Abstract
We recently reported the synthesis of NOSH-aspirin, a novel hybrid that releases both nitric oxide (NO) and hydrogen sulfide (H2S). In NOSH-aspirin, the two moieties that release NO and H2S are covalently linked at the 1, 2 positions of acetyl salicylic acid, i.e. ortho-NOSH-aspirin (o-NOSH-aspirin). In the present study, we compared the effects of the positional isomers of NOSH-ASA (o-NOSH-aspirin, m-NOSH-aspirin and p-NOSH-aspirin) to that of aspirin on growth of HT-29 and HCT 15 colon cancer cells, belonging to the same histological subtype, but with different expression of cyclooxygenase (COX) enzymes; HT-29 express both COX-1 and COX-2, whereas HCT 15 is COX-null. We also analyzed the effect of these compounds on proliferation and apoptosis in HT-29 cells. Since the parent compound aspirin, inhibits both COX-1 and COX-2, we also evaluated the effects of these compounds on COX-1 and COX-2 enzyme activities and also performed modeling of the interactions between the positional isomers of NOSH-aspirin and COX-1 and COX-2 enzymes. We observed that the three positional isomers of NOSH aspirin inhibited the growth of both colon cancer cell lines with IC50s in the nano-molar range. In particular in HT-29 cells the IC50s for growth inhibition were: o-NOSH-ASA, 0.04±0.011 µM; m-NOSH-ASA, 0.24±0.11 µM; p-NOSH-ASA, 0.46±0.17 µM; and in HCT 15 cells the IC50s for o-NOSH-ASA, m-NOSH-ASA, and p-NOSH-ASA were 0.062 ±0.006 µM, 0.092±0.004 µM, and 0.37±0.04 µM, respectively. The IC50 for aspirin in both cell lines was >5 mM at 24 h. The reduction of cell growth appeared to be mediated through inhibition of proliferation, and induction of apoptosis. All 3 positional isomers of NOSH-aspirin preferentially inhibited COX-1 over COX-2. These results suggest that the three positional isomers of NOSH-aspirin have the same biological actions, but that o-NOSH-ASA displayed the strongest anti-neoplastic potential.
Keywords: Nitric oxide, Hydrogen sulfide, NSAIDs, Aspirin, Colon cancer, Cyclooxygenase, Molecular docking
Graphical abstract
Highlights
-
•
NOSH-aspirin is a hybrid compound, releasing nitric oxide and hydrogen sulfide.
-
•
Positional isomers of NOSH-aspirin effectively inhibit colon cancer cell growth.
-
•
In colon cancer cells, NOSH-aspirin inhibits proliferation and induces apoptosis.
-
•
NOSH-aspirin preferentially inhibits COX-1 enzyme activity over COX-2.
-
•
Potency order is o-NOSH-aspirin>m-NOSH-aspirin>p-NOSH-aspirin.
1. Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) in general and aspirin in particular have anti-cancer activities [1–3]. Unfortunately, prolonged use of these agents may lead to potentially life threatening side effects; including renal, cardiovascular, and gastrointestinal, reviewed in [4]. In our efforts to develop a safer aspirin, we synthesized NOSH-aspirin [5], a hybrid molecule that releases nitric oxide (NO) and hydrogen sulfide (H2S), two gasotransmitters of physiological importance [6]. The development of NOSH-aspirin was based on the observation that NO [7–9] and H2S [10–12] have some of the same general properties as prostaglandins (PGs) within gastric mucosa and that should compensate for the reduced gastric PGs caused by aspirin, thus enhancing the local mucosal defense systems.
NOSH-aspirin has been shown to have strong anti-inflammatory properties; have IC50s for cell growth inhibition in the nano-molar range in 11 different human cancer cell lines of six different tissue origins (colon, breast, pancreas, lung, prostate and leukemia) [5]. Although highly potent, NOSH-aspirin was shown to have very limited cyto-toxicity as demonstrated by the very low (<10%) lactate dehydrogenase (LDH) release at four times its IC50 for cell growth inhibition at 24 h [5]. It was also shown to have superior gastrointestinal safety profile compared to aspirin [13] and was very efficacious in an in vivo model of human colon cancer xenografts in mice [14].
In the present study we compared the cell growth inhibitory properties of o-NOSH-aspirin (in which the two moieties that release NO and H2S are covalently linked at the 1, 2 positions of acetyl salicylic acid) to that of the 1, 3 (m-NOSH-aspirin) and the 1, 4 (p-NOSH-aspirin) positional isomers on HT-29 and HCT-15 colon cancer cell lines. We also verified the potential anti-growth activity of the positional isomers of NOSH-ASA on HT-29 colon cancer cell proliferation, and apoptosis. The effect of these agents on COX-1 and COX-2 enzymatic activity were also evaluated together with molecular modeling studies to determine their binding interactions with these enzymes.
2. Materials and methods
2.1. Reagents
NOSH-aspirin, 4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 2-((4-(nitrooxy)butanoyl)oxy)benzoate (o-NOSH-ASA), and the positional isomers, [4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 4-((4-(nitrooxy)butanoyl)oxy)benzoate] (p-NOSH-ASA) and [4-(3-thioxo-3H-1,2-dithiol-5-yl)phenyl 3-((4-(nitrooxy)butanoyl)oxy)benzoate] (m-NOSH-ASA), were synthesized as described [15] and were gifts from Avicenna Pharmaceuticals Inc (New York, NY). Stock (100 mM) solutions of the test compounds were prepared in dimethyl sulfoxide (Fisher Scientific, Fair Lawn, NJ). Traditional aspirin was obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
HT-29 (ATCC® HTB38™) and HCT-15 (ATCC® CCL-221™) human colon adenocarcinoma were obtained from American Type Tissue Collection (Manassas, VA). HT-29 cells express both COX-1 (cyclo-oxygenase) and COX-2 enzymes, instead HCT-15 cells are COX null [16]. HT-29 and HCT-15 human colon cancer cells were grown as monolayer in McCoy 5A and RPMI-1640 media, respectively. All media were supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 1% penicillin (50 U/ml) and streptomycin (50 µg/ml) (Invitrogen, Carlsbad, CA). Cells were seeded on culture dishes making a 1:5 dilution and incubated at 37 °C in 5% CO2 and 90% relative humidity. Single cell suspensions were obtained by trypsinization (0.05% trypsin/EDTA) and cells were counted using a hemocytometer. For cryopreservation of the complete growth media, the final DMSO (dimethyl sulfoxide) concentration was adjusted to 1%.
