Abstract
Background
Interleukin (IL)-8 -251 T/A and IL-10 (-1082 G/A and -819/592 C/T) polymorphisms and their expression may influence gastritis, atrophy, intestinal metaplasia (IM) and gastric cancer (GC) following H. pylori infection.
Methods
Genotyping of these genes was performed (ASO-PCR) in 200, 182 and 250 with GC, functional dyspepsia (FD) and healthy controls (HC), respectively. Anti-H. pylori IgG-antibody was tested in all and serums IL-8 and IL-10 were measured randomly in 60 subjects of each group by ELISA.
Results
Pro-(IL-8)-251 AA and anti-inflammatory (IL-10)-819 TT genotypes were commoner among GC than HC (p = 0.023, OR 1.86 [1.09–3.2] and p = 0.020, OR 2.0 [1.11–3.5]) but comparable with FD. IL-8 AA and IL-10-819 T allele carriage was also commoner in H. pylori-infected GC than HC (p = 0.011, OR 2.47 [1.23–5.0], and p = 0.018, OR 2.3 (1.16–4.59). IL-10-1082 G/A genotype and haplotypes (ACC, GCC, ATA and GTA) were comparable in all groups. Circulating levels of IL-8 and IL-10 were higher among GC than HC but comparable to FD (IL-8; 57.64 [6.44–319.46] vs. 54.35 [4.24–318.96] and 26.33 [4.67–304.54] pg/ml, p < 0.001 and IL-10; 15.47 [1.01–270.87] vs. 12.28 [0.96–64.88] and 3.79 [1.24–56.65], p < 0.001 for GC vs. HC). IL-8/IL-10 ratio was lower among GC than HC but higher than FD (3.7 [0.18–38.41] vs. 6.59 [0.98–130.2], p < 0.001 and 4.22 [0.15–61.4], p < 0.01). Circulating levels of IL-8, IL-10 and IL-8/lL-10 ratios were different among H. pylori-infected and non-infected GC than HC (p < 0.001, p < 0.001 and p < 0.01).
Conclusions
Pro-(IL-8)-251 T/A and anti-inflammatory (IL-10)-819 C/T gene polymorphisms and their circulating levels may play a role in H. pylori-associated gastric carcinogenesis in northern India.
Abbreviations: GC, Gastric cancer; FD, Functional dyspepsia; HC, Healthy control; H. pylori, Helicobacter pylori; IM, Intestinal metaplasia; IL, Interleukin; EDTA, Ethylene diamine tetra acetic acid; ELISA, Enzyme linked immune-sorbent assay; EIU, Enzyme immune unit; ARMS-PCR, Amplification refractory mutation system-polymerase chain reaction
Keywords: Interleukin, Gastric cancer, Functional dyspepsia, Genetic polymorphism, Helicobacter pylori
Highlights
-
•
IL-8-251 AA genotype was commoner in GC particularly in the presence of H. pylori and IM.
-
•
IL-10–819 TT and T carriage were frequent in GC even in the presence of H. pylori.
-
•
Circulatory IL-8 and IL-10 levels were higher; however, IL-8/IL-10 ratio was lower in GC.
-
•
IL-8-251A carriage had higher IL-8 but IL-10–1082 A carriage and ATA+ had lower IL-10.
1. Introduction
Gastric cancer (GC) is an aggressive neoplasm that is associated with an extremely poor prognosis (Berardi et al., 2004). Although the incidence of GC has been recently declining in several countries, it is still the second most common cause of cancer related mortality worldwide (Parkin et al., 2001). Helicobacter pylori (H. pylori), host genetic make-up and dietary factors are the major contributors in the pathogenesis of GC (Ghoshal et al., 2007). The annual incidence of GC is high (32–115 per 100,000 population) in some areas of the world such as Japan and China despite low sero-prevalence of H. pylori (40–50%) (Singh and Ghoshal, 2006). In contrast, in some areas of the world such as India, a lower proportion of subjects develop GC in spite of high prevalence of H. pylori infection (Singh and Ghoshal, 2006; Ghoshal et al., 2010). This incongruent observation might suggest that environmental factors (other than H. pylori infection) and host genetic factors may influence the clinical outcome of H. pylori infection (Bae et al., 2014; Oliveira and Silva, 2012).
GC develops through multiple steps and etiological factors are diverse. Pre-cancerous lesions of the stomach such as chronic atrophic gastritis, intestinal metaplasia (IM) and dysplasia precede the development of the GC (Correa, 1992; Sugano, 2013). Chronic inflammation with sustained proliferation has been generally accepted as a risk factor for cancer, including GC (Coussens and Werb, 2002; Balkwill and Mantovani, 2001). It is well known that persistent H. pylori infection causes inflammation (chronic gastritis), which is thought to progress to pre-cancerous lesions such as IM and finally to invasive GC. Chronic inflammation develops in genetically susceptible hosts with defective mucosal defense mechanisms or de-regulated immune responses by cytokines (Coussens and Werb, 2002). Genetic variations in cytokine genes can influence the inter-individual responses and disease susceptibility. The role of genes that encode pro- and anti-inflammatory cytokines and their circulating levels are well established in other gastro-duodenal diseases (Sugimoto et al., 2009). Although the link between inflammation and GC has been well established, the mechanisms involved in the process remains unclear.
Pro- and anti-inflammatory cytokines modulate the inflammatory response of the gastric mucosa. Pro-inflammatory chemotactic cytokine (IL-8) activates the inflammatory cells by the migration of neutrophils, mononuclear phagocytes, and mast cells and plays a major role in acquired immune responses (Coussens and Werb, 2002; Matsushima et al., 1992). On the other hand, anti-inflammatory cytokine (IL-10, a product of Th2 cells) is a potent factor for suppressing inflammatory and neoplastic processes by inhibiting IFN-γ production and antigen-specific T-cell activation (Bidwell et al., 1999; Howell). Balance between pro- (IL-8) and anti-inflammatory (IL-10) cytokines may influence the degree of inflammation, which is a potential factor in the development of gastritis and GC (Howell and Rose-Zerilli, 2006; Yasui et al., 2005).
