Abstract
Sulindac is chemopreventive and has utility in patients with familial adenomatous polyposis; however, side effects preclude its long-term use. NOSH-sulindac (AVT-18A) releases nitric oxide and hydrogen sulfide, was designed to be a safer alternative. Here we compare the gastrointestinal safety, anti-inflammatory, analgesic, anti-pyretic, anti-platelet, and anti-cancer properties of sulindac and NOSH-sulindac administered orally to rats at equimolar doses. Gastrointestinal safety: 6 h post-administration, number/size of hemorrhagic lesions in stomachs were counted. Tissue samples were frozen for PGE2, SOD, and MDA determination. Anti-inflammatory: 1 h after drug administration, the volume of carrageenan-induced rat paw edemas was measured for 5 h. Anti-pyretic: fever was induced by LPS (ip) an hour before administration of the test drugs, core body temperature was measured hourly for 5 h. Analgesic: time-dependent analgesic effects were evaluated by carrageenan-induced hyperalgesia. Antiplatelet: anti-aggregatory effects were studied on collagen-induced platelet aggregation of human platelet-rich plasma. Anti-cancer: We examined the effects of NOSH-sulindac on the growth properties of 12 human cancer cell lines of six different tissue origins. Both agents reduced PGE2 levels in stomach tissue; however, NOSH-sulindac did not cause any stomach ulcers, whereas sulindac caused significant bleeding. Lipid peroxidation induced by sulindac was higher than that from NOSH-sulindac. SOD activity was significantly lowered by sulindac but increased by NOSH-sulindac. Both agents showed similar anti-inflammatory, analgesic, anti-pyretic, and anti-platelet activities. Sulindac increased plasma TNFα whereas this rise was lower in the NOSH-sulindac-treated animals. NOSH-sulindac inhibited the growth of all cancer cell lines studied, with potencies of 1000- to 9000-fold greater than that of sulindac. NOSH-sulindac inhibited cell proliferation, induced apoptosis, and caused G2/M cell cycle block. These results demonstrate that NOSH-sulindac is gastrointestinal safe, and maintains the anti-inflammatory, analgesic, antipyretic, and antiplatelet properties of its parent compound sulinsac, with anti-growth activity against a wide variety of human cancer cells.
Abbreviations: NO, nitric oxide; H2S, hydrogen sulfide; NOSH, nitric oxide-hydrogen sulfide; NSAIDs, nonsteroidal anti-inflammatory drugs; SUL, sulindac; LPS, Lipopolysaccharide; PGE2, Prostaglandin E2; MDA, malondialdehyde; SOD, Superoxide dismutase
Keywords: Nitric oxide, Hydrogen sulfide, NSAIDs, Sulindac, Gastrointestinal, Ulcer, Inflammation, Pain, Cyclooxygenase, Platelet, Anti-cancer
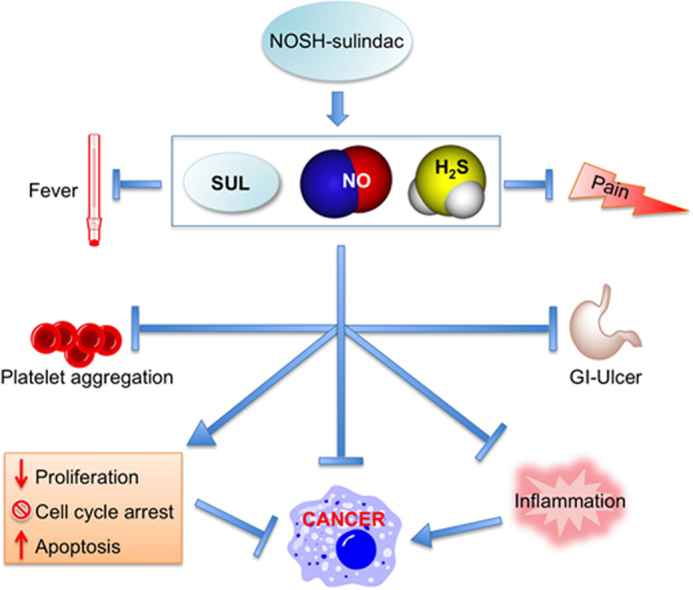
Graphical abstract
Highlights
-
•
NOSH-sulindac is a hybrid compound that releases nitric oxide and hydrogen sulfide.
-
•
NOSH-sulindac is gastrointestinal safe but inhibits COX-1.
-
•
NOSH-sulindac has anti-inflammatory, antipyretic, anti-platelet, and analgesic properties.
-
•
NOSH-sulindac inhibited growth of 12 different human cancer cell lines of 6 different tissue origins.
-
•
NOSH-sulindac inhibited proliferation, caused G2/M cell cycle arrest, inducing apoptosis.
1. Introduction
The use of anti-inflammatory drugs (NSAIDs) in cancer prevention is based on the recognition that inflammation is central to the carcinogenesis process [1]. There is considerable body of evidence suggesting that the long-term use of NSAIDs is associated with a significant reduction in many forms of cancers including, colon [2–5], breast [6–8], pancreas [9], bladder [10,11], head and neck [12], esophageal [13], ovarian [14,15], prostate [16], hepatocellular [17], and skin [18–20]. Of these, cellular and molecular mechanisms of colorectal cancer (CRC), which in many ways represent the prototypical case for cancer prevention, have been studied most extensively. From all accumulated data, what has become abundantly clear is that although NSAIDs are chemopreventive, they reduce the risk of, and mortality from, CRC by about half [21]. Furthermore, long-term use of NSAIDs may lead to significant side effects, mainly gastrointestinal, cardiovascular, and renal which obviously limits their use [1].
Familial adenomatous polyposis (FAP) is an autosomal dominant disease caused by a mutation in the adenomatous polyposis coli (APC) gene [22] which is characterized by hundreds of colorectal adenomatous polyps that eventually progress to CRC. Almost all FAP patients will develop CRC if they are not identified and treated at an early stage. Generally, management of patients with FAP is with a total colectomy [23]. Therefore, chemoprevention in this setting is of paramount importance. Sulindac (SUL) belongs to the indene class of NSAIDs [24] that has extensively been studied and utilized as a chemopreventive agents in patients with FAP [25–28]. However, a limiting factor in the long term use of SUL is its toxicity, that can affect up to 20% of patients [24]. In our efforts on improving the safety profile of SUL, we developed NOSH-sulindac (NOSH-SUL), a hybrid molecule capable of releasing nitric oxide (NO) and hydrogen sulfide (H2S), two gasotransmitters of physiological significance [29]. Our rational for developing NOSH-SUL was based on the observations that NO [30] and H2S [31] have some of the same properties as prostaglandins within the gastric mucosa, thus modulating some components of the mucosal defense systems. In the present study, we carried out a head-to-head comparison of the gastrointestinal safety, anti-inflammatory, analgesic, anti-pyretic, and anti-platelet, properties of SUL with those of NOSH-SUL. We also evaluated the effects of SUL and NOSH-SUL in 12 different cancer cell lines of 6 different tissue origins and on cell kinetics using a human colon cancer cell line.
2. Materials and methods
2.1. Chemicals
NOSH-SUL (AVT-18A), (Z)-4-(3-thioxo-3H-1,2-dithiol-5-yl) phenyl 5-(2-(5-fluoro-2-methyl-1-(4-(methylsulfinyl) benzylidene)-1H-inden-3-yl) acetoxy)-2-((4-(nitrooxy) butanoyl)oxy) benzoate was synthesized as described previously [29] and was a gift from Avicenna Pharmaceuticals Inc, (New York, NY). The structural components of the NOSH-SUL are shown in Fig. 1. Lipopolysaccharide (LPS) from Escherichia coli, SUL, and carrageenan were purchased from Sigma (St. Louis, MO, USA). Kits used for determination of PGE2, lipid peroxidation, and superoxide dismutase, were from Cayman Chemical (Ann Arbor, MI).
Fig. 1.
