ABSTRACT
Particular types of hormonal contraceptives (HCs) and genital tract infections have been independently associated with risk of HIV-1 acquisition. We examined whether immunity in women using injectable depot medroxyprogesterone acetate (DMPA), combined oral contraceptives (COC), or no HCs differs by the presence of cervicovaginal infections. Immune mediators were quantified in cervical swabs from 832 HIV-uninfected reproductive-age Ugandans and Zimbabweans. Bacterial infections and HIV were diagnosed by PCR, genital herpes serostatus by enzyme-linked immunosorbent assay (ELISA), altered microflora by Nugent score, and Trichomonas vaginalis and Candida albicans infection by wet mount. Generalized linear models utilizing Box-Cox-Power transformation examined associations between levels of mediators, infection status, and HCs. In no-HC users, T. vaginalis was associated with broadest spectrum of aberrant immunity (higher interleukin 1β [IL-1β], IL-8, macrophage inflammatory protein 3α [MIP-3α], β-defensin 2 [BD2], and IL-1 receptor antigen [IL-1RA]). In women with a normal Nugent score and no genital infection, compared to the no-HC group, COC users showed higher levels of IL-1β, IL-6, IL-8, and IL-1RA, while DMPA users showed higher levels of RANTES and lower levels of BD2, both associated with HIV seroconversion. These effects of COC were blunted in the presence of gonorrhea, chlamydia, trichomoniasis, candidiasis, and an abnormal Nugent score; however, RANTES was increased among COC users with herpes, chlamydia, and abnormal Nugent scores. The effect of DMPA was exacerbated by lower levels of IL-1RA in gonorrhea, chlamydia, or herpes, SLPI in gonorrhea, and IL-1β, MIP-3α, and IL-1RA/IL1β ratio in trichomoniasis. Thus, the effects of HC on cervical immunity depend on the genital tract microenvironment, and a weakened mucosal barrier against HIV may be a combined resultant of genital tract infections and HC use.
IMPORTANCE
In this article, we show that in young reproductive-age women most vulnerable to HIV, hormonal contraceptives are associated with altered cervical immunity in a manner dependent on the presence of genital tract infections. Through altered immunity, hormones may predispose women to bacterial and viral pathogens; conversely, a preexisting specific infection or disturbed vaginal microbiota may suppress the immune activation by levonorgestrel or exacerbate the suppressed immunity by DMPA, thus increasing HIV risk by their cumulative action. Clinical studies assessing the effects of contraception on HIV susceptibility and mucosal immunity may generate disparate results in populations that differ by microbiota background or prevalence of undiagnosed genital tract infections. A high prevalence of asymptomatic infections among HC users that remain undiagnosed and untreated raises even more concerns in light of their combined effects on biomarkers of HIV risk. The molecular mechanisms of the vaginal microbiome’s simultaneous interactions with hormones and HIV remain to be elucidated.
INTRODUCTION
Most commonly used hormonal contraceptives (HCs), including the progestin injectable depot medroxyprogesterone acetate (DMPA [also known as Depo-Provera]), injected every 3 months, and combined estrogen-progestin oral contraceptives (COC), as well as pregnancy have been variably associated with increased risk of human immunodeficiency virus (HIV) and other sexually transmitted infections (1–7). In addition, a large epidemiological study, supplemented with molecular analysis of transmitting HIV, suggested that women using certain types of HCs may confer a higher risk of HIV transmission to their male partner (5). A recent study showed that the progestin levonorgestrel may decrease the clearance of high-risk human papillomavirus (HPV) infection and possibly increase acquisition (8), thus suggesting a potential impact of HCs on cervical immunity going beyond the risk of HIV. While experts continue to debate the validity of the epidemiological associations, to which populations they may apply, and how to balance the risk of HCs versus risks of pregnancy, over 150 million women continue using hormonal contraceptives. In the African epicenter of the epidemic, the United Nations (UN) estimates that 29% of all women using a modern contraceptive method use DMPA (9), and the rates are especially high in eastern and South Africa, where the epidemic is most severe.
To date, it remains unclear whether there are biological grounds for the disparate epidemiological data on the effect of hormonal contraceptives on HIV acquisition. A reasonable biological explanation would be that microbial factors not taken into consideration by published epidemiological studies invert, suppress, or amplify hormonal influences on the cervicovaginal mucosal immune barrier.
To address this question, we focused our attention on genital inflammation because of its known role as a risk factor of HIV acquisition (10) and on the uterine cervix as a major contributor to the cervicovaginal secretory mucosal barrier (11–13). Many cytokines and other innate immunity mediators secreted by the cervix have been implicated in HIV immunopathogenesis through host cell activation or direct effects on the viral replication cycle (8, 14). Although it has been shown that female genital tract inflammation and immunity are hormonally regulated and linked to menstrual cycle phase (15–20) and to cervical ectopy, which is also hormonally regulated (15), limited clinical data exist on the effect of hormonal contraception on cervical immunity (21, 22). A small study (15 DMPA users, 18 levonorgestrel intrauterine device users, and 23 controls) limited to women with no sexually transmitted infections (STIs) showed significant gene upregulation of pathways of inflammation and immunity in the cervical transformation zone associated with progestin use (23). Analysis of cervicovaginal lavage specimens and paired serum samples from 18 DMPA users, 14 COC users, and 21 control women measured only interferon alpha (IFN-α) and showed reduced local and systemic levels with DMPA use (24). Another relatively small study of HIV-uninfected women, in which most women (>64%) belonged to the special category of HIV-discordant couples, only 16 women used oral contraceptives, and 41 used DMPA versus 171 in the no-HC group, and where STIs showed imbalanced distribution, mostly recorded in women who did not use HCs, found increased cervical levels of some but not other antibacterial cationic peptides in DMPA users (25). Earlier we utilized a much bigger study of 832 women from the Hormonal Contraception and Risk of HIV (HC-HIV) cohort to test the association between HCs, cervical immunity, and risk of HIV acquisition. We found that elevated cervical levels of the chemokine RANTES (regulated upon activation, normal T-cell expressed, and secreted) was associated with higher risk of HIV seroconversion within the next 3 months and that cervical levels of RANTES were also higher in the DMPA users (n = 307) compared to those who did not use HCs (n = 226) or those who used COC (n = 299). In addition, DMPA users had lower levels of β-defensin 2 (BD2), and COC users had higher levels of most inflammatory proteins measured (26). However, in our published analyses of the HC-HIV cohort, we did not assess immunity in association with concurrent bacterial, fungal, or viral cervicovaginal infections (CVIs) and altered microflora.
Reproductive tract infections and microbiome disturbances, and especially the syndrome clinically diagnosed as bacterial vaginosis (BV), are recognized as proinflammatory risk factors for HIV acquisition and transmission (27–29). However, studies that have attempted to establish cytokine signatures of STIs and vaginal microbiota have not specifically elucidated how microbial factors contribute to the altered cervical barrier in women using hormonal contraceptives (30, 31). We hypothesized that cervicovaginal pathogens (e.g., Trichomonas vaginalis, Neisseria gonorrhoeae, Chlamydia trachomatis, genital herpesvirus 2 [HSV-2], and Candida albicans) and disturbances in the normal vaginal microbiota that are often undiagnosed, not treated, and especially common and hazardous in women at risk of HIV (32–35) may alter or contribute to the effects of hormonal contraceptives on cervical immunity. To test this hypothesis we performed secondary analysis of the HC-HIV cohort, which provided both immunologic and microbiologic data. We assessed the combined effects of cervicovaginal infections (CVIs) and hormonal contraceptives on levels of immune mediators chosen based on their proven biological significance (36–39) and abundant production by the cervicovaginal epithelium (11, 40–44), as well as based on their reliable measurement in human cervicovaginal secretions (36, 37, 45–47). The following 10 proteins were selected to represent five major classes of immunoinflammatory mediators: (i) the cytokines interleukin-1β (IL-1β) and IL-6, (ii) chemokines XCL8 (IL-8), CCL20 (macrophage inflammatory protein 3α [MIP-3α]), and CCL5 (RANTES), (iii) vasoactive mediators and adhesion molecules acting downstream from cytokine and chemokine activation, including vascular endothelial growth factor (VEGF) and soluble intercellular adhesion molecule 1 (sICAM-1 [CD54]), (iv) the anti-inflammatory cytokine antagonist IL-1 receptor antagonist (IL-1RA), and (v) the antibacterial and antiviral proteins secretory leukocyte protease inhibitor (SLPI) and BD2.
