Abstract
Purpose
The aim of this study was to determine the effect of overlaying titanium mesh (TM) with an adjunctive collagen membrane (CM) for preserving the buccal bone when used in association with immediate implant placement in dogs.
Methods
Immediate implant placements were performed in the mesial sockets of the third premolars of five dogs. At one site the TM was attached to the fixture with the aid of its own stabilizers and then covered by a CM (CM group), while the contralateral site received only TM (TM group). Biopsy specimens were retrieved for histologic and histomorphometric analyses after 16 weeks.
Results
All samples exhibited pronounced buccal bone resorption, and a high rate of TM exposure was noted (in three and four cases of the five samples in each of the TM and CM groups, respectively). A dense fibrous tissue with little vascularity or cellularity had infiltrated through the pores of the TM irrespective of the presence of a CM. The distances between the fixture platform and the first bone-implant contact and the bone crest did not differ significantly between the TM and CM groups.
Conclusions
Our study suggests that the additional use of a CM over TM does not offer added benefit for mucosal healing and buccal bone preservation.
Graphical Abstract
Keywords: Bone regeneration, Bone resorption, Dental implants, Postoperative complications, Tooth extraction
INTRODUCTION
Immediate implantation has many benefits for both patients and clinicians with respect to reducing treatment time and the number of surgeries required. However, following tooth extraction, the thin buccal bone follows a natural remodeling process irrespective of the presence of an implant, and so various ridge-preservation procedures have been attempted [1,2]. To date there has been no consensus regarding the choice of biomaterial for immediate implant placement due to the heterogeneous methodology and outcomes of previous studies [3,4,5].
One rationale for using biomaterial around dental implants is to stabilize the blood clot [6,7], the initial stability of which may be critical for the healing around the periodontium and implant because of the many indwelling cytokines and growth factors in the blood clot [8,9]. In this context, in order to optimize wound healing, the focus should be on clot stabilization rather than the details of the biomaterial used.
Periosteal harnessing has been investigated with the aim of enhancing healing [10]. The inner cambium layer of the periosteum is rich in cellular components, including osteoblasts [11]. It has been speculated that the numerous pericytes in the abundant vasculature of the periosteum are a source of osteoprogenitor cells [12]. Thus, titanium mesh (TM) is a potential tool for maintaining the osteogenic potential of the periosteum at the site where regeneration is required. However, the concept of guided bone regeneration requires that the biomaterials used should be protected or play a physically protective role against the overlying soft tissues [13]. In clinical and preclinical studies, only limited bone regeneration and infiltration of soft tissue has been observed when a cell-occlusive barrier membrane is not used [14,15,16,17].
Lim et al. [18] installed a TM only, with immediate implant placement and observed no evidence of bone regeneration in most samples. Although they reported a high rate of TM exposure (at four of five sites), TM was suspected to be insufficient for maintaining clot stability due to the presence of pores, and the authors suggested that a system with cell-occlusive properties is required. In this respect, the addition of a barrier membrane could be advantageous; the presence of a soft-tissue layer beneath the TM in previous studies [19,20,21] and the lack of evidence of mineralization in that layer [22] highlight the need for such an additional barrier. Furthermore, when barrier membranes are used in conjunction with TM for placing implants in deficient ridges, complete bone filling is observed [23,24].
The aim of this study was to determine the effect of placing an additional collagen membrane (CM) over a TM for preserving the buccal bone in association with immediate implant placement in dogs. We used the CM to confer an occlusive property on the TM, and suspected that the CM would also enhance clot stability and tissue healing due to its tissue-friendly nature.
MATERIALS AND METHODS
This study was approved by the bioethics committee for animal selection, management, surgical protocol, and preparation at the Yonsei Medical Center, Seoul, South Korea. Five male beagle dogs (weighing 10-12 kg) were used that were generally healthy, with a healthy periodontium.
Study design
Sample-size calculation and group allocation were conducted as per a previous study [18]. In brief, the histomorphometric results by Araújo et al. [3] were used as a reference, and five dogs were required to achieve 80% power at a two-sided alpha level of 0.05%. The mesial sockets of the third premolars in both mandibular quadrants were used as recipient sites. The quadrant for CM coverage was selected randomly in the first experimental animal by an independent investigator and then assigned alternately for the remainders. After implant placement in both sockets, one socket received placement of TM only (TM group, n = 5), while the TM was covered with a CM in the contralateral socket (CM group, n = 5).