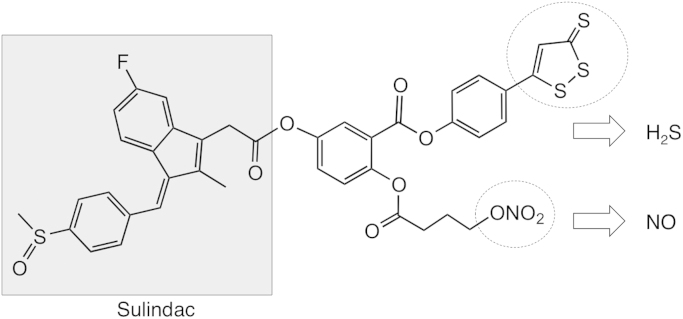
Structural components of NOSH-sulindac. The parent compound sulindac is shown in the shaded box. The parts of the molecule that releases NO and H2S are shown in the dotted ellipses.
2.2. Experimental groups and treatments
In all the protocols described below, we used at least 5 male Wistar rats per group that weighed 180–200 g. The rats were obtained from Charles River Laboratories International (Wilmington, MA) and were fed standard laboratory chow and water. All experimental procedures were approved by our institutional animal research committees and were performed in accordance with nationally approved guidelines for the treatment of laboratory animals.
2.3. Determination of ulcer index
Rats were fasted for 48 h with free access to drinking water. SUL and NOSH-SUL at equimolar concentrations, 200 mg/kg and 467 mg/kg respectively, were administered orally by gavage suspended in 1% carboxymethylcellulose (CMC) solution. Six hours post-administration, animals were euthanized in a CO2 chamber; stomachs were then removed immediately, cut along the greatest curvature, and rinsed with ice-cold distilled water. The ulcer index (UI) was determined as described by Best et al. [32]. Briefly, the number and the length of ulcers observed in each stomach were determined using a magnifying lens. Using the following scoring module, the severity of each gastric lesion was measured along its greatest length with 1 mm=rating of 1, 1–2 mm=rating of 2, and >2 mm=rating according to the measured length in mm. The “ulcer index” (UI) was then calculated by adding the total number of lengths (L, mm) in each stomach and then dividing the total by the total number of rats in each group (N=5): UI=(L1+L2+L3+L4+L5)/5.
The excised tissues from each of the stomachs were processed for measurement of Prostaglandin E2 (PGE2), malondialdehyde (MDA) and Superoxide dismutase (SOD) activity. Blood samples from each of the rats were taken by cardiac puncture into heparin-containing vials and used for determination of plasma TNFα.
2.4. Measurement of PGE2 levels
One gram of tissue from each stomach was placed in a test tube containing 5 mL of 0.1 M phosphate buffer (pH7.4), 1 mM EDTA, and 10 μM indomethacin. After homogenization, the homogenate was centrifuged at 10,000×g for 10 min at 4 °C. PGE2 content in the supernatant was determined in duplicate by an enzyme immunoassay kit following the protocol described by the manufacturer (Cayman Chemical, Ann Arbor, MI). Briefly, standard (50 µL) or homogenate (50 µL), enzymatic tracer (50 µL) and specific antiserum (50 µL) were mixed. After overnight incubation at 4 °C, the plates were washed with wash buffer and Ellman reagent (200 μL) was added into each well. The absorbance at 412 nm was measured after 1 h incubation at room temperature. Results are expressed as pg of PGE2 per mg of protein. Proteins were determined by Biorad assay.
2.5. Determination of Malondialdehyde (MDA) levels as index of lipid peroxidation
Snap frozen stomach tissue (25 mg) was sonicated for 15 s at 40 V over ice with 250 μL of radioimmunoprecipitation (RIPA) buffer (25 mM TrisHCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) with PMSF (phenylmethylsulphonyl fluoride). Homogenates were centrifuged for 10 min at 200×g at 4 °C. Thiobarbituric acid reactant substances (TBARS) was measured in the supernatant using a kit from Cayman Chemical (Ann Arbor, MI) as described by the manufacturer. Briefly, reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA) at high temperature (90–100 °C) in acidic conditions produced an adduct with a chromophore which absorbed visible light at 530–540 nm. Results are expressed as picomoles of malondialdehyde per gram protein.
2.6. Superoxide dismutase (SOD) activity
SOD activity in the gastric mucosa was assayed using a colorimetric kit from Cayman Chemical, (Ann Arbor, MI). Mucosal tissue (1 g) was homogenized with 5 mL of 20 mM HEPES buffer (pH 7.2) containing 1 mM EGTA and 300 mM of sucrose solution. Homogenates were centrifuged at 200×g for 10 min at 4 °C. SOD activity of the supernatants were measured spectrophotometrically at 460 nm. As indicated in Cayman's SOD assay kit, “this procedure utilizes a tetazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine”. SOD activity is expressed as the amount of the SOD standard showing activity equivalent to the determined activity. The results are expressed as units (U) of SOD activity/mg protein. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutaion of the superoxide radical.
2.7. Determination of plasma TNF-α
Plasma TNF-α was measured using an enzyme immunoassay kit from R&D systems (Minneapolis, MN) following the protocol described by the manufacturer. Briefly, each sample (50 μL) was incubated with antibodies specific for rat TNF-α and washed three times with assay buffer. An enzyme-linked polyclonal antibody specific for rat TNF-α conjugated to horseradish peroxidase was then added. Following washing of unbound antibody-enzyme reagent, substrate solution containing tetramethylbenzidine (TMB), plus hydrogen peroxide was then added. The enzyme reaction produced a blue product (oxidized TMB) that turned yellow when dilute hydrochloride acid (stop solution) was added. Color intensity was determined at 450 nm using a standard ELISA plate reader. Results are expressed as pg/mL.
2.8. Anti-pyretic activity
Fever was induced in animals as described previously [33]. Briefly, LPS (50 μg/kg, Sigma, St. Louis, MO, USA) was administered intra-peritoneally to the animals an hour before the administration of SUL or NOSH-SUL at equimolar doses, 200 mg/kg and 467 mg/kg respectively, given orally by gavage suspended in 1% CMC. Rectal temperature was measured by inserting a lubricated thermistor probe (external diameter: 3 mm) 2.8 cm into the rectum of the animal. The probe was linked to a digital reader, which displayed the temperature at the tip of the probe (±0.1 °C). The values displayed were manually recorded. Rectal temperatures were taken every hour for 5 h.
2.9. Anti-inflammatory activity
Carrageenan (1%, 100 μL, suspended in sterile saline solution) was subcutaneously injected into the plantar surface of the right hind paw in rat following the protocol described by Winter et al. [34]. Paw volume was measured using a water displacement plethysmometer (Model 520, IITC/Life Sciences Instruments, Woodland Hills, CA) before carrageenan injection and thereafter at 1 h intervals for 5 h. The paw volume measured just prior to carrageenan injection was used as the control volume. Data are expressed as the change in paw volume (mL) at each time point. At the end of the experiment, rats were euthanized by asphyxiation in a CO2 chamber. After cutting each hind paw at the level of the calcaneus bone, exudates (oedema fluid) were collected and processed for measurement of PGE2, as described in Section 2.4.
2.10. Analgesic activity
Hindpaw inflammation was produced by intraplantar injection of carrageenan (100 μL of 1% carrageenan in sterile saline solution) into the right paw. SUL or NOSH-SUL at equimolar concentrations, 200 mg/kg and 467 mg/kg respectively, were administered orally by gavage suspended in 0.5% CMC 1 h after carrageenan injection, and the mechanical nociceptive threshold was determined 30 min after this and thereafter every 1 h for up to 5 h. The paw hyperalgesia was measured with an electronic pressure-meter. Each hindpaw was positioned in turn under a conical probe surface (tip radius approximately 1 mm) and gradually increasing pressure applied to the hindpaw surface until the animal vocalized at which point the measurement was terminated. Mechanical nociceptive threshold for both the injected and contralateral (i.e. non-injected) hindpaw were determined. The animals were tested before and after treatments and the results are expressed by the delta reaction force (g).