RESULTS
Prevalence and distribution of lab-confirmed CVIs.
In this study, controls (633 women who remained HIV negative) were matched to cases (199 women who later HIV seroconverted) by a composite STI variable (bacterial vaginosis [BV] and chlamydia- and/or gonorrhea-positive status). As expected from this matched design driven by the high rate of STIs associated with HIV, the overall prevalence of lab-confirmed CVIs was high (86%) in our nested cohort (Table 1). The distribution of CVIs differed by HC use. The COC and DMPA users had lower rates of lab-confirmed CVIs (84.3% and 84.4%, respectively) compared to the no-HC group (91%) (P = 0.046). The same differences by HC use were observed among the nonpregnant women alone (P = 0.043), suggesting that the imbalanced distribution was not driven by the higher rates of pregnancy, which expectedly occurred among the no-HC users and is considered a risk factor for CVIs. The differences could not be explained by the prevalence of unprotected sex acts, since the prevalence of unprotected sex and the number of unprotected sexual acts were highest in COC users, followed by DMPA users and no-HC users (P < 0.001) (Fig. 1A). On the other hand, the higher rate of CVIs among the no-HC group appeared driven by HSV (which was not used in the matching composite variable in our cohort): 69% (133/473) of no-HC users were HSV+ compared with 58% (168/473) and 57% (172/473) in the COC and DMPA groups, respectively (P = 0.01).
TABLE 1 .
Distribution of CVIs stratified by hormonal contraception use, pregnancy, and breastfeeding
| Group | No. (%) of CVIs in treatment groupa |
nb | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COC |
DMPA |
No-HC |
Total |
|||||||
| CVI+ | CVI− | CVI+ | CVI− | CVI+ | CVI− | CVI+ | CVI− | |||
| Nonpregnant and nonbreastfeeding | 229 (84.50) | 42 (15.50) | 187 (84.23) | 35 (15.77) | 144 (92.31) | 12 (7.69) | 560 (86.29) | 89 (13.71) | 649 | 0.043 |
| Nonpregnant and breastfeeding | 11 (78.57) | 3 (21.43) | 67 (85.90) | 11 (14.10) | 35 (92.11) | 3 (7.89) | 113 (78.29) | 17 (13.08) | 130 | 0.400 |
| Pregnant | 7 (87.50) | 1 (12.50) | 1 (50.00) | 1 (50.00) | 25 (83.33) | 5 (16.67) | 33 (82.50) | 7 (17.50) | 40 | 0.446 |
| Total n | 247 (84.30) | 46 (15.70) | 255 (84.44) | 47 (15.56) | 204 (91.07) | 20 (8.93) | 706 (86.20) | 113 (13.80) | 819 | 0.046 |
The CVIs include T. vaginalis, N. gonorrhoeae, C. trachomatis, C. albicans, HSV-2, and abnormal microflora by Nugent score. COC, combined estrogen-progestin oral contraceptive (levonorgestrel); DMPA, injectable progestin (Depo-Provera); no-HC, no hormonal contraceptives.
Thirteen women were excluded from the analysis due to insufficient lab test data to classify their CVI status.
FIG 1 .
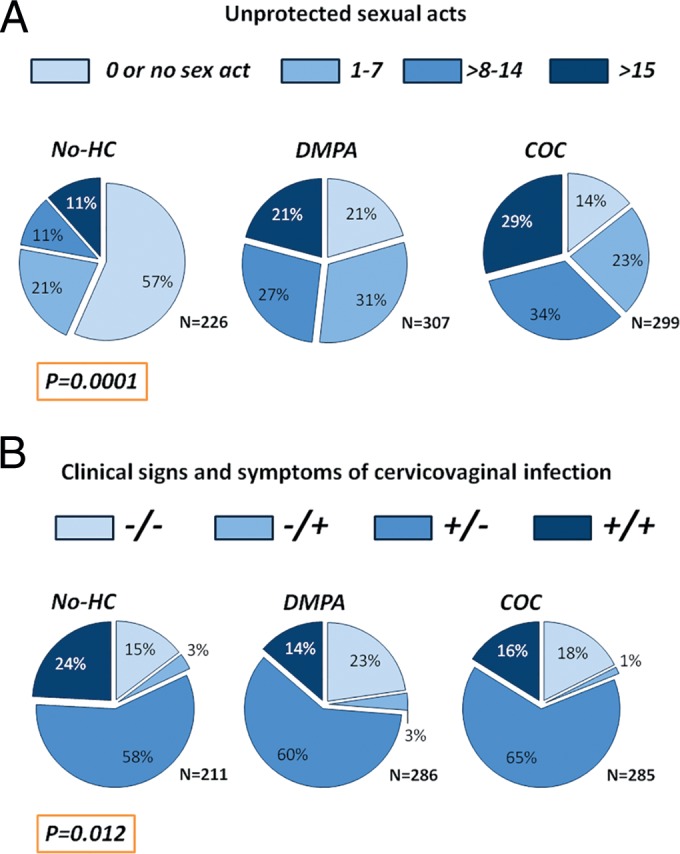
Distribution of number of sexual acts (A) and clinical signs and symptoms of cervicovaginal infections (B) among women who chose not to use hormonal contraception (no-HC) and women with majority use of combined oral contraceptives (COC) or DMPA. P values indicate differences among the HC groups.
The laboratory analysis showed a high degree of overlapping CVIs. Importantly more than half of our study cohort (473/832 [57%]) was herpes positive by serology, and over one-third was positive for BV (265/832 [32%]) and these two CVIs had an imbalanced distribution among the other CVIs, as shown in Table 2 (for the nonpregnant women only). The herpes-positive cases were evenly distributed among women with chlamydia, candidiasis, or BV but significantly (P < 0.05) more common among women positive for T. vaginalis infection or gonorrhea (74% versus 59% among the T. vaginalis infection- or gonorrhea-positive versus negative). The BV-positive status (Nugent score of 7 to 10) was evenly distributed, except among women positive for candidiasis, where BV was significantly (P < 0.05) less common (14% among candidiasis-positive versus 39% among candidiasis-negative women), which was expected based on previously reported inverse relationship between BV and candidiasis in the overall HC-HIV study (48).
TABLE 2 .
Distribution of HSV-2 and BV infections overlapping with other CVIs among all nonpregnant women
| Parameter | No. (%) of CVIs overlapping with HSV-2 and BVa |
Total n (% [n = 791]) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
T. vaginalis infection |
Chlamydia |
Candidiasis |
Nugent score |
Gonorrhea |
|||||||
| − | + | − | + | − | + | <7 | >7 | − | + | ||
| HSV-2 | |||||||||||
| − | 300.00 (40.54) | 11.00 (25.58) | 301.00 (40.08) | 10.00 (31.25) | 270.00 (39.53) | 41.00 (40.59) | 203.00 (42.56) | 93.0 (35.5) | 299.00 (40.57) | 12.00 (26.09) | 473.00 (59.8) |
| + | 440.00 (59.46) | 32.00 (74.42) | 450.00 (59.92) | 22.00 (68.75) | 413.00 (60.47) | 60.00 (59.41) | 274.00 (57.44) | 169.0 (64.5) | 438.00 (59.43) | 34.00 (73.91) | 311.00 (39.32) |
| Nugent score | |||||||||||
| <7 | 459.00 (65.01) | 22.00 (56.41) | 464.00 (65.08) | 17.00 (51.52) | 399.00 (61.29) | 82.00 (86.32) | 481.00 (100) | 0 (0) | 455.00 (64.91) | 26.00 (57.78) | 481.00 (60.81) |
| 7–10 | 247.00 (34.99) | 17.00 (43.59) | 249.00 (34.92) | 16.00 (48.48) | 252.00 (38.71) | 13.00 (13.68) | 0 (0) | 265.0 (100) | 246.00 (35.09) | 19.00 (42.22) | 265.00 (33.5) |
The CVIs include T. vaginalis, N. gonorrhoeae, C. trachomatis, and C. albicans. The boldface values show significantly imbalanced distributions (P ≤ 0.05).