2.3. Cell growth inhibition
Cell growth inhibitory effect of the positional isomers of NOSH-aspirin was measured using a colorimetric MTT assay kit (Roche, Indianapolis, IN).
HT-29 and HCT-15 cancer cells were plated in 96-well plates at a density of 30,000 cells/well. The cells were incubated for 24 h with different concentrations of positional isomers of NOSH-ASA. The absorbance of the plates was measured on a spectrophotometric plate reader at a wavelength of 570 nm.
2.4. Assay for cell proliferation
The levels of proliferating cell nuclear antigen (PCNA) was determined using an ELISA (enzyme-linked immunosorbent assay) Kit (Calbiochem, La Jolla, CA), in accordance with the manufacturers protocol. The HT-29 cells (1×106 cells/mL) were treated for 24 h with different multiples of IC50s for cell growth inhibition of each of the positional isomers of NOSH-aspirin. A polyclonal antibody, specific for the human PCNA protein, has been immobilized onto the surface of microtiter wells provided in the kit. HT-29 cancer cells containing antigen in suspension buffer (5 mM EDTA, 0.2 mM phenylmethyl sulfonyl fluoride, 1 µg/mL pepstatin, 0.5 µg/mL leupeptin and 50 mM Tris/HCl pH 8.0) were pipetted into the wells followed by incubation with biotinylated detector monoclonal antibody (clone PC10) for 2 h. Unbound material was washed away and horseradish peroxidase-conjugated streptavidin was added and incubated for 30 min at room temperature. After another 30 min of incubation with the chromogenic substrate TetraMethylBenzidine (TMB) and the addiction of the stop solution, the colored reaction product was quantified using a spectrophotometer. The absorbance of the solutions in the wells was measured at 450 nm.
2.5. Assay for apoptosis
HT-29 cells (1×106 cells/mL) were treated with the positional isomers of NOSH-aspirin at their respective IC50 for cell growth inhibition for 24 h. Cells were washed and resuspended in 500 µL of 1X Binding Buffer (Annexin V binding buffer, 0.1 M HEPES/NaOH (pH 7.4), 1.4 M NaCl, 25 mM CaCl2; BD BioSciences Pharmingen, San Diego, CA). Then, 10 µL of Annexin V-FITC (final concentration: 0.5 mg/mL) was added to the FACS tube followed by 10 µL of Propidium Iodide (PI). After 20 min of incubation in the dark at room temperature, percentage of apoptotic cells was measured by flow cytometry using a Becton Dickinson LSR II equipped with a single argon ion laser. Thus, cells that are considered viable are both Annexin V and PI negative, while cells that are in early apoptosis are Annexin V positive and PI negative, cells that are in late apoptosis are both Annexin V and PI positive and cells already dead are Annexin V negative and PI positive. For each subset, about 10,000 events were analyzed. All the parameters were collected in list mode files, and the data were analyzed by Flow Jo software.
2.6. Cyclooxygenase inhibition assay
Ovine COX-1 or COX-2 (200 units) were incubated in the presence of the positional isomers of NOSH-aspirin at their respective IC50s for cell growth inhibition, in 600 µL of reaction buffer (100 mM) Tris-maleate buffer, pH 6.5, 0.1% Tween-20, gelatin at (1 mg/mL), hematin (3 µM), and TMPD (100 µM, Sigma-Aldrich, St. Louis, MO). The concentration of DMSO was always 10% of the final volume. After incubating the samples for 30 min at 4 °C, arachidonic acid (100 µM) was added to start the enzymatic reaction, this was incubated for 5 min at 25 °C. The COX enzymes activity was measured by monitoring the oxidation of TMPD at 600 nm in a microtiter plate. The percent inhibition was determined as previously reported [17]. We also measured the degree of COX-1 and COX-2 inhibition by conventional aspirin (1 mM) and indomethacin (1 µM) as reference compound.
2.7. Molecular docking experiments
Docking experiments were performed using the computational software Discovery Studio (DS) Client version 4.0, Structure-Based-Design from Accelrys/BIOVIA Inc., San Diego, USA Software Inc. The NOSH-aspirin derivatives were constructed using the small molecules module in DS and were energy minimized by 1000 steps each of steepest descent followed by conjugate gradient minimization using a distance dependent dielectric constant and CHARMm force field. The x-ray crystal structure of the COX inhibitor meloxicam bound to COX-1 enzyme was obtained from protein data bank (pdb id: 4O1Z) [18]. The enzyme was prepared for docking studies using the macromolecules module in DS. The COX-1 active site was defined by selecting a sphere of 15 Å radius around meloxicam after which meloxicam was deleted. The NOSH-aspirin derivatives were docked using the ligand–receptor interactions module in DS. The CDOCKER protocol was used to conduct docking studies. It is based on a simulated annealing principle which generates ten top ligand binding poses. These are ranked based on CDOCKER energy and CDOCKER interaction energy in kcal/mol. The best pose obtained is further analyzed by considering the number and types of polar and nonpolar interactions with the COX-1 enzyme. It should be noted that the molecular docking experiment was conducted by considering an implicit-water function Generalized-Born with Switching Function (GBSW), present in DS, to account for the effect of hydrophilic environment on enzyme–ligand complex. A similar docking study of NOSH-aspirin derivatives with COX-2 enzyme was carried out by using the x-ray crystal structure of isoxicam bound to COX-2 enzyme (pdb id: 4M10) [18].
