Abstract
Stroke instigates regenerative responses that reorganize connectivity patterns among surviving neurons. The new connectivity patterns can be suboptimal for behavioral function. This review summarizes current knowledge on post-stroke motor system reorganization and emerging strategies for shaping it with manipulations of behavior and cortical activity to improve functional outcome.
As of 2010, there were more than 33 million stroke survivors worldwide, a population that is predicted to rise to 70 million by 2030 (63). The disability burden of stroke now falls most heavily on those between 20 and 75 years of age (110). Hemiparesis of the upper extremity (hand and arm) is the most prevalent (114), and among the most enduring and disabling (76), consequences of stroke, interfering with life quality and productivity. Despite thousands of promising preclinical studies of neuroprotective treatments that minimize tissue damage from stroke, their effective translation to clinical stroke populations has been disappointing (42, 147). Thrombolytic treatments to restore blood flow during the acute phase of stroke can be very effective in reducing impairments (166), but due to a short (3–4.5 h) treatment window and risk of hemorrhage, few stroke patients receive them (1, 62). Thus, as it stands now, the options for minimizing stroke-induced brain damage are limited, and the need to better understand and better treat the functional aftermath of this damage continues.
There is usually some degree of spontaneous improvement in function after stroke. Two general mechanisms are thought to contribute to this. 1) There is a resolution of temporary disruptions in neural activity, metabolism, and blood flow in regions connected to and surrounding the injured tissue. 2) Surviving neurons reorganize their connectivity patterns in a manner that supports partial restoration of, or compensatory substitution for, the lost functions. These are interrelated mechanisms (FIGURE 1). Despite them, most stroke survivors are left with chronic disability.
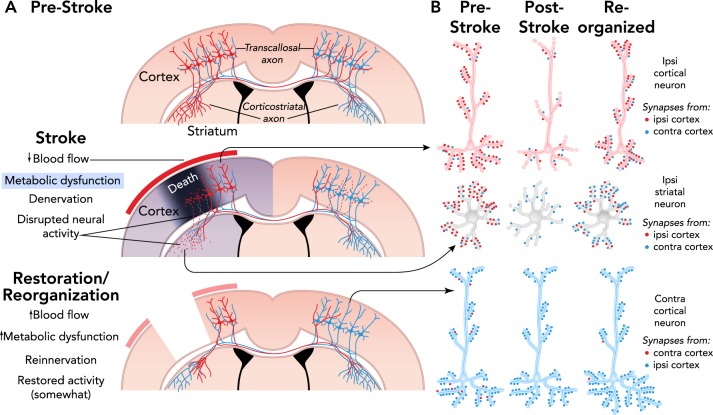
FIGURE 1.
Mechanisms of spontaneous functional improvements after stroke
A: coronal section illustrations of some of the axonal projections of cortical pyramidal neurons. Death of neurons in the core of an ischemic infarct results in denervation and disrupted activity in afferent targets, the striatum and contralateral cortex in this example. In peri-infarct cortex, there is a gradient of blood flow reductions, denervation of intracortical connections (not shown), and various degrees of dendritic retraction in surrounding neurons. Over time, blood flow and metabolic activity are restored, and denervated regions are reinnervated by axonal sprouting and synaptogenesis, resulting in reorganized connectivity. Restoration and reorganization are interrelated, e.g., reinnervation may depend on some degree of blood flow recovery and contribute to its fuller restoration. B: illustration of potential changes in neural connectivity patterns after reinnervation. The relative quantities of synapses from the contralateral vs. ipsilateral cortex may substantially change. Other sources of synaptic input to these neuronal populations can contribute to reinnervation and alter the balance of excitatory and inhibitory activity.
Neural reorganization after stroke is initiated by cellular reactions to degeneration. As neurons in an ischemic region die, their axons and synapses degenerate in widespread brain regions, instigating regenerative responses that promote the growth of new connections among surviving neurons. There is tremendous potential for variability in the patterns, and functional benefit, of the new connectivity that emerges from this process.
Several factors influence neural reorganization patterns, as reviewed below. A major one of these is the activity of the neuronal populations contributing to new connections. The intrinsic malleability of neural connections to altered neural activity underlies the capacity to learn across the lifespan. By influencing neural activity patterns, behavioral experiences drive the growth, maturation, and selective survival of synapses in relevant circuits. After stroke, behavioral experiences that influence the activity of regenerating circuits can potently shape neural reorganization patterns (90).
This creates an opportunity to use manipulations of behavior and neural activity to shape brain reorganization in a manner that optimizes functional outcome. There has been a surge of excitement in recent years over the potential to do this, as reflected in major growth in the field of stroke neurorehabilitation. However, the question of how best to do it is far from answered. More detailed understanding of what constitutes optimal neural reorganization after any given stroke, its behavioral dependencies, and other conditionalities are needed, but rapid advances in the field support optimism that this is within reach, given sufficient attention.
Below, we review recent research on neural reorganization after stroke, with a focus on motor system reorganization and its contribution to upper extremity function. Behavioral interventions such as motor rehabilitative training can shape neural reorganization to improve function. However, self-taught compensatory behavioral strategies can be a dominant force in driving reorganization patterns and can do so in a manner that interferes with motor rehabilitative training efficacy. Rehabilitative training alone is usually insufficient to restore normal function, but it has the potential to be improved by its combination with other treatments. We highlight one promising strategy for this: that of combing behavioral training with cortical stimulation.
Regenerative Responses to Stroke
Ischemic stroke is characterized by a gradient of reduced blood flow, with a core of severely reduced blood flow and severe tissue damage, and a surrounding penumbra, where blood flow reduction and degenerative reactions are less extreme (79). The loss of blood supply inititiates a cascade of metabolic failure, excitotoxicity, mitochondrial breakdown, oxidative stress, and neuroinflammation that, if not quickly reversed, results in irrevocable tissue damage (57). As neurons die in the ischemic region, connected regions undergo axonal degeneration, synapse loss, glia reactivity, and neuronal dysfunction or death to varying degrees, depending on the original connectivity with the ischemic region (15, 57, 81).
Degeneration triggers regenerative counter-reactions that can be observed on molecular to network levels, as reviewed in detail elsewhere (91, 135, 143). Briefly, degeneration elevates molecules that promote cell survival and proliferation and the structural remodeling of dendrites, axons, and synapses (157, 198). Growth inhibitory molecules that normally limit axonal plasticity in the adult brain are reduced (33, 130), and surviving neurons sprout new axon collaterals to reinnervate neurons in the penumbra and other denervated regions (17, 155). New synapses tend to be produced exuberantly followed by selective synapse pruning and maturation (174). Axonal sprouting and synaptogenesis are coordinated with dendritic remodeling in postsynaptic (2, 41, 187) and presynaptic (7, 152) neurons. The neuronal growth responses are intercoordinated with glial, vascular (10, 80), and extracellular matrix remodeling (157, 171). The whole process yields a system with new connectivity patterns, sometimes with much altered excitatory and inhibitory activity patterns (34, 96, 193).
Although regenerative responses to injury appear to be an intrinsic capacity of the adult central nervous system (CNS), they have many conditionalities and constraints. In adult CNS, axonal reinnervation is mainly accomplished by collateral sprouting of remaining projections within, or proximal to, the denervated region rather than long-distance growth of new axons (50, 51). For example, reinnervation of dentate gyrus granule cells after perforant path lesions is accomplished mainly by sprouting from remaining fibers terminating in the same layer (71). Nevertheless, even collateral sprouting can profoundly alter connectivity patterns (48). The more abundant and more active remaining afferents tend to contribute most to reinnervation (25, 32, 35, 69, 159, 165). This makes neural activity a promising therapeutic target, as discussed below. Axonal degeneration is a major trigger for remaining axons to sprout (44, 175), a relatively local signal, such that sprouting and accompanying dendritic remodeling patterns can be expected to vary tightly with degeneration patterns, which of course varies with injury territories. Regenerative plasticity also varies with injury modality; regenerative responses to ablation and traumatic brain injury are more limited compared with ischemic injury (95, 183, 186). There are numerous other contributors to variable regenerative responses, including injury severity (103), age (137, 156), individual genetic differences (154), and common stroke comorbidities, such as diabetes (80, 176).
Motor System Reorganization After Stroke
Hemiparesis is a prevalent consequence of stroke due to its tendency to involve the vascular supply of cerebral motor regions and their projection pathways, e.g., territories supplied by branches of the middle cerebral artery. Strokes are often positioned to disconnect cortex from its output to midbrain, brain stem, and spinal cord as a result of subcortical damage to descending fiber tracts, direct damage to motor cortex, or a combination of the two. As a result, axons carrying motor commands from motor cortex to subcortical targets, including spinal cord, are lost. Descending axons from surviving cortical neurons sprout to reinnervate these subcortical targets.