2.11. Inhibition of human platelet aggregation in vitro
Anti-aggregatory effects of SUL and NOSH-SUL were evaluated on collagen-induced platelet aggregation of human platelet-rich plasma (PRP). The collagen-induced aggregation occurs through a pathway dependent upon the arachidonic acid cascade [35]. Venous blood samples from healthy volunteers who had not taken any drugs for at least 2 weeks were used to prepare PRP by centrifugation of citrated blood at 200g for 20 min. Aliquots (500 μL) of PRP were added into aggregometer cuvettes, and aggregation was recorded as increased light transmission under continuous stirring (1000 rpm) at 37 °C for 10 min after the addition of the stimulus. Collagen at submaximal concentrations (1.0 μg/mL) was used as the platelet activator. Sulindac and NOSH-SUL at various concentrations were preincubated with PRP 10 min before the addition of collagen. Vehicle alone (0.5% DMSO) added to PRP did not affect platelet function in control samples. The anti-aggregatory activity of the two compounds was determined as percent inhibition of platelet aggregation compared to control samples. IC50 values were calculated by nonlinear regression analysis.
2.12. Measurement of COX enzyme activity
NOSH-SUL was compared to SUL for its ability to inhibit COX-1 and COX-2 enzyme activities in vitro as described previously [36] using a colorimetric COX (ovine, o-COX) inhibitor screening kit from Cayman Chemicals (Ann Arbor, MI).
2.13. Cell culture and MTT assay
Human colon adenocarcinoma (HT-29, SW-480 and HCT-15), human breast cancer (MDA-MB 231, SK-BR-3 and MCF-7), human pancreatic cancer (MIA PaCa-2 and BxPC-3), human lung cancer (A549 and H383), human prostate cancer (LNCAP), and human leukemia (Jurkat T) cells were obtained from American Type Tissue Collection (Manassas, VA). All cells lines were grown as monolayers except for the Jurkat T cells which was grown as suspension culture. The pancreatic and breast cancer cells were grown in Dulbecco's modified Eagle's medium, the prostate, Jurkat, SW-480 and HCT-15 colon cells were grown in RPMI 1640 medium, the lung cells were grown in F-12 and the colon HT-29 cells were grown in McCoy 5A. All media were supplemented with 10% fetal calf serum (Invitrogen, Carlsbad, CA) penicillin (50 U/mL), and streptomycin (50 µg/mL) (Invitrogen, Carlsbad, CA). Cells were incubated at 37 °C in 5% CO2 and 90% relative humidity. Single cell suspensions were obtained by trypsinization (0.05% trypsin/EDTA), and cells were counted using a hemacytometer. The final DMSO concentration was adjusted in all media to 1%. Viability was determined by the trypan blue dye exclusion method.
Cell growth inhibitory effect of SUL and NOSH-SUL was measured using a colorimetric MTT assay kit (Roche, Indianapolis, IN). Cancer cells were plated in 96-well plates at a density of 25,000 cells/well. The cells were incubated for 24 h with different concentrations of SUL and NOSH-SUL after which 10 µL of MTT dye (3-[4,5–dimethylthiazol–2–yl]-2,5-diphenyl tetrazolium bromide, 5 mg/mL in phosphate buffered saline), was added to each well, and the plates were incubated for 2 h at 37 °C. Then, the media was aspirated, and 100 μL of the solubilization solution (10% SDS in 0.01 M HCl) was added to each well. The absorbance was measured on a spectrophotometric plate reader at a wavelength of 570 nm.
2.14. Cell proliferation
PCNA antigen expression was determined using an ELISA Kit (Calbiochem, La Jolla, CA), following the manufacturers protocol. HT-29 cells (1×106 cells/mL) were incubated with serum-free media for 24 h to remove the effect of endogenous growth factors; they were then treated for 24 h with various concentrations of NOSH-SUL or vehicle as previously reported [37].
2.15. Cell cycle analysis
Cell cycle phase distributions of control and treated HT-29 cells were obtained using a Coulter Profile XL equipped with a single argon ion laser. For each subset, >10,000 events were analyzed. All parameters were collected in list mode files. Data were analyzed on a Coulter XL Elite Work station using the Software programs MultigraphTM and MulticycleTM. HT-29 Cells (0.5×106) treated with various concentrations of NOSH-SUL or vehicle were fixed in 100% methanol for 10 min at −20 °C, pelleted (5000 rpm×10 min at 4 °C), resuspended and incubated in PBS containing 1% FBS/0.5% NP-40 on ice for 5 min. Cells were washed again in 500 µL of PBS/1% FBS containing 40 µg/mL propidium iodide (used to stain for DNA) and 200 mg/mL RNase type IIA, and analyzed within 30 min by flow cytometry. The percentage of cells in G0/G1, G2/M, and S phases was determined form DNA content histograms as reported previously [37].
2.16. Assay for apoptosis
HT-29 cells (0.5×106 cells/mL) were treated for 24 h with various concentrations of NOSH-SUL or vehicle. Cells were washed with and resuspended in 1×Binding Buffer (Annexin V binding buffer, 0.1 M HEPES/NaOH (pH 7.4), 1.4 M NaCl, 25 mM CaCl2; BD BioSciences Pharmingen, San Diego, CA). Then 5 mL of Annexin V-FITC (final concentration 0.5 mg/mL) was added followed by propidium iodide as a counterstain (final concentration 20 mg/mL). The cells were then incubated at room temperature for 15 min in the dark. Finally, the cells were transferred to FACS tubes for analysis. Percentage of apoptotic cells were obtained using a Becton Dickinson LSR II equipped with a single argon ion laser. For each subset, about 10,000 events were analyzed. All parameters were collected in list mode files. Data was analyzed by Flow Jo software as reported previously [37].
2.17. Statistical analysis
In vivo treatment groups and number of animals in each group are indicated in the figure legend. In vitro data are presented as mean±SEM for at least three different sets of plates done in triplicate. Comparisons between groups were performed using a one-way analysis of variance followed by the Student-t test. P<0.05 was regarded as statistically significant.
3. Results and discussion
3.1. NOSH-SUL is gastrointestinal safe
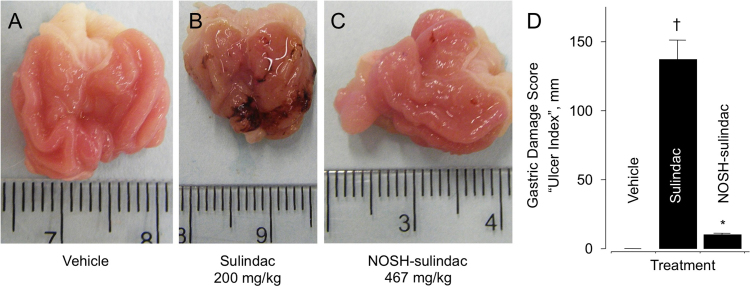
The rats receiving the vehicle (0.5% CMC solution) had a normal glandular region on the surface of their stomach, and no ulcerative damage (Fig. 2A and D). For these rats, the gastric damage score (also described in the literature as “ulcer index”, or UI), was zero (UI=0). Administration of SUL (200 mg/kg) resulted in extensive mucosal injury, UI=130 (Fig. 2B and D). NOSH-SUL (476 mg/kg) did not produce significant ulcerative damage (Fig. 2C and D), UI=10 compared to SUL at equimolar doses, which represents a remarkable reduction (P<0.01) in gastrointestinal toxicity. Thus, this modified sulindac which has been shown to releases NO and H2S [29] appears to be gastrointestinal safe. As alluded to in the introduction, SUL has extensively been utilized as a chemopreventive agent in patients with FAP [25–28]. However, a limiting factor in its long-term use is its GI toxicity. Based on the data presented here, NOSH-SUL would be an ideal drug candidate for development in such a setting.
Fig. 2.
NOSH-sulindac is gastrointestinal safe. SUL and NOSH-SUL were administered orally at equimolar doses (0.56 mmol/kg; 200 mg/kg and 467 mg/kg for SUL and NOSH-SUL, respectively) and effects on the stomach were evaluated as indicated in Section 2.3. Panel A, shows the stomach of a vehicle-treated rat; Panel B, stomach of a SUL-treated rat showing ulceration and bleeding; Panel C, stomach of a NOSH-SUL-treated rat which is essentially devoid of ulcers. Panel D, gastric damage due to SUL, UI=135±15 mm (†P<0.01 compared to vehicle), NOSH-SUL was gastric damage-sparing, UI=10±1 mm (*P<0.01 compared to SUL). Photographs in Panels A–C are representative from 5 rats in each group. Results in Panel D are mean±SEM of 5 rats in each group.