Cytokine polymorphisms are the most studied genetic factor and are associated with the risk of GC in many regions, but have not been studied extensively in an Indian population (de Oliveira et al., 2014; Won et al., 2010). Therefore, we investigated the association between pro- (IL)-8-251 T/A (rs4073) and anti-inflammatory (IL-10)-1082 G/A (rs1800896) and -819/592 C/T (rs1800871) gene polymorphisms with their circulating levels among patients with GC as compared to controls (functional dyspepsia [FD] and healthy controls [HC]), with particular attention towards the relationship between H. pylori infection and the presence of precancerous lesions such as IM and gastritis.
2. Methods
2.1. Sample size calculation and study subjects
Quanto program version 1.1.1 (http://hydra.usc.edu/gxe) was used for sample size estimation for each genetic marker. The sample size for the study on IL-8-251 T/A gene polymorphism was calculated with input of the following parameters: case–control study design (1:1), significance level (α) < 0.05 (2 sided), model of inheritance was log additive (which is the most suitable model for the polygenic diseases), genetic effect (odds ratio) ≤ 1.5 or ≥ 2.0, power 80%, proportion of population expected to have GC 0.0001% and proportion of control expected to have IL-8-251A variant allele: 39% as reported from India (Ahirwar et al., 2010). The sample size was estimated to be 198 subjects in each group.
Similarly, the sample size for the study on IL-10 gene polymorphisms was calculated keeping all the above mentioned parameters the same and IL-10-1082 “G” allele frequency as 37% and IL-10-819 “T” variant allele frequency as 35% among control population as reported from our institute. The sample size was estimated to be 200 and 204 subjects in each group, respectively (Kesarwani et al., 2009; Achyut et al., 2008).
Two hundred patients with histologically confirmed non-cardia GC were included in the study. One hundred eighty-two age- and gender-matched patients with FD were included as a disease control (Miwa et al., 2012). Subjects who had received anti-microbial therapy, H2-receptor blockers, proton-pump inhibitors and non-steroidal anti-inflammatory drugs within 15 days before were excluded. Two hundred and fifty volunteers from the community (age- and sex-matched) willing to participate in the study were included as HC. The study protocol was approved by the Institutional Ethics Committee (IEC code: 2013-69-SRF-70) and written informed consent was obtained from all the subjects.
2.2. Esophagogastroduodenoscopy and sample collection
Esophagogastroduodenoscopy (EGD) was performed using a video endoscope (Olympus Optical Co Ltd., Tokyo, Japan). Six biopsies were collected in 10% neutral buffered formalin from tumor margins. Hematoxylin and eosin (H & E)-stained sections of endoscopic biopsy or surgically resected specimens were evaluated for confirmation of malignancy. If GC was diagnosed, it was further sub-classified into intestinal or diffuse type on histology using Lauren's classification (Lauren, 1965). Two to four biopsies were also collected among the patients with GC and FD (each from the antrum and body) from normal looking areas of the stomach for evaluation of H. pylori infection, IM and gastritis (graded according to updated Sydney system) (Dixon et al., 1996; Correa, 1988). This was assessed by an expert pathologist, who was blinded about the endoscopic findings. If the scores in the two biopsies were different, the higher score was accepted.
For genotyping studies, three milliliter blood in ethylene diamine tetra acetic acid (EDTA) was collected from all the patients and controls. Blood samples were collected from all the patients at morning after overnight fasting on the day of EGD. In the healthy subjects, blood samples were also collected similarly early in the morning in fasting state.
2.3. Diagnosis of H. pylori infection
Enzyme linked immune-sorbent assay (ELISA) was performed for IgG antibodies (HpIgG ELISA) using a commercially available kit (Biohit Oyj, Finland) (Ghoshal et al., 2011). A cut-off value of antibody concentration ≥ 30 enzyme immune unit (EIU) was considered positive.
2.4. DNA extraction and quantification
Genomic DNA was extracted from blood containing EDTA using commercially available kit (AuPrep, Life technologies, India) as per manufacturer's instructions. The quantity and purity of DNA were checked by 1.0% agarose gel electrophoresis and also by the ratio of optical density (OD) at 260 nm and 280 nm. It was stored at − 40 °C until use.
2.5. Genotyping of IL-8 and IL-10 polymorphisms
Genotyping was performed by amplification refractory mutation system-polymerase chain reaction (ARMS-PCR), as described previously (Kesarwani et al., 2009; Hull et al., 2000). In this method, we have used two reactions per samples. One common primer and each allele specific primer were used for each reaction. In IL-8-251 T/A genotyping, alleles were coded as A/A if amplification was seen in A specific primers; T/T when amplification was seen in T specific primers; A/T when amplification was seen in both A as well as T specific primers and the same method was also used for genotyping of IL-10-1082 G/A and -819 C/T. PCR products were run on 2% agarose gel and stained with 5% ethidium bromide. Quality control and assessment was done at every step of the study. If genotyping could not be assessed initially, it was repeated. A molecular base pair marker was included during electrophoresis. To ensure PCR success, an internal control region was amplified from the β-globin. Ten percent of samples from patients and controls were repeated to evaluate the quality of genotyping, which showed 100% concordance.
2.6. Estimation of serum IL-8 and IL-10 levels
Venous blood was clotted for 1 h in gel clot activator based vacutainers (RS Biosciences, USA), then centrifuged for 1000 g × 15 min at room temperature. Serum was removed immediately and stored at − 40 °C. IL-8 and IL-10 levels in the serum were estimated by sandwich ELISA based commercially available kits. The assay sensitivity was 0.8 and 2 pg/ml for IL-8 and IL-10 respectively. We have also used the positive and negative controls in every reaction of ELISA (standard dilute provided by BD biosciences, USA).
2.7. Statistical analysis
Categorical data and Hardy–Weinberg's equilibrium was evaluated by Chi-squared (χ2) test. p-Values less than 0.05 were considered significant. Binary logistic regression was used to estimate risks as odds ratio (OR) with 95% confidence intervals (CI). Patients were categorized on the basis of disease group, presence and absence of H. pylori and Lauren's classification of GC. Differences among groups for cytokine levels were evaluated using the non-parametric Kruskal–Wallis H and Mann–Whitney U test. Post hoc analysis and Bonferroni's correction (a multiple-comparison correction) were applied to significant association in subgroup analysis. In addition, haplotype analysis was used to estimate the frequency of unknown combinations by online software; http://bioinfo.iconcologia.net/SNPstats and frequency of different haplotypes was compared among groups by logistic regression; SPSS ver. 15 (SPSS, Inc., Chicago, IL, USA).