2.8. Statistical analysis
Data are presented as means±SEM for at least 3–5 different sets of plates and treatment groups. Statistical comparison among the groups was performed using Student's t-test, P-values<0.05 were considered significant.
3. Results and discussion
3.1. Positional isomers of NOSH-aspirin are potent inhibitors of HT-29 and HCT 15 human colon cancer cell growth
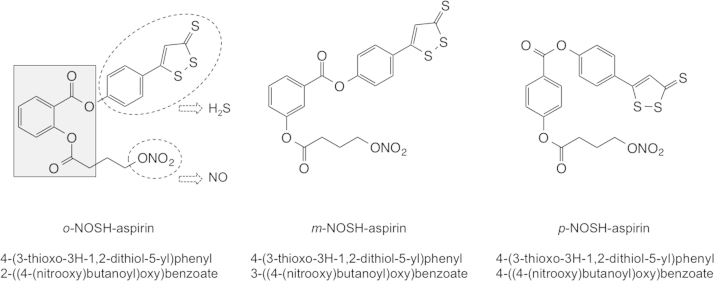
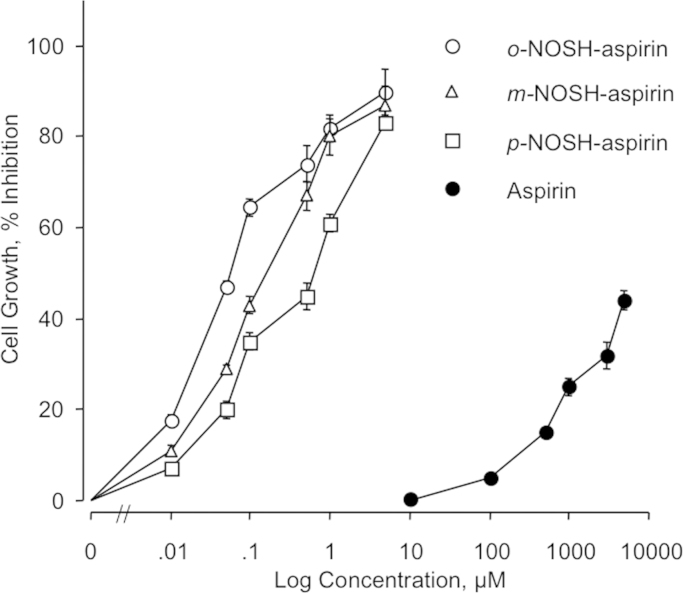
The structural components of the three positional isomers of NOSH-aspirin are shown in Fig. 1. HT-29 and HCT 15 cells were treated with various concentrations of aspirin, o-NOSH-ASA, m-NOSH-ASA, and p-NOSH-ASA for 24 h and compared with vehicle treated controls. As shown in Fig. 2, all three positional isomers of NOSH-ASA strongly inhibited the growth of the HT-29 cells in a concentration dependent manner. Similar dose–response curves were obtained for HCT 15 cells (data not shown). From such dose–response curves, IC50s for cell growth inhibition were determined and are presented in Table 1. The IC50 for the parent compound aspirin, in both cell lines was greater than 5 mM whereas the IC50s for the positional isomers of NOSH-ASA was in the nano-molar range. Specifically, the IC50s in HT-29 cells were: o-NOSH-ASA (40±11 nM), m-NOSH-ASA (240±110 nM) and p-NOSH-ASA (460±170 nM). This represents an enhanced potency of >10,000-fold for p-NOSH-ASA, >20,000-fold for m-NOSH-ASA, and >125,000-fold for o-NOSH-ASA. Similarly, the IC50s in HCT 15 cells were: o-NOSH-ASA (62±6 nM), m-NOSH-ASA (92±4 nM) and p-NOSH-ASA (370±40 nM). This represents an enhanced potency of >13,000-fold for p-NOSH-ASA, >50,000-fold for m-NOSH-ASA, and >80,000-fold for o-NOSH-ASA. This enhanced potency of the positional isomer of NOSH-ASA compared to the parent compound ASA, is unlikely to be mimicked by adding the various components of NOSH-ASA together, that is by ASA (o-, m-, or p-) plus NO plus H2S. This assertion is based on our previous studies with o-NOSH-ASA (where we showed that, the biological activity of o-ASA+SNAP (S-Nitroso-N-acetyl-penicillamine which releases NO)+ADT-OH (5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione, which releases H2S) was not the same as that of the intact o-NOSH-ASA molecule [14]. Based on recent reports, what appears more likely is that the released NO and H2S interact to produce a new species HSNO, which is a highly reactive intermediate [19,20].
Fig. 1.
The chemical structures of the positional isomers of NOSH-aspirin. The three structural components NOSH-ASA are indicated. H2S is released from degradation of the ADT-OH (5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione) moiety and NO is released from the nitrate group, both shown in the dotted ellipses, respectively. The parent compound, aspirin is shown in the shaded box.
Fig. 2.
Inhibitory effects of o-, m-, p-NOSH-ASA on HT-29 cell growth. Cells were treated with various concentrations of o-, m-, p-NOSH-ASA as described in Section 2.3. Cell viability was determined by MTT at 24 h.
Table 1.
