Abstract
The historically understood role of the central amygdala (CeA) in fear learning is to serve as a passive output station for processing and plasticity that occurs elsewhere in the brain. However, recent research has suggested that the CeA may play a more dynamic role in fear learning. In particular, there is growing evidence that the CeA is a site of plasticity and memory formation, and that its activity is subject to tight regulation. The following review examines the evidence for these three main roles of the CeA as they relate to fear learning. The classical role of the CeA as a routing station to fear effector brain structures like the periaqueductal gray, the lateral hypothalamus, and paraventricular nucleus of the hypothalamus will be briefly reviewed, but specific emphasis is placed on recent literature suggesting that the CeA 1) has an important role in the plasticity underlying fear learning, 2) is involved in regulation of other amygdala subnuclei, and 3) is itself regulated by intra- and extra-amygdalar input. Finally, we discuss the parallels of human and mouse CeA involvement in fear disorders and fear conditioning, respectively.
Fear can be defined as the neurophysiological processes that prepare an organism to perform innate or learned responses to cope with danger. In general, our understanding of the physiology of fear is based on models of fear learning including fear conditioning, extinction, and fear-potentiated startle. Fear conditioning involves the repeated pairing of a neutral conditioned stimulus (CS; e.g., an acoustic tone) with a noxious unconditioned stimulus (US; e.g., an electric foot shock) such that later presentation of the CS alone results in fear behaviors [conditioned response (CR); e.g., freezing]. Fear extinction is the process of repeatedly presenting the CS alone until it no longer elicits fear behaviors. Similarly, the fear-potentiated startle paradigm involves conditioning an animal to fear a light and then measuring its startle reflex in response to a loud noise in the presence or absence of the conditioning light (fear-conditioned animals will startle more than light-naive animals). Through the use of these behavioral methods, a conceptualization of the brain circuitry underlying fear learning and expression has emerged, and a preponderance of evidence suggests a pivotal role for the amygdala (15, 27, 29, 66a, 86, 87, 102).
Although often treated as a single entity, it is important to note that the amygdala is actually a collection of distinct nuclei (often subdivided further into subnuclei; FIGURE 1) that are thought to play separate but complementary roles in the acquisition, expression, and extinction of fear. Of these nuclei, the lateral/basolateral nucleus, the putative site of plasticity underlying the learned association between the CS and US (34), and the medial nucleus, which is thought to play a modulatory role in the process of predator odor-induced fear learning (146), have been the subject of extensive research and multiple reviews. However, the central amygdala (CeA), the putative output station of the fear circuit, has only recently become the focus of extensive research and currently lacks a focused and recent review on its role in fear processing (129). A review of the function of the CeA is especially timely, given emerging literature that has greatly expanded our understanding of its role in fear learning and expression. Classically, the CeA was considered to be a single homogenous structure that served as a passive relay station of fear output to fear effector brain sites, such as the lateral/paraventricular hypothalamus and the periaqueductal gray (78). However, recent research has suggested that the CeA's structure and function are not so simple. Emerging evidence indicates that its medial and lateral subdivisions are not only anatomically distinct but also functionally separable in their contributions to fear output. Furthermore, there is evidence that plasticity occurs within the CeA and that these small-scale functional changes contribute to the acquisition, expression, and extinction of conditioned fear. Therefore, in the following review, we will discuss the evidence for the CeA's involvement in each of these functions, the implications of these findings for the current model of fear processing, and necessary future experiments that should further refine our understanding of the CeA's role in the fear circuit.
FIGURE 1.
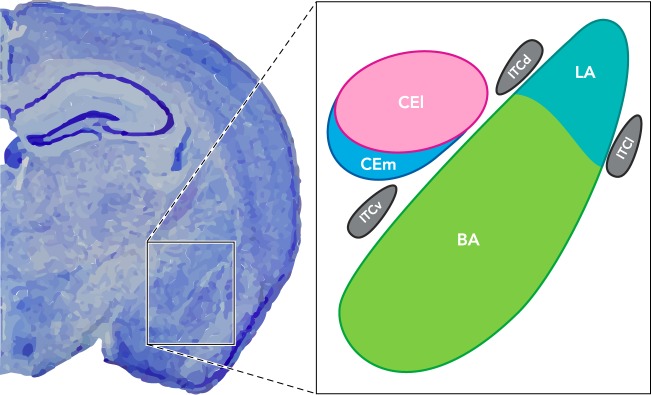
Subdivisions of the mouse amygdala
The basolateral complex (BLA) is composed of the basal (BA) and lateral (LA) nuclei. The central amygdala (CeA) is composed of the centromedial (CEm) and centrolateral (CEl) nuclei. The intercalated cell masses include the dorsal (ITCd), ventral (ITCv), and lateral (ITCl) clusters (87).
Before beginning our functional discussion, we will briefly outline the major divisions of the amygdala and the connectivity of the CeA, although we note that an exhaustive anatomical discussion is beyond the scope of this review (please see Refs. 31, 80).
Divisions of the Amygdala
The amygdala is a highly conserved set of brain nuclei present in a wide range of vertebrates including mice and humans (98). It is functionally divided into four sections: the basolateral complex (BLA), the intercalated cells (ITC), the central nucleus, and the medial nucleus (MeA) (87) (FIGURE 1). The BLA is further divided into the basolateral (BL), basomedial (BM) [collectively known as the basal nucleus (BA)], and lateral (LA) nuclei (87).
Overview of the Anatomy, Histology, and Connectivity of the CeA
Anatomy and Histology
The CeA is located medial to the BLA and is bounded laterally and ventrally by the longitudinal association bundle and medially by the stria terminalis (76). It is subdivided into lateral (CEl), medial (CEm), capsular (CEc), and intermediate (CEi) divisions (124). The entire CEl, as well as the posterior part of the CEm, contains many GABAergic neurons, and while the GABAergic cells of the CEl are medium spiny neurons that show considerable dendritic branching, GABAergic CEm cells have minimally branched dendrites and few spines (19, 91, 141).
Connectivity
The CeA receives input from a variety of amygdalar (102) and extra-amygdalar (90) sites (FIGURE 2A). Within the amygdala, not only do both CEl and CEm cells receive inhibitory GABAergic input from ITC cells (103, 123), but CEl cells also inhibit CEm cells (33, 53). Additionally, LA cells project directly to the CEl, and BL and BM cells project to the CEm (76, 104), possibly bypassing the ITC cells. Extra-amygdalar sources of input to the CeA include the dysgranular insula (DI) (135), which projects to the CEl, the agranular insula (AI) (135), which projects to the CEl and CEm, and the infralimbic cortex (IL) (152) and bed nucleus of the stria terminalis (BNST) (14), both of which project to the CEm. The CEl also receives input from the auditory cortex (90), the auditory thalamus (79), and the pontine parabrachial nucleus (9, 62).
FIGURE 2.