3. Results
3.1. Demographic data
All the patients and controls were comparable in gender. Patients with GC were also comparable to HC in age. Body weight (BW) was lower among the patients with GC than FD and HC (Table 1).
Table 1.
Demographical and histological characteristics in patients and controls.
| GC (n = 200) | FD (n = 182) | HC (n = 250) | p-Value | |
|---|---|---|---|---|
| Age in years (mean ± S.D.) | 53.3 ± 10.38 | 49.19 ± 9.67 | 51.54 ± 10.38 | GC vs. FD < 0.001 |
| GC vs. HC = 0.090 | ||||
| Gender | ||||
| Male | 142 (71.0%) | 118 (64.8%) | 176 (70.4%) | GC vs. FD = 0.789 |
| Female | 58 (29.0%) | 64 (35.2%) | 74 (29.6%) | GC vs. HC = 0.197 |
| BW in kg (mean ± S.D.) | 46.05 ± 15.17 | 62.17 ± 21.25 | 65.12 ± 15.36 | < 0.001 |
| Intestinal metaplasiaa | 64/178 (36.0%) | 16/179 (8.9%) | – | < 0.001 |
| Presence of gastritisa | 115/176 (65.3%) | 131/175 (74.9%) | – | 0.062 |
| Severity of gastritis | ||||
| Mild | 85 (73.9%) | 108 (82.4%) | – | 0.984 |
| Moderate | 24 (20.9%) | 20 (15.3%) | 0.797 | |
| Severe | 6 (5.2%) | 3 (2.3%) | 0.749 | |
| Lauren classification of GCa | ||||
| Diffuse | 75 (42.6%) | – | – | |
| Intestinal | 92 (52.3%) | |||
| Unclassified | 9 (5.1%) | |||
| H. pylori IgG seropositivity | ||||
| IgG ELISA | 105/176 (59.7%) | 121/182 (66.5%) | 168/250 (67.2%) | 0.235 |
| Anti-H. pylori IgG levels in EIU among sero-positive subjects (mean ± S.D.) | 55.11 ± 38.5 | 56.3 ± 35.12 | 42.17 ± 30.05 | GC vs. FD = 0.752 |
| GC vs. HC < 0.001 |
Abbreviations used: GC, gastric cancer; FD, functional dyspepsia; HC, healthy control; BW, body weight; kg, kilogram; S.D., standard deviation; IgG ELISA, anti-H. pylori IgG enzyme linked immunosorbent assay; EIU, enzyme immuno unit.
Bold data indicates significant at P-value < 0.05.
Histology data were not available in some patients.
3.2. Histology of gastric biopsy in patients with GC and FD
Of the 367 patients (both GC and FD), 80/357 (22.4%) had IM and 246/351 (70.1%) had gastritis. Patients with GC had higher frequency of IM than those with FD (64/178 [36.0%] vs. 16/179 [8.9%], p < 0.001) but had similar frequency of gastritis (115/176 [65.3%] vs. 131/175 [74.9%], p = 0.062). Severity of gastritis was similar among patients with GC and FD (Table 1). By Lauren's classification (n = 176), 92 (52%) had intestinal and 75 (43%) had diffuse type of tumor. In nine (5%), the tumor was unclassified.
3.3. H. pylori seropositivity
In all, 105 of 176 (59.7%) patients with GC, 121/182 (66.5%) with FD and 168/250 (67.2%) HC were positive for anti-H. pylori IgG serology. Anti-H. pylori IgG levels were similar among patients with GC and FD but higher than HC (Table 1). Seropositivity of IgG ELISA for H. pylori was comparable among different groups (p = 0.235).
3.4. Association between pro-inflammatory (IL-8)-251 T/A genotypes and risk of GC
Frequency of genotypes, and alleles were in Hardy–Weinberg equilibrium among controls (p = 0.801 for FD [disease control] and 0.618 for HC). Homozygous variant (AA) was commoner among patients with GC and conferred up to twofold risk in reference to homozygous wild (TT) genotype [47/200 (23.5%) vs. 35/250 (14%); p = 0.023, OR 1.86, 95% CI 1.09–3.19] in comparison to HC. Heterozygous (TA) and dominant (TA + AA) models were comparable among all the three groups (Table 2).
Table 2.
Genotype frequency of pro- (IL-8-251 T/A) and anti- (IL-10-1082 G/A & -819/592 C/T) inflammatory polymorphism and risk of gastric cancer.
| GC (n = 200) |
FD (n = 182) |
HC (n = 250) |
GC vs. FD OR (95% CI) | GC vs. HC OR (95% CI) | |
|---|---|---|---|---|---|
| IL-8-251 T/A | |||||
| TT (under-producer)⁎ | 67 (33.5%) | 58 (31.9%) | 93 (37.2%) | 1(Reference) | 1(Reference) |
| TA (intermediate) | 86 (43%) | 88 (48.4%) | 122 (48.8%) | 0.85 (0.53–1.34) | 0.98 (0.64–1.49) |
| AA (over-producer) | 47 (23.5%) | 36 (19.8%) | 35 (14%) | 1.13 (0.65–1.98) | 1.86 (1.09–3.19)a |
| A allele carriers vs. non-carriers | 133 (66.5%) | 124 (68.1%) | 157 (62.8%) | 0.93 (0.61–1.42) | 1.1 4 (0.78–1.68) |
| IL-10–1082 G/A | |||||
| GG (over-producer)⁎⁎ | 30 (15%) | 25 (13.7%) | 43 (17.2%) | 1(Reference) | 1(Reference) |
| GA (intermediate) | 96 (48%) | 90 (49.5%) | 122 (48.8%) | 0.89 (0.48–1.72) | 1.13 (0.66–1.93) |
| AA (under-producer) | 74 (37%) | 67 (36.8%) | 85 (34%) | 0.92 (0.49–1.72) | 1.25 (0.71–2.19) |
| A allele carriers vs. non-carriers | 170 (85%) | 157 (86.3%) | 207 (82.8%) | 0.90 (0.51–1.6) | 1.18 (0.71–1.9) |
| IL-10–819/592 C/T | |||||
| Homozygous wild (CC)⁎⁎⁎ | 61 (30.5%) | 51 (28%) | 101 (40.4%) | 1(Reference) | 1(Reference) |
| Heterozygous (CT) | 103 (51.5%) | 95 (52.2%) | 119 (47.6%) | 0.91 (0.57–1.44) | 1.43 (0.95–2.17) |
| Homozygous variant (TT) | 36 (18%) | 36 (19.8%) | 30 (12%) | 0.84 (0.46–1.51) | 1.99 (1.11–3.55)b |
| T allele carriers vs. non-carriers | 139 (69.5%) | 131 (72%) | 149 (59.6%) | 0.88 (0.57–1.38) | 1.55 (1.04–2.29)c |
Abbreviations used: GC, gastric cancer; FD, functional dyspepsia; HC, healthy control; OR, age and gender matched odds ratio; 95% CI = 95% confidence interval; *TT, **GG and ***CC taken as a reference for IL-8, IL-10–1082 and − 819/592 respectively: for risk analysis assuming no associations with disease outcome (OR = 1).