Distribution of clinical signs and symptoms.
The prevalence of clinical signs and symptoms of CVIs were also differentially distributed among the various contraceptive methods (P = 0.012) (Fig. 1B). This analysis excluded 50 women who answered some questions about symptoms with “don’t know” or for whom no assessment was done due to menses. The greatest proportion of women had asymptomatic clinically manifested signs of infections (61%), which were most prevalent among the COC users (65%), followed by the DMPA (60%) and no-HC users (58%). In contrast, symptomatic presence of signs of CVIs was more common in the no-HC group than in the COC and DMPA groups (24% versus 16% and 14%, respectively; P = 0.009).
To investigate the potential impact of the uneven distribution of symptoms on our immunologic analyses, we compared levels of immune mediators between women with lab-confirmed CVIs who did or did not report symptoms. No significant differences were found except for BD2 (higher in symptomatic women [P < 0.01]) and SLPI (lower in symptomatic women [P = 0.05]) (see Table S1 in the supplemental material).
Cervical immunity by CVI status and HC use.
To control for the uneven distribution for overlapping infections, signs, and symptoms, we assessed the differences in cervical immune mediators by CVI status and HC use using a multivariable analysis adjusting for overlapping individual CVIs and for clinical signs and symptoms of CVI via generalized linear models.
The concentrations of each biomarker in no-HC users stratified by CVI are shown in Table S2 in the supplemental material. To illustrate the combined effect of CVIs and HCs, we used the average of the CVI-free no-HC group as a baseline and calculated differences between this baseline and each combination of CVI with no-HC, DMPA, or COC (Fig. 2).
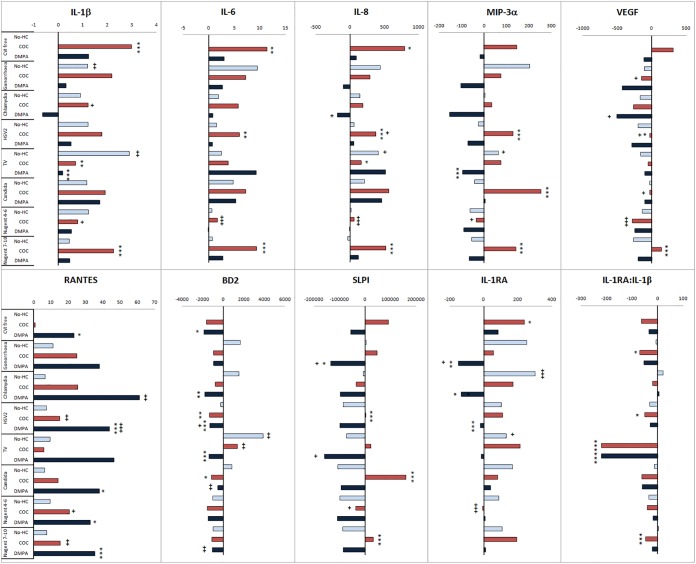
FIG 2 .
Combined effects of cervicovaginal infections (CVIs) and hormonal contraception (HC) on markers of cervical immunity. Women were stratified by CVI status and HC use within each CVI stratum, and levels of immune biomarkers were compared by multivariable analysis via generalized linear models and Wilcoxon tests after adjustment for other individual CVIs. Bars represent the differences between the average concentrations calculated for each of the combined CVI-plus-HC category listed on the left and the average concentration calculated for the physiologic CVI-free no-HC baseline (15 women who were infection free, had a normal Nugent score, and who did not use any HC). Consequently, the CVI-free, no-HC baseline average was set to 0 in each bar plot. P values with asterisks (*, P < 0.05; **, P < 0.01; and ***, P < 0.001) signify differences between no-HC and combined oral contraceptive (COC) or DMPA users within each CVI stratum. P values with plus signs (+, P < 0.05; ++, P < 0.01; and +++, P < 0.001) signify differences between each CVI-positive group and the CVI-free group matched by HC (e.g., no-HC, COC, and DMPA). The P values for the two comparisons and the number of women in each group are shown in Tables S1 and S2 in the supplemental material.
First assessed were differences between no HCs and the two types of HCs within each CVI group (see Table S3 in the supplemental material). In the CVI-free group (normal Nugent score of <4 and lab-confirmed negative results for chlamydia, gonorrhea, herpes, or T. vaginalis and Candida infection), COC users compared to no-HC users showed higher levels of IL-1β (P < 0.001), IL-6 (P < 0.01), and IL-8 and IL-1RA (P < 0.05). In the CVI-free group, compared to the no-HC group, DMPA users showed higher RANTES and lower BD2 levels (P < 0.05).
The effects of COC were different within the CVI-positive groups. The above immunostimulatory effects of COC use observed compared to those of no HC use among the CVI-free women were blunted or even reversed in the presence of most CVIs, with a few exceptions. Compared to the no-HC group, IL-1β was significantly increased by COC use only within the BV+ group (P < 0.001), IL-6 and IL-8 were increased only within the BV+ (P < 0.001) and HSV-2 + groups (P < 0.001 and P = 0.01, respectively), and in addition, in these two groups, COC use showed higher levels of MIP-3α (P < 0.001), VEGF (P = 0.04 for herpes and P < 0.001 for BV), and SLPI (P < 0.001). MIP-3α and SLPI were also increased (P < 0.001) by COC use within the candidiasis-positive group, and BD2 was decreased in the candidiasis-positive (0 = 0.04) and HSV-2+ (P < 0.001) groups. Levels of IL-1β (P = 0.01) and IL-8 (P = 0.04) were even decreased in COC users with T. vaginalis infection. COC use failed to increase IL-1RA compared to no-HC use in any of the CVI+ groups, and in addition, the anti-inflammatory ratio of IL-1RA to IL-1β was decreased by COC use in women with T. vaginalis infection, BV (P < 0.001), gonorrhea (P = 0.01), and herpes (P = 0.04).
Similarly, the effects of DMPA use were influenced by the presence of CVIs. RANTES remained significantly increased by DMPA use only in women positive for herpes, BV (P < 0.001), or candidiasis (P = 0.04) and with an abnormal Nugent score of 4 to 6 (P = 0.05). Lower BD2 remained associated with DMPA use only if positive for herpes, T. vaginalis infection (P < 0.001), or chlamydia (P = 0.01). The immunosuppressive effect of DMPA was exacerbated by additional lower levels of IL-1RA in women with gonorrhea (P = 0.01), chlamydia (P = 0.05), or HSV-2 (P < 0.001), SLPI in women with gonorrhea (P = 0.05), or IL-1β, MIP-3α, and IL-1RA/IL-1β ratio in those with T. vaginalis infection (P < 0.001).
We next examined differences by CVI status within each HC-use stratum (Fig. 2; see Table S4 in the supplemental material). In the no-HC group, T. vaginalis infection was the only CVI to show significant changes detectable by increased levels of multiple immune mediators, including IL-1β (P < 0.001), BD2 (P = 0.01), IL-8, IL-1RA (P = 0.02), and MIP-3α (P = 0.04). Of the other CVIs lab confirmed within the no-HC group, only gonorrhea and chlamydia showed an immunostimulatory effect limited to increased IL-1β levels (P < 0.001) or IL-1RA (P < 0.001), respectively.