Most animal studies of subcortical reinnervation have examined projections arising from the uninjured (contralesional) primary motor cortex (M1), which after cortical infarcts or middle cerebral artery occlusions can sprout midline-crossing fibers that reinnervate striatum (35, 129), red nucleus, and spinal cord (113, 115, 121, 151, 191). The focus on sprouting from contralesional cortex reflects, in part, the ease in detecting it in the quantity of fibers crossing at midline. However, surviving neurons of the ipsilesional cortex can contribute as well. Starkey et al. (173) found that, after infarcts of the MI forelimb region in rats, corticospinal projections from the hindlimb region, which normally terminate in lower spinal cord levels, reinnervate the cervical spinal cord (upper limb region). Liu et al. (121) found that both hemispheres contribute to spinal cord reinnervation after middle cerebral artery occlusion in mice. It is likely that projections from the contralesional cortex necessarily predominate after injuries that spare little of the ipsilesional pathways (20) (FIGURE 2).
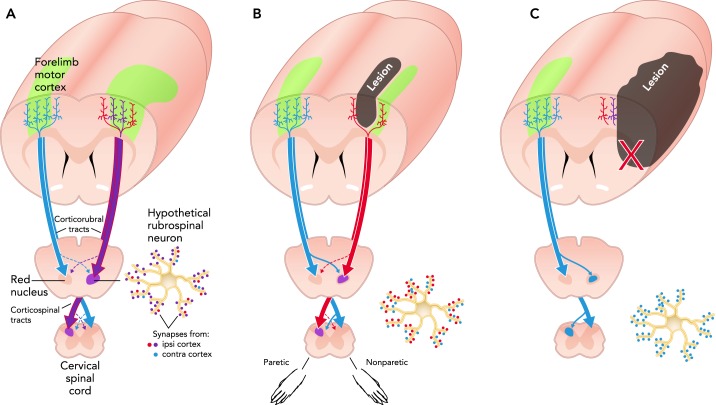
FIGURE 2.
Neuroantomical reorganization after stroke
A: illustration of a rat brain sectioned coronally near the rostral edge of the MI forelimb representation region (green). Callosal, corticorubral, and corticospinal tract projections of forelimb MI are illustrated in an intact brain. B: after subtotal infarcts of the forelimb area, remaining neurons of the forelimb region and surrounding motor cortex of the injured hemisphere can contribute to reinnervation. C: larger infarcts can severely damage descending projection pathways or the cortical pyramidal neurons that give rise to them, leaving crossed collaterals of contralesional projections as a primary source of reinnervation. The rubrospinal neuron illustration shows potential alterations in relative quantities of synaptic input from ipsi- and contralesional M1.
It seems reasonable to think that the capacity of the new subcortical connections to mediate the return of movement varies with the neuronal populations that supply them, but this has yet to be conclusively determined. There is strong evidence that sprouting from either hemisphere contributes to improved function, as supported by correlations and gain- and loss-of-function manipulations (e.g., Refs. 115, 151, 173, 188). However, Liu et al.'s study mentioned above found that final behavioral improvements were correlated with the contribution of ipsi- but not contralesional cortex to subcortical reinnervation. There is also suspicion that, when contralesional projections dominate reinnervation, it contributes to the development of abnormal muscle synergies in the paretic limb (128).
Direct damage to M1 results in extensive dendritic remodeling of remaining cortical neurons and reorganization of their connectivity. The dendrites and spines of pyramidal neurons near a cortical infarct partially degenerate followed by varying degrees of regrowth over time (22, 40, 109). In adult rats, M1 infarcts lead to sprouting of new intracortical axons that travel from the ipsilesional rostral motor cortex (analogous to primate premotor cortex) to synapse in the peri-infarct M1 (17, 32, 119). Similarly, after infarcts of the M1 hand representation in monkeys, axons from the ipsiventral premotor cortex sprout into peri-infarct M1 and somatosensory cortex (48). Transcallosal projections from the contralesional motor cortex also contribute to reinnervation of peri-infarct cortex (32, 36, 121).
Axonal sprouting and synaptogenesis create new patterns of synaptic connectivity, which can be expected to alter neural activity patterns (e.g., Refs. 31, 61). On a larger scale, neural reorganization is reflected in the organization of cortical maps of body movements (movement representations) and skin surfaces (somatosensory representations), and in cortical activation patterns related to movement and sensation (17, 45, 46, 75, 143). In animal and clinical studies, paretic limb function has been strongly linked to the reorganization of movement (68, 144, 148, 167, 181) and somatosensory (23, 43) cortical representations in the injured hemisphere and with the return to more normal patterns of activity across motor regions (75, 101, 141, 161). That the sprouting of new axonal connections contributes to at least some of these changes is suggested by close spatial relationships between regions of somatosensory (43) and motor (48) cortical map reorganization, and regions providing and receiving new projections.
There is bilateral neuroanatomical reorganization of motor cortex after unilateral motor system injury (91, 169). Unilateral damage to M1 results in degeneration of transcallosal cortical projections, which instigates dendritic remodeling of layer V pyramidal neurons of the contralesional cortex in rats (87, 88, 92). If the injuries sufficiently impair one forelimb, leading to compensatory reliance on the other (nonparetic) forelimb, this promotes a major increase in dendrites and synapses in layer V of the contralesional cortex (2, 27, 94). Pyramidal neurons in contralesional M1 that contribute to subcortical reinnervation also grow more dendrites after middle cerebral artery occlusions in rats (152). In both rodents and humans, the contralesional motor cortex has increased excitability (170, 193), increased fMRI activation during stimulation or movement of the paretic side (54, 75), and reduced functional connectivity (interdependent activity patterns) with the injured hemisphere (101, 161), effects that vary with stroke locus and severity and may contribute to paretic limb impairments (30, 72, 122, 172, 190).
At present, there is still a piecemeal understanding of how these various facets of neuroanatomical reorganization unfold over time, but there is every indication that, while the process takes a long time overall (months at least), it is particularly dynamic earlier after injury (33, 75, 81, 82, 135, 161, 164). Thus it can be predicted that there is both a protracted time period for modulating the reorganization process therapeutically and a time-sensitivity in the potency of this modulation (10).
Many elements of motor system reorganization are sensitive to manipulations of behavioral experience and neural activity. For example, electrical stimulation of contralesional cortex increases sprouting of contralesional corticospinal projections (25, 31). Sprouting of intracortical axons is increased by forced use of the paretic forelimb (149). Compensation with the nonparetic limb promotes dendritic growth in the contralateral cortex (94). Motor map reorganization is enhanced by training the paretic forelimb, as described below. Glial and vascular remodeling responses also are highly sensitive to behavioral manipulations (reviewed in Refs. 10, 89).
Motor system reorganization is also linked with changes in behavioral function, although not always to improvements. As noted above, the reorganization of motor (145) and somatosensory maps (43), intracortical sprouting (150), synaptogenesis in peri-infarct cortex (3), and corticospinal sprouting from either hemisphere (115, 121, 173) are positively correlated with functional improvements. However, the contralesional dendritic growth in layer V pyramidal neurons that we have observed has no known benefit (83), and contralesional M1 activity can have disruptive influences on paretic limb function (47, 126, 134, 141). Sprouting can also be maladaptive. For example, sprouting of proprioceptive afferents from the muscles into denervated spinal cord contributes to hyperreflexia (177), and sprouting in the hippocampus contributes to seizure susceptibility (59). Thus neural reorganization after stroke can be beneficial, irrelevant, suboptimal, or maladaptive for functional outcome. This leads to the need to drive these responses in beneficial directions. Their sensitivity to behavioral experience and neural activity provide obvious tools for this purpose.
Wrangling Behavioral Experience to Optimize Motor System Reorganization
The Trouble With Compensation
The natural response to disability is to learn new ways of accomplishing daily activities, i.e., to develop compensatory behaviors. Stroke survivors with upper extremity impairments typically learn to rely on the nonparetic hand and arm for daily activities. This encourages disuse of the paretic side, known as “learned-nonuse,” which has long been believed to exacerbate impairments (179). Our studies in rodent models indicate that learning new skills with the nonparetic side also can subvert neural mechanisms of functional improvements in the paretic forelimb.
We have modeled the effects of compensatory skill learning with the nonparetic forelimb in rodents using skilled reaching tasks that require movements resembling those used by humans (192) and permit control over lateralization and quantity of experience (8, 97). Following unilateral ischemic M1 lesions, if rats or mice receive a period of daily training with the nonparetic limb training (NPT) on a novel (for the limb) reaching task, disuse and dysfunction of the paretic forelimb is exacerbated (8, 9, 11, 98), regardless of whether the reaching skill is entirely novel to either limb (11) or had been established in the paretic limb before the infarct (8). In contrast, training one limb of intact rats has no detrimental effect on the other limb. MacLellan et al. (124) found that the deleterious effects of NPT persist long after the training ceases. The functional improvements that can be achieved with subsequent rehabilitative reach training focused on the paretic forelimb are also lessened (8, 9, 98), although prior NPT does not diminish activity with the paretic forelimb during rehabilitation.