p-Value = 0.023.
p-Value = 0.020.
p-Value = 0.030.
3.5. Association between anti-inflammatory (IL-10-1082 G/A and -819/592 C/T) genotypes, their haplotypes and risk of GC
Frequency of genotypes and alleles were in Hardy–Weinberg equilibrium in controls (ϰ2 test: p-value for patients with FD = 0.547, 0.491; HC = 0.945, 0.574 for polymorphisms of -1082 G/A and -819/592 C/T, respectively). Genotyping distribution of IL-10-1082 G/A was comparable in patients with GC than FD and HC (Table 2). In case of IL-10-819/592 C/T polymorphism, homozygous variant (TT) was commoner among patients with GC and conferred twofold risk in reference to homozygous wild (CC) genotype [36/200 (18%) vs. 30/250 (12%); p = 0.020, OR 1.99, 95% CI 1.11–3.55] in comparison to HC. On applying dominant model, T allele carriage (CT + TT) was also commoner in patients with GC than HC [139/200 (69.5%) vs. 149/250 (59.6%); p = 0.030, OR 1.54, 95% CI 1.04–2.29] in reference to CC (Table 2).
Haplotypes were evaluated for IL-10-1082 G/A, -819 C/T and -592 C/A (-819 and -592 are in linkage disequilibrium) polymorphisms located in the same gene with emphasis on combination of variants, which might be more likely to influence change in IL-10 level. Four haplotypes, over-producers (GCC and GTA), intermediate (ACC) and under-producer (ATA) were constructed. Frequency of haplotypes was comparable among cases and controls as shown in Fig. 1.
Fig. 1.
Frequency of IL-10-1082 G/A and -819 (C/T)/592 (C/A) haplotypes among cases and controls. GC = gastric cancer, FD = functional dyspepsia, HC = healthy control and vs. = versus; *Haplotype number (n) represents total number of chromosomes; frequency of combination equivalent to zero in any cell for IL-10-1082 or IL-10-819/592 was not included in analysis; frequency of haplotypes was comparable among case and controls.
3.6. Association between pro- (IL-8) and anti-inflammatory (IL-10) gene polymorphisms and risk of GC in relation to H. pylori infection
Among H. pylori-infected subjects, frequency of IL-8-251 AA genotypes was commoner among patients with GC than HC (28/105 [26.7%] vs. 22/168 [13.1%], p = 0.011, OR = 2.47 [95% CI = 1.23–4.96]). The remaining genotypes had comparable frequency among different groups (Table 3).
Table 3.
Genotype frequency of IL-8 and IL-10 in relation to H. pylori seropositivity.
| GC (n = 176) |
FD (n = 182) |
HC (n = 250) |
||||
|---|---|---|---|---|---|---|
| H. pylori serology | + ve (n = 105) N | -ve (n = 71) N | + ve (n = 121) N, OR (95% CI) | -ve (n = 61) N, OR (95% CI) | + ve (n = 168) N, OR (95% CI) | -ve (n = 82) N, OR (95% CI) |
| IL-8-251 T/A | ||||||
| TT* | 33 | 26 | 37, 1 (ref) | 21, 1 (ref) | 64, 1 (ref) | 29, 1 (ref) |
| TA | 44 | 30 | 59, 1.26 (0.61–2.57) | 29, 1.1 (0.42–2.89) | 82, 1.04 (0.59–1.82) | 40, 0.84 (0.41–1.29) |
| AA | 28a | 15 | 25, 0.84 (0.45–1.54) | 11, 0.84 (0.39–1.8) | 22, 2.47 (1.2–4.96)a | 13, 1.29 (0.52–3.2) |
| A allele carriers | 72 | 45 | 84, 0.96 (0.55–1.69) | 40, 1.7 (0.48–2.76) | 104, 1.34 (0.8–2.25) | 53, 1.78 (0.69–1.64) |
| IL-10-1082 G/A | ||||||
| GG** | 17 | 6 | 16, 1 (ref) | 9, 1 (ref) | 31, 1 (ref) | 12, 1 (ref) |
| GA | 54 | 29 | 59, 0.86 (0.39–1.9) | 31, 1.4 (0.44–4.43) | 78, 1.05 (0.51–2.17) | 44, 2.77 (0.92–8.34) |
| AA | 34 | 36 | 46, 0.69 (0.31–1.57) | 21, 2.57 (0.8–8.21) | 59, 1.26 (0.64–2.51) | 26, 1.32 (0.45–3.91) |
| A allele carriers | 88 | 65 | 105, 0.79 (0.38–1.6) | 52, 1.87 (0.63–5.61) | 137, 1.17 (0.61–2.24) | 70,1.86 (0.66–5.24) |
| IL-10–819/592 C/T | ||||||
| CC*** | 33 | 18 | 36, 1 (ref) | 15, 1 (ref) | 65, 1 (ref) | 36, 1 (ref) |
| CT | 61 | 32 | 65, 0.86 (0.47–1.6) | 30, 1.17 (0.51–2.68) | 94, 1.97 (0.94–4.11) | 28, 2.44 (0.86–6.9) |
| TT | 11 | 21 | 16, 1.14 (0.53–2.5) | 20, 0.57 (0.2–1.6) | 9, 1.49 (0.98–4.65) | 18, 0.26 (0.73–2.2) |
| T allele carriers | 72b | 53 | 46, 0.92 (0.52–1.6) | 85, 0.96 (0.43–2.12) | 103, 2.3 (1.16–4.59)b | 46, 1.44 (0.86–2.4) |
Abbreviations used: + ve, seropositive; − ve, seronegative; N, number of genotype; OR, odds ratio; 95% CI = 95% confidence interval; ref., reference; GC, gastric cancer; FD, functional dyspepsia; HC, healthy control; *TT, **GG and ***CC taken as a reference for IL-8, IL-10–1082 and − 819/592 respectively: for risk analysis assuming no associations with disease outcome (OR = 1).