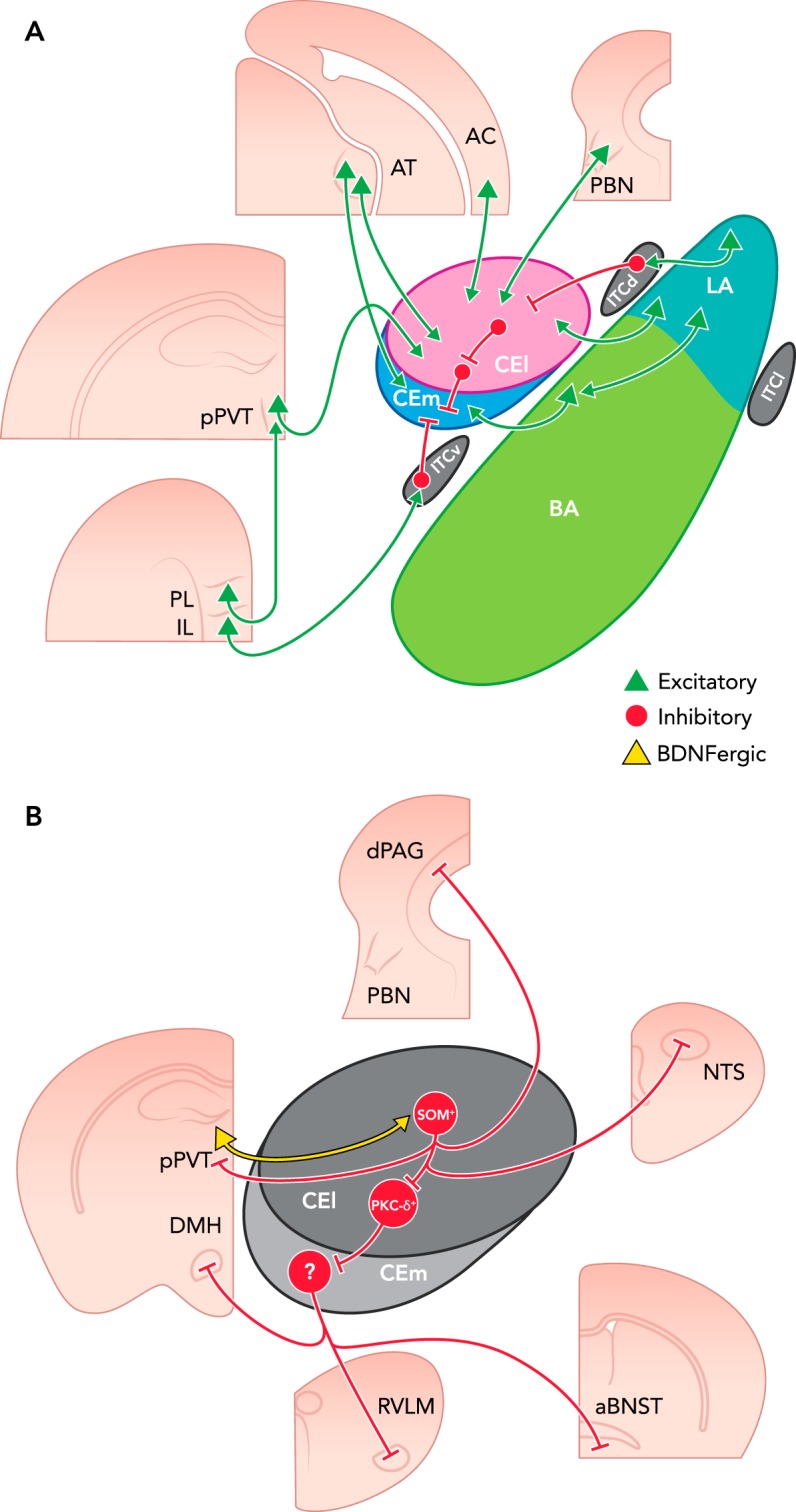
Inputs and outputs of the rodent CeA
A: intra- and extra-amygdalar CeA inputs. The centrolateral amygdala (CEl) receives direct excitatory input from the auditory cortex (AC), auditory thalamus (AT), pontine parabrachial nucleus (PBN), and lateral nucleus of the amygdala (LA). It also receives indirect excitatory input from the prelimbic cortex (PL) via the posterior paraventricular thalamus (pPVT) as well as indirect inhibitory input from the LA via the dorsal intercalated cell mass (ITCd). The centromedial amygdala (CEm) receives direct excitatory input from the AT and direct inhibitory input from the CEl. It also receives indirect excitatory input from the LA via the BA, as well as indirect inhibitory input from the infralimbic cortex (IL) via the ventral intercalated cell mass (ITCv). B: intra- and extra-amygdalar CeA outputs. The somatostatin-expressing (SOM+) cells of the CEl directly inhibit the pPVT, dorsal periaqueductal gray (dPAG), and nucleus tractus solitarius (NTS), as well as the protein kinase C-delta-expressing (PKC-δ+) cells of the CEl, which directly inhibit the output neurons of the CEm. The CEm cells directly inhibit the dorsomedial hypothalamus (DMH), rostral ventrolateral medulla (RVLM), and anterior bed nucleus of the stria terminalis (aBNST). Of note, the excitatory projections from the pPVT to the CEl have been shown to preferentially innervate SOM+ CEl cells and to release BDNF onto them (107).
The CeA sends output to areas throughout the brain that are involved in mediating a wide variety of autonomic functions (28) (FIGURE 2B). The CeA has known projections to the lateral hypothalamus (LH) (75), paraventricular nucleus of the hypothalamus (PVN) (47), dorsomedial hypothalamus (DMH) (97), medial preoptic area (mPOA) (97), and periaqueductal gray (PAG) (48). These areas are involved in the cardiovascular effects, corticosteroid release, and freezing observed during fear conditioning and extinction (28). In addition to the direct projections to these areas, there also may be an indirect projection from the CeA to the PVN via the BNST that similarly mediates the neuroendocrine response to stress (21, 28, 142).
The Role and Pathways of the CeA as an Output Station of the Fear Circuit
The CeA as the “Output Station” of the Fear Circuit
Early clues to a potential role for the CeA in fear learning came from electrical stimulation and lesioning studies showing that the CeA plays a role in generating stress responses (8, 61, 118, 148), such as those observed during open-field testing (158) and heart-rate conditioning (41-43, 65). However, the idea that the CeA is an output station for the fear circuit was based on work by LeDoux et al., who tested the effects electrolytic and ibotenic lesions of the bed nucleus of the stria terminalis (BNST), lateral hypothalamus, and periaqueductal gray–all of which receive projections from the CeA (59, 75, 76)–on fear conditioning. Their findings suggest that lesions of the LH interfere with the autonomic responses elicited by fear conditioning (e.g., changes in blood pressure) but do not affect the behavioral response (i.e., freezing). Conversely, lesions of the PAG do not affect the autonomic responses, but do interfere with the behavioral response. Interestingly, lesions of the BNST do not affect either the autonomic or the behavioral responses (78). Later, direct lesions of the CeA in models of conditioned fear (i.e., fear conditioning and fear-potentiated startle) were shown to induce similar behavioral and autonomic deficits (17, 25, 55, 56, 94, 121, 131). Taken together, these results lead to the notion that the CeA is a critical site for the expression of fear and that it mediates these effects through its projections to downstream areas.