3.2. Gastric mucosal exudate prostaglandin E2 content
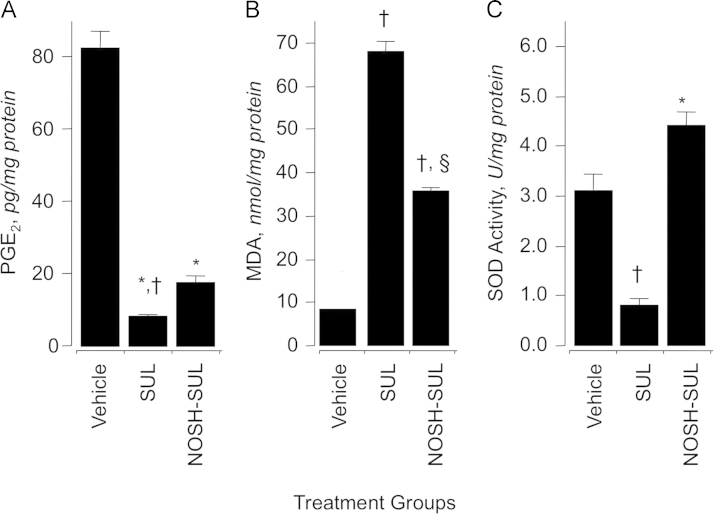
We investigated the effect of SUL and NOSH-SUL on prostaglandin E2 (PGE2) content in gastric mucosa (Fig. 3A). Rats treated with SUL (200 mg/kg) produced about 88% less PGE2 than rats in the control group. NOSH-SUL (467 mg/kg) also reduced PGE2 levels but not to the same extent as SUL, the reduction being around 75% (Fig. 3A). Prostaglandins are the main product of cyclooxygenase-mediated arachidonic acid metabolism in gastric mucosa, therefore, comparison of PGE2 content between control and drug-treated groups showed a clear and significant COX inhibition by both SUL and NOSH-SUL. In order to confirm that indeed COX enzyme activity was being inhibited, we evaluated the effects of these two compounds on ovine COX-1 and COX-2 enzymatic activity at their respective IC50s for cell growth inhibition, in HT-29 colon cancer cells (see Section 3.9). As shown in Table 1, NOSH-SUL at a concentration of 90 nM inhibited COX-1 enzymatic activity of more than that of COX-2, the respective values being 44±1% and 14±1%. SUL at 800 µM inhibited COX-1 by 82±2% and COX-2 by 68±1%. Therefore, SUL at its IC50s for cell growth inhibition inhibits both COX-1 and COX-2 more than NOSH-SUL at IC50s for cell growth inhibition. Since NOSH-SUL is significantly more potent than SUL, this strongly suggests that targets other than COX must be contributing to its mode of action. We also measured the degree of COX-1 and COX-2 inhibition by indomethacin (1 µM) a nonselective COX inhibitor [38] as a reference compound in order to ensure that there were no anomalies with our assay system. Indomethacin inhibited COX-1 and COX-2 by 74±2% and 68±1%, respectively (Table 1).
Fig. 3.
Effects of sulindac and NOSH-sulindac on gastric PGE2 levels, lipid peroxidation (MDA) and superoxide dismutase (SOD). At the end of the gastrointestinal safety evaluations as described in Section 2.3, tissues from the excised stomachs of each rat was snap frozen in liquid nitrogen and processed as described in Sections 2.4–2.6. SUL and NOSH-SUL caused a significant reduction in gastric mucosal PGE2 levels (panel A). Results are mean±SEM of 5 rats in each group, *P<0.05 vs vehicle group, †P<0.05 vs NOSH-SUL group. SUL caused an almost 9-fold increase in MDA levels, for NOSH-SUL-treated rats, MDA levels were about 2-fold higher (panel B). Results are mean±SEM for 5 rats in each group, †P<0.01 vs vehicle group, §P<0.05 vs SUL group. SUL caused a significant reduction in SOD activity, whereas NOSH-SUL did not have an effect (panel C). Results are mean±SEM of 5 rats in each group, †P<0.05 vs vehicle group, †P<0.01 vs SUL group.
Table 1.
NOSH-SUL inhibits cyclooxygenase enzyme activity.
| Treatment | COX-1 % Inhibition | COX-2 % Inhibition |
|---|---|---|
| SUL, 800 µM | 82±2 | 68±1 |
| NOSH-SUL, 90 nM | 44±1 | 14±1 |
| Indomethacin, 1 µM | 74±2 | 68±1 |
Pure ovine COX enzymes were treated with SUL or NOSH-SUL at their respective IC50s for cell growth inhibition in HT-29 colon cancer cell line for 15 min at 4 °C after which o-COX-1 and o-COX-2 enzyme activity were determined. Results are mean±range of two independent studies performed in duplicate.
3.3. Effect of NOSH-SUL on lipid peroxidation and superoxide dismutase activity
Measuring the concentration of MDA in intact mucosa 6 h post-administration of SUL and NOSH-SUL at 200 mg/kg and 476 mg/kg respectively was used to assess oxidative stress in gastric tissue. MDA levels were 8±1 nmol/mg protein in the vehicle treated rats (Fig. 3B), this was increased to 68±2 nmol/mg protein in the SUL treated rats but was significantly less in the NOSH-SUL treated animals, 32±1 nmol/mg protein, (Fig. 3B). Samples from the same gastric tissues were used to measure SOD activity. In the intact mucosa (control group) SOD activity was 3.2±0.3 U/mg protein. Following administration of SUL a significant decrease in SOD activity was observed (0.9±0.1 U/ mg protein, †P<0.05 compared to vehicle). However, in the NOSH-SUL treated rats, SOD activity was significantly increased to 4.4±0.3 U/mg protein (⁎P<0.01 compared to SUL, Fig. 3C). SOD is an antioxidative marker. Its activity was significantly lowered in the SUL-treated animals, this may explain the high levels of MDA and ulcerations observed in the stomachs. SOD activity was significantly higher in the NOSH-SUL-treated animals, which correlated with lower MDA levels and essentially no ulcerations to the stomachs. Thus, some if not all of the changes in the gastric mucosal tissue may be as the result of the antioxidative effects of NOSH-SUL.
3.4. Carrageenan-induced paw swelling
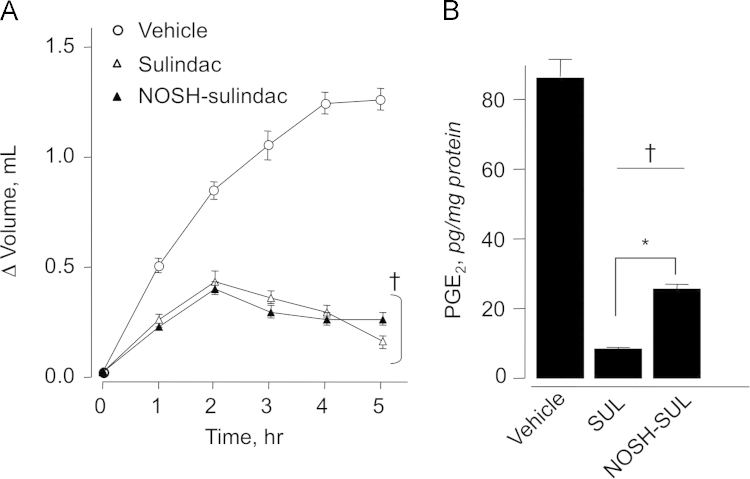
Sulindac is mainly used for treating inflammatory conditions. We therefore wanted to compare the COX-dependent anti-inflammatory activity of SUL to that of NOSH-SUL. For this, we used the carrageenan-induced edema model [34]. After inducing inflammation, animals receiving vehicle showed a fast time-dependent increase in paw volume (ΔV=0.5 mL) within 1 h, and gradual increase to 1.3 mL over the course of the experiment (5 h) (Fig. 4A). In contrast, animals receiving SUL or NOSH-SUL showed a weak inflammatory response, ΔV=0.2–0.3 mL by 1 h, which peaked to ΔV=0.40–0.45 mL at 2 h and then decreased over the next 3 h (Fig. 4A). The anti-inflammatory effect registered in animals dosed with NOSH-SUL was comparable to those treated with SUL. Prostaglandins (PGE2) are the main products of cyclooxygenase-mediated arachidonic acid metabolism [1]. Comparison of PGE2 content of paw exudates showed a clear and significant COX inhibition by SUL and NOSH-SUL (Fig. 4B). PGE2 levels in control vehicle-treated rats were 85±4 pg mg−1 and in the SUL and NOSH-SUL-treated rats it went down to 9±1 pg mg−1 and 24±2 pg mg−1, respectively. This is equivalent to reduction of 89% and 72% by SUL and NOSH-SUL, respectively.