p-Value = 0.011; IL-8 (-251 T/A): GC vs. HC.
p-Value = 0.018; IL-10 (− 819 C/T): GC vs. HC.
Frequency of IL-10-1082 G/A genotypes was similar among groups with and without H. pylori infection (Table 3). In the case of IL-10-819/592 C/T genotypes, though the IL-10-819 allele T carriage was associated with higher risk of GC in the presence of H. pylori (p = 0.018, 2.3 [1.16–4.5]), the IL-10-819 genotypes did not confer any risk in the absence of H. pylori (Table 3).
3.7. Relationship between pro- IL-8 (-251 T/A) and anti-inflammatory IL-10 (-1082 G/A and -819 C/T) genotypes and serum IL-8 and IL-10 levels
Subjects with heterozygous (TA) and A allele carriage had higher levels of serum IL-8 than those with TT genotypes (median [min.–max.]; TA vs. TT; 56.1 [4.24–319.46] vs. 32.23 [4.67–311.03], p < 0.001 and A allele carriage vs. non-carriage; 54.55 [4.24–319.46] vs. 32.23 [4.67–311.03], p < 0.001, respectively). However, homozygous variant (AA) was comparable to TT variant (AA; 45.61 [6.44–234.46], p = 0.148, Fig. 2A).
Fig. 2.
Comparison of serum cytokine levels with their genotypes and haplotypes; (A) serum IL-8 and IL-8-251 T/A genotypes, (B) serum IL-10 and IL-10-1082 G/A genotypes, (C) serum IL-10 and -819 C/T genotypes, (D) serum IL-10 and haplotypes of IL-10-1082 G/A, -819/592 C/T (C/A). Levels were indicated by median [min–max]. Statistical test: Kruskal–Wallis post hoc analysis was used to compare the parameters among groups.
Subjects having the IL-10-1082 G/A with homozygous variant (AA) and A allele carriage had lower IL-10 levels than those with the homozygous wild (GG) genotype (8.99 [0.19–94.02] and 10.29 [0.15–98.45] vs. 14.9 [1.1–88.36], p = 0.023 and 0.037, Fig. 2B; respectively). However, IL-10 levels were comparable among IL-10-819 C/T genotypes (Fig. 2C). Variations in serum IL-10 levels were also observed at the IL-10 (-1082 G/A, -819/-592 C/T [C/A]) haplotypes level (Fig. 2D), i.e., subjects having ATA + haplotypes had lower IL-10 levels than did GCC + (7.75 [0.15–94.32] vs. 13.23 [1.1–97.54], p = 0.038).
3.8. Serum levels of IL-8, IL-10 and IL-8/IL-10 ratio among cases and controls in relation to H. pylori infection
Serum levels of IL-8, IL-10 and IL-8/IL-10 ratio were different among cases and controls (p < 0.001). Patients with GC had higher level of cytokines than HC (58.31 [6.44–319.45] vs. 26.33 [4.67–277.62], p = 0.001 for IL-8 and 16.83 [1.1–98.45] vs. 4.22 [0.15–61.4], p < 0.001 for IL-10) but comparable to FD (55.09 [4.24–318.96] and 20.49 [2.46–98.09] pg/ml, p = ns), respectively. However, IL-8/IL-10 ratio was lower among patients with GC than HC but higher than FD (3.7 [0.18–38.41] vs. 6.59 [0.98–130.2], p < 0.001 and 4.22 [0.15–61.4], p < 0.01; respectively, Fig. 3).
Fig. 3.
Comparison of cytokine levels with their ratio among cases and controls; GC = gastric cancer, FD = functional dyspepsia, HC = healthy control (A) serum IL-8 level, (B) serum IL-10, (C) serum IL-8/IL-10 ratio among patients with GC, FD and HC. Levels were indicated by median [min–max]. Statistical test: Kruskal–Wallis post hoc analysis was used to compare the parameters among groups.
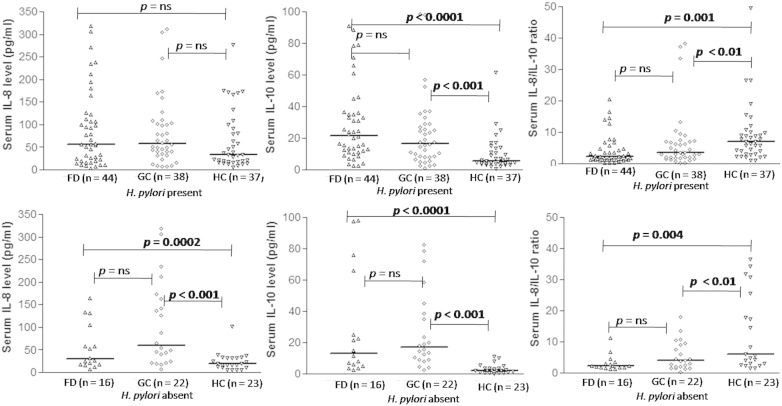
Serum IL-10 and IL-8/IL-10 ratio were higher among H. pylori-infected and non-infected GC than HC, though serum IL-8 was only higher among H. pylori non-infected GC (Fig. 4A and B). Serums IL-8 and IL-10 were comparable among H. pylori-infected and non-infected GC than FD.