IC50 values at 24 h for cell growth inhibition in HT-29 and HCT 15 human colon cancer cell lines.
| Origin/Cell line, IC50 (nM) |
||
|---|---|---|
| Compound | Colon, HT-29 | Colon, HCT 15 |
| Aspirin | >5,000,000 | >5,000,000 |
| o-NOSH-aspirin | 40±11 | 62±6 |
| Enhanced potency | >125,000 | >80,000 |
| m-NOSH-aspirin | 240±110 | 92±4 |
| Enhanced potency | >20,000 | >50,000 |
| p-NOSH-aspirin | 460±170 | 370±40 |
| Enhanced potency | >10,000 | >13,000 |
HT-29 (COX-1/ -2 positive) and HCT 15 (COX null) human colon cancer cell lines were treated with various concentrations of the positional isomers of NOSH-ASA as described under Section 2.3. Cell numbers were determined at 24 h from which IC50 values were calculated. Results are mean±S.E.M. of five different experiments done in triplicate. For HT-29 cells, significance between the treatment groups were: o vs m, P>0.05; o vs p, P<0.05; m vs p, P>0.05. For HCT 15 cells, significance between the treatment groups were: o vs m, P<0.01; o vs p, P<0.001; m vs p, P<0.001.
From the results presented here there are two important observations that can be made. One, o-NOSH-ASA is the most potent positional isomer in both HT-29 and HCT 15 colon cancer cell lines. The order of potency being o-NOSH-ASA>m-NOSH-ASA>p-NOSH-ASA. Two, the magnitude of the IC50s for each positional isomer appears to be same in both cell line, no statistical differences. This strongly suggests that the effect of these positional isomers of NOSH-ASA on cell growth inhibition is independent of COX status as HT-29 express both COX-1 and COX-2 whereas HCT 15 are COX null [16]. Based on the IC50s determined here, we chose 50 nM for o-NOSH-ASA, 250 nM for m-NOSH-ASA, and 500 nM for p-NOSH-ASA as our standard IC50 concentrations, and used multiple of these in all our other studies.
3.2. Effects of positional isomers of NOSH-ASA on HT-29 colon cancer cell kinetics
Two determinants of cell growth or cellular mass are cell renewal and cell death. Therefore, we evaluated the effects of the positional isomers of NOSH-ASA on cell proliferation and apoptosis. For proliferation, we chose two concentrations of each compound, their respective IC50 and 2×IC50 for cell growth inhibition. For apoptosis, we evaluated each compound at its IC50 as a function of treatment time.
3.2.1. Cell proliferation
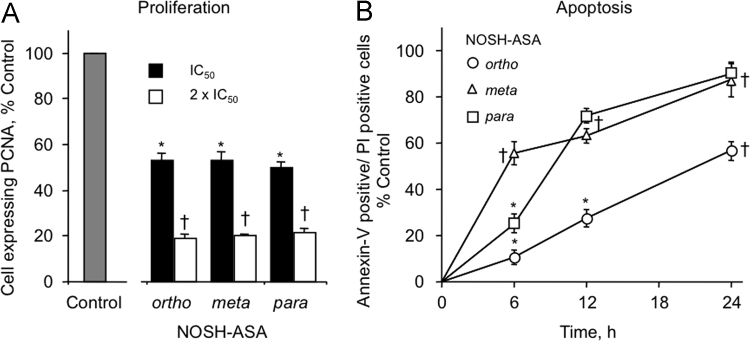
At 24 h, all positional isomers of NOSH-ASA reduced PCNA expression in a dose-dependent manner. Ortho, meta and para NOSH-aspirins qualitatively had similar effects on proliferation. These results are graphically represented in Fig. 3A. For o-NOSH-ASA, the proliferation decreased to 53.3±3% at its IC50 (50 nM) and 18.7±2% at 2 x IC50 (100 nM); the latter is the highest antiproliferative effect amongst the three compounds. m-NOSH-ASA decreased cell proliferation to 53.2±4% and 20.1±1%, when treated at its IC50 (250 nM) and 2×IC50 (500 nM), respectively. For p-NOSH-ASA, PCNA expression was reduced to 49.7±3% and 21.5±2% at its IC50 (500 nM) and 2×IC50 (1000 nM), respectively, compared to the untreated control.
Fig. 3.
Positional isomers of NOSH-aspirin inhibit proliferation and increase cell death. (A) For proliferation, HT-29 cells were treated with o-NOSH-ASA, m-NOSH-ASA and p-NOSH-ASA at the concentration corresponding to their IC50s and 2×IC50s for cell growth inhibition, for 24 h, compared to control. PCNA expression was determined by flow cytometry and expressed as percentage positive cells. Results are mean±SEM of three different experiments. *P<0.05, †P<0.01 compared with untreated cells. (B) Cells were treated with the positional isomers of NOSH-ASA at their respective IC50 for cell growth inhibition and analyzed at different times for apoptosis by Annexin V- PI staining and flow cytometry. All the positional isomers of NOSH-aspirin induce apoptosis in a time-dependent manner. *P<0.05, †P<0.01 compared with untreated control cells.
3.2.2. Apoptosis
The proportion of cells undergoing apoptosis increased in a time dependent manner for all positional isomers of NOSH-ASA, as determined by Annexin V- FITC and propidium iodide staining, followed by flow cytometry, Fig. 3B. For o-NOSH-ASA at its IC50 (50 nM), the percent of cells in late apoptotic phase increased from 0 in control cells to about 10.7% by 6 h, to 27.5% by 12 h, and to 56.6% by 24 h. For m-NOSH-ASA at its IC50 (250 nM), the percent of cells undergoing apoptosis increased from 55% at 6 h to 63.3 by 12 h, and 87.3 by 24 h. For p-NOSH-ASA at its IC50 (500 nM), the percent of cells undergoing apoptosis increased from 25.3% to 71.7% to 90.3% at 6, 12, 24 h respectively. It therefore appears that, the p-NOSH-ASA isomer caused the most amount of cell death at all time points and the ortho isomer caused the least.