Animal experiments
Details of the animal surgery are described elsewhere [18]. In brief, a subcutaneous injection of atropine (0.05 mg/kg; Kwangmyung Pharmaceutical, Seoul, South Korea), and a subsequent intravenous injection of a combination of xylazine (Rompun, Bayer Korea, Seoul, South Korea) and Zoletil (Virbac, Carros, France) were used to induce general anesthesia. Anesthesia was maintained throughout surgery via inhalation of enflurane (Gerolan, Choongwae Pharmaceutical, Seoul, South Korea). In addition, local anesthesia was applied to the surgical sites.
Sulcular incisions and two vertical incisions were performed mesially and distally in the second and third premolar areas. The premolars were extracted cautiously by hemisectioning of the teeth without damaging the surrounding bone. The thicknesses of the buccal and lingual bone were measured at 1 mm below the level of the ridge crest. A fixture (3.5 mm × 8.5 mm; IS type, Neobiotech, Seoul, South Korea) was placed leaning against the lingual wall; the final vertical position of the fixture platform was 1 mm below the buccal bone crest. The distance between the outer cortex of the buccal bone and the implant surface at the top of the platform was measured (Table 1).
Table 1. Histometric Measurements (mm; mean±SD).
| PB(B) | PB(L) | PC(B) | PC(L) | GAP(B) | GAP(L) | |
|---|---|---|---|---|---|---|
| CM (n = 5) | 3.60 ± 1.25a) | 0.79 ± 0.52 | 3.60 ± 1.25a) | 0.62 ± 0.68 | 0 | 0.16 ± 0.09 |
| TM (n = 5) | 3.54 ± 2.00a) | 1.05 ± 0.85 | 3.85 ± 2.22a) | 0.88 ± 1.10 | 0 | 0.25 ± 0.31 |
a)Statistically significant difference between the buccal and lingual aspects (P < 0.05)
CM, titanium mesh and overlaying collagen membrane; TM, titanium mesh; PB, vertical distance between the fixture platform and the most coronal level of bone-implant contact level; P-C, vertical distance between the fixture platform and alveolar bone crest; (B), buccal; (L), lingual.
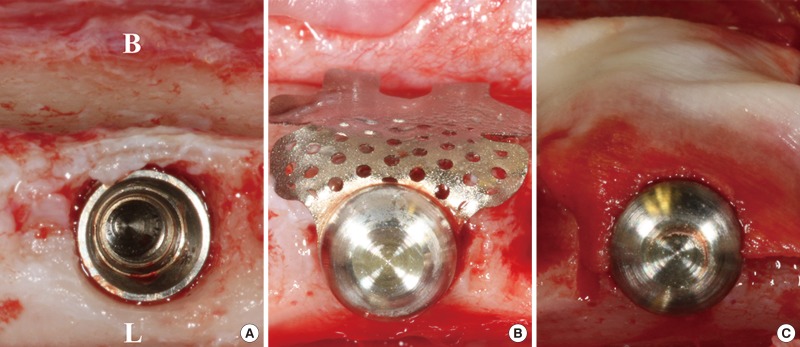
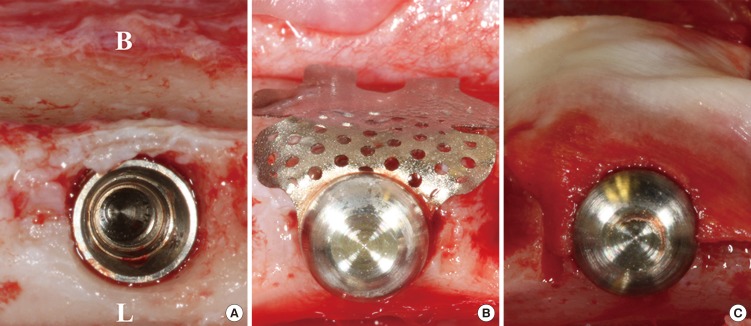
After implant placement, the TM (CTi-mem A1 type, Neobiotech, Seoul, South Korea) was bent to fit the outline of the buccal bone and then installed using stabilizers (spacers and caps). For the CM group, a small hole was made adjacent to one side of a CM (Bio-Gide, Geistlich, Wolhusen, Switzerland) using a rubber dam punch, and the membrane was fixed to the TM with stabilizers, covering it completely (Fig. 1). The flaps were fully released by periosteal releasing incisions on the buccal and lingual flaps, and primary flap closure was readily achieved using interrupted sutures (4-0 Monosyn, Aesculap, B-Braun, Center Valley, PA, USA).
