 2001;11:173–183. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 128.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos C.X., Nabeebaccus A.A., Shah A.M., Camargo L.L., Filho S.V., Lopes L.R. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxid. Redox Signal. 2014;20:121–134. doi: 10.1089/ars.2013.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma N., Nehru B. Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem. Int. 2015 doi: 10.1016/j.neuint.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 132.Sharov V.S., Dremina E.S., Galeva N.A., Williams T.D., Schöneich C. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochem. J. 2006;394:605–615. doi: 10.1042/BJ20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shen W.W., Frieden M., Demaurex N. Remodelling of the endoplasmic reticulum during store-operated calcium entry. Biol. Cell. 2011;103:365–380. doi: 10.1042/BC20100152. [DOI] [PubMed] [Google Scholar]
- 134.Sienaert I., De Smedt H., Parys J.B., Missiaen L. Regulation of Ca2+-release channels by luminal Ca2. In: Verkhratsky A., Toescu E., editors. Plenum Press; New York: 1998. pp. 131–161. [Google Scholar]
- 135.Smaili S., Hirata H., Ureshino R., Monteforte P.T., Morales A.P., Muler M.L., Terashima J., Oseki K., Rosenstock T.R., Lopes G.S., Bincoletto C. Calcium and cell death signaling in neurodegeneration and aging. An. Acad. Bras. Cienc. 2009;81:467–475. doi: 10.1590/s0001-37652009000300011. [DOI] [PubMed] [Google Scholar]
- 136.Sohal R.S., Allen R.G. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1995;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 137.Steinckwich N., Schenten V., Melchior C., Bréchard S., Tschirhart E.J. An essential role of STIM1, Orai1, and S100A8-A9 proteins for Ca2+ signaling and FcγR-mediated phagosomal oxidative activity. J. Immunol. 2011;186:2182–2191. doi: 10.4049/jimmunol.1001338. [DOI] [PubMed] [Google Scholar]
- 138.Streeter J., Schickling B.M., Jiang S., Stanic B., Thiel W.H., Gakhar L., Houtman J.C., Miller F.J., Jr Phosphorylation of Nox1 regulates association with NoxA1 activation domain. Circ. Res. 2014;115:911–918. doi: 10.1161/CIRCRESAHA.115.304267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sullivan L.B., Chandel N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2(17) doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sun Q.A., Hess D.T., Nogueira L., Yong S., Bowles D.E., Eu J., Laurita K.R., Meissner G., Stamler J.S. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel by NADPH oxidase 4. Proc. Natl. Acad. Sci. USA. 2011;108:16098–16103. doi: 10.1073/pnas.1109546108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun F.C., Shyu H.Y., Lee M.S., Lee M.S., Lai Y.K. Involvement of calcium-mediated reactive oxygen species in inductive GRP78 expression by geldanamycin in 9L rat brain tumor cells. Int. J. Mol. Sci. 2013;14:19169–19185. doi: 10.3390/ijms140919169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Susankova K., Tousova K., Vyklicky L., Teisinger J., Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol. Pharmacol. 2006;70:383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 143.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tabet F., Savoia C., Schiffrin E.L., Touyz R.M. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 145.Tadic V., Prell T., Lautenschlaeger J., Grosskreutz J. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front. Cell Neurosci. 2014;30(8):147. doi: 10.3389/fncel.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takahashi N., Mori Y. TRP channels as sensors and signal integrators of redox status changes. Front. Pharmacol. 2011;2:58. doi: 10.3389/fphar.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Terentyev D., Györke I., Belevych A.E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E.C., Khanna S., Sen C.K., Cardounel A.J., Carnes C.A., Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tong X., Ying J., Pimentel D.R., Trucillo M., Adachi T., Cohen R.A. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J. Mol. Cell Cardiol. 2008;44:361–369. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Trebak M., Ginnan R., Singer H.A., Jourd’heuil D. Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid. Redox Signal. 2010;12:657–674. doi: 10.1089/ars.2009.