3.3. Positional isomers of NOSH-ASA inhibit cyclo-oxygenase enzyme activity
We determined the percent of enzyme inhibition exerted by o-NOSH-ASA, m-NOSH-ASA, and p-NOSH-ASA at their respective IC50s for cell growth inhibition. As shown in Table 2, all three positional isomers of NOSH-ASA inhibited the enzymatic activities of COX-1 more than that of COX-2. The extent of COX-1 inhibition for all three compounds was in the range 40–50% and for COX-2, about 20–27%. Since we could not determine an IC50 for ASA at 24 h in HT-29 or HCT 15 cells even though we went up to 5 mM levels, we decided to evaluate the effects of ASA on COX-1 and COX-2 enzymatic activity at 1 mM based on our previous studies with ASA and COX inhibition [14]. At this concentration, both COX-1 and COX-2 were inhibited to the same extent, 65±5 and 60±3 for COX-1 and COX-2, respectively (Table 2). At lower concentrations, ASA is a significantly more potent inhibitor of COX-1 relative to COX-2 [21]. In order to ensure that there were no anomalies with our assay system, we also evaluated the inhibitory effects of indomethacin (1 µM), a nonselective COX inhibitor as a reference compound [22]. Indomethacin inhibited COX-1 and COX-2 by 75±2% and 69±3%, respectively (Table 2).
Table 2.
Inhibition of cyclooxygenase enzyme activity by positional isomers of NOSH-aspirin.
| % Enzyme Inhibition | ||
|---|---|---|
| Treatment | COX-1 | COX-2 |
| Aspirin, 1 mM | 65±5 | 60±3 |
| o-NOSH-aspirin, 50 nM | 52±4* | 21±2 |
| m-NOSH-aspirin, 250 nM | 45±3* | 27±3 |
| p-NOSH-aspirin, 500 nM | 40±3* | 22±2 |
| Indomethacin, 1 µM | 75±2 | 69±3 |
Pure ovine COX enzymes were treated with o-NOSH-ASA, m-NOSH-ASA, and p-NOSH-ASA at their respective IC50 for cell growth inhibition for 15 min at 4 °C after which o-COX-1 and o-COX-2 enzyme activity were determined. Results are mean±SEM of three independent determinations.
P<0.05 compared to COX-2.
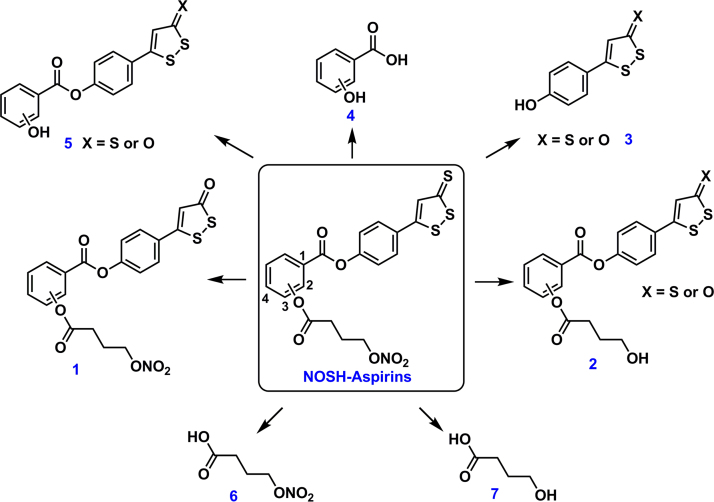
Differential inhibition of COX-1 versus COX-2 might suggest adverse gastrointestinal side effects since that would lead to significantly lower PG levels within the gastric mucosa. However, we have shown that o-NOSH-ASA was devoid of any GI side effects even though it inhibited PGs within the gastric mucosa [13]. This observation is most likely due to the released NO and H2S which have been shown to be cytoprotective within the stomach [7–12]. Recently we also reported that positional isomers of aspirin (o-ASA, m-ASA, p-ASA) were equally potent in inhibiting colon cancer cell growth but had differences in the mode by which they inhibited COX-1 and COX-2 activity [23]. o-ASA (conventional aspirin) was shown to be an irreversible inhibitor of COX-1 and COX-2, whereas m-ASA and p-ASA were about 30–35% reversible. These data may also explain the differential IC50s seen in the present study for the positional isomers of NOSH-ASA. Also, based on the plausible metabolic pathway suggested (Fig. 6), it appears that these molecules may not be able to cause irreversible acetylation of Ser present in the COX active site. Additional studies are needed to determine the metabolites formed for these NOSH-aspirins.
Fig. 6.
The potential in vivo metabolites of o-, m-, p-NOSH-ASA derivatives.
3.4. Docking results
The binding interactions of o-NOSH-ASA, m-NOSH-ASA, and p-NOSH-ASA with COX-1 and COX-2 enzymes were investigated by conducting molecular modeling studies. The x-ray crystal structures of ovine COX-1 and murine COX-2 were used to determine the binding modes of NOSH-aspirins [18].