Figure 1. Clinical photographs of the surgery. (A) The implant was installed after extraction. (B) A ready-made titanium mesh (TM) and its stabilizer were affixed to the fixture (TM group). (C) The collagen membrane (CM) fully covered the TM (CM group). B, buccal; L, lingual.
Antibiotic (cefazoline, 20 mg/kg; Yuhan, Seoul, South Korea) was administered intramuscularly for three days postoperatively and wounds were disinfected daily. Animals were provided with a soft diet for the entire 16-week healing period, after which they were euthanized by an overdose injection of pentobarbital sodium (90-120 mg/kg).
Histologic preparation
Bone blocks containing each experimental group site were harvested and immersed in 10% neutral buffered formalin solution, then dehydrated with ethanol solutions and embedded in resin. The specimens were sectioned buccolingually (Exakt, Apparatebau, Norderstedt, Germany), and the central sections were selected, reduced to a final thickness of about 20 µm, and then stained with hematoxylin-eosin.
Histologic and histomorphometric analyses
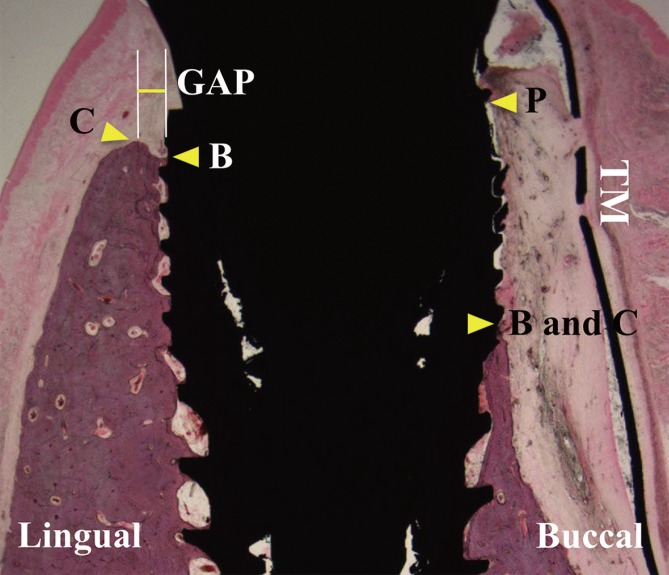
Histologic and histomorphometric observations were made with the aid of a stereomicroscope (MZFLIII, Leica, Wetzlar, Germany) and light microscope (DM-LB, Leica). Images of the histologic specimens were captured (cellSens Standard 1.11, Olympus, Center Valley, PA, USA) and histomorphometric analysis was conducted using an image-processing system (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA). In line with a previous study [18], the fixture platform (P) was selected as a reference level (Fig. 2). From the level (P), vertical distances to the most-coronal level of bone-implant contact (B) and the alveolar bone crest (C) were measured on both the buccal [PB(B), PC(B)] and lingual sides [PB(L), PC(L)]. The width of the remaining horizontal gap at the level of C (GAP) was measured on both the buccal and lingual sides; GAP (B) and GAP (L).
Figure 2. Illustration of the measurements, indicating the vertical distances between the fixture platform (P) and the most-coronal level of bone-implant contact (B), and between P and the level of the alveolar bone crest (C), and the width of the horizontal defect at the level of C (GAP).
Statistical analysis
Histologic specimens were examined twice with an interval of three weeks by one experienced examiner (H.C.L.) in a blind manner. The intraexaminer reliability was defined by the intraclass correlation coefficient (ICC). The calculated ICC was 0.999 with P < 0.001. Histomorphometric results are presented as mean ± SD values. Nonparametric evaluation was performed due to the small sample size. The Wilcoxon signed-rank test was used to assess statistical significance, and the null hypothesis was rejected at P < 0.05 (SPSS 20.0, Chicago, IL, USA).