However, the common issue among many of these studies was that they treated the CeA as a single, homogenous entity, even though it is known that the CeA's medial and lateral nuclei are histologically, anatomically, and functionally distinct. While many of the details of these differences are beyond the scope of this review (please see Refs. 18, 143), a few major themes relating to fear conditioning merit mention. In particular, based on tracing studies, the CeA's medial division is thought to be the major source of the CeA's output projections, whereas its lateral division is thought to be composed primarily of local inhibitory circuits. The latter observation indicates that, rather than being a simple output station for the fear processing that occurs in upstream areas, the CeA may play an active role in regulating fear output.
Recent research has focused on parsing out the roles of the CEl and CEm in fear conditioning. Using optogenetics, Ciocchi et al. (26) showed that direct activation of CEm neurons leads to expression of fear behavior (freezing) and that, while injection of the GABAA receptor agonist muscimol into either the CEm alone or the entire CeA does not change freezing behavior, injection into the CEl alone results in unconditioned freezing. Additionally, inactivation of the CEl and CeA, but not the CEm, during fear conditioning results in fear-expression deficits. Finally, inactivation of the CEm and CeA, but not the CEl, 24 h after training results in impaired fear expression. Taken together, these findings suggest that the CEl plays a role in fear learning, whereas the CEm is more involved in fear output (26). However, even this model is likely incomplete. Past neural tracing studies have shown that the CEl also sends direct projections to fear effector brain regions (20, 49, 109, 150), and Penzo et al. (106) recently used slice electrophysiology to show that these CEl projections [predominantly from somatostatin-expressing (SOM+) neurons] to the PAG and the paraventricular nucleus of the thalamus (PVT) show increases in the frequencies and amplitudes of their miniature excitatory postsynaptic potentials after fear conditioning. These findings establish the potential that CEl neurons are also involved in fear expression independently of CEm output (106). Thus a confluence of evidence suggests that the CeA is not a homogenous structure that simply relays information to other areas of the brain that generate fear behaviors but rather is a complex structure with multiple interconnected functional divisions that is capable of information processing. Future studies should attempt to characterize the ways in which the various CeA cell populations are distinct from each other on genetic and epigenetic levels, and the ways in which these different populations contribute to the various aspects of fear expression. Ultimately, a clear understanding of how various physiological and pathological functions map onto genetically defined cell populations could allow for more targeted interventions for treating disorders of dysregulated fear expression.
Output Pathways of the CeA and Their Roles in Fear Learning
Not only have recent studies begun to parse out the roles of the various CeA sub-nuclei, but they have also started to identify the relative contributions of the CeA's output pathways to fear expression. It is well known that the efferents of the CeA are extensive and reach widespread brain stem structures that are essential for mediating fear expression (76, 114). As a whole, the central threat response system can be considered to have four main output targets: the periaqueductal gray in the midbrain, the lateral hypothalamus and paraventricular nucleus of the hypothalamus in the forebrain, and the rostral ventrolateral medulla (RVLM) and nucleus tractus solitarius (NTS) in the brain stem (57). The following section discusses output projections from the CeA to these regions and their roles in fear conditioning.
CeA-Periaqueductal Gray
Several groups have provided evidence that the CeA is a source of efferent projections to the PAG, the structure responsible for generating the most commonly used measure of conditioned fear: behavioral freezing (63, 69). Consistent with these prior results, Johansen et al. reported that pharmacological inactivation of the PAG before fear conditioning reduced the expression of conditioned fear and unconditioned reflex responses (63). Furthermore, these authors hypothesized that if the PAG is only involved in fear expression, then PAG inactivation should impair fear expression but not fear learning. Contrary to this idea, they found that fear acquisition was impaired by pretraining inactivation of the PAG. Therefore, these and more recent data (69) suggest that the PAG plays multiple roles during fear conditioning, contributing to both the expression and the learning of fear via reciprocal connections with the amygdala. Finally, in considering the subdivisions of the CeA, it is notable that both the CEl and CEm appear to have connections to the PAG; however, the relative contributions of CEl-PAG and CEm-PAG projections remain poorly understood.
CeA-Hypothalamus: Lateral and Paraventricular Areas
Efferent projections from the CeA to the lateral hypothalamus and brain stem are the primary neural pathways that mediate the autonomic and somatic motor components of the conditioned fear response (44, 54, 74, 78, 157). More specifically, the CEm and CEc predominantly innervate the dorsolateral and caudolateral regions of the hypothalamus (78, 108, 144). In addition to these direct projections to the LH, the CeA has a strong projection to the BNST, which also innervates hypothalamic nuclei and plays a major role in fear conditioning (156). Although these CeA-hypothalamic circuits have been well mapped out, recent studies indicate the existence and importance of independent hypothalamic circuits involved in the expression of different types of fear (i.e., social vs. predator) (137). In addition, it has been proposed that a bias toward active or passive fear responses is mediated by a neuronal switch in the CeA (46), which could therefore lead to differential regulation of CeA-hypothalamic-brain stem interactions involved in fear expression. Viviani et al. demonstrated that the neurohormone oxytocin selectively gates output from different parts of the amygdala to downstream hypothalamic and brain stem sites that result in the differential modulation of autonomic parameters such as blood pressure and heart rate (154).
As mentioned earlier, excitatory interactions between the CeA and downstream structures can be facilitated via indirect projections and the relay of information via brain stem pathways. The interaction between the CeA and paraventricular nucleus of the hypothalamus is thought to function in such a way (160). Although the PVN is best known for its role in the stress response and hypothalamic-pituitary-adrenal axis activation via glucocorticoid secretion, its role in the fear response remains poorly understood. Recent evidence, however, identifies the PVN as an important modulator of fear expression and extinction (83, 84, 152, 153), which may involve indirect connection between the CeA and PVN via brain stem structures.
CeA-Brain Stem: Nucleus Tractus Solitarius and Ventrolateral Medulla
CeA lesioning (70) and stimulation (40) studies have shown that disrupting the fear output pathway at the level of the CeA impairs the cardiovascular and motor (freezing) responses to fear conditioning, suggesting that the CeA is an important link between upstream centers for fear processing and downstream brain stem sites involved in regulating autonomic activity. Tracing studies have established that CeA neurons project to brain regions that modulate sympathetic tone, including the nucleus tractus solitarius and rostral ventrolateral medulla (23, 127). In particular, the NTS has been shown to play a key role in modulating cardiorespiratory responses and has been implicated in cardiovascular disorders such as hypertension (6, 120). The NTS receives both direct (126, 128) and indirect input from the CeA. Afferent fibers from arterial baroreceptors and chemoreceptors also terminate in the NTS, and there is evidence to suggest that GABAergic CeA-NTS projections attenuate the baroreceptor reflex control of blood pressure (125). Future studies should add cell-type information to the anatomical and functional maps of the CeA projections using viral tract tracing and optogenetic projection targeting methods, respectively.