Fig. 4.
Anti-inflammatory properties of sulindac and NOSH-sulindac. Rat paw edema was induced by carrageenan injection as described in Section 2.9. SUL and NOSH-SUL were both equally effective in reducing paw volume at all time points (panel A). Results are mean±SEM of 5 rats in each group, †P<0.05 vs vehicle treated rats at all time points. SUL and NOSH-SUL also caused a significant reduction in PGE2 levels in the paw exudate (panel B). Results are mean±SEM for 5 rats in each group, †P<0.01 vs vehicle, *P <0.05 vs NOSH-SUL.
3.5. Plasma TNFα levels
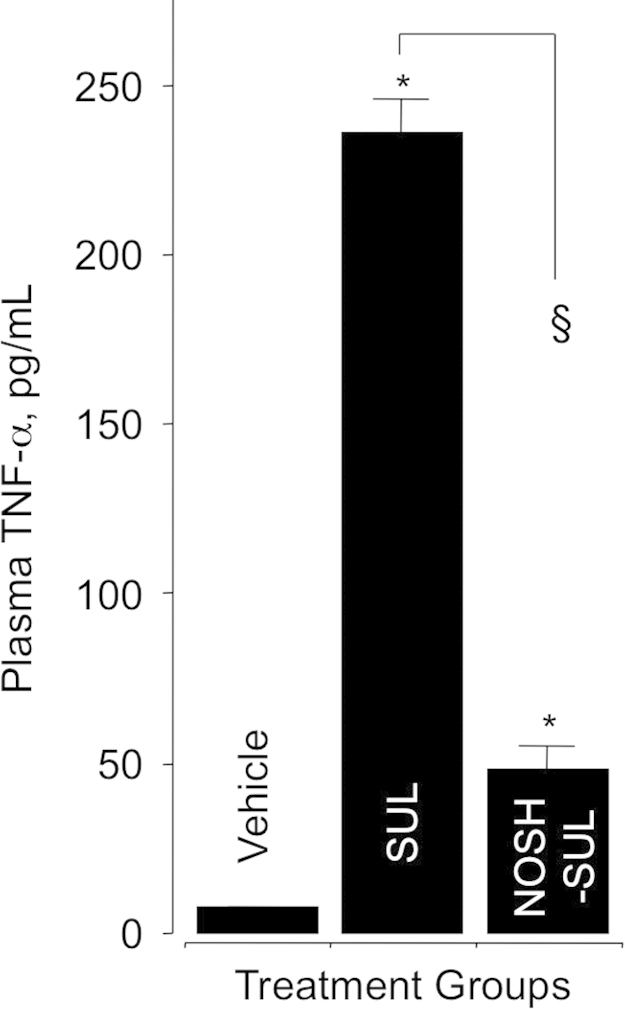
We determined the inhibitory effect of SUL and NOSH-SUL on the proinflammatory cytokine tumor necrosis factor-α in plasma obtained from control and drug-treated animals at the end of the gastrointestinal safety experiments, 6 h post-administration (Section 2.3). Administration of SUL (200 mg/kg, 0.56 mmol/kg) increased TNFα concentration by about 25-fold (9.5±0.3 control and 230±5 pg/mL SUL). However, this rise was considerably lower in the NOSH-SUL-treated (476 mk/g, 0.56 mmol/kg) animals, 50±3 pg/mL (Fig. 5).
Fig. 5.
Effect of sulindac and NOSH-sulindac on plasma TNF-α. At the end of the gastrointestinal safety evaluations as described in Section 2.3, blood was drawn and processed as described in Section 2.7 for determination of plasma TNF-α. SUL caused a significant rise in plasma TNF-α, however, this rise was significantly less in the NOSH-SUL-treated rats. Results are mean±SEM for 5 rats in each group, *P<0.001 vs vehicle, §P<0.01 vs SUL.
3.6. Antipyretic activity
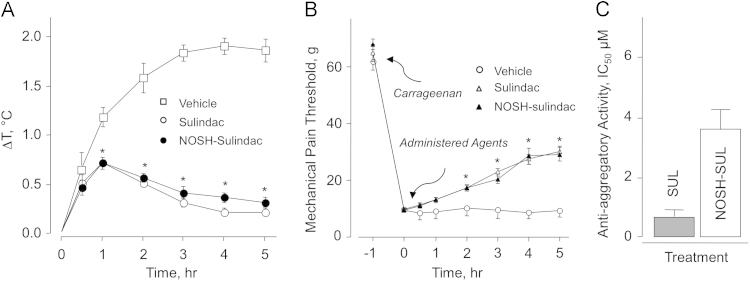
It is well known that NSAIDs exert a moderate antipyretic effect when administered orally; although SUL is seldom used for that purpose. Nevertheless for comparison considerations, we wanted to determine the decrease in body temperature induced by NOSH-SUL compared to that obtained with SUL. Experimental drugs, SUL and NOSH-SUL were administered orally at equimolar doses (0.56 mmol/kg; 200 mg/kg and 467 mg/kg for SUL and NOSH-SUL, respectively) 30 min before injecting the rats with LPS (50 μg/kg ip). In this regard, control animals showed a time-dependent increase in body temperature which leveled off between 3 and 4 h with ΔT=1.8 °C and this was maintained until the end of the screen (5 h). However, SUL and NOSH-SUL-treated animals showed only about a half-degree increase in body temperature at 30 min after LPS injection, this increased to ΔT=0.7 °C by 1 h thereafter gradually decreased (Fig. 6A).
Fig. 6.
Sulindac and NOSH-sulindac reduce LPS-induced fever, raise the threshold for hyperalgesia, and show anti-platelet activity. Panel A: LPS (50 μg/kg, ip) was administered to the rats one hour before administration of SUL or NOSH-SUL at equimolar doses (0.56 mmol/kg; 200 mg/kg and 467 mg/kg for SUL and NOSH-SUL, respectively). Core body temperature was recorded at 30 min and thereafter hourly for 5 h. Results are mean±SEM for 5 rats in each group, *P<0.01 vs vehicle for both SUL and NOSH-SUL from 1–5 h. Panel B: Mechanical pain threshold was increased in a time-dependent manner by SUL and NOSH-SUL. Results are mean±SEM for 5 rats in each group. *P<0.05 vs vehicle for SUL and NOSH-SUL from 2–5 h.Panel C: SUL and NOSH-SUL were equally effective in inhibiting human platelet aggregation. Results are mean±range for two individuals.
3.7. Carrageenan-induced mechanical hyperalgesia
This assay measures the ability of the test drugs to decreased threshold to a painful stimuli produced by injection of carrageenan onto the plantar surface of the right hind paw. The mechanical pain threshold was increased upon time by administering of SUL and NOSH-SUL (Fig. 6B). Pain threshold was markedly reduced from 65 g to about 10 g in animals receiving vehicle (control group), indicating a higher sensitivity to mechanical stimuli (non-painful at normal conditions). Hyperalgesia was decreased in animals receiving SUL and NOSH-SUL to the same extent, about 32 g or ~50% reduction compared to the initial response. Another NO- and H2S-releasing NSAID, NOSH-aspirin (NBS 11–20) was recently shown to have greater potency than aspirin in reducing inflammatory pain in several clinically relevant models [39]. The enhanced antinociceptive effect of NOSH-aspirin appeared to be due to its ability to reduce the production of pronociceptive cytokines such as IL-1β. NOSH-aspirin was also shown to reduce hyperalgesia, caused by a directly acting hyperalgesic mediator in a mechanism dependent on modulation of KATP channels. The latter effect is presumably due to the released H2S as this gasotransmitter in known to affect [1,40].