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tu B.P., Weissman J.S. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell. Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Uchida K., Dezaki K., Damdindorj B., Inada H., Shiuchi T., Mori Y., Yada T., Minokoshi Y., Tominaga M. Lack of TRPM2 impaired insulin secretion and glucose metabolisms in mice. Diabetes. 2011;60:119–126. doi: 10.2337/db10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Valencia A., Kochevar I.E. Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J. Invest. Dermatol. 2008;128:214–222. doi: 10.1038/sj.jid.5700960. [DOI] [PubMed] [Google Scholar]
- 154.Vazquez G. TRPC channels as prospective targets in atherosclerosis: terra incognita. Front. Biosci. 2012;4:157–166. doi: 10.2741/258. [DOI] [PubMed] [Google Scholar]
- 155.Wang Y., Deng X., Hewavitharana T., Soboloff J., Gill D.L. Stim, ORAI and TRPC channels in the control of calcium entry signals in smooth muscle. Clin. Exp. Pharmacol. Physiol. 2008;35:1127–1133. doi: 10.1111/j.1440-1681.2008.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang C.L., Liu C., Niu L.L., Wang L.R., Hou L.H., Cao X.H. Surfactin-induced apoptosis through ROS-ERS-Ca2+-ERK pathways in HepG2 cells. Cell Biochem. Biophys. 2013;67:1433–1439. doi: 10.1007/s12013-013-9676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Webster K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Future Cardiol. 2012;8:863–884. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weinberg S.E., Chandel N.S. Targeting mitochondria metabolism for cancer therapy. Nat. Chem. Biol. 2015;11:9–15. doi: 10.1038/nchembio.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weissmann N., Sydykov A., Kalwa H., Storch U., Fuchs B., Mederos y Schnitzler M., Brandes R.P., Grimminger F., Meissner M., Freichel M., Offermanns S., Veit F., Pak O., Krause K.H., Schermuly R.T., Brewer A.C., Schmidt H.H., Seeger W., Shah A.M., Gudermann T., Ghofrani H.A., Dietrich A. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat. Commun. 2012;3:649. doi: 10.1038/ncomms1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu R.F., Ma Z., Liu Z., Terada L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell Biol. 2010;30:3553–3568. doi: 10.1128/MCB.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xu S.Z., Sukumar P., Zeng F., Li J., Jairaman A., English A., Naylor J., Ciurtin C., Majeed Y., Milligan C.J., Bahnasi Y.M., Al-Shawaf E., Porter K.E., Jiang L.H., Emery P., Sivaprasadarao A., Beech D.J. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., Kaneko S., Lange I., Fleig A., Penner R., Nishi M., Takeshima H., Mori Y. TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 2008;14:738–7471. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yan Y., Liu J., Wei C., Li K., Xie W., Wang Y., Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc. Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 164.Yu W., Dittenhafer-Reed K.E., Denu J.M. SIRT3 protein deacetylates isocitrate dehydrogenase 2(IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 2012;287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zaidi A. Plasma membrane Ca-ATPases: targets of oxidative stress in brain aging and neurodegeneration. World J. Biol. Chem. 2010;1:271–280. doi: 10.4331/wjbc.v1.i9.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang L., Kanq P.T., Chen C.L., Green K.B., Chen Y.R. Oxidative modifications of mitochondria complex II. Methods Mol. Biol. 2013;1005:143–156. doi: 10.1007/978-1-62703-386-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zima A.V., Blatter L.A. Redox regulation of cardiac calcium channels and transporters. Cardiovasc. Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 168.Zimmerman M.C., Takapoo M., Jagadeesha D.K., Stanic B., Banfi B., Bhalla R.C., Miller F.J., Jr Activation of NADPH oxidase 1 increases intracellular calcium and migration of smooth muscle cells. Hypertension. 2011;58:446–453. doi: 10.1161/HYPERTENSIONAHA.111.177006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.L.K. Seidlmayer, V.V.Juettner, S. Kettlewell, E.V. Pavlov, L.A. Blatter, E.N. Dedkova, (2015). Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc Res. pii: cvv097. [Epub ahead of print] [DOI] [PMC free article] [PubMed]