The effect of NPT on rehabilitation efficacy is linked with reduced neuronal activation (8) and loss of forelimb movement representations (104) in peri-lesion M1 (FIGURE 3), a region known to mediate functional improvements in the paretic limb, as explained below. Animals that receive rehabilitative training after NPT have a greater increase in synaptic densities in the residual forelimb region compared with rehabilitative training alone (104). That is, NPT does not block neural reorganization of peri-infarct cortex but rather alters it in a manner that is maladaptive for the paretic forelimb. The promotion of synapse addition in peri-infarct cortex by NPT may interfere with subsequent synaptic changes that can be driven by rehabilitative training.
FIGURE 3.
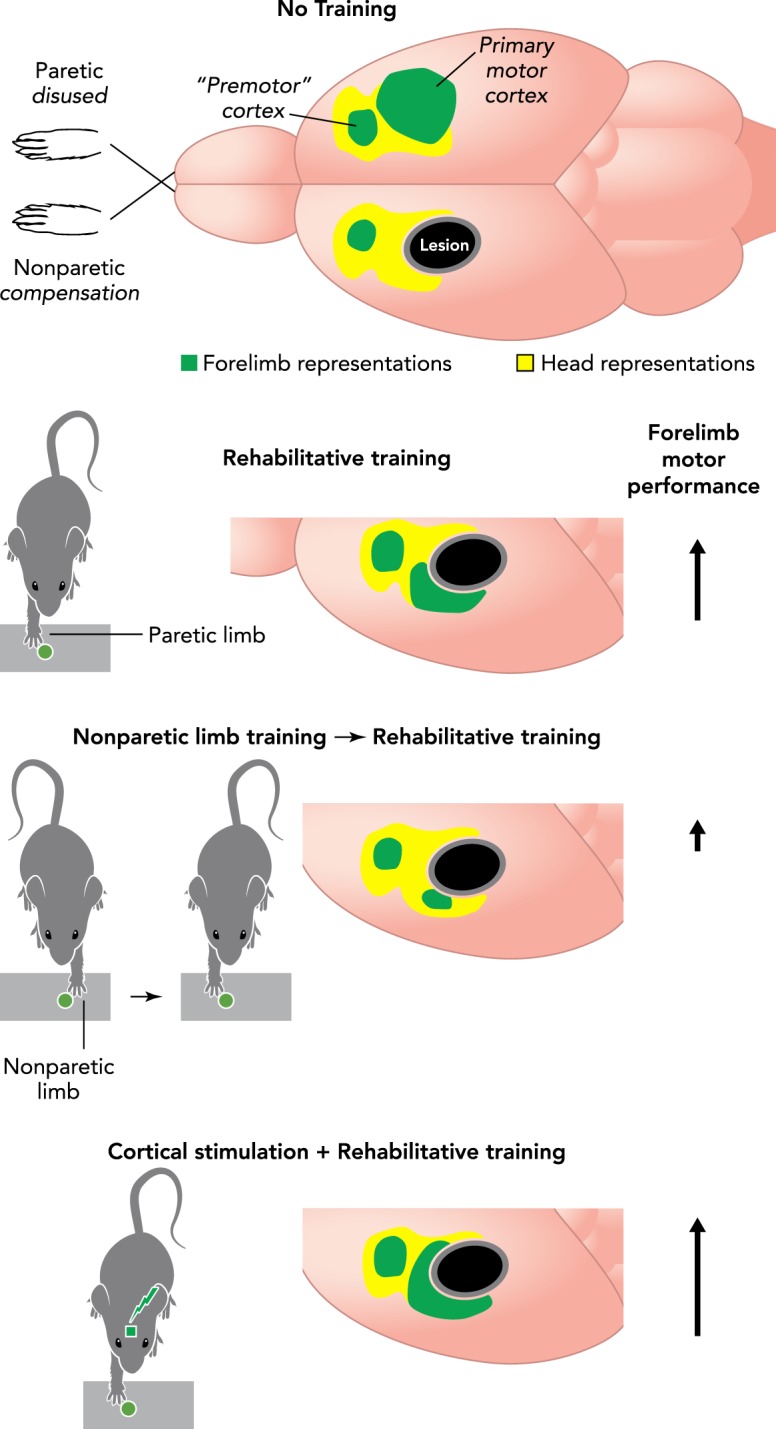
Skill training and cortical stimulation effects on motor maps of the paretic limb
Motor skill training focused on the paretic forelimb (“rehabilitative training”) promotes the maintenance and restoration of forelimb movement representations, as well as the growth of dendrites and maturation of synapses in motor cortex. The effects of rehabilitative training are disrupted by prior training of the nonparetic limb (to model learning to compensate with this limb). The effects of rehabilitative training are amplified when electrical stimulation is delivered to cortex concurrently with training. Arrows indicate the magnitude of improvement in skilled motor function relative to no training.
Together, these results suggest that, at least after direct M1 damage, learning to rely on the nonparetic side can interfere with the capacity of peri-infarct cortex to change in a manner that benefits paretic limb function. This is problematic given that such compensation tends to develop quickly after stroke and is a dominant strategy for dealing with impairments. However, it appears to be specifically deleterious to learn new unimanual skills with the nonparetic forelimb while disusing the paretic forelimb. Impairments in the paretic limb are not exacerbated if both limbs are trained in an alternating fashion (8) or if NPT is paired with greater skilled bimanual activity in the home cage (98). Thus it is possible that the deleterious effects of compensating with the nonparetic side could be minimized with sufficient involvement of the paretic side in skilled unimanual or bimanual activity.
Transcallosal projections of the contralesional M1 are involved in the deleterious effects of NPT on paretic forelimb function (9). If callosal fibers are severed or the contralesional M1 is damaged before the onset of NPT, it has no deleterious effect on the paretic limb. The involvement of interhemispheric connections in NPT effects makes it seem likely the compensation with the nonparetic limb contributes to the clinical observations of abnormal interhemispheric activity and disruptive influences of contralesional M1 on paretic limb movement (134, 141). It also raises the possibility that NPT effects would vary depending on the severity of stroke-induced damage to transcallosal projection territory, a possibility that we have not yet fully tested.
That the contralesional M1 mediates the deleterious effects of NPT hardly precludes it from making beneficial contributions to paretic limb function. There are ample suggestions in the literature that contralesional cortex can contribute to functional improvements in the paretic limb, e.g., in reinnervating spinal cord (115, 151, 188). In addition to its effects on peri-infarct cortex, NPT promotes dendritic and synaptic growth in the contralesional M1 (2, 26, 123), but these effects do not appear to be directly responsible for the maladaptive effects of NPT. Callosal transections do not block the promotion of contralesional dendritic growth by NPT, but they do block its deleterious effects on paretic limb function (9). Behavioral training that improves function in the paretic limb can also increase dendritic growth (18) and synaptogenesis (93) in contralesional M1.
Nevertheless, it is quite possible that the contralesional dendritic growth resulting from NPT is coupled with changes in the axonal projections from the same region that are maladaptive. For example, NPT might drive greater transcallosal connectivity in peri-infarct cortex that competes with intracortical reinnervation, e.g., from premotor cortex. As noted above, after larger infarcts, the contralesional hemisphere is likely to be a predominant source of new neural connections in denervation regions. It may matter most that these new connections are driven by behavioral experience to subserve functional improvements in the paretic rather than exclusively the nonparetic forelimb.
While it is not feasible to replicate our rodent behavioral manipulations in a similarly controlled fashion in humans, our findings are highly consistent with behavioral and neural phenomena of learned-nonuse suggested by Taub et al. (178, 179). Since experience with the weakness and ineptitude of the paretic side leads to reliance on the nonparetic limb, this reduces practice with the paretic limb, reducing its influence on neural reorganization. Once this compensatory pattern is well established and, as our findings suggest, its influence on neural reorganization is established, behavioral interventions probably need to work harder to counteract it.
The clinical rehabilitation approach, Constraint-Induced Movement Therapy (CIMT), was developed to counter learned-nonuse (133). This involves constraining the nonparetic limb for most waking hours during a period of intense motor rehabilitative training of the paretic limb. Clinical trials strongly support the efficacy of CIMT for improving motor function after stroke (111, 112, 194). Our finding that NPT promotes synapse addition in peri-infarct cortex (104) suggests another potential mechanism for CIMT efficacy, one based on synaptic competition. Synapses that more effectively activate a postsynaptic neuron are selectively maintained and matured at the expense of less active ones (12, 78). It may be that constraining the nonparetic limb reduces activity at synapses that were created in response to learning to compensate with this limb. In the converging neural circuitry of the two limbs (e.g., peri-infarct motor cortex), this would be expected to facilitate the formation, maturation, and survival of synapses activated by experiences of the paretic limb. That is, CIMT could be effective, in part, because it confers a competitive edge at the synaptic level to experiences of the paretic, over the nonparetic, limb.