Within the COC user group, increased levels were seen only for RANTES by herpes, intermediary Nugent score, and BV and BD2 by T. vaginalis infection (P = 0.01). In contrast, chlamydia decreased IL-1β levels (P = 0.03), herpes decreased IL-8 (P = 0.05) and VEGF (P = 0.02) levels, gonorrhea (P = 0.03) and candidiasis (P = 0.04) decreased VEGF, and an intermediary Nugent score was associated with the broadest immunosuppressive effect demonstrated by decreased levels of IL-1RA, IL-6, IL-8, VEGF (P < 0.001), IL-1β (P = 0.01), MIP-3α (0.02), and SLPI (P = 0.05).
Within the DMPA user group, gonorrhea was associated with decreased SLPI (P = 0.04) and IL-1RA (P = 0.02), chlamydia with reduced levels of IL-8 (P = 0.05) and VEGF (P = 0.02) but increased levels of RANTES (P = 0.01), T. vaginalis infection with decreased levels of SLPI (P = 0.02), and herpes (0.04), candidiasis, and BV (P = 0.01) with increased levels of BD2.
DISCUSSION
The HC-HIV study had previously found that women who used DMPA, but not those who used COC, were at significantly increased risk of HIV acquisition compared to women not using hormonal contraception (1, 7), and we had shown that this risk may be mediated by differential effects of DMPA and COC on cervical innate immunity (22). More specifically, in the nested cohort of 199 HIV seroconverters and 633 controls, HIV seroconversion was associated with preceding higher levels of RANTES and BD2 and lower levels of SLPI (22). We now demonstrate that cervicovaginal infections modified these three markers of HIV seroconversion risk in a manner dependent on HC use. Of special concern is our new finding that even though COC use had no effect on RANTES in CVI-free women, RANTES was increased among the COC users in association with herpes and abnormal vaginal microbiota (Nugent score of ≥4), both highly prevalent in women at HIV risk. Thus, dependent on the CVI and microbiota status, COC use may convey risk of HIV. RANTES was increased by DMPA in CVI-free women, but this effect was amplified by some CVIs, such as chlamydia and herpes. SLPI, which is a major antibacterial and antiviral effector in the cervicovaginal environment, previously shown to be reduced by BV and T. vaginalis infection (49), was decreased by T. vaginalis infection and gonorrhea among the DMPA users and among the COC users—by intermediary abnormal microbiobiota (Nugent score of 4 to 6). The third marker of HIV seroconversion risk, decreased BD2, was increased among no-HC and COC users by T. vaginalis infection and among DMPA users by herpes, candidiasis, and BV. Thus, the differential effects of COC and DMPA on the risk of HIV acquisition may be explained by immune factors potentiated by population differences in the prevalence of abnormal microbiota and bacterial, viral, or protozoan sexually transmitted infections.
In addition to RANTES, SLPI, and BD2, which were associated with subsequent HIV seroconversion and altered by DMPA or DMPA when combined with specific CVIs but also similarly by COC use only when combined with certain CVIs, the profiles of other proinflammatory mediators differed by HC use. Overall, COC increased levels of the proinflammatory mediators IL-1β, IL-6, and IL-8, while DMPA did not change or decreased their levels when combined with CVIs (e.g., chlamydia). In CVI-free women, COC but not DMPA use was associated with higher levels of IL-1β, IL-6, and IL-8. In agreement with our findings, an experimental study showed upregulation of IL-6 in ectocervical and vaginal epithelial cells when treated with COC but not DMPA (50). Another in-vitro study showed that a DMPA dose of 10−7 M induced downregulation of a broad spectrum of immune mediators in peripheral blood mononuclear cells (24). In the same study, IL-6 was suppressed by DMPA but only at a very high dose of 10−6 M, which may not be maintained at the cervical tissue level.
The biological grounds for differences observed in the effects of DMPA and COC can be attributed to differential regulation of inflammatory pathways by different progestins like DMPA and levonorgestrel (24), as well as to differences between progestins alone and progestins combined with estrogens. In another experimental study, natural estrogen, as well as natural progesterone combined with estrogen, but not progesterone alone decreased BD2 expression by vaginal epithelial cells in vitro (51). In contrast, in our study, DMPA, which represents progestin action alone, suppressed BD2 levels, while COC, which is an estrogen-progestin combination, did not suppress BD2 unless combined with herpes or candidiasis. These differences between the natural hormones and DMPA and COC can be explained by the fact that synthetic progestins, unlike the natural reproductive hormones, promiscuously bind to multiple steroid receptors (52–54), leading to activation of different transcription factors and cofactors leading to transactivation or repression of a myriad of immune response genes (55–57).
We found that in comparison to CVI-free women, women positive for BV and herpes experienced broader proinflammatory effects with COC use, including higher levels of IL-6, IL-8, and/or IL-1β, and in addition, higher levels of MIP-3α and VEGF and lower values of the anti-inflammatory IL-1RA/IL-1β ratio. These changes may lead to increased risk of HIV due to inflammatory tissue damage and HIV host cell activation. Higher cervicovaginal levels of IL-1β, IL-6, and IL-8 and lower levels of IL-1RA correlated with cervicovaginal epithelial tissue damage and inflammatory infiltration in a recent prospective randomized trial assessing vaginal mucosal safety of cellulose sulfate, nonoxynol-9, and the universal hydroxyethyl cellulose (HEC) placebo (58). Preexisting high cervicovaginal levels of IL-1β and IL-8 predicted tenofovir gel failure to prevent HIV in a case-control study of HIV seroconverters and HIV-negative controls (59). The recent CAPRISA 004 trial showed that the effectiveness of the tenofovir gel, the first vaginal microbicide to show promise for HIV prevention, was diminished by preceding innate immune activation measurable not only at the cervical but also at the systemic level (60). Increased IL-6 was among the systemic markers predicting HIV seroconversion in the CAPRISA trials (60). Finally, IL-1β, IL-6, and IL-8 were inversely correlated with systemic CD4 counts in cervicovaginal specimens from women with acute HIV infection (61).
Some aspects of the innate immune barrier amplified by COC and DMPA in uninfected women (e.g., higher IL-8 and IL-1RA by COC use and higher RANTES by DMPA use) may also be protective against some forms of BV or BV persistence. In the HC-HIV study, both DMPA and COC were associated with a reduced prevalence of BV (6, 48, 62). The reduction in BV with HC use was also seen in the Mombasa sex worker study (6).
On the other hand, once established, the abnormal intermediary vaginal microbiota was associated with a broadly suppressed cervical innate immunity among the COC users in our study (lower levels of IL-1β, IL-6, IL-8, MIP-3α, VEGF, SLPI, and IL-1RA). This immunosuppressed state in the presence of intermediary vaginal microbiota is especially concerning since this condition of the vaginal microenvironment is not routinely diagnosed and treated, and it may facilitate the survival of other sexually transmitted pathogens, especially in women using COC. In addition, other aspects of innate and acquired immunity not measured in our study can be suppressed by HCs. In murine models of STIs, progesterone suppressed Th17 cell responses to gonorrhea, shifting the balance to immune tolerance (63), and estradiol downregulated Th17 responses to C. albicans infection (64). In our study, COC use was associated with suppressed BD2 among the women positive for candidiasis and BD2 is essential for antifungal defense and for killing C. albicans in particular (65). Suppressed immune responses to C. albicans infection may explain why COC use was associated with more candidiasis in the HC-HIV study (48).
In conclusion, using clinical specimens that were obtained from women attending reproductive health clinics in Uganda and Zimbabwe, we showed that cervicovaginal pathogens and altered vaginal microbiota contribute to the differences in the effects of HCs on the cervical mucosal immune environment in HIV-negative women. The high prevalence of asymptomatic infections especially among COC and DMPA users that remain likely undiagnosed and untreated raises even more concerns in light of their combined effects on cervical immunity and biomarkers associated with risk of HIV. A deeper understanding of the pathogen-HC interactions may provide further insights for development of targeted interventions based on HC use and CVI status to improve reproductive health in women. Awareness of this fact can facilitate the design of clinical trials and meta-analyses to better define the role of different types of HCs on HIV risk. HCs can alter the risk of HIV acquisition and transmission differently based upon the background differences in concurrent CVIs. Epidemiological studies assessing the effect of HCs on mucosal immunity may generate different results in populations that differ by CVI prevalence or microbiota characteristics. In the future, these findings should be accounted for when assessing the risk of HIV acquisition/transmission and designing multipurpose technologies to better prevent pregnancy and HIV acquisition in women.