RESULTS
Clinical findings
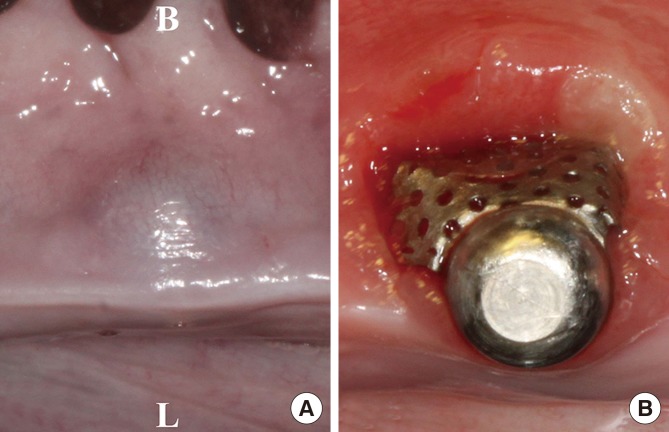
After 16 weeks of healing, three of the five sites in the TM group and four of the five sites in the CM group exhibited varying degrees of TM exposure, generally extending from the gingival crest to the buccal side. In addition, plaque accumulation was observed on the surface of the TM. However, none of the implants were lost or mobile. The gingival tissues around the exposed TM exhibited a tube-like morphology and slight redness. In the nonexposed sites, the stabilizer could be seen through the thin gingiva. No sites presented with severe adverse signs such as pus discharge or an allergic reaction (Fig. 3).
Figure 3. Clinical photographs taken after 16 weeks of recovery. (A) A nonexposed site. (B) An exposed site. Gingival tissues around the exposed TM exhibited mucosal deformity with slight redness. In the nonexposed sites, the gingiva above the stabilizer became thin. B, buccal; C, Lingual.
Histologic and histomorphometric analyses
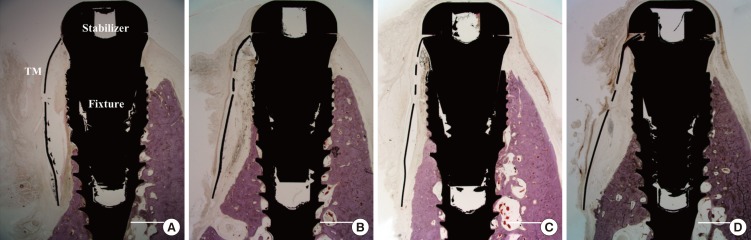
All implants became integrated with mature bone and the TM remained affixed to the implant by stabilizers. Compared with the corresponding lingual wall, the buccal walls were markedly resorbed in all samples, irrespective of the presence or absence of TM exposure. No residual CM could be detected over the TM, even in the unexposed site. A soft-tissue layer was generally observed under the TM, and a void could be seen in some exposed specimens (Fig. 4).
Figure 4. Photomicrographs of exposed sites in the TM group (A) and CM group (B), and of nonexposed sites in the TM group (C) and the CM group (D). The buccal walls were significantly resorbed in all samples, irrespective of the presence or absence of TM exposure. Hematoxylin-eosin stain; scale bars = 2 mm.
Histomorphometric data obtained after 16 weeks of healing are presented in Table 1. There was no statistically significant difference in PB(B) (3.5 ± 2.0 mm vs. 3.6 ± 1.3 mm) or PC(B) (3.9 ± 2.2 mm vs. 3.6 ± 1.3 mm) between the TM and CM groups. However, PB(B) and PC(B) were significantly greater than PB(L) and PC(L), respectively, in both groups (P = 0.043 for all).In terms of GAP, there was almost no discernible residual defect on the buccal side. GAP did not differ significantly between groups or between the buccal and lingual sides.