Motor Rehabilitation
It is now well established that practicing motor skills with the paretic upper extremity enhances behaviorally relevant neural reorganization after stroke in both humans (28, 56, 86, 120, 168) and animal models (18, 93, 145). For example, the improvements in upper limb motor function resulting from CIMT are associated with enlargement of the motor map of the hand in MI (120, 168). In animals with subtotal lesions of the forelimb region of M1, training the paretic forelimb in skilled reaching tasks after resurrects, maintains, and reorganizes movement representations in the remaining forelimb territory of MI (37, 145, 160). Without rehabilitative training, forelimb movement representations near the infarct are lost (104, 144). Disrupting motor cortical reorganization prevents the training-induced functional gains (160). Rehabilitative training also reduces the size of movement representations of the nonparetic forelimb in contralesional M1 (13).
While motor map reorganization is strongly correlated with functional improvements, it is delayed relative to these improvements (144). Thus it is better said to reflect, rather than explain, the mechanisms of functional improvement. It is nevertheless a very useful neural correlate because it bridges findings from animal and clinical studies. In intact animals, motor skill training instigates in motor cortex time-dependent changes in gene expression, protein synthesis, synaptic potentiation, and synapse addition, which is localized to regions of motor map reorganization (4, 108). After M1 infarcts, there is close correspondence between cortical map reorganization and axonal sprouting patterns (43, 48), and rehabilitative training also increases synaptic densities and synapse maturation in forelimb movement representations (3, 104). Thus it may be that the reorganization of movement representations in peri-infarct cortex reflects that substantial underlying changes in cortical connectivity have already occurred.
Rehabilitative training effects vary with training intensity (16), age (181), and timing (10, 196). In animal studies, rehabilitative training initiated within the first weeks after CNS injury results in greater functional gains and more profound neural changes than does later training (14, 19, 153). Early skill practice with the paretic limb also counteracts maladaptive NPT effects (98). Clinical studies also support that earlier behavioral interventions are more effective than later ones (122, 162). For example, Lang et. al (112) found that constraint-induced movement therapy (CIMT) is more effective for improving motor performance when initiated 3–9 mo post-stroke compared with later onsets. These findings are consistent with the idea that earlier interventions can interact with more dynamic phases of neural remodeling to promote better reorganization. However, there is also potential for early interventions to worsen impairments (58, 195). There is a need for a more detailed understanding of the neural bases of time-sensitivities in rehabilitation efficacy (10).
One possibility is that early, but not too early, interventions are most effective in shaping neural reorganization. Lee et al. (116) found that axonal sprouting from contralesional M1 in rats is increased by reach training initiated at 5 days, but not 1 or 14 days, after cortical infarcts, potentially reflecting that there are optimal stages of axonal reinnervation to target. Consistent with this, Wahl et al. (188) found that motor rehabilitative training in rats with cortical infarcts could be improved by combined treatment with an antibody (Nogo-A) that promotes corticospinal tract sprouting, but only if the antibody treatment preceded the training rather than being administered concurrently. The sequential treatment resulted in a more orderly pattern of spinal cord reinnervation, suggesting that its superior efficacy could be a result of the rehabilitative training being timed to stabilize and refine the new connections of sprouting axons.
Although the knowledge needed to wield them is incomplete, we consider behavioral manipulations to be a core tool set for promoting functionally useful neural reorganization after stroke. They rely on the intrinsic brain mechanisms for gaining new functionality, the experience-dependent neural plasticity underlying learning (106). At present, rehabilitation strategies are often far from sufficient to normalize function. There has been a growing focus on strategies for enhancing rehabilitation efficacy by combining it with neural plasticity-facilitating treatments.
Modulating Cortical Activity to Improve Function
Epidural Cortical Stimulation
In rats and monkeys, combining training of the paretic forelimb on skilled reaching tasks with concurrent high-frequency (50–100 Hz) cortical stimulation (CS) delivered via epidural or subdural electrodes over peri-infarct M1 enhances performance improvements compared with training alone (5, 6, 38, 107, 132, 158, 182, 199). In most studies, CS was delivered at 50% of movement thresholds continuously during reach training. The performance improvements were linked with increases in dendritic and synaptic densities (3, 5, 199), forelimb movement representation area (107, 158), and motor cortical evoked potentials (182) in peri-infarct M1. CS also reduces peri-injury gliosis (73, 199) and promotes anti-apoptotic signaling (200). The functional improvements appear to be extremely long lasting. Performance improvements endured for 9–10 mo posttreatment in CS-treated rats compared with training alone (146).
In our studies, the improvements in skilled reaching resulting from CS compared with training alone could be attributed to greater normalization of reaching movement practiced during CS delivery (3) as well as to the promotion of functionally useful compensatory movement patterns in the paretic limb (146). The performance improvements did not generalize to other motor behaviors that were not practiced during CS delivery (3, 5, 6). CS effects are also timing-dependent. Most of the studies above tested treatments initiated within 3 wk post-infarct, but we recently found no clear influence over training alone when it was initiated 3 mo post-infarct (146). One possibility is that the earlier treatments influence neural reorganization in a manner that cannot be accomplished later. The findings that CS promotes corticofugal sprouting (31), dendritic plasticity (6, 200), and synaptogenesis (3) are generally consistent with this possibility. However, it also remains possible that the timing dependency could be overcome with different stimulation frequencies, different current intensities, or more robust behavioral training.
Injury severity is a major variable in the efficacy of epidural CS. CS is less effective in improving rehabilitation efficacy in rats with more severe behavioral impairment levels (3), and effective stimulation parameters vary between small and large infarcts (132). Findings from clinical trials are consistent with the possibility that CS efficacy varies with injury severity. While two early clinical studies (phase I and II trials) of epidural CS combined with motor practice supported its safety and efficacy to improve motor function (24, 117), a larger phase III trial failed to demonstrate its efficacy 4 wk posttreatment when all participants were included (118). The main difference between the animal and earlier clinical studies and the phase III trial was in the proportion of participants in which hand movements could be evoked by CS. In the phase I and II studies, movements were evoked in 100% and 42% of subjects, respectively. In the phase III study, only 16% of subjects had stimulation-evoked movements in the hand. In follow-up analyses, significant improvements were found in this participant subset (118). In addition, a greater proportion of the entire CS cohort maintained functional improvements out to 24 wk posttreatment compared with controls. These findings seem to imply that the efficacy of CS for increasing functional gains, but not for promoting the persistence of these gains, depends strongly on a minimum level of integrity in descending corticospinal pathways.
Transcranial Cortical Stimulation
The non-invasive stimulation approaches of transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have emerging potential for improving motor function after stroke. In healthy humans, transcranial stimulation over motor cortex modulates cortical excitability to improve motor speed and accuracy (49, 185), general hand function (21), and motor learning (29, 139, 162, 180). There is now a major effort to determine the post-stroke therapeutic potential of these stimulation approaches.
Repetitive TMS (rTMS) uses 5- to 20-Hz pulse trains to facilitate neural activation or ∼0.2- to 1-Hz trains to inhibit neural activation, referred to as high- and low-frequency rTMS, respectively. rTMS over motor cortex increases motor-evoked potential amplitudes in acute stroke (53), reduces spasticity and hemiparesis in chronic stroke subjects (125), and improves grasping function after subcortical stroke (142). The size of TMS coils makes it challenging to deliver during rehabilitation regimens, but it alters cortical excitation for a period after stimulation to facilitate learning “off-line” (39, 65, 84).
tDCS uses relatively weak electric currents (∼1–2 mA) that modulate neural activity via effects on ion channel activation (64, 65, 140). Excitatory tDCS (anodal) enhances motor learning, likely by strengthening synaptic connections through NMDA receptor-dependent long-term potentiation (LTP)-like effects (70, 131, 138). After stroke, excitatory tDCS delivered over the affected motor cortex or inhibitory tDCS delivered over the contralesional motor cortex improves motor performance on standardized tests of motor function (66, 84, 85, 102).
Many transcranial stimulation studies have focused on restoring the balance of interhemispheric activity after stroke, based on findings of increased excitability of the contralesional hemisphere, which may overly inhibit activity in the injured hemisphere (30, 55, 77, 170, 193). Animal studies also support an increase in GABAergic activity in peri-lesion cortex (34, 197). It follows that balancing interhemispheric activity, either by exciting the injured hemisphere or by inhibiting the contralesional cortex, might improve function. Consistent with this, facilitatory stimulation (high-frequency TMS or anodal tDCS) over the stroke-affected motor cortex or disruptive stimulation (low-frequency TMS or cathodal tDCS) can acutely improve performance of the paretic side (52, 66, 67, 84, 85, 99, 100, 105, 127). However, the influence of excitability in the contralesional cortex, and hence the efficacy of disruptive stimulation in this hemisphere, is likely to vary with stroke subtypes (e.g., cortical vs. subcortical) (30, 72, 75). The efficacy of stimulating either hemisphere may vary with timing, stimulation parameters, impairment severity, and integrity of ipsilesional M1 (64).
Many of the effects described above were only evident for a short period of time after stimulation, but more enduring motor improvements result from stimulation coupled with motor training (105, 136, 184, 189). For example, tDCS paired with 10 consecutive occupation therapy sessions improved paretic upper limb function compared with therapy alone for up to 6 mo, as assessed with the Fugl-Meyer Score (102). Vestito et. al (184) found that tDCS paired with practice in naming in aphasic patients improved naming performance for 16 wk compared with controls. There are emerging applications for transcranial stimulation in combination with robot-assisted training (60, 74) and brain-machine interfaces (73).