MATERIALS AND METHODS
Study design.
For this nested study, we utilized samples and data from 18- to 35-year-old HIV-uninfected participants in the HC-HIV study (n = 823) enrolled from family planning clinics in Uganda and Zimbabwe (7). The nested study included 199 women (51 Ugandan and 148 Zimbabwean) who became HIV infected, sampled at the study visit just prior to the one at which HIV seroconversion was documented (median of 3 months). These samples were matched with samples from 633 controls (160 Ugandan and 473 Zimbabwean), who remained HIV uninfected during a 6-month follow-up. The population characteristics of the cohort studied here are presented in detail elsewhere (66). Briefly, contraceptive group designation was based on the primary contraceptive method women used during the time between their previous study visit and the selected visit. Women in the non-hormonal contraceptive (no-HC) group used only condoms or no contraception. Women who chose to use hormonal contraceptives received from study clinicians either DMPA (150 mg injected every 3 months) or COC (30 µg ethinyl estradiol [EE] and 150 µg levonorgestrel). All women were asked to abstain from sexual intercourse 48 h prior to cervical swab specimen collection. No specimens were collected during menstrual bleeding, and friable cervix/visible blood was rarely recorded during swab collection.
Cases and controls were matched by study site, age, a composite sexually transmitted infection (STI) variable (see below), and time in study, with up to 4 matched controls for each case. The composite STI variable was set to 1 if a participant was diagnosed with C. trachomatis, N. gonorrhoeae (both confirmed by PCR), or had bacterial vaginosis (BV) at either the visit at which HIV infection was detected or at the previous visit (the last HIV-negative visit). The composite STI variable was set to 0 for women who tested negative for all 3 of these conditions at both visits. The composite STI variable was chosen to control for exposures rather than to detect interactions among specific pathogens.
Ethics statement.
The study was carried in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The parent HC-HIV study was carried with subjects’ informed consent and Institutional Review Board approval for human subject research at participating institutions in the United States and Africa. The biomarker sub study protocol received a nonhuman subject determination (use of deidentified data) from the Office of International Research Ethics at FHI 360 and the Institutional Review Board at Brigham and Women’s Hospital.
Clinical and laboratory diagnosis of infection.
A CVI-free status was defined as having no laboratory-diagnosed infection. Laboratory diagnosis of CVIs included PCR (Roche Amplicor) for C. trachomatis and N. gonorrhoeae, antibody enzyme-linked immunosorbent assay (ELISA) for herpes simplex virus 2 (HSV-2), wet mount for T. vaginalis and Candida, and Nugent scoring for BV. HIV-negative status was ascertained by PCR.
Clinical signs of CVIs were defined as positive (+) when any one of the following findings was recorded by clinicians on a physical exam: inflammation or ulcers of the vulva; yellow/green, white/creamy/grey, or mixed vaginal discharge; positive whiff test; presence of clue cells; abnormal vaginal epithelium; abnormal cervical epithelium; or yellow/green cervical mucus.
Information on symptoms of CVIs were obtained through a structured interview during which subjects were asked if they had abnormal vaginal discharge, genital itching, lower abdominal pain, or pain during sex and were given the options to answer with “yes,” “no,” or “don’t know.” Symptoms were defined as positive (+) when abnormal vaginal discharge, genital itching, lower abdominal pain, or pain during sex was reported. Bleeding between periods was not considered a symptom of CVI. For this analysis, only those who answered “no” to all of the above were considered symptom free (those who answered “don’t know” were excluded).
Biomarkers of cervical immunity.
For biomarkers of cervical immunity, we utilized cervical Dacron swabs, which were collected in Amplicor lysis buffer (Roche Diagnostics) and processed as previously described (67).
Eight biomarkers (IL-1β, IL-1RA, IL-6, IL-8, RANTES, MIP-3α, VEGF, and sICAM-1) were measured simultaneously using the Meso Scale Discovery (MSD) multiplex platform and Sector Imager 2400 (MSD, Gaithersburg, MD). This MSD detection platform has been validated for accuracy and precision of cytokine recovery using international standards by comparisons with traditional ELISA (68) and has shown high clinical content validity for all eight biomarkers in large clinical cohorts (69–78). The MSD 8-plex was custom designed and optimized to allow detection of each biomarker within the linearity concentration range of the eluted cervical swab samples. SLPI and BD2 were measured by ELISA (Quantikine human SLPI assay from R&D Systems, Minneapolis, MN, and human β-defensin 2 assay from Phoenix Pharmaceuticals, Inc., Burlingame, CA).
Each sample was tested in duplicate, and the average value was normalized to average milligram total protein concentration obtained from duplicate measurement using the Pierce bicinchoninic acid (BCA) protein assay (Fisher Scientific, Pittsburgh, PA). The ELISA and BCA assays were read using a Victor2 reader (PerkinElmer, Boston, MA). The percentages of coefficient of variation (CV) of duplicate values obtained by this method were <10%. A quality control sample pool that showed values within the linearity range was split into aliquots, and one aliquot was tested on each assay plate showing interplate variation of <25% for all immunoassays and proteins. Spiking of the Amplicor lysis buffer and diluent with known concentrations of the test proteins confirmed no assay interferences at the chosen lowest sample dilutions (2-fold for the MSD 8-plex, 80-fold for SLPI, and 25-fold for BD2). All samples showed values above the low limits of detection (LLD) for each assay as follows: IL-1β, 1.2 pg/ml; IL-1RA, 0.16 ng/ml; IL-6, 1.7 pg/ml; IL-8, 0.8 pg/ml; RANTES, 1.8 pg/ml; MIP-3α, 16.4 pg/ml; VEGF, 0.12 pg/ml; and sICAM-1, 3.6 pg/ml. For SLPI, the LLD was 0.46 ng/ml, and for BD2 the LLD was 12.2 pg/ml.
Statistical analysis.
The Box-Cox power transformation approach was used to transform biomarker concentrations into normal distributions for statistical modeling analyses. Descriptive statistics, including medians and ranges were used to summarize biomarker levels. The associations between levels of mediators and CVIs, clinical signs and symptoms of CVIs, and HC use were evaluated by the Wilcoxon tests and Kruskal-Wallis tests. Fisher’s exact test was used to evaluate the association between CVIs, HC use, and women’s characteristics. Bivariate and multivariable analyses controlling for other CVIs via generalized linear models were used to examine the effects of individual CVIs and HC use on levels of mediators. This study was based on a secondary data analysis to explore the impact of CVIs on the relationship between HC use and cervical safety/immunity biomarkers. Due to the post hoc nature of the analysis power calculation were not applicable (79). Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
SUPPLEMENTAL MATERIAL
Median (range) of immune biomarkers (pg/mg total protein) and total protein (mg/ml) in women with or without symptoms of cervicovaginal infections.
Median and range of immune biomarkers (pg/mg total protein) and total protein (mg/ml) in women not using hormonal contraception with and without cervicovaginal infections.
Summary of combined effects of individual cervicovaginal infections (CVI) and hormonal contraception (HC) on markers of cervical immunity after being adjusted for other individual CVIs, symptoms, and signs (multivariable analysis via generalized linear models). The highlighted boxes show statistically significant differences (P < 0.05) between COC and no-HC and DMPA and no-HC compared within the same CVI group. For P values, ↑ and ↓ indicate significant increases or decreases, respectively, in the levels of these mediators.