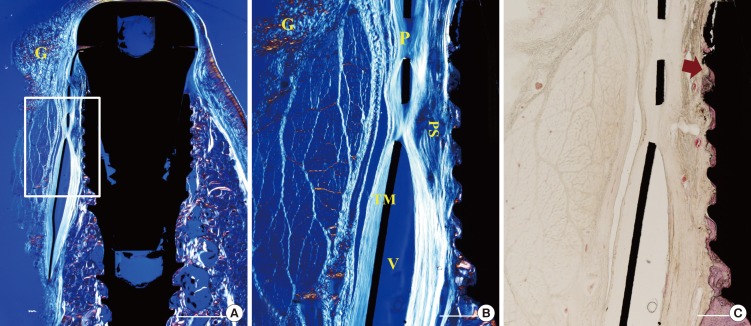
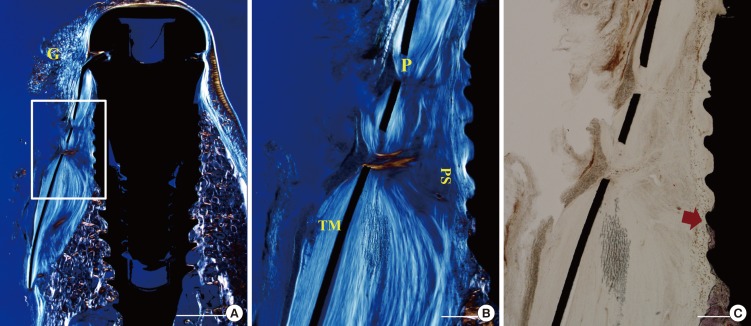
The gingival and connective tissues were observed to project into the space through the pores of the TM. A void was occasionally observed immediately below the TM. The tissue fibers appeared to wrap around the mesh through pores near the TM. The orientation of the fibers adjacent to the fixture was generally parallel to the fixture surface. In some samples the fibers were aligned perpendicular to the fixture surface. The infiltrated tissue was dense and fibrotic, like scar tissue. Few blood vessels were observed (Figs. 5 and 6).
Figure 5. Photomicrographs of a nonexposed site in the TM group. (A, B) Polarizing photomicrographs. (B, C) Higher magnification views of the boxed area in A. The tissue fibers appeared to infiltrate through pores and wrap around them. Hematoxylin-eosin stain; scale bar (A) = 2 mm, (B,C) =500 µm, respectively. G, gingival tissue; P, pore; V, void; PS, pseudoperiosteum; arrow, the most-coronal level of bone-implant contact.
Figure 6. Photomicrographs of a nonexposed site in the CM group. (A, B) Polarizing photomicrographs. (B, C) Higher magnification views of the boxed area in A. Collagen membrane did not prevent the infiltration of tissue fiber through the pores. Hematoxylin-eosin stain; scale bar (A) = 2 mm, (B,C) = 500 µm, respectively. G, gingival tissue; P, pore; PS, pseudoperiosteum.
DISCUSSION
The presence of pores in TM may influence wound healing, but this has yet to be confirmed. The pores may act as pathways for transferring osteogenic potential from the periosteum [19,25]. Under this assumption, full or partial contact between the periosteum and the underlying bone substitute or blood coagulum would be expected to enhance bone regeneration. However, it may alternatively be detrimental to bone regeneration, since the space beneath the TM can become occupied by soft tissue infiltrating through its pores before the bone can regenerate [14].
In the present study it was expected that an adjunctive CM overlaid upon TM could occlude the pores to exclude the unwanted soft tissue. Moreover, enhanced mucosal healing was anticipated due to the tissue-friendly nature of the CM. For close adaptation and minimization of mobility, the CM was affixed to the customized TM by the stabilizer through the small hole made by punching. However, a high rate of TM exposure was noted, irrespective of the adjunctive CM, and the buccal bone beneath the TM underwent a resorptive remodeling process in both the TM and CM groups.
The placement of an adjunctive cell-occlusive barrier can be beneficial for minimizing the formation of an unwanted tissue layer. Assenza et al. [23] used TM with an expanded polytetrafluoroethylene (e-PTFE) membrane and observed no residual bone defects, and their histologic examination revealed the new bone to be in close contact with the TM. A resorbable membrane was also used with TM by Degidi et al. [24]. While these authors similarly reported significant bone regeneration, their histologic observations were somewhat different: the TM was surrounded by a dense connective tissue with few cellular components, and new bone in close contact with the TM was observed in only a few areas. In the nonexposed specimen of the present study, the space under the TM was largely filled with dense fibrous tissue, which in accordance with our previous findings contained few blood vessels or cellular components [18]. The reason for these histologic differences between the studies is unclear, but they may be due to variations in, and possible imbalance between, the rates of bone formation and dissolution of the CM.