3.8. Platelet anti-aggregatory activity
Sulindac is not used as an anti-aggregatory agent whereas aspirin is frequently employed for this purpose. This is primarily because aspirin is an irreversible inhibitor of COX-1 whereas SUL is not [24]. Nevertheless, since SUL does inhibit COX-1 we wanted to compare the anti-aggregatory effects of NOSH-SUL to that of SUL for complete characterization of these two compounds. We used collagen-induced platelet aggregation of human platelet-rich plasma (PRP) for the comparison. The results expressed as IC50s are shown in Fig. 6C. Analysis of the data does not show any statistical differences between SUL and NOSH-SUL. It should be noted that NOSH-SUL releases NO and H2S [29] both of which can have independent anti-platelet properties [41–43].
3.9. NOSH-SUL inhibits the growth of various human cancer cell lines
We investigated the effects of SUL and NOSH-SUL on the growth properties of 12 different cancer cell lines of six different histological subtypes. The cell lines were that of colon (HT-29: COX-1 and COX-2 positive, HCT 15: COX null, and SW480: COX-1 positive, low levels of endogenous COX-2), breast (MCF7: [ER(+)], MDA MB-231 and SKBR3: [ER(−)]); pancreatic (BxPC3: both COX-1 and COX-2 positive, MIAPaCa-2: COX-null), lung (A549, H383), prostate (LNCaP), and T-cell leukemia (Jurkat). NOSH-SUL was extremely effective in inhibiting the growth of these cell lines (Table 2). The IC50s for cell growth inhibition at 24 h for NOSH-SUL ranged from 0.09±0.01 to 0.32±0.03 µM and that for SUL was 212±37 to 935±35 µM. The growth inhibition by NOSH-SUL versus SUL was very high in the panel of cancer cell lines studied. In a fold comparison study of the IC50 values (SUL/NOSH-SUL), NOSH-SUL was at least 1000-fold to 9000-fold more potent than SUL in various cell lies (Table 2). Such fold increases imply that the NO and H2S-related structural modifications of the SUL molecule imparts a differential enhancement in potency. Furthermore, our data strongly suggests that this effect may be tissue-type independent since NOSH-SUL was effective against adenomatous, epithelial, and lymphocytic cancer cell lines. Here we studied 12 cell lines originating from six different tissues, therefore, it may be envisaged that our findings are part of a generalized effect. An interesting aspect of growth inhibition also emerges with respect to COX expression in the cell lines examined. NOSH-SUL showed similar effects on two colon cancer cell lines, HT-29 (expresses COX-1 and COX-2) and HCT 15 (no COX expression) [44] and on two pancreatic cancer cell lines, BxPC-3 (expresses COXs) and MIA PaCa-2 (no COX expression) [45] suggesting a COX-independent effect.
Table 2.
IC50 (µM) values at 24 h for cell growth inhibition in different cancer cell lines.
| Agent | Colon |
Breast |
Pancreas |
Lung |
Prostate |
Leukemia |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HT-29 | HCT 15 | SW480 | MDA MB 231 | SKBR3 | MCF-7 | MIAPaCa2 | BxPC3 | A549 | H383 | LNCAP | Jurkat | |
| SUL | 835±75 | 850±55 | 710±65 | 935±35 | 845±55 | 975±80 | 792±68 | 960±58 | 212±37 | 532±38 | 810±80 | 650±45 |
| NOSH-SUL | 0.095± 0.01 | 0.11±0.01 | 0.11±0.009 | 0.098±0.017 | 0.12±0.01 | 0.10±0.007 | 0.098±0.018 | 0.12±0.01 | 0.18±0.015 | 0.088±0.01 | 0.09±0.01 | 0.32±0.03 |
| Enhanced Potency | ~8700 | ~7700 | ~6400 | ~9500 | ~7000 | ~9700 | ~8000 | ~8000 | ~1100 | ~6000 | ~9000 | ~2000 |
Colon, breast, pancreas, lung, prostate, and leukemia cancer cell lines were treated with various concentrations of sulindac (SUL) and NOSH-sulindac (NOSH-SUL) as described in Section 2.13. Cell numbers were determined at 24 h from which IC50 values were calculated. The ratios of SUL/NOSH-SUL represent fold-enhancement in potency of NOSH-SUL over SUL. Results are mean±SEM of three independent determinations. In all cell lines, P<0.001 for NOSH-SUL vs SUL.
Currently we cannot explain the underlying mechanism(s) for the enhanced potency of NOSH-SUL observed in these studies. We do not yet know anything about the kinetics of NO and H2S release and their potential interactions. However, we do know that both contribute towards the potency of the intact molecule. This is based on our earlier observations where we showed that the biological activity of aspirin plus SNAP (S-Nitroso-N-acetyl-penicillamine, which releases NO) plus ADT-OH (5-(4-hydroxyphenyl)-3H-1, 2-dithiole-3-thione, which releases H2S) was not the same as the biological activity of the intact NOSH-aspirin molecule [46]. Thus the sum of parts did not equal the whole. The same was observed for NOSH-naproxen [29]. So, we suspect the same will hold true for NOSH-SUL. However, recent reports indicate that NO can react with H2S to produce HSNO which is a highly reactive intermediate [47,48]. Furthermore, NO and H2S signaling pathways appear to be intimately intertwined with mutual potentiation of biological responses [49].
3.10. Effect of NOSH-SUL on cell growth kinetics
The effects of NOSH-SUL on cell proliferation, apoptosis, and cell cycle transition, all of which affect cell growth were also examined in HT-29 colon cancer cells.
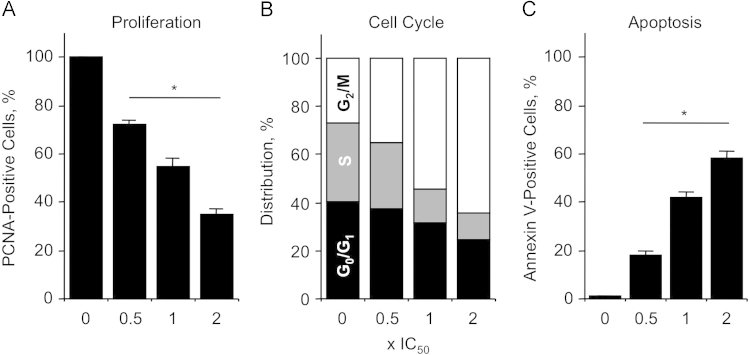
To determine the antiproliferative effects, HT-29 colon cancer cells were treated with different concentration of NOSH-SUL for 24 h, followed by PCNA quantification. The concentrations we used were, 0.5×IC50 (50 nM), 1×IC50 (100 nM), and 2×IC50 (200 nM). NOSH-SUL reduced proliferation in a dose-dependent manner, as measured by the expression of PCNA, (Fig. 7A). Proliferation decreased to 72±2%, to 55±3% and to 35±2% at 0.5×IC50, 1×IC50, and 2×IC50, respectively.
Fig. 7.
Effect of NOSH-sulindac on colon cancer cell kinetics. NOSH-SUL inhibits proliferation by altering cell cycle progression and inducing apoptosis. HT-29 cells were treated with vehicle, 0.5x IC50 (50 nM), 1×IC50 (100 nM) or 2×IC50 (200 nM) NOSH-SUL for 24 h and analyzed for (A) proliferation by PCNA antigen expression; (B) cell cycle phases by PI staining and flow cytometry; (C) apoptosis by Annexin V staining and flow cytometry. In (A) and (C), results are mean±SEM for 3 different experiments performed in duplicate, *P<0.05 compared to control. In (B), results are representative of two different experiments.