P values calculated using generalized linear models for cervicovaginal infection (CVI)-positive compared to CVI-free nonpregnant women within the same HC group. The highlighted boxes indicate significant increase (↑) and decrease (↓) in levels of immune mediators (multivariable analysis adjusting for individual overlapping CVIs and signs and symptoms).
ACKNOWLEDGMENTS
The study was funded with United States federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, through an Interagency Agreement with the United States Agency for International Development (USAID) (GHO-A-00-09-00016-00) and from funds from USAID provided to CONRAD (GPO-A-00-08-00005-00). The cytokine testing at the Laboratory of Genital Tract Biology was supported by PPA-10-068 (to R.N.F.) from Contraception Research and Development (CONRAD) Eastern Virginia Medical School, under a Cooperative Agreement with the US Agency for International Development (USAID). The study was also supported by 1R01HD077888-01 from NICHD provided to FHI 360 and Brigham and Women’s Hospital.
The views expressed by the authors do not necessarily reflect those of the sponsors.
We thank the study participants and the laboratory team who processed and analyzed the cervical specimens in R. N. Fichorova’s laboratory (Olimpia Suciu, Hassan Dawood, Hidemi Yamamoto, Ryan Murray, Bi Yu Li, Yujin Lee, Raymond Wong, Tai Nguyen, Xenia Chepa-Lotrea, and Yoshika Yamamoto).
R.N.F., C.S.M., C.M., P.C., and G.D. designed the study. R.N.F. wrote the first draft of the paper and generated hypotheses to be tested. K.M., P.L.C., C.S.M., C.M., G.D., C.K., T.C., and R.S. contributed to the writing of the paper. P.C., R.N.F., C.K., and C.S.M. analyzed the HC-HIV data. R.N.F. directed the measurements of immune biomarkers. All authors agreed with the manuscript’s results and conclusions and have reviewed and approved the final version of the manuscript.
None of the authors has any conflict of interest with this study.
Footnotes
Citation Fichorova RN, Chen P-L, Morrison CS, Doncel GF, Mendonca K, Kwok C, Chipato T, Salata R, Mauck C. 2015. The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. mBio 6(5):e00221-15. doi:10.1128/mBio.00221-15.
REFERENCES
- 1.Morrison CS, Chen PL, Kwok C, Richardson BA, Chipato T, Mugerwa R, Byamugisha J, Padian N, Celentano DD, Salata RA. 2010. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS 24:1778–1781. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison CS, Turner AN, Jones LB. 2009. Highly effective contraception and acquisition of HIV and other sexually transmitted infections. Best Pract Res Clin Obstet Gynaecol 23:263–284. doi: 10.1016/j.bpobgyn.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Mugo NR, Heffron R, Donnell D, Wald A, Were EO, Rees H, Celum C, Kiarie JN, Cohen CR, Kayintekore K, Baeten JM, Partners in Prevention HSV/HIV Transmission Study Team . 2011. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS 25:1887–1895. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Nalugoda F, Kiddugavu M, Sewankambo N, Quinn TC, Reynolds SJ, Wawer MJ. 2005. Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. Lancet 366:1182–1188. doi: 10.1016/S0140-6736(05)67481-8. [DOI] [PubMed] [Google Scholar]
- 5.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, Coombs RW, Baeten JM, Partners in Prevention HSV/HIV Transmission Study Team . 2012. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis 12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Nyange PM, Richardson BA, Lavreys L, Chohan B, Martin HL, Mandaliya K, Ndinya-Achola JO, Bwayo JJ, Kreiss JK. 2001. Hormonal contraception and risk of sexually transmitted disease acquisition: results from a prospective study. Am J Obstet Gynecol 185:380–385. doi: 10.1067/mob.2001.115862. [DOI] [PubMed] [Google Scholar]
- 7.Morrison CS, Richardson BA, Mmiro F, Chipato T, Celentano DD, Luoto J, Mugerwa R, Padian N, Rugpao S, Brown JM, Cornelisse P, Salata RA, Hormonal Contraception and the Risk of HIV Acquisition (HC-HIV) Study Group . 2007. Hormonal contraception and the risk of HIV acquisition. AIDS 21:85–95. doi: 10.1097/QAD.0b013e3280117c8b. [DOI] [PubMed] [Google Scholar]
- 8.Lekovich JP, Amrane S, Pangasa M, Pereira N, Frey MK, Varrey A, Holcomb K. 2015. Comparison of human papillomavirus infection and cervical cytology in women using copper-containing and levonorgestrel-containing intrauterine devices. Obstet Gynecol 125:1101–1105. doi: 10.1097/AOG.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 9.United Nations 2011. World contraceptive use. Department of Economic and Social Affairs, Population Division, United Nations, New York, NY. [Google Scholar]
- 10.Masson L, Passmore JS, Liebenberg LJ, Werner L, Baxter C, Arnold KB, Williamson C, Little F, Mansoor LE, Naranbhai V, Lauffenburger DA, Ronacher K, Walzl G, Garrett NJ, Williams BL, Couto-Rodriguez M, Hornig M, Lipkin WI, Grobler A, Abdool Karim Q. 2015. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 61:260–269. doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fichorova RN, Anderson DJ. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod 60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 12.Fichorova RN, Rheinwald JG, Anderson DJ. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol Reprod 57:847–855. doi: 10.1095/biolreprod57.4.847. [DOI] [PubMed] [Google Scholar]
- 13.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. 2005. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol 53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 14.Alfano M, Poli G. 2002. The cytokine network in HIV infection. Curr Mol Med 2:677–689. doi: 10.2174/1566524023361925. [DOI] [PubMed] [Google Scholar]
- 15.Kyongo JK, Jespers V, Goovaerts O, Michiels J, Menten J, Fichorova RN, Crucitti T, Vanham G, Ariën KK. 2012. Searching for lower female genital tract soluble and cellular biomarkers: defining levels and predictors in a cohort of healthy Caucasian women. PLoS One 7:e43951. doi: 10.1371/journal.pone.0043951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Harthi L, Kovacs A, Coombs RW, Reichelderfer PS, Wright DJ, Cohen MH, Cohn J, Cu-Uvin S, Watts H, Lewis S, Beckner S, Landay A, WHS 001 Study Team . 2001. A menstrual cycle pattern for cytokine levels exists in HIV-positive women: implication for HIV vaginal and plasma shedding. AIDS 15:1535–1543. doi: 10.1097/00002030-200108170-00011. [DOI] [PubMed] [Google Scholar]
- 17.Al-Harthi L, Wright DJ, Anderson D, Cohen M, Matity Ahu D, Cohn J, Cu-Unvin S, Burns D, Reichelderfer P, Lewis S, Beckner S, Kovacs A, Landay A. 2000. The impact of the ovulatory cycle on cytokine production: evaluation of systemic, cervicovaginal, and salivary compartments. J Interferon Cytokine Res 20:719–724. doi: 10.1089/10799900050116426. [DOI] [PubMed] [Google Scholar]
- 18.Wira CR, Fahey JV. 2008. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS 22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gargiulo AR, Fichorova RN, Politch JA, Hill JA, Anderson DJ. 2004. Detection of implantation-related cytokines in cervicovaginal secretions and peripheral blood of fertile women during ovulatory menstrual cycles. Fertil Steril 82(Suppl 3):1226–1234. doi: 10.1016/j.fertnstert.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. 2003. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril 79:856–863. doi: 10.1016/S0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 21.Barousse MM, Theall KP, Van Der Pol B, Fortenberry JD, Orr DP, Fidel PL. 2007. Susceptibility of middle adolescent females to sexually transmitted infections: impact of hormone contraception and sexual behaviors on vaginal immunity. Am J Reprod Immunol 58:159–168. doi: 10.1111/j.1600-0897.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 22.Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, Salata R, Doncel GF. 2014. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 66:109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 23.Goldfien GA, Barragan F, Chen J, Takeda M, Irwin JC, Perry J, Greenblatt RM, Smith-McCune KK, Giudice LC. 2015. Progestin-containing contraceptives alter expression of host defense-related genes of the endometrium and cervix. Reprod Sci 22:814–828. doi: 10.1177/1933719114565035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huijbregts RP, Helton ES, Michel KG, Sabbaj S, Richter HE, Goepfert PA, Hel Z. 2013. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology 154:1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthrie BL, Introini A, Roxby AC, Choi RY, Bosire R, Lohman-Payne B, Hirbod T, Farquhar C, Broliden K. 2015. Depot medroxyprogesterone acetate use is associated with elevated innate immune effector molecules in cervicovaginal secretions of HIV-1-uninfected women. J Acquir Immune Defic Syndr 69:1–10. doi: 10.1097/QAI.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauck CK, Chen P, Morrison C, Fichorova R, Kwok C, Chipato T, Salata R, Doncel G. 2014. Biomarkers of cervical inflammation and immunity associated with cervical HIV-1 RNA. AIDS Res Hum Retroviruses 30(Suppl 1):A181. doi: 10.1089/aid.2014.5386.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell C, Marrazzo J. 2014. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 71:555–563. doi: 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 29.Laga M, Manoka A, Kivuvu M, Malele B, Tuliza M, Nzila N, Goeman J, Behets F, Batter V, Alary M, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95–102. doi: 10.1097/00002030-199301000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Masson L, Mlisana K, Little F, Werner L, Mkhize NN, Ronacher K, Gamieldien H, Williamson C, Mckinnon LR, Walzl G, Abdool Karim Q, Abdool Karim SS, Passmore JA. 2014. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 90:580–587. doi: 10.1136/sextrans-2014-051601. [DOI] [PubMed] [Google Scholar]