3.4.1. Molecular docking of the positional isomers of NOSH-ASA with COX-1 enzyme
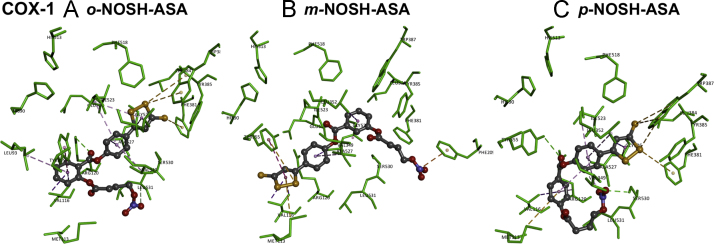
The binding interactions of o-NOSH-ASA with COX-1 enzyme shows that it binds in the COX-1 active site in an open U-shaped conformation (Fig. 4, panel A) and underwent contacts with most of the residues that line the binding site. The 5-phenyl-3H-1,2-dithiole-3-thione substituent ortho to the (nitrooxybutanoyloxy)benzoate group was in a hydrophobic region consisting of Val349, Leu352, Phe381, Tyr385, Trp387, Phe518, Ile523 and Gly526. The 1,2-dithiole-3-thione moiety was oriented closer to the catalytic site at the apex of COX-1 active site and underwent van der Waal's contact with side chains of Leu352 and Ile523 respectively (π-alkyl interaction, distance<5 Å). Hydrophobic π-sulfur interactions were seen with the disulfide sulfurs and aromatic rings of Phe381 and Trp387 (distance<5 Å). The 5-phenyl ring attached to 1,2-dithiole-3-thione moiety was in van der Waal's contact with side chains of Val349 and Ala527 (distance<5 Å). The benzoate ring with (nitrooxy)butanoyloxy substituent was oriented closer to the entrance of COX-1 active site and was surrounded by Leu93, Met113, Val116, Arg120, Val349, Leu531, and Tyr355. The benzoate C=O (COO) linked to 5-phenyl ring underwent electrostatic interaction with the guanidine side chain of Arg120 (distance<3.0 Å) whereas the benzoate aromatic ring underwent T-shaped π–π interaction with aromatic ring of Tyr355 (distance<5 Å). Moving the 5-phenyl-3H-1,2-dithiole-3-thione substituent from ortho to meta-position in m-NOSH-ASA had a dramatic effect on its binding mode within COX-1 compared to o-NOSH-ASA and exhibits an l-shaped conformation (Fig. 4, panel B). The 1,2-dithiole-3-thione moiety was oriented closer to the entrance of COX-1 active site in a nonpolar region comprised of Met113, Val116, Tyr355 and Leu359. The sulfur atom (dithiole ring) underwent π-sulfur and π–π hydrophobic interaction with Tyr355 (distance<5 Å). In addition, π-sulfur interaction was seen with Met113 (distance<5 Å). The 5-phenyl ring next to 1,2-dithiole-3-thione was in van der Waal's contact with side chains of Val349, Ile523 and Ala527 (distance<5 Å). The benzoate ring was oriented closer to the apex of COX-1 active site (distance<5 Å). Interestingly, the charged nitrooxy group (ON+OO−) underwent cation-π interaction with aromatic ring of Phe209 (distance<4 Å). The binding interactions of p-NOSH-ASA shows that it binds in a closed U-shaped conformation such that the 1,2-dithiole-3-thione was oriented closer to the apex of COX-1 binding site (Phe381, Leu384, Tyr385 and Trp387) as seen with the o-NOSH-ASA (Fig. 4, panel C). The 1,2-dithiole-3-thione underwent π-sulfur interactions with Phe381 and Trp387 (distance<5.5 Å). The 5-phenyl ring linked to 1,2-dithiole-3-thione moiety underwent a number of van der Waal's contact with side chains of Val349, Leu352, Ile523 and Ala527 (distance<5 Å). Similar to o-NOSH-ASA the benzoate ring was oriented closer to the entrance of COX-1 active site and underwent hydrophobic contacts with Met113 and Val116. The benzoate C=O (COO) linked to 5-phenyl ring underwent hydrogen bonding interaction with Arg120 and Tyr355 (distance<3 Å). However unlike o-NOSH-ASA, the (nitrooxy)butanoyloxy substituent in p-NOSH-ASA was not in proximity to the COX-1 entrance. Instead it was closer to Ser530 and Leu531 (distance<3 Å). Interestingly the nitrooxy group (ON+OO−) underwent polar contact with OH of Ser530 (distance=2.7 Å), the acetylation site of aspirin [24]. These investigations show that positional isomers o-, m-, p-NOSH-ASA exhibit different binding modes in the COX-1 active site. In this regard, the orientation of 5-membered 1,2-dithiole-3-thione for regioisomers o-NOSH-ASA and p-NOSH-ASA was similar to the methylthiazole substituent present in meloxicam which suggests that lipophilic, planar 5-membered rings could orient closer to Phe381, Tyr385 and Trp387 at the apex of COX-1 enzyme [18].
Fig. 4.
Docking of positional isomers of NOSH-ASA to the active site of cyclooxygenase-1. Hydrogen atoms are not shown for clarity. Polar and nonpolar interactions are colored coded and details are provided in text.