To determine whether cells were undergoing apoptosis in addition to inhibition of cell proliferation, apoptotic population was evaluated by Annexin V-FITC and propidium iodide staining, followed by flow cytometry. As shown in Fig. 7C, NOSH-SUL caused a significant increase in the number of cells undergoing apoptosis. The percentage of apoptotic cells increased from 18±2% at 0.5×IC50 to 42±2% at 1×IC50, and 58±3% at 2×IC50 compared to control.
We also determined the effect of the NOSH-SUL on the distribution of cells in G0/G1, S, and G2/M phases of the cell cycle. Cells were exposed to NOSH-SUL at concentrations of 0.5×IC50, 1×IC50, and 2×IC50 for 24 h, and analyzed for cell cycle phases by flow cytometry. DMSO-treated control cells proceeded through a normal cell cycle. Increasing concentrations of NOSH-SUL were associated with dose-dependent decreases in the percentage of cells in G0/G1 and S phases, and a corresponding increase in the percentages of cells in G2/M phase (Fig. 7B), suggesting a G2/M phase cell cycle block. For example, at 1×IC50 the population cells in G0/G1 phase of the cell cycle decreased from 40.6% to 31.4% and the S phase was reduced from 32.7% to 14%, while the cells in G2/M increased from 26.7% to 54.6%. This mode of cell cycle arrest has been reported for the parent drug sulindac in SW480 human colon cancer cells [50]. Thus, NOSH-SUL inhibits proliferation of HT-29 colon cancer cells by a combined induction of G2/M arrest and apoptosis.
Summary and future directions
In the present study, proof-of-concept animal studies demonstrated that NOSH-sulindac is essentially devoid of any gastrointestinal side effects even though it reduces gastric tissue PGE2 levels. The hybrid molecule retains all the positive pharmacological attributes of its parent NSAID, sulindac. That is, it is a potent anti-inflammatory and analgesic that has anti-pyretic and anti-platelet activity. In addition to its GI safety, NOSH-sulindac might also prove to have enhanced cardiovascular and renal safety profiles. This is because NO and H2S have protective roles in the cardiovascular and renal system [51–53]. NOSH-sulindac is also potentially useful as a chemopreventive agent against many types of cancer. In this regard, we are currently evaluating its utility in different animal models of cancer such as the APCMin/+ mice. We are also deciphering its mechanism of action, focusing on molecular targets that are relevant to inflammation and cancer and to possible interactions between NO and H2S in producing a new signaling entity.
Authorship contributions
Participated in research design: Kashfi, Chattopadhyay, Kodela.
Conducted experiments: Kodela, Chattopadhyay.
Performed data analysis: Kashfi, Chattopadhyay, Kodela.
Wrote or contributed to the writing of the manuscript: Kashfi, Chattopadhyay, Kodela.
Grant support
This work was supported in part by the National Institutes of Health National Institute [Grant R24 DA018055]. The funding agency had no role in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Conflict of interest
The authors have nothing to disclose except for KK, who has an equity position in Avicenna Pharmaceuticals, Inc. the supplier of NOSH-sulindac used in these studies.
References
- 1.Kashfi K. Anti-inflammatory agents as cancer therapeutics. Adv. Pharmacol. 2009;57:31–89. doi: 10.1016/S1054-3589(08)57002-5. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell P.M., Price J.F., Fowkes F.G., Zanchetti A., Roncaglioni M.C., Tognoni G. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 3.Baron J.A., Cole B.F., Sandler R.S., Haile R.W., Ahnen D., Bresalier R. A randomized trial of aspirin to prevent colorectal adenomas. N. Engl. J. Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 4.Sandler R.S., Halabi S., Baron J.A., Budinger S., Paskett E., Keresztes R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N. Engl. J. Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 5.Cuzick J., Thorat M.A., Bosetti C., Brown P.H., Burn J., Cook N.R. Estimates of benefits and harms of prophylactic use of aspirin in the general population. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol./ESMO. 2015;26:47–57. doi: 10.1093/annonc/mdu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Pedro M., Baeza S., Escudero M.T., Dierssen-Sotos T., Gomez-Acebo I., Pollan M. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res. Treat. 2015;149:525–536. doi: 10.1007/s10549-015-3267-9. [DOI] [PubMed] [Google Scholar]
- 7.Yiannakopoulou E.C. Aspirin and NSAIDs for breast cancer chemoprevention. Eur. J. Cancer Prev.: Off. J. Eur. Cancer Prev. Organ. (ECP) 2014;24:416–421. doi: 10.1097/CEJ.0000000000000098. [DOI] [PubMed] [Google Scholar]
- 8.Takkouche B., Regueira-Mendez C., Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J. Natl. Cancer Inst. 2008;100:1439–1447. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 9.Cui X.J., He Q., Zhang J.M., Fan H.J., Wen Z.F., Qin Y.R. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43:135–140. doi: 10.1097/MPA.0b013e3182a8d41f. [DOI] [PubMed] [Google Scholar]
- 10.Nicastro H.L., Grubbs C.J., Margaret Juliana M., Bode A.M., Kim M.S., Lu Y. Preventive effects of NSAIDs, NO-NSAIDs, and NSAIDs plus difluoromethylornithine in a chemically induced urinary bladder cancer model. Cancer Prev. Res. 2014;7:246–254. doi: 10.1158/1940-6207.CAPR-13-0164. [DOI] [PubMed] [Google Scholar]
- 11.Daugherty S.E., Pfeiffer R.M., Sigurdson A.J., Hayes R.B., Leitzmann M., Schatzkin A. Nonsteroidal antiinflammatory drugs and bladder cancer: a pooled analysis. Am. J. Epidemiol. 2011;173:721–730. doi: 10.1093/aje/kwq437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker C., Wilson J.C., Jick S.S., Meier C.R. Non-steroidal anti-inflammatory drugs and the risk of head and neck cancer: a case-control analysis. Int. J. Cancer J. Int. Cancer. 2015 doi: 10.1002/ijc.29601. 10.1002/ijc.29601. [DOI] [PubMed] [Google Scholar]
- 13.Sun L., Yu S. Meta-analysis: non-steroidal anti-inflammatory drug use and the risk of esophageal squamous cell carcinoma. Dis. Esophagus: Off. J. Int. Soc. Dis. Esophagus/ISDE. 2011;24:544–549. doi: 10.1111/j.1442-2050.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- 14.Baandrup L., Kjaer S.K., Olsen J.H., Dehlendorff C., Friis S. Low-dose aspirin use and the risk of ovarian cancer in Denmark. Ann. Oncol.: Off. J. Eur. Soc. Med. Oncol./ESMO. 2015;26:787–792. doi: 10.1093/annonc/mdu578. [DOI] [PubMed] [Google Scholar]
- 15.Trabert B., Ness R.B., Lo-Ciganic W.H., Murphy M.A., Goode E.L., Poole E.M. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, and acetaminophen use and risk of invasive epithelial ovarian cancer: a pooled analysis in the Ovarian Cancer Association Consortium. J. Natl. Cancer Inst. 2014;106:1–11. doi: 10.1093/jnci/djt431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shebl F.M., Sakoda L.C., Black A., Koshiol J., Andriole G.L., Grubb R. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO study. Br. J. Cancer. 2012;107:207–214. doi: 10.1038/bjc.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahasrabuddhe V.V., Gunja M.Z., Graubard B.I., Trabert B., Schwartz L.M., Park Y. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J. Natl. Cancer Inst. 2012;104:1808–1814. doi: 10.1093/jnci/djs452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clouser M.C., Roe D.J., Foote J.A., Harris R.B. Effect of non-steroidal anti-inflammatory drugs on non-melanoma skin cancer incidence in the SKICAP-AK trial. Pharmacoepidemiol. Drug Saf. 2009;18:276–283. doi: 10.1002/pds.1718. [DOI] [PubMed] [Google Scholar]