The Expanding Role of the CeA in Fear Learning
The Background of the CeA as a Site of Plasticity with an Expanding Role in Fear Learning
Recent research has suggested that the CeA may also be directly involved in fear learning. Early evidence suggested that the CeA exhibits synaptic plasticity in response to fear conditioning, conditioned taste aversion, appetitive taste aversion, and exposure to painful stimuli (129). Such studies showed that changes in neural activity in response to the CS occur with fear conditioning (7, 105), that CREB expression increases following fear-expression testing (51), and that infusions of NMDA receptor antagonists into the CeA block fear learning (45, 161), all of which suggest that the CeA undergoes persistent functional changes in response to fear conditioning. Further results suggested that the CeA is capable of processing fear-related information in its own right and does not rely solely on input from the BLA to generate fear responses (68). Additionally, this work established the possibility that plasticity can occur in the CeA as well as in the BLA. These assertions began to seem even more plausible when thalamic inputs were shown to drive LTP in the CEm, even in the absence of connections between the BLA and CEm, indicating that CeA input is not limited to projections from the BLA but can also come from other subcortical structures (130).
These studies, which challenged the assumption that the CeA was a passive relay station, raised several questions. To what extent was the CeA involved in the acquisition, consolidation, expression, and extinction of fear memories? Was the plasticity of the CeA reflective of its ability to form CS-US associations? Furthermore, how did the CEm and CEl differ in their plastic properties and roles? A series of drug and optogenetic studies would shed further light on the circuit-level implications of the CeA's potential for plasticity.
Recent Literature on the Expanding Role of the CeA in Fear Learning
Wilensky et al. (159) used inactivation with the GABAA receptor agonist muscimol and the protein synthesis inhibitor anisomycin to determine the extent to which the CeA plasticity is involved in fear learning and consolidation. They observed that inactivating the CeA using muscimol impairs both fear learning and expression, and blocking protein synthesis in the CeA impairs fear memory consolidation. Additional studies used muscimol inactivation to show that CeA activity is necessary for the acquisition and expression of over-trained fear (88, 117, 162). Together, these results provided evidence that the CeA is involved in multiple aspects of fear learning and consolidation and that it may even be capable of forming CS-US associations (32).
The CEm is thought to be the origin of most of the output projections to the brain stem and hypothalamus that are involved in generating fear expression behaviors (59, 78, 133, 150). The CEl, on the other hand, while having some long-distance projections to such effector sites also has GABAergic cells that inhibit the CEm (18, 92, 109, 143). Furthermore, the CEl and CEc also receive input from extra-amygdalar brain regions, including sensory input from the auditory cortex and thalamus (90, 147) and nociceptive input from the pontine parabrachial nucleus (9, 62, 151). This led to the suggestion that the CEl is the site of plasticity and that the CEm acts as the output station (129), although this model does not take into account the host of projections to the CEm, such as those from the auditory thalamus (85, 147), or the evidence that LTP can be induced in the CEm following high-frequency stimulation (130). Clearly, both sub-nuclei receive input from extra-amygdalar structures and are capable of plasticity. At least two schema have been proposed for how these regions contribute to fear learning (33).
The first is that the connections between the sensory regions of the thalamus (e.g., the medial geniculate nucleus) and the CEm are excitatory and plastic, and that these synapses are strengthened during fear acquisition. This proposal is in line with the evidence showing projections from the medial geniculate to the CEm (85, 147) and evidence of LTP in the CEm as generated by high-frequency stimulation of thalamic neurons (130). However, the projections from the medial geniculate, in particular to the CEm, have not been tested for potential plasticity, and these experiments have not been conducted in vivo.
The second possibility is that the inhibitory control of the CEl over the CEm is plastic, and that tuning the level of inhibition of the CEm can cause corresponding shifts in the activities of the neurons projecting to the brain stem and hypothalamus. Support for this mechanism comes from the observation that the CEl receives many intra- and extra-amygdalar inputs, which could tune the activities of its inhibitory projections onto the CEm (60). Recent studies have tested the dynamics of the CEl-CEm inhibitory hypothesis. For example, Ciocchi et al. (26) were able to dissociate the functional roles of the CEl and CEm in fear learning and expression. First, they showed that optogenetic stimulation of the CEm results in unconditioned freezing. Conversely, inactivation of the entire CeA or just the CEm with muscimol does not induce freezing, but inactivation of the CEl alone does, presumably via disinhibition of the CEm. These results suggest that the CEm is independently capable of inducing unconditioned freezing and that it is normally inhibited by the CEl.
Additionally, inactivation of either the entire CeA or just the CEl, but not the CEm, during fear conditioning prevents animals from acquiring learned fear. Conversely, inactivation of either the entire CeA or just the CEm, but not the CEl, impairs fear expression. Thus the CEl may contribute to fear learning, whereas the CEm may contribute to fear expression. Using electrophysiological approaches, it was found that CEl neurons' activity patterns in response to presentations of the CS fall into three categories: those showing increased activity (CElon), decreased activity (CEloff), or no change in activity. Notably, both CElon and CEloff neurons are able to cause inhibition of CEm neurons. Furthermore, following fear conditioning, CEm output neurons projecting to brain stem targets show a biphasic response to the CS, with the first component having a short-latency onset and brief duration (similar to the observed responses of CElon neurons), and the second component having a delayed onset but prolonged duration (similar to the responses of CEloff neurons). Therefore, it was proposed that an initial direct input from the thalamus excites the CEm, which is then quickly and transiently inhibited by the CElon neurons, and then the CEloff neurons disinhibit the CEm (by removing the CEl's tonic inhibition on the CEm neurons), allowing for the delayed but protracted component of the response (26).
Further work revealed that most of the GABAergic CEloff neurons express PKC-δ (PKC-δ+), and beyond their role of disinhibiting the CEm for fear expression they also make reciprocal inhibitory connections onto PKC-δ− neurons. Through the employment of optogenetic, transgenic, and neural tracing tools, these PKC-δ+ CEl neurons were shown to synapse onto CEm output neurons that project to the PAG, which is known for its role in generating freezing behavior (53). In follow-up work, it was found that fear conditioning results in an increase in the proportion of CEloff cells, but not CElon cells, and that extinction training results in a decrease in the proportion of CElon cells (32). Clearly, shifts in the activities of these CeA microcircuits are important for many aspects of fear learning and expression, yet there is only preliminary evidence on how these shifts occur.
The broader implications of these observed changes in CeA activity with fear conditioning are that activity-dependent synaptic plasticity may occur in the CEl and its afferents during fear learning, and that this plasticity is contributing to the storage of fear-related information in a manner that is at least partially independent of BLA activity. Furthermore, at least two in vitro studies (one focused on the parabrachial nucleus-to-CEl pathway and one focused on the BLA/LA-to-CEl pathway) provide evidence for LTP in these connections (37). Of these two pathways, the projections from the BLA to the CEl have been studied more extensively. Li et al. (81) studied another subset of CEl neurons, those that express the neuropeptide somatostatin (SOM+), which are largely distinct from the PKC-δ+ population. They used transgenic techniques to fluorescently label these SOM+ neurons in mice and then used slice physiology to simultaneously record from SOM+ and SOM− neurons while stimulating the LA (using electrical and optogenetic stimulation in independent experiments). Their results suggest that not only do shifts in the excitability of SOM+ and SOM− neurons occur during fear conditioning but that these shifts are 1) mediated by activity-dependent strengthening of the synapses from LA cells onto the SOM+ cells of the CEl and 2) necessary for fear acquisition. To better characterize the connectivity of these SOM+ neurons, they showed that <15% of the projection neurons from the CEl to the CEm are SOM+. Furthermore, stimulation of these SOM+ CEl neurons resulted in robust IPSCs in all recorded CEl neurons but only in a few of the recorded CEm neurons and in none of the PAG-projecting SOM+ neurons. Finally, in an in vivo model, they showed that optogenetic stimulation of SOM+ CEl neurons in untrained animals results in unconditioned freezing. Furthermore, optogenetic inhibition of SOM+ CEl neurons during fear-expression testing abolishes conditioned freezing, indicating that the activity of these neurons is necessary for fear expression (81). Taken together, these results suggest that SOM+ CEl neurons inhibit the SOM− CEl neurons (a majority of which are PKC-δ+) that are normally responsible for tonic inhibition of the CEm and suppression of fear expression.