Fig. 4.
Comparison of cytokine levels with their ratio among patients and controls in relation to anti-H. pylori IgG serology. (A.1) serum IL-8 level, (A.2) serum IL-10, and (A.3) serum IL-8/IL-10 ratio in the presence of H. pylori. (B.1) serum IL-8 level, (B.2) serum IL-10, and (B.3) serum IL-8/IL-10 ratio in the absence of H. pylori. Levels were indicated by median [min–max]. Statistical test: Kruskal–Wallis post hoc analysis was used to compare the parameters among groups.
3.9. Association between gene polymorphisms, serum levels and histological findings
Carriage of IL-8 A, IL-10 A and -819 T alleles had no relationship with the presence of GC (intestinal or diffuse) and gastritis among patients with GC. IL-8-251 A allele carriage was more frequent among patients with GC having IM than those without (49 [76.6%] vs. 68 [59.6%], p = 0.007, OR = 2.24 [2.0–4.91]) though the other genotypes were comparable. Serum levels of pro- and anti-inflammatory cytokines and their ratio were also comparable among patients with GC with or without IM and gastritis.
4. Discussion
The present study shows that (1) homozygous variant (AA) of pro-inflammatory (IL-8)-251 T/A gene polymorphism was commoner among patients with GC than HC, particularly in the presence of H. pylori and IM than those without, (2) patients with GC more often had homozygous variant (TT) and T allele carriage of anti-inflammatory (IL-10) -819/592 C/T polymorphism even in the presence of H. pylori infection, (3) circulatory IL-8 and IL-10 were higher; however, serum IL-8/IL-10 ratio was lower among patients with GC than HC but higher than FD, (4) subjects with IL-8-251 T/A, heterozygous (TA) and A allele carriage had higher levels of IL-8 whereas subjects having the IL-10-1082 G/A, homozygous (AA), A allele carriage [GA + AA]) and ATA+ haplotypes had lower levels of IL-10.
Genetic factors may play an important role in pathogenesis of GC (El-Omar et al., 2000). The presence of pro-inflammatory cytokine (IL)-8-251 AA genotype increased transcriptional activity of the IL-8 promoter site along with H. pylori infection in in-vitro assay (Crabtree, 1994). Several recent studies showed an association between IL-8 gene promoter -251 T/A polymorphism and GC (Wang et al., 2010). However, the results are contradictory. We found that homozygous IL-8 AA variant was commoner among patients with GC, a finding similar to two recent meta-analysis by Wang et al. (2010) and Lu et al. (2007). In another meta-analysis, Asian studies also showed higher risk of GC but the pooled effect of studies on Europeans showed conflicting results (Wang et al., 2010). IL-8-251 AA genotypes were also higher among patients with GC having H. pylori infection and IM in our study, similar to the finding of a meta-analysis (Liu et al., 2010). Furthermore, this genotype was comparable among patients with GC and controls in relation to gastritis, Lauren's type of tumor and in the absence of H. pylori. However, two meta-analyses showed results discordant with our observations (Wang et al., 2010; Xue et al., 2012a). Possible reasons for such discordant findings might be related to ethnic variations. There were differences in genetic make-up among Asians including Indians, and other races (Ghoshal et al., 2010). These observations suggest that Asians (China, Japan, Korea) including Indians have similar relationship between IL-8-251 T/A genotypes and GC, though the incidence of GC is lower among Indians than other Asian countries.
It is suggested that the ability of individuals to produce high levels of IL-8 is partly determined by the IL-8-251 A allele carriage (act as an over-producer), which might increase the risk of GC. We observed that patients with GC had higher levels of IL-8 than HC though comparable to FD as shown by Song et al. (2009). In our study, subjects with heterozygous IL-8-251TA, A allele carriage, H. pylori infection and IM had higher levels of IL-8 than those without. These observations were also supported by some other studies (Yamaoka et al., 1999; Takagi et al., 2002). These findings may suggest that IL-8 cytokine may help in promoting the development of H. pylori-associated gastritis, IM and GC as suggested by Correa (1992). These observations also suggest that GC and FD show similar progression of IL-8 related inflammation. Our findings suggest that IL-8-251 AA genotype and its serum levels may be an important pro-inflammatory biomarker of GC susceptibility among Indians.
Anti-inflammatory IL-10 cytokine is an immune-regulatory and immune-suppressive cytokine (Mocellin et al., 2005). It has been reported that IL-10 SNPs -1082 A/G, -819(-592) C/T(C/A) may influence immune function and alter the disease progression (Kesarwani et al., 2009; Rad et al., 2004). In our study, frequency of IL-10-1082 G/A genotype was similar among patients with GC and controls. Homozygous TT and T allele carriage of IL-10-819 C/T polymorphism was frequent among patients with GC than HC. Our result was similar to Caucasian studies; it was, however, contradictory to other Asian data (Ni et al., 2012; El-Omar et al., 2003; Wu et al., 2003; Xue et al., 2012b). We have also found that four haplotypes (GCC, ACC, GTA and ATA) of these polymorphisms of IL-10 were comparable in all groups.
Cytokine (IL-10) gene polymorphisms with H. pylori infection also influence the development of pre-cancerous lesions like chronic gastritis, IM and finally progression of GC (Rad et al., 2004). In our study, IL-10-819 T allele carriage was commoner among H. pylori sero-positive patients but similar in sero-negative patients with GC. A recent meta-analysis reported that IL-10-819 TT variant was not associated with GC in the absence of H. pylori but protective against GC susceptibility among individuals infected with H. pylori (Xue et al., 2012b). These observations might suggest that H. pylori infection is not a sole factor for development of GC. Some other factors, especially host genetic background, may also play a significant role. We have also observed that there was no association between IL-10 gene polymorphisms and IM, gastritis and Lauren's tumor type among patients with GC, similar to a Chinese study and a meta-analysis (Xue et al., 2012b; Leung et al., 2006). However, a few studies did contradict our observation (Ni et al., 2012; Kang et al., 2009). Racial and genetic difference and type II statistical error might explain this discordance. However, IL-10-1082 A allele carriage was commoner among patients with gastritis (patients with GC and FD both) than those without in our analysis. This observation is supported by one study from our center among patients with pre-cancerous lesions (gastritis) of prostate cancer (Kesarwani et al., 2009). Briefly, high frequency of IL-10-1082 A and -819 T alleles (lower secretor of IL-10) promote inflammation and risk of cancer development.