There are clearly many details to work out to optimize and tailor parameters of transcranial stimulation for treating stroke disability (64, 163). Nevertheless, these studies together with those of epidural CS converge to support that extrinsic modulation of cortical activity can be used to improve the short- and long-term functional gains from rehabilitation.
Conclusions and Future Perspectives
Motor system stroke instigates a dramatic and widespread reorganization of the connectivity of surviving neurons, involving extensive axonal sprouting, dendritic remodeling, and synapse formation in either hemisphere. This is linked with reorganization of motor and sensory cortical maps, and bilateral changes in neural activity and functional connectivity patterns. Some, but hardly all, of these changes are functionally beneficial.
The current excitement over the potential to drive optimal reorganization with behavioral manipulations and neural activity modulators is well founded, but the neural focus of the efforts could stand to be much better informed. Much of our understanding about the functional relevance of motor system reorganization is based on correlations between brain and behavioral change. In the context of the dramatic remodeling response instigated by stroke, the potential to mistakenly infer causality from coincidence is high. The behavioral relevance of reorganization involving the contralesional motor cortex is especially murky. Excitability changes in contralesional cortex are sometimes, but not always, linked with worsened function. Dendritic growth and synaptogenesis in contralesional cortex is increased by manipulations that improve paretic limb function and by those that worsen it. We think that the devil is likely to be found in the details: for neural changes in either hemisphere to subserve functional improvements in the paretic upper limb requires the right input, in the form of experiences of the paretic limb, at the right time.
It is probably typical for experiences of the nonparetic forelimb to be a dominant force in driving post-stroke brain reorganization, because the compensatory reliance on this limb involves major new skill learning, which is heavily practiced, the sort of experience that is very effective at promoting plasticity even in intact brains. At least after M1 injury, this learning counteracts the capacity to remodel the injured hemisphere to better subserve function of the paretic limb. The job of improving paretic limb function via motor rehabilitative training may become all the more challenging as a result. Earlier onsets of rehabilitative treatments may be more effective not only because they interact with early dynamic phases of neural remodeling but also because they rein in maladaptive effects of compensating with the nonparetic side.
Even with early onset rehabilitation, there is still much room for improvement. Although the process of optimizing and tailoring them is ongoing, cortical stimulation approaches are showing major promise for their potential to do this. There are many hints that cortical stimulation efficacy can vary with post-stroke timing, injury locus, and injury severity. The efforts to tailor and optimize these approaches would benefit from a clearer distinction between the neural remodeling events in either hemisphere that are adaptive, maladaptive, and irrelevant for paretic limb function and how they vary after different strokes.
Footnotes
The authors are supported by National Institutes of Health Grants NS-056839 and NS-078791 (T.A.J.), and NINDS NS-065866 and P20 GM-109040 (D.L.A.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: T.A.J. prepared figures; T.A.J. and D.L.A. drafted manuscript; T.A.J. and D.L.A. edited and revised manuscript; T.A.J. approved final version of manuscript.
References
- 1.Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke 42: 1952–1955, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins DL, Bury SD, Jones TA. Laminar-dependent dendritic spine alterations in the motor cortex of adult rats following callosal transection and forced forelimb use. Neurobiol Learn Mem 78: 35–52, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Adkins DL, Hsu JE, Jones TA. Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp Neurol 212: 14–28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol 101: 1776–1782, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol 200: 356–370, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res 25: 780–788, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Allegra Mascaro AL, Cesare P, Sacconi L, Grasselli G, Mandolesi G, Maco B, Knott GW, Huang L, De Paola V, Strata P, Pavone FS. In vivo single branch axotomy induces GAP-43-dependent sprouting and synaptic remodeling in cerebellar cortex. Proc Natl Acad Sci USA 110: 10824–10829, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol 210: 172–181, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allred RP, Cappellini CH, Jones TA. The “good” limb makes the “bad” limb worse: experience-dependent interhemispheric disruption of functional outcome after cortical infarcts in rats. Behav Neurosci 124: 124–132, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allred RP, Kim SY, Jones TA. Use it and/or lose it-experience effects on brain remodeling across time after stroke. Front Hum Neurosci 8: 379, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allred RP, Maldonado MA, Hsu JE, Jones TA. Training the ‘less-affected’ forelimb after unilateral cortical infarcts interferes with functional recovery of the impaired forelimb in rats. Restorative Neurol Neurosci 23: 297–302, 2005. [PubMed] [Google Scholar]
- 12.Antonini A, Gillespie DC, Crair MC, Stryker MP. Morphology of single geniculocortical afferents and functional recovery of the visual cortex after reverse monocular deprivation in the kitten. J Neurosci 18: 9896–9909, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbay S, Guggenmos DJ, Nishibe M, Nudo RJ. Motor representations in the intact hemisphere of the rat are reduced after repetitive training of the impaired forelimb. Neurorehabil Neural Repair 27: 381–384, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbay S, Plautz EJ, Friel KM, Frost SB, Dancause N, Stowe AM, Nudo RJ. Behavioral and neurophysiological effects of delayed training following a small ischemic infarct in primary motor cortex of squirrel monkeys. Exp Brain Res 169: 106–116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron JC, Yamauchi H, Fujioka M, Endres M. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 34: 2–18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell JA, Wolke ML, Ortez RC, Jones TA, Kerr AL. Training intensity affects motor rehabilitation efficacy following unilateral ischemic insult of the sensorimotor cortex in C57BL/6 mice. Neurorehabil Neural Repair 29: 590–598, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz LI, Carmichael ST. Promoting axonal rewiring to improve outcome after stroke. Neurobiol Dis 37: 259–266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci 21: 5272–5280, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci 24: 1245–1254, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biernaskie J, Szymanska A, Windle V, Corbett D. Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur J Neurosci 21: 989–999, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Boggio PS, Castro LO, Savagim EA, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MT, Fregni F. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett 404: 232–236, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke 39: 1286–1291, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci 29: 1719–1734, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery 58: 464–473, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci 27: 13793–13801, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci 22: 8597–8606, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bury SD, Adkins DL, Ishida JT, Kotzer CM, Eichhorn AC, Jones TA. Denervation facilitates neuronal growth in the motor cortex of rats in the presence of behavioral demand. Neurosci Lett 287: 85–88, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Butefisch CM. Neurobiological bases of rehabilitation. Neurol Sci 27, Suppl 1: S18–S23, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol 91: 2110–2116, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22: 4–21, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Carmel JB, Martin JH. Motor cortex electrical stimulation augments sprouting of the corticospinal tract and promotes recovery of motor function. Front Integr Neurosci 8: 51, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist 9: 64–75, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol 59: 735–742, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Carmichael ST. Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol 69: 161–167, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci 22: 6062–6070, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmichael ST, Wei L, Rovainen CM, Woolsey TA. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis 8: 910–922, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Castro-Alamancos MA, Borrel J. Functional recovery of forelimb response capacity after forelimb primary motor cortex damage in the rat is due to the reorganization of adjacent areas of cortex. Neuroscience 68: 793–805, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Chang WH, Kim H, Sun W, Kim JY, Shin YI, Kim YH. Effects of extradural cortical stimulation on motor recovery in a rat model of subacute stroke. Restor Neurol Neurosci. In press. [DOI] [PubMed] [Google Scholar]