3.4.2. Molecular docking of the positional isomers of NOSH-ASA with COX-2 enzyme
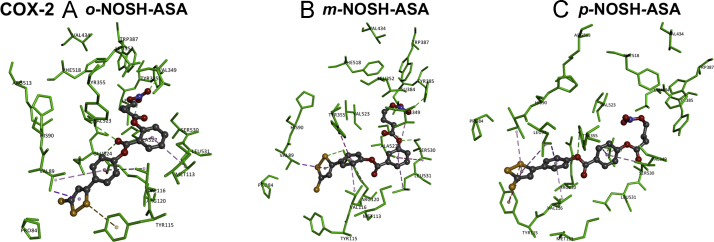
In the murine COX-2 enzyme, o-NOSH-ASA exhibits a completely different binding mode compared to COX-1 (Fig. 4, panel D). The (nitrooxy)butanoyloxy substituent was closer to the apex of COX-2 (Leu384, Tyr385 and Trp387) with the nitrooxy moiety (ON+OO−) undergoing hydrogen bonding interaction with Tyr385 OH (distance=2.1 Å). The butanoyloxy group was in van der Waal's contact with side chains of Leu352, Val523 and Ala527 (distance<5 Å). The benzoate aromatic ring underwent π-alkyl interactions with side chains of Ala527 and Leu531 (distance<5 Å) and the benzoate C=O (COO) formed hydrogen bond with Arg120 side chain (distance<2.5 Å). The 5-phenyl-3 H-1,2-dithiole-3-thione was oriented closer to the COX-2 entrance in a region comprised of Pro84, Val89, Tyr115 and Arg120 (distance<5 Å). The 5-phenyl aromatic ring underwent cation-π interactions with Arg120 and hydrophobic interactions with Val89 and Val116 respectively (distance<5 Å). The sulfur containing 1,2-dithiole-3-thione ring was closer to Pro84, Val89 and Tyr115 (distance<5.5 Å). The binding interaction of m-NOSH-ASA shows an open hair pin conformation (Fig. 4, panel E). Similar to its binding in COX-1 active site, the 5-phenyl-1,2-dithiole-3-thione was oriented closer to the COX-2 entrance where 5-phenyl substituent underwent π-alkyl and π-π interactions with Val116 and Tyr355 respectively (distance<5 Å). The 1,2-dithiole-3-thione formed π-alkyl and cation-π interactions with Val89 and Arg120 side chains respectively (distance<5 Å). However, unlike its binding in COX-1, the benzoate with its nitrooxy(butanoyloxy) substituent was oriented closer to Val349, Ser530 and Leu531 whereas the nitrooxy moiety (ON+OO−) underwent hydrogen bonding interaction with Tyr385 OH (distance=2.2 Å) as seen with o-NOSH-ASA within COX-2 enzyme (Fig. 4 panel D). Interestingly the butanoyloxy oxygen (OCO) was forming a hydrogen bonding interaction with Ser530 (distance=2.1 Å). The binding interaction of p-NOSH-ASA which contains a para 5-phenyl-3H-1,2-dithiole-3-thione ring, showed an almost linear conformation unlike that seen with either o-NOSH-ASA or m-NOSH-ASA (Fig. 4, panel F). The nitrooxy (butanoyloxy) substituent was closer to Tyr385 and Trp387. Interestingly the nitrooxy moiety (ON+OO−) underwent hydrogen bonding interaction with backbone NH of Ala527 (distance=2.7 Å) which was not seen for either o-NOSH-ASA or m-NOSH-ASA (Fig. 4 panels D and E) and the benzoate aromatic ring was in van der Waal's contact with Val349 and Ala527 (distance<5 Å). Interestingly as seen with m-NOSH-ASA, the butanoyloxy oxygen (OCO) was forming a hydrogen bonding interaction with Ser530 (distance=1.8 Å) whereas the benzoate oxygen atom underwent hydrogen bonding interaction with Arg120 (distance=2.7 Å). The entire 5-phenyl-3H-1,2-dithiole-3-thione substituent was oriented beyond the entrance of COX-2 active site in a region comprised of Leu93, Val89, Tyr115 and Val116. The 1,2-dithiole-3-thione underwent π-alkyl, π–π and π-sulfur interactions with Val89, Leu93 and Tyr115 respectively (distance<5 Å).
Based on these studies it appears that the binding inhibition and potency of o-NOSH-ASA, m-NOSH-ASA, and p-NOSH-ASA toward COX-1 enzymes was sensitive to regioisomeric placement of 5-phenyl-3H-1,2-dithiole-3-thione substituent. In this regard, the presence of a bulkier isoleucine (Ile523) in COX-1 active site instead of a smaller valine (Val523) affects the binding orientation and stability of ligand–enzyme complex of o-, m-, p-NOSH-ASA with the ortho regioisomer (o-NOSH-ASA) exhibiting better stability (CDOCKER energy=−15.05 kcal/mol) compared to meta (m-NOSH-ASA) and para (p-NOSH-ASA) regioisomers (CDOCKER energies=−12.76 and −4.24 kcal/mol respectively). In contrast, the presence of a smaller valine (Val523) in COX-2 active site makes it slightly larger. This factor appears to accommodate the regioisomeric NOSH-aspirin derivatives (o-, m-, p-NOSH-ASA) such that they all exhibit a similar binding mode within COX-2 active site where the 5-phenyl-3H-1,2-dithiole-3-thione substituent is oriented closer to COX-2 entrance despite their regioisomeric differences. The ligand–enzyme complex of o-NOSH-ASA with COX-2 exhibited better stability (CDOCKER energy=−24.0 kcal/mol) compared to m-NOSH-ASA (CDOCKER energy=−7.34 kcal/mol) and p-NOSH-ASA (CDOCKER energy=−22.16 kcal/mol). The in vitro COX inhibition studies indicate that NOSH-aspirins exhibit better activity toward COX-1 enzyme which is supported by the modeling studies where it is seen that the lipophilic 1,2-dithiole-3-thione substituent is generally buried in a solvent exposed polar environment beyond COX-2 entrance which was not seen with the COX-1 binding modes.
It should be noted that in vivo, the positional isomers of NOSH-ASA may form a number of metabolites as suggested in Fig. 5. Among them, proposed metabolites 1–5 are capable of exhibiting COX inhibition on their own. Furthermore, depending on the dose, frequency and routes of administration, a fraction of intact NOSH-aspirin derivatives can interact and inhibit the COX enzymes (Fig.6).
Fig. 5.
Docking of positional isomers of NOSH-ASA to the active site of cyclooxygenase-2. Hydrogen atoms are not shown for clarity. Polar and nonpolar interactions are colored coded and details are provided in text.
In summary, in the present study, we demonstrated that all three positional isomers of NOSH aspirin inhibit the growth of HT-29 and HCT-15 colon cancer cell lines with different IC50s, suggesting an important role for positional isomerism in modulating the potential anti-cancer properties of these compounds. However, since the IC50s in each cell line were about same nano-molar range, we can conclude that this effect is independent COX status. All three positional isomers of NOSH-aspirin, inhibited proliferation of HT-29 cells and induced apoptosis, two determinants of cell mass. All three compounds also preferentially inhibited COX-1 enzyme activity. Our results also demonstrate that positional isomerism markedly affects the anti-neoplastic potential of NOSH-ASA in colon cancer. In particular, o-NOSH-ASA appears to be 5-fold more potent than m-NOSH-ASA and 10-fold more potent than p-NOSH-ASA. We are currently evaluating the many potential molecular targets of these novel compounds in various models of cancer.