However, not all of the SOM+ CEl neurons project locally. There is evidence that some of these neurons project to the PAG and the paraventricular nucleus of the thalamus (PVT), both of which are involved in generating fear behaviors. Exploring these projections further with slice preparations, Penzo et al. (106) showed that optogenetic activation of these projections results in IPSCs in the neurons of the PAG but not the PVT. Furthermore, when animals underwent fear conditioning, there was a notable increase in the frequency and amplitude of EPSCs from the SOM+ neurons projecting to either the PAG or the PVT (106). However, the role that these projections play in fear learning has yet to be elucidated.
Very few studies have investigated the molecules that mediate this balance of excitation and inhibition in CeA microcircuits. Pitts et al. showed that corticotropin-releasing factor (CRF) in the CeA is an important mediator for contextual fear conditioning (112, 113). Similarly, Kamprath et al. showed that cannabinoid signaling in the CeA is essential for short-term plasticity and adaptation with fear conditioning (64). For fear memory consolidation, Andero et al. showed the importance of the Tac2 gene and its product neurokinin B within the CeA (5).
The Roles of Cortical and Subcortical Inputs to the CeA in Fear Learning
To convincingly argue that the CeA is capable of processing fear-related information independently of BLA input, it must first be shown that the CeA receives input from extra-amygdalar areas. Given that the CeA is both a source of fear output and a site of plasticity for fear learning, it is not surprising that it receives input from numerous cortical and subcortical structures (104, 138). While many of these projections have not been studied extensively, there have been several recent attempts to characterize the inputs to the CeA from two primary areas of the medial prefrontal cortex: the infralimbic and the prelimbic cortices (via the intercalated cells and paraventricular thalamus, respectively).
Infralimbic Cortex-ITC-CEm
The medial prefrontal cortex (mPFC), with its diffuse pattern of connectivity (50), has long been implicated in controlling fear responses (1, 96). In particular, it has been studied extensively in the framework of fear extinction, in which case it is thought to serve an inhibitory role by decreasing the activities of amygdala circuits involved in fear memory recall (115, 119). Within this context, Quirk et al. showed that lesions of the infralimbic (IL) portion of the mPFC resulted in a loss of memory of extinction (116). Furthermore, Milad and Quirk showed that response patterns of the IL were increased with tone presentations after extinction (95). Clearly, IL activity is involved in fear extinction, but the systems-level mechanisms by which the IL exerts these effects remain unclear. However, growing evidence suggests a role for IL control of the CeA in this process.
mPFC stimulation was found to robustly reduce the responsiveness of CEm neurons, indicating a functional link between the two areas (116). However, the direction of this effect was unexpected; given that most mPFC projections are excitatory, it was surprising to observe a decrease in the activity of one of its afferent targets in response to mPFC stimulation. This observation led to the hypothesis that mPFC-mediated inhibition of the CEm occurs via activation of intermediate inhibitory neurons. Currently, these neurons are thought to be the intercalated (ITC) cells of the amygdala (12), which project onto and inhibit the CEm output neurons (2, 103, 123), with increases in IL c-Fos expression correlated with increases in ITC c-Fos (12, 72). Furthermore, ITC cells respond to IL stimulation (3), and IL projections predominantly innervate medial ITC cells (although there are also direct projections from IL to lateral CEc) (111). Future work should aim to genetically and functionally (i.e., in the context of fear learning) characterize the projections from the IL to the ITC and from the ITC to the CEm.
Prelimbic Cortex-Paraventricular Thalamus-CEl
Previous work has suggested that the PL is involved in fear memory consolidation (24) and expression (16), but it remains unclear by what pathways the PL mediates these effects. Given that the PL projects densely to the dorsal midline thalamus (dMT) (83) and that the dMT is necessary for fear expression 24 h after conditioning (100), it was hypothesized that the PL-dMT projection may contribute to the PL's role in fear expression. The observation, based on c-Fos time course studies, that the PVT (a subregion of the dMT) only came online >24 h after fear conditioning suggested that its involvement in fear expression depended on plastic changes associated with memory consolidation. Additionally, PVT neurons respond more robustly to the conditioned stimulus 24 h after conditioning than at earlier time points. Furthermore, retrograde tracing and c-Fos labeling techniques showed that the PVT neurons active during fear expression were the ones that projected to the CeA.
Optogenetic inhibition of the cell bodies of PL neurons resulted in a decrease in the firing rates of some PL cells and an increase in the firing rates of others. Similarly, using the same technique to inhibit PVT-projecting PL terminals resulted in a decrease in the firing rates of some PVT cells and an increase in the firing rates of others. Furthermore, inhibiting PL projections to the PVT or PVT projections to the CeA only affected expression 7 days after conditioning. Taken together, these results suggest that the circuits recruited for fear expression change as a function of time and that projections from the PVT to the CeA are involved in the expression of distant, but not recent, fear memories. Although they generate interest in the PL-PVT-CeA pathway in the context of fear expression, these results alone do not reveal how the activity of this pathway influences the microcircuit-level reciprocal inhibition patterns that have previously been implicated in the gating of fear output from the CeA.
Based on the previous observation that the PVT projects strongly to the CEl (82), Penzo et al. isolated two non-overlapping sets of projections from the PVT to the CEl and BLA. Then, using a chemogenetic approach, they introduced an engineered inhibitory G-protein-coupled receptor into the CEl-projecting PVT cells, which when activated during either fear conditioning or fear expression testing resulted in decreased conditioned freezing. Interestingly, chemogenetic inhibition during fear conditioning of the PVT neurons that synapse onto the SOM+ cells did not reduce the synaptic potentiation observed (81) at 3 h after conditioning, but it did abolish the potentiation at 24 h, suggesting that PVT input is critical for the maintenance of this SOM+ CEl potentiation but not its induction. Next, given their finding that PVT neurons innervate SOM+ cells twice as heavily as SOM− ones, they used optogenetic stimulation to explore the functional differences between PVT projections onto SOM+ and SOM− CEl neurons. Surprisingly, brief optogenetic stimulation did not evoke fast synaptic responses in SOM+ or SOM− cells, but high-frequency stimulation evoked slow inward currents in SOM+ cells and increased the frequency of spontaneous inhibitory postsynaptic currents in SOM− cells. The observation that stimulation of PVT-CEl projections evoked slow synaptic currents in SOM+ CEl cells indicated that transmission at these synapses was mediated by a neuromodulator. They then demonstrated that brain-derived neurotrophic factor (BDNF) is enriched in CEl-projecting PVT neurons and that the BDNF receptor TrkB is expressed by SOM+ CEl neurons. Deleting the TrkB receptor from SOM+ CEl cells abolished the PVT-driven slow inward currents, and bath-applying a BDNF scavenger abolished the PVT-driven increase in inhibition of SOM− CEl cells. Finally, using in vivo transgenic and viral techniques, they showed that both BDNF knockout from PVT neurons and TrkB receptor knockout from SOM+ CEl neurons resulted in impaired fear conditioning. Furthermore, knockout of the TrkB receptor from SOM+ CEl cells attenuated the presynaptic potentiation previously seen with fear conditioning, and injection of BDNF into the CEl facilitated fear conditioning(107). Together, these findings suggest that the plasticity of the SOM+ neurons in the CEl caused by fear conditioning can be attributed to input from the PL-PVT-CEl circuit and that BDNF released by projections from the PVT is necessary for this plasticity.