- 39.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Tran S, Sigler A, Murphy TH. Automated and quantitative image analysis of ischemic dendritic blebbing using in vivo 2-photon microscopy data. J Neurosci Methods 195: 222–231, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Cheng HW, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp Neurol 147: 287–298, 1997. [DOI] [PubMed] [Google Scholar]
- 42.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx 1: 36–45, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarkson AN, Lopez-Valdes HE, Overman JJ, Charles AC, Brennan KC, Thomas Carmichael S. Multimodal examination of structural and functional remapping in the mouse photothrombotic stroke model. J Cereb Blood Flow Metab 33: 716–723, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collyer E, Catenaccio A, Lemaitre D, Diaz P, Valenzuela V, Bronfman F, Court FA. Sprouting of axonal collaterals after spinal cord injury is prevented by delayed axonal degeneration. Exp Neurol 261: 451–461, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol 63: 272–287, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Dancause N. Plasticity in the motor network following primary motor cortex lesion. Adv Exp Med Biol 782: 61–86, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Dancause N, Touvykine B, Mansoori BK. Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog Brain Res 218: 361–387, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Dancause N, Barbay S, Frost SB, Plautz EJ, Chen D, Zoubina EV, Stowe AM, Nudo RJ. Extensive cortical rewiring after brain injury. J Neurosci 25: 10167–10179, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Date S, Kurumadani H, Watanabe T, Sunagawa T. Transcranial direct current stimulation can enhance ability in motor imagery tasks. Neuroreport 26: 613–617, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Deller T, Frotscher M. Lesion-induced plasticity of central neurons: sprouting of single fibres in the rat hippocampus after unilateral entorhinal cortex lesion. Prog Neurobiol 53: 687–727, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Deller T, Del Turco D, Rappert A, Bechmann I. Structural reorganization of the dentate gyrus following entorhinal denervation: species differences between rat and mouse. Prog Brain Res 163: 501–528, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Di Lazzaro V, Pilato F, Dileone M, Profice P, Capone F, Ranieri F, Musumeci G, Cianfoni A, Pasqualetti P, Tonali PA. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol 119: 715–723, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Di Lazzaro V, Pilato F, Dileone M, Profice P, Capone F, Ranieri F, Musumeci G, Cianfoni A, Pasqualetti P, Tonali PA. Modulating cortical excitability in acute stroke: a repetitive TMS study. Clin Neurophysiol 119: 715–723, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci 23: 510–517, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Domann R, Hagemann G, Kraemer M, Freund HJ, Witte OW. Electrophysiological changes in the surrounding brain tissue of photochemically induced cortical infarcts in the rat. Neurosci Lett 155: 69–72, 1993. [DOI] [PubMed] [Google Scholar]
- 56.Dong Y, Winstein CJ, Albistegui-DuBois R, Dobkin BH. Evolution of FMRI activation in the perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke: a pilot study. Neurorehabil Neural Repair 21: 412–428, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology 55: 310–318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL, Edwards DF. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology 73: 195–201, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dudek FE, Staley KJ. The time course and circuit mechanisms of acquired epileptogenesis. In: Jasper's Basic Mechanisms of the Epilepsies, edited by Noebels JL, Avoli M, Rogawski MA, Olsen RW, Escueta-Delgado AV. Bethesda, MD: National Center for Biotechnoogy Information, 2012. [PubMed] [Google Scholar]
- 60.Edwards DJ, Krebs HI, Rykman A, Zipse J, Thickbroom GW, Mastaglia FL, Pascual-Leone A, Volpe BT. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. Restor Neurol Neurosci 27: 199–207, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Emerick AJ, Kartje GL. Behavioral recovery and anatomical plasticity in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. Behav Brain Res 152: 315–325, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med 5: 406–409, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA. Global burden of stroke: an underestimate. Authors' reply. Lancet 383: 1205–1206, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Floel A. tDCS-enhanced motor and cognitive function in neurological diseases. Neuroimage 85: 934–947, 2014. [DOI] [PubMed] [Google Scholar]
- 65.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 3: 383–393, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJ, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 16: 1551–1555, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Fregni F, Boggio PS, Valle AC, Rocha RR, Duarte J, Ferreira MJ, Wagner T, Fecteau S, Rigonatti SP, Riberto M, Freedman SD, Pascual-Leone A. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 37: 2115–2122, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127: 747–758, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Friel KM, Martin JH. Bilateral activity-dependent interactions in the developing corticospinal system. J Neurosci 27: 11083–11090, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66: 198–204, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frotscher M, Heimrich B, Deller T. Sprouting in the hippocampus is layer-specific. Trends Neurosci 20: 218–223, 1997. [DOI] [PubMed] [Google Scholar]
- 72.Gerloff C, Bushara K, Sailer A, Wassermann EM, Chen R, Matsuoka T, Waldvogel D, Wittenberg GF, Ishii K, Cohen LG, Hallett M. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain 129: 791–808, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Gharabaghi A, Kraus D, Leao MT, Spuler M, Walter A, Bogdan M, Rosenstiel W, Naros G, Ziemann U. Coupling brain-machine interfaces with cortical stimulation for brain-state dependent stimulation: enhancing motor cortex excitability for neurorehabilitation. Front Hum Neurosci 8: 122, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giacobbe V, Krebs HI, Volpe BT, Pascual-Leone A, Rykman A, Zeiarati G, Fregni F, Dipietro L, Thickbroom GW, Edwards DJ. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: the dimension of timing. Neuro Rehabil 33: 49–56, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grefkes C, Ward NS. Cortical reorganization after stroke: how much and how functional? Neuroscientist 20: 56–70, 2014. [DOI] [PubMed] [Google Scholar]
- 76.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989–1990. Stroke 33: 1034–1040, 2002. [DOI] [PubMed] [Google Scholar]
- 77.Harris-Love ML, Perez MA, Chen R, Cohen LG. Interhemispheric inhibition in distal and proximal arm representations in the primary motor cortex. J Neurophysiol 97: 2511–2515, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron 38: 785–796, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Heiss WD. The ischemic penumbra: how does tissue injury evolve? Ann NY Acad Sci 1268: 26–34, 2012. [DOI] [PubMed] [Google Scholar]
- 80.Hermann DM, Buga AM, Popa-Wagner A. Neurovascular remodeling in the aged ischemic brain. J Neural Transm. In press. [DOI] [PubMed] [Google Scholar]
- 81.Hinman JD. The back and forth of axonal injury and repair after stroke. Curr Opin Neurol 27: 615–623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoff SF, Scheff SW, Benardo LS, Cotman CW. Lesion-induced synaptogenesis in the dentate gyrus of aged rats: I. Loss and reacquisition of normal synaptic density. J Comp Neurol 205: 246–252, 1982. [DOI] [PubMed] [Google Scholar]
- 83.Hsu JE, Jones TA. Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur J Neurosci 22: 2069–2080, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 128: 490–499, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain 128: 1122–1138, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Jones TA. Multiple synapse formation in the motor cortex opposite unilateral sensorimotor cortex lesions in adult rats. J Comp Neurol 414: 57–66, 1999. [PubMed] [Google Scholar]
- 88.Jones TA, Schallert T. Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res 581: 156–160, 1992. [DOI] [PubMed] [Google Scholar]
- 89.Jones TA, Greenough WT. Behavioral experience-dependent plasticity of glial-neuronal interactions. In: Glia in Synaptic Transmission, edited by Volterra A, Magistretti P, Haydon PG. Oxford, UK: Oxford Univ; Press, 2002, p. 248–265. [Google Scholar]
- 90.Jones TA, Adkins DL. Behavioral influences on neuronal events after stroke. In: Brain Repair After Stroke, edited by Cramer SC, Nudo RJ. Cambridge, UK: Cambridge Univ; Press, 2010, p. 23–34. [Google Scholar]
- 91.Jones TA, Jefferson SC. Reflections of experience-expectant development in repair of the adult damaged brain. Dev Psychobiol 53: 466–475, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones TA, Kleim JA, Greenough WT. Synaptogenesis and dendritic growth in the cortex opposite unilateral sensorimotor cortex damage in adult rats: a quantitative electron microscopic examination. Brain Res 733: 142–148, 1996. [DOI] [PubMed] [Google Scholar]
- 93.Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci 19: 10153–10163, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones TA, Allred RP, Adkins DL, Hsu JE, O'Bryant A, Maldonado MA. Remodeling the brain with behavioral experience after stroke. Stroke 40: 136–138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones TA, Liput DJ, Maresh EL, Donlan N, Parikh TJ, Marlowe D, Kozlowski DA. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma 29: 1455–1468, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelley MS, Steward O. Injury-induced physiological events that may modulate gene expression in neurons and glia. Rev Neurosci 8: 147–177, 1997. [DOI] [PubMed] [Google Scholar]