We also found that patients with GC had higher IL-10 level than HC though comparable to patients with FD. Two previous studies (one from Japan and the other from Italy) with similar findings suggested that elevated levels of IL-10 was associated with a worse prognosis of GC, independent of their tumor stage (De Vita et al., 1999; Tesse et al., 2012). Subjects having IL-10-1082 AA homozygous, A allele carriage and haplotype ATA+ had lower IL-10 levels in our study; these findings are in accordance with the study by Rad et al. (2004). In our data, serum IL-10 was also higher in H. pylori sero-positive subjects. These suggest that H. pylori-induced host immune response may influence the development of pre-cancerous lesions and its progression, where IL-10 cytokine may play an important role.
Imbalance between pro- and anti-inflammatory cytokines plays an important role in the development of chronic atrophic gastritis, IM and their progression to GC (Kidd, 2003). This may be the first study to determine the IL-8/IL-10 ratio as a biomarker of gastric carcinogenesis. In our study, patients with GC had lower IL-8/IL-10 ratio than HC but had higher ratio than FD. Furthermore, IL-8/IL-10 ratio was also lower among patients with GC in relation to H. pylori infection than those without. These observations suggest that IL-8/IL-10 ratio can be used as a biomarker of gastric carcinogenesis.
In summary, our findings suggest that IL-8 and IL-10 gene polymorphisms and their serum levels may lead to an imbalance between the pro-inflammatory and anti-inflammatory cytokine responses which might influence the susceptibility to GC. Ethnic differences in the frequency of host cytokine gene polymorphisms may explain variation in frequency of GC in spite of high frequency of H. pylori infection in different parts of the world. More multi-ethnic studies addressing this issue are needed.
Acknowledgments
We thank all the patients and volunteers who participated in our study. SK thanks the Indian Council of Medical Research for providing fellowship (No. 3/2/2/209/2013/NCD-III).
References
- Achyut B.R., Tripathi P., Ghoshal U.C., Moorchung N., Mittal B. Interleukin-10 (− 819 C/T) and tumor necrosis factor-alpha (− 308 G/A) gene variants influence gastritis and lymphoid follicle development. Dig. Dis. Sci. 2008;53:622–629. doi: 10.1007/s10620-007-9925-y. [DOI] [PubMed] [Google Scholar]
- Ahirwar D.K., Mandhani A., Mittal R.D. IL-8-251T > A polymorphism is associated with bladder cancer susceptibility and outcome after BCG immunotherapy in a northern Indian cohort. Arch. Med. Res. 2010;41:97–103. doi: 10.1016/j.arcmed.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Bae M., Jang S., Lim J.W., Kang J., Bak E.J., Cha J.H. Protective effect of Korean red ginseng extract against Helicobacter pylori-induced gastric inflammation in Mongolian gerbils. J. Ginseng Res. 2014;38:8–15. doi: 10.1016/j.jgr.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F., Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Berardi R., Scartozzi M., Romagnoli E., Antognoli S., Cascinu S. Gastric cancer treatment: a systematic review. Oncol. Rep. 2004;11:911–916. [PubMed] [Google Scholar]
- Bidwell J., Keen L., Gallagher G., Kimberly R., Huizinga T., McDermott M.F. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3–19. doi: 10.1038/sj.gene.6363645. [DOI] [PubMed] [Google Scholar]
- Correa P. Chronic gastritis: a clinico-pathological classification. Am. J. Gastroenterol. 1988;83:504–509. [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. The role of cytokines in Helicobacter pylori infection. Mucosal Immunol. Update. 1994:5–7. [Google Scholar]
- de Oliveira J.G., Rossi A.F., Nizato D.M., Miyasaki K., Silva A.E. Profiles of gene polymorphisms in cytokines and toll-like receptors with higher risk for gastric cancer. Dig. Dis. Sci. 2014;58:978–988. doi: 10.1007/s10620-012-2460-5. [DOI] [PubMed] [Google Scholar]
- De Vita F., Orditura M., Galizia G., Romano C., Infusino S., Auriemma A. Serum interleukin-10 levels in patients with advanced gastrointestinal malignancies. Cancer. 1999;86:1936–1943. [PubMed] [Google Scholar]
- Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- El-Omar E.M., Carrington M., Chow W.H., McColl K.E., Bream J.H., Young H.A. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- El-Omar E.M., Rabkin C.S., Gammon M.D., Vaughan T.L., Risch H.A., Schoenberg J.B. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- Ghoshal U.C., Chaturvedi R., Correa P. The enigma of Helicobacter pylori infection and gastric cancer. Indian J. Gastroenterol. 2010;29:95–100. doi: 10.1007/s12664-010-0024-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal U.C., Kumar S., Krishnani N., Kumari N., Chourasia D., Tripathi S. Serological assessment of gastric intestinal metaplasia and atrophy using pepsinogen-I, pepsinogen-II and gastrin-17 levels in a low incidence area of gastric cancer endemic for H. pylori infection. Trop. Gastroenterol. 2011;32:292–298. [PubMed] [Google Scholar]
- Ghoshal U.C., Tripathi S., Ghoshal U. The Indian enigma of frequent H. pylori infection but infrequent gastric cancer: is the magic key in Indian diet, host's genetic make up, or friendly bug? Am. J. Gastroenterol. 2007;102:2113–2114. doi: 10.1111/j.1572-0241.2007.01324_13.x. [DOI] [PubMed] [Google Scholar]
- W.M. Howell. Interleukin-10 gene polymorphisms and cancer. In: FM M, editor. Interleukin-10. p. 1–17.