Some authors have proposed that primary flap closure is not crucial in ridge-preservation procedures [26,27], following observations that in nonmolars and molars, intentional membrane exposure and secondary healing did not reduce bone fill [26,27]. Moreover, membrane exposure after ridge preservation does not relate to clinical complications [28], and when TM is exposed, the resulting inflammatory response has been demonstrated to be minimal [29]. However, after TM exposure, whether intentional or unintentional, secondary healing above the TM is unlikely, and mucosal deformity occurs. We hypothesized that the presence of an adjunctive CM over the TM might enhance mucosal healing; however, such an effect was not observed in the present study. Such findings suggest that if an overlaid CM layer is exposed, the subsequent exposure of TM may be inevitable.
The exposure rate of TM has been a major concern in previous studies [30,31,32]. The rate of reported TM exposure varies widely, with some studies demonstrating no exposure, but others finding an exposure rate of 80% [31,32]. Mucosal irritation from TM has been suggested to be a causal factor of the exposure [19], which can be aggravated in sites with poor-quality soft tissue [32]. The present study targeted postextraction sockets where soft tissue conditions might not be optimal for TM.
Weng et al. [15] demonstrated that bone formation under a TM was inferior to that under a combination of TM and an e-PTFE membrane. In histologic sections from the present study, a dense soft-tissue layer was found across the pores, and no mineralization was observed in that layer. This soft-tissue layer found under the TM has been labeled a "pseudoperiosteum" by some authors [21,33]. This term may connote the expectation that the layer has potential to form bone. Although the eventual function of this layer has yet to be fully discovered, the evidence for bone-forming potential appears weak thus far [22]. The role of the pseudoperiosteum may be the prevention of graft infection and resorption [32], such that its formation could protect the graft and aid secondary intention healing in the case of TM exposure [22,32,34]. The pseudoperiosteum is suggested to take 2-6 weeks to form [34,35,36], so it is therefore important to not irritate the wound during the early healing period when a TM is used.
In this study the peri-implant gap was filled with blood coagulum alone, without a bone substitute. Previous studies used TM to protect the underlying bone materials in unfavorable ridge deficiencies [19,21,37,38]. Autogenous bone, and a combination of autogenous and bovine bone, could successfully function beneath the TM [19,21,37,38]. However, a consistent finding has been the formation of a soft-tissue layer around the TM. The formation of a so-called pseudoperiosteum under the TM thus seems to be inevitable during wound healing. In the present study, the gap between the buccal bone and the implant surface was less than 1 mm. Given the thickness of the pseudoperiosteum, providing sufficient space under the TM would secure the bone tissue from the pseudoperiosteum.
In conclusion, it was not possible to determine the effect of placing a CM over a stabilized TM in this study because of the high rate of TM exposure. The benefit from the placement of a collagen membrane may be questionable with respect to improving mucosal healing and facilitating buccal bone preservation.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A120822).