The Role of CeA Neuropeptides in Fear Learning
The CeA, via direct and indirect projections, can regulate the activity of the hypothalamic PVN and influence the synthesis and secretion of key neuropeptides involved in the physiological response to fear, such as corticotropin-releasing factor (CRF), adrenocorticotropic hormone, arginine vasopressin, and oxytocin (140). The following discussion will highlight the more extensively studied neuropeptides CRF, estrogen, vasopressin, and angiotensin II, all of which have been implicated in fear learning and fear-related behavioral disorders such as PTSD.
CRF is distributed throughout the brain in regions such as the CeA, the PVN, and portions of the extended amygdala including the BNST (13, 132, 145). The amygdala has been shown to be a major extra-hypothalamic source of CRF-containing neurons, with high expression levels of CRF receptors (101, 149), and stress has been show to increase CRF mRNA expression in the CeA (134). Overexpression of CRF in the CeA has profound effects on hypothalamic-pituitary-adrenal axis regulation, with associated behavioral, physiological, and reproductive consequences, all of which are involved in the development of fear disorders (66). Additionally, plasticity in CRF-expressing neurons has been implicated in fear conditioning and extinction. Mice with a GABAA receptor deletion confined to CRF-expressing neurons have increased baseline anxiety and impaired extinction of conditioned fear (39). A more recent study suggests that glutamatergic modulation of CRF neurons also contributes to changes in fear behavior (38). Although the CRF-expressing neurons of the CeA have been repeatedly implicated in fear learning and expression, the molecular mechanisms remain unclear. Future studies should investigate how changes in the excitability of CRF-expressing CeA neurons influences CRF regulation and should characterize the functional heterogeneity of the CRF-expressing neuron populations.
In addition to CRF, other neuropeptides also act on CeA cells to modulate the acquisition and expression of fear. Hormones like oxytocin and vasopressin have recently been shown to stimulate distinct neuronal populations in the CeA that regulate autonomic fear responses (60). In particular, work has shown that oxytocin excites CEl neurons that in turn inhibit the CEm, whereas vasopressin appears to stimulate the CEm directly (155). Following up on these findings, Knobloch et al. (73) used optogenetic stimulation of CEl-projecting oxytocinergic hypothalamic axons to show that endogenous oxytocin release causes a reduction in conditioned fear expression. Future work likely will continue to parse out the contributions of CeA neuropeptides to fear learning and expression. For example, the octapeptide angiotensin II, most notably known for its role in blood pressure regulation, and its receptors AT1, AT2, and AT4 are expressed in the amygdala and other brain regions. Recently, this peptide has been shown to play a role in fear expression and extinction retention, and it is possible that these effects are mediated by its action on CeA cells (67, 89).
The CeA in Humans and Mice
Given the importance of the CeA in fear learning in rodents, it is possible that the CeA plays a role in human fear disorders as well. However, while many studies have used functional magnetic resonance imaging to examine the human amygdala (30, 58, 110), including its functional connectivity (122), responses to fearful faces (52), and even fear conditioning and extinction (77), none has yet been able to dissociate the responses of amygdala sub-nuclei, likely because existing non-invasive imaging techniques do not have adequate spatial resolution to resolve such small structures (136).
Instead, a growing number of studies have used single-nucleotide polymorphism analyses to associate genetic variants with differences in susceptibility to developing fear and anxiety disorders. For example, the Oprl1 gene and the nociceptin/orphan FQ receptor (NOP-1) have been implicated in both mouse fear conditioning and human PTSD. In particular, injection of a NOP-1 agonist into the CeA impairs consolidation of conditioned fear memory, and a single-nucleotide polymorphism within the Oprl1 gene is associated with increased severity of PTSD symptoms after a traumatic event, physiological changes in startle responses, and changes in the functional connectivity between the amygdala and insula (4). Additionally, a SNP in the promoter region of the serotonin transporter gene has been shown to convey enhanced sensitivity to fearful stimuli (52). Future studies using epigenomic and transcriptomic profiling of healthy and diseased postmortem human amygdala tissue will facilitate a better understanding of normal and pathological fear, and may inspire new avenues for treatment of fear disorders.
Conclusions and Future Directions
The CeA has had an evolving history with respect to fear learning. While initially believed to be primarily a final common output pathway of the fear circuit to the behavioral and autonomic effector regions of the brain, it is becoming increasingly clear that the CeA is involved in the acquisition, expression, and even consolidation of conditioned fear. Current evidence suggests that the role of the output station can be subscribed to the CEm, whereas the site of plasticity is the CEl. Specifically, it appears that the CEl is able to gate the activity of the CEm, which results in the expression of behavioral and autonomic responses. Furthermore, the CEl receives input from many extra-amygdalar sources, including the mPFC, pPVT, auditory thalamus and cortex, and several brain stem areas. Additionally, it appears that several of these projections release neuromodulators onto CeA cells, such as glucocorticoids, estrogen, CRF, and oxytocin. Together, these findings lead to the working hypothesis that local inhibitory circuits in the CEl gate fear-related information as it passes from the BLA to the CEm and that cortical and subcortical inputs directly onto CEl cells tune the activities of these local inhibitory circuits using neuromodulators. Beyond studies in rodents, recent work has suggested that the human CeA plays an important role in PTSD.
However, several unanswered questions remain. Over the past several years, our structural maps of the inputs, internal connectivity, and outputs of the CeA have grown increasingly sophisticated. Additionally, in some cases, we have been able to overlay basic functional information (i.e., electrophysiological characteristics, such as whether projections tend to be excitatory or inhibitory) onto this structural map. In even fewer cases, we have even been able to add more detailed functional information, such as which genes are expressed and which neurotransmitters and neuromodulators are released by these cells. However, as FIGURE 2B illustrates, for the majority of anatomically defined circuits, we still have little of this advanced functional information. Therefore, the most urgent priority for future studies attempting to understand the circuit- and cellular-level contributions of the CeA to fear learning is to obtain sophisticated functional data that can be superimposed onto our existing structural framework. Fortunately, an increasing number of genetically encoded fluorescent sensors (22, 35, 36, 139) and actuators (10, 11, 71, 93) (for optical recording and control of neural populations, respectively) are becoming available, and these approaches, in combination with intersectional genetic methods (99), have the power to extract this critical information.