- 97.Kerr AL, Tennant KA. Compensatory limb use and behavioral assessment of motor skill learning following sensorimotor cortex injury in a mouse model of ischemic stroke. J Vis Exp 10: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kerr AL, Wolke ML, Bell JA, Jones TA. Post-stroke protection from maladaptive effects of learning with the non-paretic forelimb by bimanual home cage experience in C57BL/6 mice. Behav Brain Res 252: 180–187, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology 65: 466–468, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Khedr EM, Shawky OA, El-Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, Tohamy AM. Effect of anodal versus cathodal transcranial direct current stimulation on stroke rehabilitation: a pilot randomized controlled trial. Neurorehabil Neural Repair 27: 592–601, 2013. [DOI] [PubMed] [Google Scholar]
- 101.Kim D, Kim RG, Kim HS, Kim JM, Jun SC, Lee B, Jo HJ, Neto PR, Lee MC, Kim HI. Longitudinal changes in resting-state brain activity in a capsular infarct model. J Cereb Blood Flow Metab 35: 11–19, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim DY, Lim JY, Kang EK, You DS, Oh MK, Oh BM, Paik NJ. Effect of transcranial direct current stimulation on motor recovery in patients with subacute stroke. Am J Phys Med Rehabil 89: 879–886, 2010. [DOI] [PubMed] [Google Scholar]
- 103.Kim SY, Jones TA. Lesion size-dependent synaptic and astrocytic responses in cortex contralateral to infarcts in middle-aged rats. Synapse 64: 659–671, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim SY, Allred RP, Adkins DL, Tennant KA, Donlan NA, Kleim JA, Jones TA. Experience with the “good” limb induces aberrant synaptic plasticity in the perilesion cortex after stroke. J Neurosci 35: 8604–8610, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, Yoo WK, Hallett M. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke 37: 1471–1476, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res 51: S225–S239, 2008. [DOI] [PubMed] [Google Scholar]
- 107.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res 25: 789–793, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem 77: 63–77, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Kolb B, Brown R, Witt-Lajeunesse A, Gibb R. Neural compensations after lesion of the cerebral cortex. Neural Plast 8: 1–16, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krishnamurthi RV, Moran AE, Forouzanfar MH, Bennett DA, Mensah GA, Lawes CM, Barker-Collo S, Connor M, Roth GA, Sacco R, Ezzati M, Naghavi M, Murray CJ, Feigin VL; Global Burden of Diseases, Injuries, and Risk Factors 2010 Study Stroke Expert Group. The global burden of hemorrhagic stroke: a summary of findings from the GBD 2010 study. Glob Heart 9: 101–106, 2014. [DOI] [PubMed] [Google Scholar]
- 111.Kwakkel G, Veerbeek JM, van Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurol 14: 224–234, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lang KC, Thompson PA, Wolf SL. The EXCITE Trial: reacquiring upper-extremity task performance with early versus late delivery of constraint therapy. Neurorehabil Neural Repair 27: 654–663, 2013. [DOI] [PubMed] [Google Scholar]
- 113.Lapash Daniels CM, Ayers KL, Finley AM, Culver JP, Goldberg MP. Axon sprouting in adult mouse spinal cord after motor cortex stroke. Neurosci Lett 450: 191–195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lawrence ES, Coshall C, Dundas R, Stewart J, Rudd AG, Howard R, Wolfe CD. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke 32: 1279–1284, 2001. [DOI] [PubMed] [Google Scholar]
- 115.Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci 24: 6209–6217, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee KH, Kim JH, Choi DH, Lee J. Effect of task-specific training on functional recovery and corticospinal tract plasticity after stroke. Restor Neurol Neurosci 31: 773–785, 2013. [DOI] [PubMed] [Google Scholar]
- 117.Levy R, Ruland S, Weinand M, Lowry D, Dafer R, Bakay R. Cortical stimulation for the rehabilitation of patients with hemiparetic stroke: a multicenter feasibility study of safety and efficacy. J Neurosurg 108: 707–714, 2008. [DOI] [PubMed] [Google Scholar]
- 118.Levy RM, Harvey RL, Kissela BM, Winstein CJ, Lutsep HL, Parrish TB, Cramer SC, Venkatesan L. Epidural electrical stimulation for stroke rehabilitation: results of the prospective, multicenter, randomized, single-blinded everest trial. Neurorehabil Neural Repair. In press. [DOI] [PubMed] [Google Scholar]
- 119.Li S, Overman JJ, Katsman D, Kozlov SV, Donnelly CJ, Twiss JL, Giger RJ, Coppola G, Geschwind DH, Carmichael ST. An age-related sprouting transcriptome provides molecular control of axonal sprouting after stroke. Nat Neurosci 13: 1496–1504, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke 31: 1210–1216, 2000. [DOI] [PubMed] [Google Scholar]
- 121.Liu Z, Zhang RL, Li Y, Cui Y, Chopp M. Remodeling of the corticospinal innervation and spontaneous behavioral recovery after ischemic stroke in adult mice. Stroke 40: 2546–2551, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lotze M, Beutling W, Loibl M, Domin M, Platz T, Schminke U, Byblow WD. Contralesional motor cortex activation depends on ipsilesional corticospinal tract integrity in well-recovered subcortical stroke patients. Neurorehabil Neural Repair 26: 594–603, 2012. [DOI] [PubMed] [Google Scholar]
- 123.Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse 54: 187–199, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Maclellan CL, Langdon KD, Botsford A, Butt S, Corbett D. A Model of persistent learned nonuse following focal ischemia in rats. Neurorehabil Neural Repair 27: 900–907, 2013. [DOI] [PubMed] [Google Scholar]
- 125.Mally J, Dinya E. Recovery of motor disability and spasticity in post-stroke after repetitive transcranial magnetic stimulation (rTMS). Brain Res Bull 76: 388–395, 2008. [DOI] [PubMed] [Google Scholar]
- 126.Mansoori BK, Jean-Charles L, Touvykine B, Liu A, Quessy S, Dancause N. Acute inactivation of the contralesional hemisphere for longer durations improves recovery after cortical injury. Exp Neurol 254: 18–28, 2014. [DOI] [PubMed] [Google Scholar]
- 127.Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64: 1802–1804, 2005. [DOI] [PubMed] [Google Scholar]
- 128.McMorland AJ, Runnalls KD, Byblow WD. A neuroanatomical framework for upper limb synergies after stroke. Front Hum Neurosci 9: 82, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.McNeill TH, Brown SA, Hogg E, Cheng HW, Meshul CK. Synapse replacement in the striatum of the adult rat following unilateral cortex ablation. J Comp Neurol 467: 32–43, 2003. [DOI] [PubMed] [Google Scholar]
- 130.Mironova YA, Giger RJ. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci 36: 363–373, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6: 424–432, 2013. [DOI] [PubMed] [Google Scholar]
- 132.Moon SK, Shin YI, Kim HI, Kim H, Lee JO, Lee MC. Effect of prolonged cortical stimulation differs with size of infarct after sensorimotor cortical lesions in rats. Neurosci Lett 460: 152–155, 2009. [DOI] [PubMed] [Google Scholar]
- 133.Morris DM, Crago JE, Deluca SC, Pidikiti RD, Taub E. Constraint-induced movement therapy for moter recovery after stroke. Neuro Rehabilitation 9: 29–43, 1997. [DOI] [PubMed] [Google Scholar]
- 134.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10: 861–872, 2009. [DOI] [PubMed] [Google Scholar]
- 136.Nair DG, Hamelin S, Pascual-Leone A, Schlaug G, . Direct current stimulation in combination with occupational therapy for 5 consecutive days improves motor function in chronic stroke patients. Stroke 38: 517, 2007. [Google Scholar]
- 137.Nieto-Sampedro M, Nieto-Diaz M. Neural plasticity: changes with age. J Neural Transm 112: 3–27, 2005. [DOI] [PubMed] [Google Scholar]
- 138.Nitsche MA, Liebetanz D, Antal A, Lang N, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation: technical, safety and functional aspects. Suppl Clin Neurophysiol 56: 255–276, 2003. [DOI] [PubMed] [Google Scholar]
- 139.Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15: 619–626, 2003. [DOI] [PubMed] [Google Scholar]
- 140.Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stimul 1: 206–223, 2008. [DOI] [PubMed] [Google Scholar]
- 141.Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair 23: 641–656, 2009. [DOI] [PubMed] [Google Scholar]
- 142.Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, Fink GR. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol 65: 741–747, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci 7: 887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996. [DOI] [PubMed] [Google Scholar]
- 145.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272: 1791–1794, 1996. [DOI] [PubMed] [Google Scholar]
- 146.O'Bryant AJ, Adkins DL, Sitko AA, Combs HL, Nordquist SK, Jones TA. Enduring poststroke motor functional improvements by a well-timed combination of motor rehabilitative training and cortical stimulation in rats. Neurorehabil Neural Repair. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol 59: 467–477, 2006. [DOI] [PubMed] [Google Scholar]
- 148.O'Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron 54: 479–490, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Overman JJ, Carmichael ST. Plasticity in the injured brain: more than molecules matter. Neuroscientist 20: 15–28, 2014. [DOI] [PubMed] [Google Scholar]
- 150.Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proc Natl Acad Sci USA 109: E2230–E2239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Papadopoulos CM, Tsai SY, Guillen V, Ortega J, Kartje GL, Wolf WA. Motor recovery and axonal plasticity with short-term amphetamine after stroke. Stroke 40: 294–302, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Papadopoulos CM, Tsai SY, Cheatwood JL, Bollnow MR, Kolb BE, Schwab ME, Kartje GL. Dendritic plasticity in the adult rat following middle cerebral artery occlusion and Nogo-a neutralization. Cereb Cortex 16: 529–536, 2006. [DOI] [PubMed] [Google Scholar]