- 31.Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung’u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. 2015. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Wijgert JH, Mason PR, Gwanzura L, Mbizvo MT, Chirenje ZM, Iliff V, Shiboski S, Padian NS. 2000. Intravaginal practices, vaginal flora disturbances, and acquisition of sexually transmitted diseases in Zimbabwean women. J Infect Dis 181:587–594. doi: 10.1086/315227. [DOI] [PubMed] [Google Scholar]
- 33.van de Wijgert JH, Morrison CS, Brown J, Kwok C, Van Der Pol B, Chipato T, Byamugisha JK, Padian N, Salata RA. 2009. Disentangling contributions of reproductive tract infections to HIV acquisition in African women. Sex Transm Dis 36:357–364. doi: 10.1097/OLQ.0b013e3181a4f695. [DOI] [PubMed] [Google Scholar]
- 34.Brown JM, Wald A, Hubbard A, Rungruengthanakit K, Chipato T, Rugpao S, Mmiro F, Celentano DD, Salata RS, Morrison CS, Richardson BA, Padian NS. 2007. Incident and prevalent herpes simplex virus type 2 infection increases risk of HIV acquisition among women in Uganda and Zimbabwe. AIDS 21:1515–1523. doi: 10.1097/QAD.0b013e3282004929. [DOI] [PubMed] [Google Scholar]
- 35.Buve A, Jespers V, Crucitti T, Fichorova RN. 2014. The vaginal microbiota and susceptibility to HIV. AIDS 28:2333–2344. doi: 10.1097/QAD.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 36.Fichorova RN. 2004. Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr 37(Suppl 3):S184–S193. doi: 10.1097/00126334-200410013-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fichorova RN, Lai JJ, Schwartz JL, Weiner DH, Mauck CK, Callahan MM. 2011. Baseline variation and associations between subject characteristics and five cytokine biomarkers of vaginal safety among healthy non-pregnant women in microbicide trials. Cytokine 55:134–140. doi: 10.1016/j.cyto.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Anderson DJ, Politch JA, Tucker LD, Fichorova R, Haimovici F, Tuomala RE, Mayer KH. 1998. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res Hum Retroviruses 14(Suppl 1):S43–S49. [PubMed] [Google Scholar]
- 39.Fichorova RN, Bajpai M, Chandra N, Hsiu JG, Spangler M, Ratnam V, Doncel GF. 2004. Interleukin (IL)-1, IL-6, and IL-8 predict mucosal toxicity of vaginal microbicidal contraceptives. Biol Reprod 71:761–769. doi: 10.1095/biolreprod.104.029603. [DOI] [PubMed] [Google Scholar]
- 40.Fichorova RN, Desai PJ, Gibson FC, Genco CA. 2001. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fichorova RN, Lee Y, Yamamoto HS, Takagi Y, Hayes GR, Goodman RP, Chepa-Lotrea X, Buck OR, Murray R, Kula T, Beach DH, Singh BN, Nibert ML. 2012. Endobiont viruses sensed by the human host—beyond conventional antiparasitic therapy. PLoS One 7:e48418. doi: 10.1371/journal.pone.0048418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fichorova RN, Tucker LD, Anderson DJ. 2001. The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis 184:418–428. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 43.Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. 2011. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. mBio 2(6):e00168-11. doi: 10.1128/mBio.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fichorova RN, Trifonova RT, Gilbert RO, Costello CE, Hayes GR, Lucas JJ, Singh BN. 2006. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun 74:5773–5779. doi: 10.1128/IAI.00631-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mauck CK, Ballagh SA, Creinin MD, Weiner DH, Doncel GF, Fichorova RN, Schwartz JL, Chandra N, Callahan MM. 2008. Six-day randomized safety trial of intravaginal lime juice. J Acquir Immune Defic Syndr 49:243–250. doi: 10.1097/QAI.0b013e318186eae7. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz JL, Mauck C, Lai JJ, Creinin MD, Brache V, Ballagh SA, Weiner DH, Hillier SL, Fichorova RN, Callahan M. 2006. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind phase I safety study. Contraception 74:133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Dezzutti CS, Hendrix CW, Marrazzo JM, Pan Z, Wang L, Louissaint N, Kalyoussef S, Torres NM, Hladik F, Parikh U, Mellors J, Hillier SL, Herold BC. 2011. Performance of swabs, lavage, and diluents to quantify biomarkers of female genital tract soluble mucosal mediators. PLoS One 6:e23136. doi: 10.1371/journal.pone.0023136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Wijgert JH, Morrison CS, Cornelisse PG, Munjoma M, Moncada J, Awio P, Wang J, Van der Pol B, Chipato T, Salata RA, Padian NS. 2008. Bacterial vaginosis and vaginal yeast, but not vaginal cleansing, increase HIV-1 acquisition in African women. J Acquir Immune Defic Syndr 48:203–210. doi: 10.1097/QAI.0b013e3181743936. [DOI] [PubMed] [Google Scholar]
- 49.Huppert JS, Huang B, Chen C, Dawood HY, Fichorova RN. 2013. Clinical evidence for the role of Trichomonas vaginalis in regulation of secretory leukocyte protease inhibitor in the female genital tract. J Infect Dis 207:1462–1470. doi: 10.1093/infdis/jit039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Africander D, Louw R, Verhoog N, Noeth D, Hapgood JP. 2011. Differential regulation of endogenous pro-inflammatory cytokine genes by medroxyprogesterone acetate and norethisterone acetate in cell lines of the female genital tract. Contraception 84:423–435. doi: 10.1016/j.contraception.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Patel MV, Fahey JV, Rossoll RM, Wira CR. 2013. Innate immunity in the vagina. Part I. Estradiol inhibits HBD2 and elafin secretion by human vaginal epithelial cells. Am J Reprod Immunol 69:463–474. doi: 10.1111/aji.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Africander DJ, Storbeck KH, Hapgood JP. 2014. A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A). J Steroid Biochem Mol Biol 143:404–415. doi: 10.1016/j.jsbmb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Bamberger CM, Else T, Bamberger AM, Beil FU, Schulte HM. 1999. Dissociative glucocorticoid activity of medroxyprogesterone acetate in normal human lymphocytes. J Clin Endocrinol Metab 84:4055–4061. doi: 10.1210/jcem.84.11.6091. [DOI] [PubMed] [Google Scholar]
- 54.Africander D, Louw R, Hapgood JP. 2013. Investigating the anti-mineralocorticoid properties of synthetic progestins used in hormone therapy. Biochem Biophys Res Commun 433:305–310. doi: 10.1016/j.bbrc.2013.02.086. [DOI] [PubMed] [Google Scholar]
- 55.Louw-du Toit R, Hapgood JP, Africander D. 2014. Medroxyprogesterone acetate differentially regulates interleukin (IL)-12 and IL-10 in a human ectocervical epithelial cell line in a glucocorticoid receptor (GR)-dependent manner. J Biol Chem 289:31136–31149. doi: 10.1074/jbc.M114.587311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Govender Y, Avenant C, Verhoog NJ, Ray RM, Grantham NJ, Africander D, Hapgood JP. 2014. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS One 9:e96497. doi: 10.1371/journal.pone.0096497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koubovec D, Vanden Berghe W, Vermeulen L, Haegeman G, Hapgood JP. 2004. Medroxyprogesterone acetate downregulates cytokine gene expression in mouse fibroblast cells. Mol Cell Endocrinol 221:75–85. doi: 10.1016/j.mce.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 58.Mauck C.Assessing markers of inflammation after vaginal product use: nonoxynol-9, cellulose sulfate, and HEC placebo comparative double-blind phase I trial. Microbicides 2010, Pittsburgh, PA. [Google Scholar]
- 59.Karim SA, Karim QA. 2012. 6th N’Galy-Mann lecture: CAPRISA: partnering for scientific innovation in HIV prevention and treatment—opening session. In CROI2012. Seattle. http://retroconference.org/static/webcasts/2012/.