- Howell W.M., Rose-Zerilli M.J. Interleukin-10 polymorphisms, cancer susceptibility and prognosis. Familial Cancer. 2006;5:143–149. doi: 10.1007/s10689-005-0072-3. [DOI] [PubMed] [Google Scholar]
- Hull J., Thomson A., Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55:1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.M., Kim N., Lee D.H., Park J.H., Lee M.K., Kim J.S. The effects of genetic polymorphisms of IL-6, IL-8, and IL-10 on Helicobacter pylori-induced gastroduodenal diseases in Korea. J. Clin. Gastroenterol. 2009;43:420–428. doi: 10.1097/MCG.0b013e318178d1d3. [DOI] [PubMed] [Google Scholar]
- Kesarwani P., Ahirwar D.K., Mandhani A., Singh A.N., Dalela D., Srivastava A.N. IL-10-1082 G > A: a risk for prostate cancer but may be protective against progression of prostate cancer in North Indian cohort. World J. Urol. 2009;27:389–396. doi: 10.1007/s00345-008-0361-1. [DOI] [PubMed] [Google Scholar]
- Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003;8:223–246. [PubMed] [Google Scholar]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Leung W.K., Chan M.C., To K.F., Man E.P., Ng E.K., Chu E.S. H. pylori genotypes and cytokine gene polymorphisms influence the development of gastric intestinal metaplasia in a Chinese population. Am. J. Gastroenterol. 2006;101:714–720. doi: 10.1111/j.1572-0241.2006.00560.x. [DOI] [PubMed] [Google Scholar]
- Liu L., Zhuang W., Wang C., Chen Z., Wu X.T., Zhou Y. Interleukin-8-251 A/T gene polymorphism and gastric cancer susceptibility: a meta-analysis of epidemiological studies. Cytokine. 2010;50:328–334. doi: 10.1016/j.cyto.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Lu Y., Wang Z.D., Shen J., Xu Y.C. Meta-analysis on the relationship between IL8-251 gene polymorphism and gastric cancer. Zhonghua Yu Fang Yi Xue Za Zhi. 2007;41:39–42. (Suppl) [PubMed] [Google Scholar]
- Matsushima K., Baldwin E.T., Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem. Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- Miwa H., Ghoshal U.C., Fock K.M., Gonlachanvit S., Gwee K.A., Ang T.L. Asian consensus report on functional dyspepsia. J. Gastroenterol. Hepatol. 2012;27:626–641. doi: 10.1111/j.1440-1746.2011.07037.x. [DOI] [PubMed] [Google Scholar]
- Mocellin S., Marincola F.M., Young H.A. Interleukin-10 and the immune response against cancer: a counterpoint. J. Leukoc. Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- Ni P., Xu H., Xue H., Lin B., Lu Y. A meta-analysis of interleukin-10-1082 promoter polymorphism associated with gastric cancer risk. DNA Cell Biol. 2012;31:582–591. doi: 10.1089/dna.2011.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira J.G.D.M., Silva A.E. IL-1ra anti-inflammatory cytokine polymorphism is associated with risk of gastric cancer and chronic gastritis in a Brazilian population, but the TNF-β pro-inflammatory cytokine is not. Mol. Biol. Rep. 2012;39:7617–7625. doi: 10.1007/s11033-012-1596-x. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Rad R., Dossumbekova A., Neu B., Lang R., Bauer S., Saur D. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53:1082–1089. doi: 10.1136/gut.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Ghoshal U.C. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J. Gastroenterol. 2006;12:1346–1351. doi: 10.3748/wjg.v12.i9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B., Zhang D., Wang S., Zheng H., Wang X. Association of interleukin-8 with cachexia from patients with low-third gastric cancer. Comp. Funct. Genomics. 2009:212345. doi: 10.1155/2009/212345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano K. Premalignant conditions of gastric cancer. J. Gastroenterol. Hepatol. 2013;28:906–911. doi: 10.1111/jgh.12209. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Furuta T., Yamaoka Y. Influence of inflammatory cytokine polymorphisms on eradication rates of Helicobacter pylori. J. Gastroenterol. Hepatol. 2009;24:1725–1732. doi: 10.1111/j.1440-1746.2009.06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A., Deguchi R., Kobayashi K., Miwa T. Cytokine expressions and H. pylori-associated gastric mucosal lesion. Keio J. Med. 2002;51(Suppl. 2):51–52. doi: 10.2302/kjm.51.supplement2_51. [DOI] [PubMed] [Google Scholar]
- Tesse R., Del Vecchio G.C., De Mattia D., Sangerardi M., Valente F., Giordano P. Association of interleukin-(IL)10 haplotypes and serum IL-10 levels in the progression of childhood immune thrombocytopenic purpura. Gene. 2012;505:53–56. doi: 10.1016/j.gene.2012.05.050. [DOI] [PubMed] [Google Scholar]
- Wang J., Pan H.F., Hu Y.T., Zhu Y., He Q. Polymorphism of IL-8 in 251 allele and gastric cancer susceptibility: a meta-analysis. Dig. Dis. Sci. 2010;55:1818–1823. doi: 10.1007/s10620-009-0978-y. [DOI] [PubMed] [Google Scholar]
- Won H.H.K.J., KIM M.J., KIm S., Park J.H. Interleukin 10 polymorphisms differentially influence the risk of gastric cancer in East Asians and Caucasians. Cytokine. 2010;51:73–77. doi: 10.1016/j.cyto.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Wu M.S., Wu C.Y., Chen C.J., Lin M.T., Shun C.T., Lin J.T. Interleukin-10 genotypes associate with the risk of gastric carcinoma in Taiwanese Chinese. Int. J. Cancer. 2003;104:617–623. doi: 10.1002/ijc.10987. [DOI] [PubMed] [Google Scholar]
- Xue H., Lin B., An J., Zhu Y., Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. doi: 10.1186/1471-2407-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H., Liu J., Lin B., Wang Z., Sun J., Huang G. A meta-analysis of interleukin-8-251 promoter polymorphism associated with gastric cancer risk. PLoS One. 2012;7:e28083. doi: 10.1371/journal.pone.0028083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Kita M., Imanishi J., Kashima K., Graham D.Y. Relation between clinical presentation, Helicobacter pylori density, interleukin 1beta and 8 production, and cagA status. Gut. 1999;45:804–811. doi: 10.1136/gut.45.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui W., Oue N., Aung P.P., Matsumura S., Shutoh M., Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86–94. doi: 10.1007/s10120-005-0320-0. [DOI] [PubMed] [Google Scholar]