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Chen ST, Buser D. Clinical and esthetic outcomes of implants placed in postextraction sites. Int J Oral Maxillofac Implants. 2009;24(Suppl):186–217. [PubMed] [Google Scholar]
- 2.Wang RE, Lang NP. Ridge preservation after tooth extraction. Clin Oral Implants Res. 2012;23(Suppl 6):147–156. doi: 10.1111/j.1600-0501.2012.02560.x. [DOI] [PubMed] [Google Scholar]
- 3.Araújo MG, Linder E, Lindhe J. Bio-Oss collagen in the buccal gap at immediate implants: a 6-month study in the dog. Clin Oral Implants Res. 2011;22:1–8. doi: 10.1111/j.1600-0501.2010.01920.x. [DOI] [PubMed] [Google Scholar]
- 4.De Buitrago JG, Avila-Ortiz G, Elangovan S. Quality assessment of systematic reviews on alveolar ridge preservation. J Am Dent Assoc. 2013;144:1349–1357. doi: 10.14219/jada.archive.2013.0070. [DOI] [PubMed] [Google Scholar]
- 5.Favero G, Botticelli D, Favero G, García B, Mainetti T, Lang NP. Alveolar bony crest preservation at implants installed immediately after tooth extraction: an experimental study in the dog. Clin Oral Implants Res. 2013;24:7–12. doi: 10.1111/j.1600-0501.2011.02365.x. [DOI] [PubMed] [Google Scholar]
- 6.Kostopoulos L, Karring T. Augmentation of the rat mandible using guided tissue regeneration. Clin Oral Implants Res. 1994;5:75–82. doi: 10.1034/j.1600-0501.1994.050203.x. [DOI] [PubMed] [Google Scholar]
- 7.Simion M, Dahlin C, Rocchietta I, Stavropoulos A, Sanchez R, Karring T. Vertical ridge augmentation with guided bone regeneration in association with dental implants: an experimental study in dogs. Clin Oral Implants Res. 2007;18:86–94. doi: 10.1111/j.1600-0501.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang HL, Boyapati L. "PASS" principles for predictable bone regeneration. Implant Dent. 2006;15:8–17. doi: 10.1097/01.id.0000204762.39826.0f. [DOI] [PubMed] [Google Scholar]
- 9.Wikesjö UM, Claffey N, Egelberg J. Periodontal repair in dogs. Effect of heparin treatment of the root surface. J Clin Periodontol. 1991;18:60–64. doi: 10.1111/j.1600-051x.1991.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin Z, Fateh A, Salem DM, Intini G. Periosteum: biology and applications in craniofacial bone regeneration. J Dent Res. 2014;93:109–116. doi: 10.1177/0022034513506445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 1992:280–286. [PubMed] [Google Scholar]
- 13.Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672–676. doi: 10.1097/00006534-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Lee KW, Oh TJ, Misch CE, Shotwell J, Wang HL. Effect of absorbable membranes on sandwich bone augmentation. Clin Oral Implants Res. 2008;19:32–41. doi: 10.1111/j.1600-0501.2007.01408.x. [DOI] [PubMed] [Google Scholar]
- 15.Weng D, Hürzeler MB, Quiñones CR, Ohlms A, Caffesse RG. Contribution of the periosteum to bone formation in guided bone regeneration. A study in monkeys. Clin Oral Implants Res. 2000;11:546–554. doi: 10.1034/j.1600-0501.2000.011006546.x. [DOI] [PubMed] [Google Scholar]
- 16.Jung UW, Lee JS, Lee G, Lee IK, Hwang JW, Kim MS, et al. Role of collagen membrane in lateral onlay grafting with bovine hydroxyapatite incorporated with collagen matrix in dogs. J Periodontal Implant Sci. 2013;43:64–71. doi: 10.5051/jpis.2013.43.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung UW, Lee JS, Park WY, Cha JK, Hwang JW, Park JC, et al. Periodontal regenerative effect of a bovine hydroxyapatite/collagen block in one-wall intrabony defects in dogs: a histometric analysis. J Periodontal Implant Sci. 2011;41:285–292. doi: 10.5051/jpis.2011.41.6.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim HC, Kim MS, Yang C, Lee JS, Hong JY, Choi SH, et al. The effectiveness of a customized titanium mesh for ridge preservation with immediate implantation in dogs. Clin Implant Dent Relat Res. 2015 doi: 10.1111/cid.12302. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 19.Her S, Kang T, Fien MJ. Titanium mesh as an alternative to a membrane for ridge augmentation. J Oral Maxillofac Surg. 2012;70:803–810. doi: 10.1016/j.joms.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg. 2008;66:235–245. doi: 10.1016/j.joms.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006;32:237–247. doi: 10.1563/1548-1336(2006)32[237:UOTMFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 22.Gutta R, Baker RA, Bartolucci AA, Louis PJ. Barrier membranes used for ridge augmentation: is there an optimal pore size? J Oral Maxillofac Surg. 2009;67:1218–1225. doi: 10.1016/j.joms.