- 153.Park JW, Bang MS, Kwon BS, Park YK, Kim DW, Shon SM, Jeong SW, Lee DK, Kim DE. Early treadmill training promotes motor function after hemorrhagic stroke in rats. Neurosci Lett 471: 104–108, 2010. [DOI] [PubMed] [Google Scholar]
- 154.Pearson-Fuhrhop KM, Burke E, Cramer SC. The influence of genetic factors on brain plasticity and recovery after neural injury. Curr Opin Neurol 25: 682–688, 2012. [DOI] [PubMed] [Google Scholar]
- 155.Perederiy JV, Westbrook GL. Structural plasticity in the dentate gyrus: revisiting a classic injury model. Front Neural Circuits 7: 17, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Petcu EB, Sfredel V, Platt D, Herndon JG, Kessler C, Popa-Wagner A. Cellular and molecular events underlying the dysregulated response of the aged brain to stroke: a mini-review. Gerontology 54: 6–17, 2008. [DOI] [PubMed] [Google Scholar]
- 157.Phillips LL, Chan JL, Doperalski AE, Reeves TM. Time dependent integration of matrix metalloproteinases and their targeted substrates directs axonal sprouting and synaptogenesis following central nervous system injury. Neural Regen Res 9: 362–376, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res 25: 801–810, 2003. [DOI] [PubMed] [Google Scholar]
- 159.Raisman G, Field PM. Synapse formation in the adult brain after lesions and after transplantation of embryonic tissue. J Exp Biol 153: 277–287, 1990. [DOI] [PubMed] [Google Scholar]
- 160.Ramanathan D, Conner JM, Tuszynski MH. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc Natl Acad Sci USA 103: 11370–11375, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rehme AK, Grefkes C. Cerebral network disorders after stroke: evidence from imaging-based connectivity analyses of active and resting brain states in humans. J Physiol 591: 17–31, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reis J, Robertson EM, Krakauer JW, Rothwell J, Marshall L, Gerloff C, Wassermann EM, Pascual-Leone A, Hummel F, Celnik PA, Classen J, Floel A, Ziemann U, Paulus W, Siebner HR, Born J, Cohen LG. Consensus: Can transcranial direct current stimulation and transcranial magnetic stimulation enhance motor learning and memory formation? Brain Stimul 1: 363–369, 2008. [DOI] [PubMed] [Google Scholar]
- 164.Reperant J, Rio JP, Ward R, Miceli D, Vesselkin NP, Hergueta S, Lemire M. Sequential events of degeneration and synaptic remodelling in the viper optic tectum following retinal ablation, A degeneration, radioautographic and immunocytochemical study. J Chem Neuroanat 4: 397–413, 1991. [DOI] [PubMed] [Google Scholar]
- 165.Salimi I, Friel KM, Martin JH. Pyramidal tract stimulation restores normal corticospinal tract connections and visuomotor skill after early postnatal motor cortex activity blockade. J Neurosci 28: 7426–7434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saver JL, Yafeh B. Confirmation of tPA treatment effect by baseline severity-adjusted end point reanalysis of the NINDS-tPA stroke trials. Stroke 38: 414–416, 2007. [DOI] [PubMed] [Google Scholar]
- 167.Sawaki L, Butler AJ, Leng X, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, Wittenberg GF. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair 22: 505–513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sawaki L, Butler AJ, Xiaoyan L, Wassenaar PA, Mohammad YM, Blanton S, Sathian K, Nichols-Larsen DS, Wolf SL, Good DC, Wittenberg GF. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair 22: 505–513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schallert T, Fleming SM, Woodlee MT. Should the injured and intact hemispheres be treated differently during the early phases of physical restorative therapy in experimental stroke or parkinsonism? Phys Med Rehabil Clin N Am 14: S27–S46, 2003. [DOI] [PubMed] [Google Scholar]
- 170.Shimizu T, Hosaki A, Hino T, Sato M, Komori T, Hirai S, Rossini PM. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain 125: 1896–1907, 2002. [DOI] [PubMed] [Google Scholar]
- 171.Soleman S, Filippov MA, Dityatev A, Fawcett JW. Targeting the neural extracellular matrix in neurological disorders. Neuroscience 253: 194–213, 2013. [DOI] [PubMed] [Google Scholar]
- 172.Song J, Young BM, Nigogosyan Z, Walton LM, Nair VA, Grogan SW, Tyler ME, Farrar-Edwards D, Caldera KE, Sattin JA, Williams JC, Prabhakaran V. Characterizing relationships of DTI, fMRI, and motor recovery in stroke rehabilitation utilizing brain-computer interface technology. Front Neuroeng 7: 31, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Starkey ML, Bleul C, Zorner B, Lindau NT, Mueggler T, Rudin M, Schwab ME. Back seat driving: hindlimb corticospinal neurons assume forelimb control following ischaemic stroke. Brain 135: 3265–3281, 2012. [DOI] [PubMed] [Google Scholar]
- 174.Steward O. Reorganization of neuronal connections following CNS trauma: principles and experimental paradigms. J Neurotrauma 6: 99–152, 1989. [DOI] [PubMed] [Google Scholar]
- 175.Steward O. Signals that induce sprouting in the central nervous system: sprouting is delayed in a strain of mouse exhibiting delayed axonal degeneration. Exp Neurol 118: 340–351, 1992. [DOI] [PubMed] [Google Scholar]
- 176.Sweetnam D, Holmes A, Tennant KA, Zamani A, Walle M, Jones P, Wong C, Brown CE. Diabetes impairs cortical plasticity and functional recovery following ischemic stroke. J Neurosci 32: 5132–5143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci 32: 12896–12908, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Taub E, Uswatte G, Mark VW. The functional significance of cortical reorganization and the parallel development of CI therapy. Front Hum Neurosci 8: 396, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys 42: 241–256, 2006. [PubMed] [Google Scholar]
- 180.Tecchio F, Zappasodi F, Assenza G, Tombini M, Vollaro S, Barbati G, Rossini PM. Anodal transcranial direct current stimulation enhances procedural consolidation. J Neurophysiol 104: 1134–1140, 2010. [DOI] [PubMed] [Google Scholar]
- 181.Tennant KA, Kerr AL, Adkins DL, Donlan N, Thomas N, Kleim JA, Jones TA. Age-dependent reorganization of peri-infarct “premotor” cortex with task-specific rehabilitative training in mice. Neurorehabil Neural Repair 29: 193–202, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res 25: 794–800, 2003. [DOI] [PubMed] [Google Scholar]
- 183.Uryu K, MacKenzie L, Chesselet MF. Ultrastructural evidence for differential axonal sprouting in the striatum after thermocoagulatory and aspiration lesions of the cerebral cortex in adult rats. Neuroscience 105: 307–316, 2001. [DOI] [PubMed] [Google Scholar]
- 184.Vestito L, Rosellini S, Mantero M, Bandini F. Long-term effects of transcranial direct-current stimulation in chronic post-stroke aphasia: a pilot study. Front Hum Neurosci 8: 785, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects' non-dominant hand compared with uni-hemisphere stimulation. BMC Neurosci 9: 103, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Voorhies AC, Jones TA. The behavioral and dendritic growth effects of focal sensorimotor cortical damage depend on the method of lesion induction. Behav Brain Res 133: 237–246, 2002. [DOI] [PubMed] [Google Scholar]
- 187.Vuksic M, Del Turco D, Vlachos A, Schuldt G, Muller CM, Schneider G, Deller T. Unilateral entorhinal denervation leads to long-lasting dendritic alterations of mouse hippocampal granule cells. Exp Neurol 230: 176–185, 2011. [DOI] [PubMed] [Google Scholar]
- 188.Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, Ommer B, Schwab ME. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science 344: 1250–1255, 2014. [DOI] [PubMed] [Google Scholar]
- 189.Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair 26: 222–230, 2012. [DOI] [PubMed] [Google Scholar]
- 190.Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129: 809–819, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wenk CA, Thallmair M, Kartje GL, Schwab ME. Increased corticofugal plasticity after unilateral cortical lesions combined with neutralization of the IN-1 antigen in adult rats. J Comp Neurol 410: 143–157, 1999. [DOI] [PubMed] [Google Scholar]
- 192.Whishaw IQ, Pellis SM, Gorny BP. Skilled reaching in rats and humans: evidence for parallel development or homology. Behav Brain Res 47: 59–70, 1992. [DOI] [PubMed] [Google Scholar]
- 193.Witte OW, Bidmon HJ, Schiene K, Redecker C, Hagemann G. Functional differentiation of multiple perilesional zones after focal cerebral ischemia. J Cereb Blood Flow Metab 20: 1149–1165, 2000. [DOI] [PubMed] [Google Scholar]
- 194.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 296: 2095–2104, 2006. [DOI] [PubMed] [Google Scholar]
- 195.Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restor Neurol Neurosci 22: 153–161, 2004. [PubMed] [Google Scholar]
- 196.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol 26: 609–616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zeiler SR, Gibson EM, Hoesch RE, Li MY, Worley PF, O'Brien RJ, Krakauer JW. Medial premotor cortex shows a reduction in inhibitory markers and mediates recovery in a mouse model of focal stroke. Stroke 44: 483–489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol 8: 491–500, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zheng J, Liu L, Xue X, Li H, Wang S, Cao Y, Zhao J. Cortical electrical stimulation promotes neuronal plasticity in the peri-ischemic cortex and contralesional anterior horn of cervical spinal cord in a rat model of focal cerebral ischemia. Brain Res 1504: 25–34, 2013. [DOI] [PubMed] [Google Scholar]
- 200.Zhou Q, Zhang Q, Zhao X, Duan YY, Lu Y, Li C, Li T. Cortical electrical stimulation alone enhances functional recovery and dendritic structures after focal cerebral ischemia in rats. Brain Res 1311: 148–157, 2010. [DOI] [PubMed] [Google Scholar]