Authorship Contributions
Participated in research design: Kashfi, Chattopadhyay, Kodela,
Conducted experiments: Vannini, Kodela, Chattopadhyay, Rao,
Performed data analysis: Vannini, Chattopadhyay, Kashfi, Rao,
Wrote or contributed to the writing of the manuscript: Vannini, Kashfi, Rao
Grant support
This work was supported in part by NIH Grant R24 DA018055 and NSERC-Discovery Grant, Canada RGPIN-03830-2014 (PR). The funding agencies had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Conflict of interest
The authors have nothing to disclose except for KK, who has an equity position in Avicenna Pharmaceuticals, Inc. the supplier of NOSH-aspirins used in these studies.
Footnotes
This article belongs to a special issue on Nitric Oxide and Cancer, edited by Jordi Muntané and Benjamin Bonavida.
References
- 1.Baron J.A., Cole B.F., Sandler R.S., Haile R.W., Ahnen D., Bresalier R. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell P.M., Price J.F., Fowkes F.G., Zanchetti A., Roncaglioni M.C., Tognoni G. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 3.Sandler R.S., Halabi S., Baron J.A., Budinger S., Paskett E., Keresztes R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 4.Kashfi K. Anti-inflammatory agents as cancer therapeutics. Adv. Pharmacol. 2009;57:31–89. doi: 10.1016/S1054-3589(08)57002-5. [DOI] [PubMed] [Google Scholar]
- 5.Kodela R., Chattopadhyay M., Kashfi K. NOSH-Aspirin: A Novel Nitric Oxide-Hydrogen Sulfide-Releasing Hybrid: A New Class of Anti-inflammatory Pharmaceuticals. ACS Med Chem Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo Faro M.L., Fox B., Whatmore J.L., Winyard P.G., Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47. doi: 10.1016/j.niox.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Wallace J.L., Miller M.J. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 8.Brzozowski T., Konturek S.J., Drozdowicz D., Dembinski A., Stachura J. Healing of chronic gastric ulcerations by l-arginine. Role of nitric oxide, prostaglandins, gastrin and polyamines. Digestion. 1995;56:463–471. doi: 10.1159/000201277. [DOI] [PubMed] [Google Scholar]
- 9.Ma L., Wallace J.L. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G341–G346. doi: 10.1152/ajpgi.2000.279.2.G341. [DOI] [PubMed] [Google Scholar]
- 10.Fiorucci S. Prevention of nonsteroidal anti-inflammatory drug-induced ulcer: looking to the future. Gastroenterol. Clin. N. Am. 2009;38:315–332. doi: 10.1016/j.gtc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Fiorucci S., Antonelli E., Distrutti E., Rizzo G., Mencarelli A., Orlandi S. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by anti-inflammatory nonsteroidal drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 12.Wallace J.L., Dicay M., McKnight W., Martin G.R. Hydrogen sulfide enhances ulcer healing in rats. FASEB J. 2007;21:4070–4076. doi: 10.1096/fj.07-8669com. [DOI] [PubMed] [Google Scholar]
- 13.Nia K.V., Kodela R., Chattopadhyay M., Kashfi K. The dual nitric oxide and hydrogen sulfide-releasing nonsteroidal anti-inflammatory drugs, NOSH-aspirin, NOSH-naproxen, and NOSH-sulindac are safe to the stomach and have strong anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Gastroenterology. 2013;144:S-596. [Google Scholar]
- 14.Chattopadhyay M., Kodela R., Olson K.R., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem. Biophys. Res. Commun. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 15.F. Vannini, A.C. MacKessack-Leitch, E.K. Eschbach, M. Chattopadhyay, R. Kodela, K. Kashfi, Synthesis and anti-cancer potential of the positional isomers of NOSH-aspirin (NBS-1120) a dual nitric oxide and hydrogen sulfide releasing hybrid. Bioorg Med Chem Lett 2015;(in press) 10.1016/j.bmcl.2015.08.023 [DOI] [PMC free article] [PubMed]
- 16.Hanif R., Pittas A., Feng Y., Koutsos M.I., Qiao L., Staiano-Coico L. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 17.Kodela R., Chattopadhyay M., Goswami S., Gan Z.Y., Rao P.P., Nia K.V. Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: differences in mode of cyclooxygenase inhibition. J. Pharmacol. Exp. Ther. 2013;345:85–94. doi: 10.1124/jpet.112.201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu S., Hermanson D.J., Banerjee S., Ghebreselasie K., Clayton G.M., Garavito R.M. Oxicams bind in a novel mode to the cyclooxygenase active site via a two-water-mediated H-bonding Network. J. Biol. Chem. 2014;289:6799–6808. doi: 10.1074/jbc.M113.517987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortese-Krott M.M., Fernandez B.O., Kelm M., Butler A.R., Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric oxide: Biol. Chem. 2015;46:14–24. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Filipovic M.R., Miljkovic J., Nauser T., Royzen M., Klos K., Shubina T. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meade E.A., Smith W.L., DeWitt D.L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 1993;268:6610–6614. [PubMed] [Google Scholar]
- 22.Riendeau D., Charleson S., Cromlish W., Mancini J.A., Wong E., Guay J. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can. J. Physiol. Pharmacol. 1997;75:1088–1095. [PubMed] [Google Scholar]
- 23.Kodela R., Chattopadhyay M., Goswami S., Gan Z.Y., Rao P.P.N., Nia K.V. Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: Differences in mode of cyclooxygenase inhibition. J. Pharmacol. Exp. Ther. 2013 doi: 10.1124/jpet.112.201970. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loll P.J., Picot D., Garavito R.M. The structural basis of aspirin activity inferred from the crystal structure of inactivated prostaglandin H2 synthase. Nat. Struct. Biol. 1995;2:637–643. doi: 10.1038/nsb0895-637. [DOI] [PubMed] [Google Scholar]