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Assenza B, Piattelli M, Scarano A, Lezzi G, Petrone G, Piattelli A. Localized ridge augmentation using titanium micromesh. J Oral Implantol. 2001;27:287–292. doi: 10.1563/1548-1336(2001)027<0287:LRAUTM>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 24.Degidi M, Scarano A, Piattelli A. Regeneration of the alveolar crest using titanium micromesh with autologous bone and a resorbable membrane. J Oral Implantol. 2003;29:86–90. doi: 10.1563/1548-1336(2003)029<0086:ROTACU>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Lundgren D, Nyman S, Mathisen T, Isaksson S, Klinge B. Guided bone regeneration of cranial defects, using biodegradable barriers: an experimental pilot study in the rabbit. J Craniomaxillofac Surg. 1992;20:257–260. doi: 10.1016/s1010-5182(05)80438-x. [DOI] [PubMed] [Google Scholar]
- 26.Engler-Hamm D, Cheung WS, Yen A, Stark PC, Griffin T. Ridge preservation using a composite bone graft and a bioabsorbable membrane with and without primary wound closure: a comparative clinical trial. J Periodontol. 2011;82:377–387. doi: 10.1902/jop.2010.090342. [DOI] [PubMed] [Google Scholar]
- 27.Kim DM, De Angelis N, Camelo M, Nevins ML, Schupbach P, Nevins M. Ridge preservation with and without primary wound closure: a case series. Int J Periodontics Restorative Dent. 2013;33:71–78. doi: 10.11607/prd.1463. [DOI] [PubMed] [Google Scholar]
- 28.Zubillaga G, Von Hagen S, Simon BI, Deasy MJ. Changes in alveolar bone height and width following post-extraction ridge augmentation using a fixed bioabsorbable membrane and demineralized freeze-dried bone osteoinductive graft. J Periodontol. 2003;74:965–975. doi: 10.1902/jop.2003.74.7.965. [DOI] [PubMed] [Google Scholar]
- 29.von Arx T, Hardt N, Wallkamm B. The TIME technique: a new method for localized alveolar ridge augmentation prior to placement of dental implants. Int J Oral Maxillofac Implants. 1996;11:387–394. [PubMed] [Google Scholar]
- 30.Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57:3–14. doi: 10.1016/j.jpor.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 31.von Arx T, Kurt B. Implant placement and simultaneous peri-implant bone grafting using a micro titanium mesh for graft stabilization. Int J Periodontics Restorative Dent. 1998;18:117–127. [PubMed] [Google Scholar]
- 32.Lizio G, Corinaldesi G, Marchetti C. Alveolar ridge reconstruction with titanium mesh: a three-dimensional evaluation of factors affecting bone augmentation. Int J Oral Maxillofac Implants. 2014;29:1354–1363. doi: 10.11607/jomi.3417. [DOI] [PubMed] [Google Scholar]
- 33.Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985;43:87–91. doi: 10.1016/0278-2391(85)90054-0. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto I, Funaki K, Yamauchi K, Kodama T, Takahashi T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: computed tomography-based evaluations of augmented bone quality and quantity. Clin Implant Dent Relat Res. 2012;14:304–311. doi: 10.1111/j.1708-8208.2009.00257.x. [DOI] [PubMed] [Google Scholar]
- 35.Corinaldesi G, Pieri F, Sapigni L, Marchetti C. Evaluation of survival and success rates of dental implants placed at the time of or after alveolar ridge augmentation with an autogenous mandibular bone graft and titanium mesh: a 3- to 8-year retrospective study. Int J Oral Maxillofac Implants. 2009;24:1119–1128. [PubMed] [Google Scholar]
- 36.Pieri F, Corinaldesi G, Fini M, Aldini NN, Giardino R, Marchetti C. Alveolar ridge augmentation with titanium mesh and a combination of autogenous bone and anorganic bovine bone: a 2-year prospective study. J Periodontol. 2008;79:2093–2103. doi: 10.1902/jop.2008.080061. [DOI] [PubMed] [Google Scholar]
- 37.Proussaefs P, Lozada J. The use of resorbable collagen membrane in conjunction with autogenous bone graft and inorganic bovine mineral for buccal/labial alveolar ridge augmentation: a pilot study. J Prosthet Dent. 2003;90:530–538. doi: 10.1016/s0022-3913(03)00521-3. [DOI] [PubMed] [Google Scholar]
- 38.Roccuzzo M, Ramieri G, Bunino M, Berrone S. Autogenous bone graft alone or associated with titanium mesh for vertical alveolar ridge augmentation: a controlled clinical trial. Clin Oral Implants Res. 2007;18:286–294. doi: 10.1111/j.1600-0501.2006.01301.x. [DOI] [PubMed] [Google Scholar]