Footnotes
This work was supported by National Institutes of Health Grants R21 MH-102191, R01 MH-096764, R01 MH-071537, and R00 HL-107675-03. This project was also supported in part by the Office of Research Infrastructure Programs/OD P51OD011132 (formerly NCRR P51RR000165).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: O.P.K., R.C.H., and P.J.M drafted manuscript; R.C.H. made figures; K.J.R. edited and revised manuscript; K.J.R. and P.J.M. approved final version of manuscript.
References
- 1.al Maskati HA, Zbrozyna AW. Stimulation in prefrontal cortex area inhibits cardiovascular and motor components of the defence reaction in rats. J Auton Nerv Syst 28: 117–125, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci 13: 489–494, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105: 3054–3066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andero R, Brothers SP, Jovanovic T, Chen YT, Salah-Uddin H, Cameron M, Bannister TD, Almli L, Stevens JS, Bradley B, Binder EB, Wahlestedt C, Ressler KJ. Amygdala-dependent fear is regulated by Oprl1 in mice and humans with PTSD. Sci Transl Med 5: 188ra173, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andero R, Dias BG, Ressler KJ. A role for Tac2, NkB, and Nk3 receptor in normal and dysregulated fear memory consolidation. Neuron 83: 444–454, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andresen MC, Mendelowitz D. Sensory afferent neurotransmission in caudal nucleus tractus solitarius: common denominators. Chem Senses 21: 387–395, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res 238: 457–462, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Applegate CD, Kapp BS, Underwood MD, McNall CL. Autonomic and somatomotor effects of amygdala central N. stimulation in awake rabbits. Physiol Behav 31: 353–360, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 63: 473–490, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 344: 420–424, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci 12: 229–234, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience 132: 943–953, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyer HS, Matta SG, Sharp BM. Regulation of the messenger ribonucleic acid for corticotropin-releasing factor in the paraventricular nucleus and other brain sites of the rat. Endocrinology 123: 2117–2123, 1988. [DOI] [PubMed] [Google Scholar]
- 14.Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Struct Funct 218: 187–208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol 81: 281–290, 1972. [DOI] [PubMed] [Google Scholar]
- 16.Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 15: 2301–2311, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann NY Acad Sci 877: 217–241, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Cassell MD, Gray TS. Morphology of peptide-immunoreactive neurons in the rat central nucleus of the amygdala. J Comp Neurol 281: 320–333, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol 246: 478–499, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Champagne D, Beaulieu J, Drolet G. CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. J Neuroendocrinol 10: 119–131, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499: 295–300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiou RJ, Kuo CC, Yen CT. Comparisons of terminal densities of cardiovascular function-related projections from the amygdala subnuclei. Auton Neurosci 181: 21–30, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. PNAS 107: 2675–2680, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi JS, Brown TH. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. J Neurosci 23: 8713–8721, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468: 277–282, 2010. [DOI] [PubMed] [Google Scholar]
- 27.Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and the fear-potentiated startle effect. Pharmacol Ther 47: 147–165, 1990. [DOI] [PubMed] [Google Scholar]
- 28.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15: 353–375, 1992. [DOI] [PubMed] [Google Scholar]
- 29.Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58: 175–198, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry 6: 13–34, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Duvarci S, Pare D. Amygdala microcircuits controlling learned fear. Neuron 82: 966–980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. J Neurosci 31: 289–294, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron 62: 757–771, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23: 229–232, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Flytzanis NC, Bedbrook CN, Chiu H, Engqvist MK, Xiao C, Chan KY, Sternberg PW, Arnold FH, Gradinaru V. Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons. Nat Commun 5: 4894, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fosque BF, Sun Y, Dana H, Yang CT, Ohyama T, Tadross MR, Patel R, Zlatic M, Kim DS, Ahrens MB, Jayaraman V, Looger LL, Schreiter ER Neural circuits . Labeling of active neural circuits in vivo with designed calcium integrators. Science 347: 755–760, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Fu Y, Shinnick-Gallagher P. Two intra-amygdaloid pathways to the central amygdala exhibit different mechanisms of long-term potentiation. J Neurophysiol 93: 3012–3015, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Gafford G, Jasnow AM, Ressler KJ. Grin1 receptor deletion within CRF neurons enhances fear memory. PLos One 9: e111009, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)α1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci USA 109: 16330–16335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galeno TM, Brody MJ. Hemodynamic responses to amygdaloid stimulation in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 245: R281–R286, 1983. [DOI] [PubMed] [Google Scholar]
- 41.Gallagher M, Kapp BS, Frysinger RC, Rapp PR. Beta-adrenergic manipulation in amygdala central n. alters rabbit heart rate conditioning. Pharmacol Biochem Behav 12: 419–426, 1980. [DOI] [PubMed] [Google Scholar]
- 42.Gallagher M, Kapp BS, McNall CL, Pascoe JP. Opiate effects in the amygdala central nucleus on heart rate conditioning in rabbits. Pharmacol Biochem Behav 14: 497–505, 1981. [DOI] [PubMed] [Google Scholar]
- 43.Gentile CG, Jarrell TW, Teich A, McCabe PM, Schneiderman N. The role of amygdaloid central nucleus in the retention of differential pavlovian conditioning of bradycardia in rabbits. Behav Brain Res 20: 263–273, 1986. [DOI] [PubMed] [Google Scholar]
- 44.Goosens KA, Maren S. Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats. Learn Mem 8: 148–155, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci 117: 738–750, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwarz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron 67: 656–666, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology 50: 433–446, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Gray TS, Magnuson DJ. Galanin-like immunoreactivity within amygdaloid and hypothalamic neurons that project to the midbrain central grey in rat. Neurosci Lett 83: 264–268, 1987. [DOI] [PubMed] [Google Scholar]
- 49.Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol 262: 365–374, 1987. [DOI] [PubMed] [Google Scholar]
- 50.Gutman DA, Keifer OP Jr, Magnuson ME, Choi DC, Majeed W, Keilholz S, Ressler KJ. A DTI tractography analysis of infralimbic and prelimbic connectivity in the mouse using high-throughput MRI. Neuroimage 63: 800–811, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci 13: 1453–1458, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400–403, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci 100: 11–22, 1986. [DOI] [PubMed] [Google Scholar]
- 55.Hitchcock JM, Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci 105: 826–842, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Hitchcock JM, Sananes CB, Davis M. Sensitization of the startle reflex by footshock: blockade by lesions of the central nucleus of the amygdala or its efferent pathway to the brainstem. Behav Neurosci 103: 509–518, 1989. [DOI] [PubMed] [Google Scholar]
- 57.Holstege G. The basic, somatic, and emotional components of the motor system in mammals. In: The Rat Nervous System, edited by Paxinos G. Sydney, Australia: Academic, 1995, p. 137–154. [Google Scholar]
- 58.Holzschneider K, Mulert C. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci 13: 453–461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 32: 529–547, 1978. [DOI] [PubMed] [Google Scholar]
- 60.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308: 245–248, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Iwata J, Chida K, LeDoux JE. Cardiovascular responses elicited by stimulation of neurons in the central amygdaloid nucleus in awake but not anesthetized rats resemble conditioned emotional responses. Brain Res 418: 183–188, 1987. [DOI] [PubMed] [Google Scholar]