- 60.Naranbhai V, Abdool Karim SS, Altfeld M, Samsunder N, Durgiah R, Sibeko S, Abdool Karim Q, Carr WH, CAPRISA004 TRAPS Team . 2012. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis 206:993–1001. doi: 10.1093/infdis/jis465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bebell LM, Passmore JA, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, Karim QA, Karim SA. 2008. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis 198:710–714. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 62.van de Wijgert JH, Verwijs MC, Turner AN, Morrison CS. 2013. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS 27:2141–2153. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 63.Xu L, Dong B, Wang H, Zeng Z, Liu W, Chen N, Chen J, Yang J, Li D, Duan Y. 2013. Progesterone suppresses Th17 cell responses, and enhances the development of regulatory T cells, through thymic stromal lymphopoietin-dependent mechanisms in experimental gonococcal genital tract infection. Microbes Infect 15:796–805. doi: 10.1016/j.micinf.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Lasarte S, Elsner D, Guía-González M, Ramos-Medina R, Sánchez-Ramón S, Esponda P, Muñoz-Fernández MA, Relloso M. 2013. Female sex hormones regulate the Th17 immune response to sperm and Candida albicans. Hum Reprod 28:3283–3291. doi: 10.1093/humrep/det348. [DOI] [PubMed] [Google Scholar]
- 65.Vylkova S, Nayyar N, Li W, Edgerton M. 2007. Human β-defensins kill Candida albicans in an energy-dependent and salt-sensitive manner without causing membrane disruption. Antimicrob Agents Chemother 51:154–161. doi: 10.1128/AAC.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, Salata R, Doncel GF. 2014. Cervical inflammation and immunity associated with hormonal contraception, pregnancy and HIV-1 seroconversion. J Acquir Immune Defic Syndr 66:109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 67.Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, Salata R, Doncel GF. 2014. Cervical inflammation and immunity associated with hormonal contraception, pregnancy and HIV-1 seroconversion. J Acquir Immune Defic Syndr 66:109–117. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 68.Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L. 2008. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study. Anal Chem 80:4741–4751. doi: 10.1021/ac702628q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McElrath TF, Fichorova RN, Allred EN, Hecht JL, Ismail MA, Yuan H, Leviton A, ELGAN Study Investigators . 2011. Blood protein profiles of infants born before 28 weeks differ by pregnancy complication. Am J Obstet Gynecol 204:418.e1–418e 12. doi: 10.1016/j.ajog.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 70.Leviton A, Kuban KC, Allred EN, Fichorova RN, O’Shea TM, Paneth N, ELGAN Study Investigators . 2011. Early postnatal blood concentrations of inflammation-related proteins and microcephaly two years later in infants born before the 28th post-menstrual week. Early Hum Dev 87:325–330. doi: 10.1016/j.earlhumdev.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 71.Leviton A, Kuban K, O’Shea TM, Paneth N, Fichorova R, Allred EN, Dammann O. 2011. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J Pediatr 158:897–903.e1-5. doi: 10.1016/j.jpeds.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 72.Leviton A, Hecht JL, Allred EN, Yamamoto H, Fichorova RN, Dammann O, ELGAN Study Investigators . 2011. Persistence after birth of systemic inflammation associated with umbilical cord inflammation. J Reprod Immunol 90:235–243. doi: 10.1016/j.jri.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 73.Leviton A, Fichorova R, Yamamoto Y, Allred EN, Dammann O, Hecht J, Kuban K, McElrath T, O’Shea TM, Paneth N. 2011. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine 53:66–73. doi: 10.1016/j.cyto.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leviton A, Allred EN, Kuban KC, Dammann O, Fichorova RN, O'Shea TM, Paneth N, ELGAN Study Co-Investigators . 2011. Blood protein concentrations in the first two postnatal weeks associated with early postnatal blood gas derangements among infants born before the 28th week of gestation. The ELGAN Study. Cytokine 56:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A, ELGAN Study Investigators . 2011. Relationship between neonatal blood protein concentrations and placenta histologic characteristics in extremely low GA newborns. Pediatr Res 69:68–73. doi: 10.1203/PDR.0b013e3181fed334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fichorova RN, Onderdonk AB, Yamamoto H, Delaney ML, DuBois AM, Allred E, Leviton A, Extremely Low Gestation Age Newborns (ELGAN) Study Investigators . 2011. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. mBio 2(1):e00280-10. doi: 10.1128/mBio.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faupel-Badger JM, Fichorova RN, Allred EN, Hecht JL, Dammann O, Leviton A, McElrath TF. 2011. Cluster analysis of placental inflammatory proteins can distinguish preeclampsia from preterm labor and premature membrane rupture in singleton deliveries less than 28 weeks of gestation. Am J Reprod Immunol 66:488–494. doi: 10.1111/j.1600-0897.2011.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bose C, Laughon M, Allred EN, Van Marter LJ, O’Shea TM, Ehrenkranz RA, Fichorova R, Leviton A, ELGAN Study Investigators . 2011. Blood protein concentrations in the first two postnatal weeks that predict bronchopulmonary dysplasia among infants born before the 28th week of gestation. Pediatr Res 69:347–353. doi: 10.1203/PDR.0b013e31820a58f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levine M, Ensom MH. 2001. Post hoc power analysis: an idea whose time has passed? Pharmacotherapy 21:405–409. doi: 10.1592/phco.21.5.405.34503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Median (range) of immune biomarkers (pg/mg total protein) and total protein (mg/ml) in women with or without symptoms of cervicovaginal infections.
Median and range of immune biomarkers (pg/mg total protein) and total protein (mg/ml) in women not using hormonal contraception with and without cervicovaginal infections.
Summary of combined effects of individual cervicovaginal infections (CVI) and hormonal contraception (HC) on markers of cervical immunity after being adjusted for other individual CVIs, symptoms, and signs (multivariable analysis via generalized linear models). The highlighted boxes show statistically significant differences (P < 0.05) between COC and no-HC and DMPA and no-HC compared within the same CVI group. For P values, ↑ and ↓ indicate significant increases or decreases, respectively, in the levels of these mediators.
P values calculated using generalized linear models for cervicovaginal infection (CVI)-positive compared to CVI-free nonpregnant women within the same HC group. The highlighted boxes indicate significant increase (↑) and decrease (↓) in levels of immune mediators (multivariable analysis adjusting for individual overlapping CVIs and signs and symptoms).