- 19.Elmets C.A., Viner J.L., Pentland A.P., Cantrell W., Lin H.Y., Bailey H. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J. Natl. Cancer Inst. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman J.R., Grossman D. Aspirin and other NSAIDs as chemoprevention agents in melanoma. Cancer Prev. Res. 2014;7:557–564. doi: 10.1158/1940-6207.CAPR-14-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiff S.J., Rigas B. Nonsteroidal anti-inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology. 1997;113:1992–1998. doi: 10.1016/s0016-5085(97)99999-6. [DOI] [PubMed] [Google Scholar]
- 22.Kinzler K.W., Nilbert M.C., Su L.K., Vogelstein B., Bryan T.M., Levy D.B. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 23.Vasen H.F., Moslein G., Alonso A., Aretz S., Bernstein I., Bertario L. Guidelines for the clinical management of familial adenomatous polyposis (FAP) Gut. 2008;57:704–713. doi: 10.1136/gut.2007.136127. [DOI] [PubMed] [Google Scholar]
- 24.Grosser T., Smyth E.M., FitzGerald G.A. Anti-inflammatory, Antipyretic, and Analgesic Agents; Pharmacology of Gout. In: Bruton L.L., editor. Goodman & Gilman's Pharmacological Basis of Therapeutics. McGraw Hill; New York: 2012. pp. 959–1004. [Google Scholar]
- 25.Kim K.Y., Jeon S.W., Park J.G., Yu C.H., Jang S.Y., Lee J.K. Regression of colonic adenomas after treatment with sulindac in familial adenomatous polyposis: a case with a 2-year follow-up without a prophylactic colectomy. Ann. Coloproctol. 2014;30:201–204. doi: 10.3393/ac.2014.30.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto T., Nakamura S., Esaki M., Yao T., Iida M. Effect of the non-steroidal anti-inflammatory drug sulindac on colorectal adenomas of uncolectomized familial adenomatous polyposis. J. Gastroenterol. Hepatol. 2006;21:251–257. doi: 10.1111/j.1440-1746.2006.04181.x. [DOI] [PubMed] [Google Scholar]
- 27.Waddell W.R., Loughry R.W. Sulindac for polyposis of the colon. J. Surg. Oncol. 1983;24:83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- 28.Giardiello F.M., Hamilton S.R., Krush A.J., Piantadosi S., Hylind L.M., Celano P. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N. Engl. J. Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 29.Kodela R., Chattopadhyay M., Kashfi K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. Med. Chem. Commun. 2013;4:1472–1481. doi: 10.1039/C3MD00185G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace J.L., Miller M.J. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–520. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 31.Fiorucci S. Prevention of nonsteroidal anti-inflammatory drug-induced ulcer: looking to the future. Gastroenterol. Clin. N. Am. 2009;38:315–332. doi: 10.1016/j.gtc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Best R., Lewis D.A., Nasser N. The anti-ulcerogenic activity of the unripe plantain banana (Musa species) Br. J. Pharmacol. 1984;82:107–116. doi: 10.1111/j.1476-5381.1984.tb16447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto L., Borreli F., Bomberdelli E., Cristonic A., Capasso F. Antiinflammatory, analgesis and antipyretic effects of glaucine in rats and mice. Pharm. Pharmacol. Commun. 1998;4:502–505. [Google Scholar]
- 34.Winter C.A., Risley E.A., Nuss G.W. Carrageenan-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 35.Nieswandt B., Watson S.P. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 36.Kulmacz R.J., Lands W.E. Requirements for hydroperoxide by the cyclooxygenase and peroxidase activities of prostaglandin H synthase. Prostaglandins. 1983;25:531–540. doi: 10.1016/0090-6980(83)90025-4. [DOI] [PubMed] [Google Scholar]
- 37.Chattopadhyay M., Kodela R., Nath N., Barsegian A., Boring D., Kashfi K. Hydrogen sulfide-releasing aspirin suppresses NF-kappaB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem. Pharmacol. 2012;83:723–732. doi: 10.1016/j.bcp.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Riendeau D., Charleson S., Cromlish W., Mancini J.A., Wong E., Guay J. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can. J. Physiol. Pharmacol. 1997;75:1088–1095. [PubMed] [Google Scholar]
- 39.Fonesca M.D., Cunha F.Q., Kashfi K., Cunha T.M. NOSH-aspirin (NBS-1120), a dual nitric oxide and hydrogen sulfide-releasing hybrid, reduces inflammatory pain. Pharmacol. Res. Perspect. 2015;3:1–12. doi: 10.1002/prp2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteman M., Winyard P.G. Hydrogen sulfide and inflammation: the good, the bad, the ugly and the promising. Expert Rev. Clin. Pharmacol. 2011;4:13–32. doi: 10.1586/ecp.10.134. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa H., Hayashi H., Kubo S., Tsubota-Matsunami M., Sekiguchi F., Kawabata A. Inhibition by hydrogen sulfide of rabbit platelet aggregation and calcium mobilization. Biol. Pharm. Bull. 2013;36:1278–1282. doi: 10.1248/bpb.b13-00018. [DOI] [PubMed] [Google Scholar]
- 42.Olas B., Kontek B. The possible role of hydrogen sulfide as a modulator of hemostatic parameters of plasma. Chem. Biol. Interact. 2014;220:20–24. doi: 10.1016/j.cbi.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Riddell D.R., Owen J.S. Nitric oxide and platelet aggregation. Vitam. Horm. 1999;57:25–48. doi: 10.1016/s0083-6729(08)60639-1. [DOI] [PubMed] [Google Scholar]
- 44.Hanif R., Pittas A., Feng Y., Koutsos M.I., Qiao L., Staiano-Coico L. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem. Pharmacol. 1996;52:237–245. doi: 10.1016/0006-2952(96)00181-5. [DOI] [PubMed] [Google Scholar]
- 45.Kashfi K., Ryan Y., Qiao L.L., Williams J.L., Chen J., Del Soldato P. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J. Pharmacol. Exp. Ther. 2002;303:1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 46.Chattopadhyay M., Kodela R., Olson K.R., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid is a potent inhibitor of colon cancer cell growth in vitro and in a xenograft mouse model. Biochem. Biophys. Res. Commun. 2012;419:523–528. doi: 10.1016/j.bbrc.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 47.Cortese-Krott M.M., Fernandez B.O., Kelm M., Butler A.R., Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide: Biol. Chem./Off. J. Nitric Oxide Soc. 2015;46:14–24. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Filipovic M.R., Miljkovic J., Nauser T., Royzen M., Klos K., Shubina T. Chemical characterization of the smallest S-nitrosothiol, HSNO; cellular cross-talk of H2S and S-nitrosothiols. J. Am. Chem. Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortese-Krott M.M., Kuhnle G.G., Dyson A., Fernandez B.O., Grman M., DuMond J.F. Key bioactive reaction products of the NO/H2S interaction are S/N-hybrid species, polysulfides, and nitroxyl. Proc. Natl. Acad. Sci. USA. 2015 doi: 10.1073/pnas.1509277112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao D., Deguchi A., Gundersen G.G., Oehlen B., Arnold L., Weinstein I.B. The sulindac derivatives OSI-461, OSIP486823, and OSIP487703 arrest colon cancer cells in mitosis by causing microtubule depolymerization. Mol. Cancer Ther. 2006;5:60–67. doi: 10.1158/1535-7163.MCT-05-0260. [DOI] [PubMed] [Google Scholar]
- 51.Wallace J.L., Ignarro L.J., Fiorucci S. Potential cardioprotective actions of no-releasing aspirin. Nat. Rev. Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- 52.Huledal G., Jonzon B., Malmenas M., Hedman A., Andersson L.I., Odlind B. Renal effects of the cyclooxygenase-inhibiting nitric oxide donator AZD3582 compared with rofecoxib and naproxen during normal and low sodium intake. Clin. Pharmacol. Therap. 2005;77:437–450. doi: 10.1016/j.clpt.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Rossoni G., Manfredi B., Tazzari V., Sparatore A., Trivulzio S., Del Soldato P. Activity of a new hydrogen sulfide-releasing aspirin (ACS14) on pathological cardiovascular alterations induced by glutathione depletion in rats. Eur. J. Pharmacol. 2010;648:139–145. doi: 10.1016/j.ejphar.2010.08.039. [DOI] [PubMed] [Google Scholar]