- 62.Jasmin L, Burkey AR, Card JP, Basbaum AI. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. J Neurosci 17: 3751–3765, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci 13: 979–986, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamprath K, Romo-Parra H, Haring M, Gaburro S, Doengi M, Lutz B, Pape HC. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 36: 652–663, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav 23: 1109–1117, 1979. [DOI] [PubMed] [Google Scholar]
- 66.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry 14: 37–50, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66a.Keifer OP Jr., Hurt RC, Gutman DA, Keilholz SD, Gourley SL, Ressler KJ. Voxel-based morphometry predicts shifts in dendritic spine density and morphology with auditory fear conditioning. Nat Commun 6: 7582, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 73: 849–855, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Killcross S, Robbins TW, Everitt BJ. Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala. Nature 388: 377–380, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Kim EJ, Horovitz O, Pellman BA, Tan LM, Li Q, Richter-Levin G, Kim JJ. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proc Natl Acad Sci USA 110: 14795–14800, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim M, Davis M. Electrolytic lesions of the amygdala block acquisition and expression of fear-potentiated startle even with extensive training but do not prevent reacquisition. Behav Neurosci 107: 580–595, 1993. [DOI] [PubMed] [Google Scholar]
- 71.Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat Neurosci 14: 513–518, 2011. [DOI] [PubMed] [Google Scholar]
- 72.Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 16: 486–493, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566, 2012. [DOI] [PubMed] [Google Scholar]
- 74.Koo JW, Han JS, Kim JJ. Selective neurotoxic lesions of basolateral and central nuclei of the amygdala produce differential effects on fear conditioning. J Neurosci 24: 7654–7662, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 178: 225–254, 1978. [DOI] [PubMed] [Google Scholar]
- 76.Krettek JE, Price JL. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol 178: 255–280, 1978. [DOI] [PubMed] [Google Scholar]
- 77.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20: 937–945, 1998. [DOI] [PubMed] [Google Scholar]
- 78.LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8: 2517–2529, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ledoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol 264: 123–146, 1987. [DOI] [PubMed] [Google Scholar]
- 80.Lee S, Kim SJ, Kwon OB, Lee JH, Kim JH. Inhibitory networks of the amygdala for emotional memory. Front Neural Circuits 7: 129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat Neurosci 16: 332–339, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506: 263–287, 2008. [DOI] [PubMed] [Google Scholar]
- 83.Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct 217: 257–273, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Li Y, Dong X, Li S, Kirouac GJ. Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front Behav Neurosci 8: 94, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linke R, Braune G, Schwegler H. Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res 134: 520–532, 2000. [DOI] [PubMed] [Google Scholar]
- 86.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35: 24–35, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24: 897–931, 2001. [DOI] [PubMed] [Google Scholar]
- 88.Maren S. Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci 19: 8696–8703, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, Ressler KJ. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry 75: 864–872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol 55: 257–332, 1998. [DOI] [PubMed] [Google Scholar]
- 91.McDonald AJ. Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 208: 401–418, 1982. [DOI] [PubMed] [Google Scholar]
- 92.McDonald AJ, Augustine JR. Localization of GABA-like immunoreactivity in the monkey amygdala. Neuroscience 52: 281–294, 1993. [DOI] [PubMed] [Google Scholar]
- 93.McIsaac RS, Engqvist MK, Wannier T, Rosenthal AZ, Herwig L, Flytzanis NC, Imasheva ES, Lanyi JK, Balashov SP, Gradinaru V, Arnold FH. Directed evolution of a far-red fluorescent rhodopsin. Proc Natl Acad Sci USA 111: 13034–13039, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melia KR, Sananes CB, Davis M. Lesions of the central nucleus of the amygdala block the excitatory effects of septal ablation on the acoustic startle reflex. Physiol Behav 51: 175–180, 1992. [DOI] [PubMed] [Google Scholar]
- 95.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420: 70–74, 2002. [DOI] [PubMed] [Google Scholar]
- 96.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett 163: 109–113, 1993. [DOI] [PubMed] [Google Scholar]
- 97.Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct 219: 1287–1303, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pabba M. Evolutionary development of the amygdaloid complex. Front Neuroanat 7: 27, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci 16: 805–815, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padilla-Coreano N, Do-Monte FH, Quirk GJ. A time-dependent role of midline thalamic nuclei in the retrieval of fear memory. Neuropharmacology 62: 457–463, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palkovits M, Brownstein MJ, Vale W. Corticotropin releasing factor (CRF) immunoreactivity in hypothalamic and extrahypothalamic nuclei of sheep brain. Neuroendocrinology 37: 302–305, 1983. [DOI] [PubMed] [Google Scholar]
- 102.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pare D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience 57: 1077–1090, 1993. [DOI] [PubMed] [Google Scholar]
- 104.Pare D, Smith Y, Pare JF. Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: Phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience 69: 567–583, 1995. [DOI] [PubMed] [Google Scholar]
- 105.Pascoe JP, Kapp BS. Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit. Behav Brain Res 16: 117–133, 1985. [DOI] [PubMed] [Google Scholar]
- 106.Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci 34: 2432–2437, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, Darvas M, Parada LF, Palmiter RD, He M, Huang ZJ, Li B. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519: 455–559, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev 38: 247–289, 2001. [DOI] [PubMed] [Google Scholar]
- 109.Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res 763: 247–254, 1997. [DOI] [PubMed] [Google Scholar]
- 110.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348, 2002. [DOI] [PubMed] [Google Scholar]
- 111.Pinard CR, Mascagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205: 112–124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Mem 95: 86–91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pitts MW, Todorovic C, Blank T, Takahashi LK. The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci 29: 7379–7388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci 1: 1242–1259, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem 9: 402–407, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rabinak CA, Maren S. Associative structure of fear memory after basolateral amygdala lesions in rats. Behav Neurosci 122: 1284–1294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reis DJ, Oliphant MC. Bradycardia and tachycardia following electrical stimulation of the amygdaloid region in monkey. J Neurophysiol 27: 893–912, 1964. [DOI] [PubMed] [Google Scholar]
- 119.Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process 1: 88–96, 1975. [PubMed] [Google Scholar]
- 120.Rogers RF, Paton JF, Schwaber JS. NTS neuronal responses to arterial pressure and pressure changes in the rat. Am J Physiol Regul Integr Comp Physiol 265: R1355–R1368, 1993. [DOI] [PubMed] [Google Scholar]
- 121.Roozendaal B, Koolhaas JM, Bohus B. Differential effect of lesioning of the central amygdala on the bradycardiac and behavioral response of the rat in relation to conditioned social and solitary stress. Behav Brain Res 41: 39–48, 1990. [DOI] [PubMed] [Google Scholar]
- 122.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci 19: 10575–10583, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev 83: 803–834, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Saha S. Role of the central nucleus of the amygdala in the control of blood pressure: descending pathways to medullary cardiovascular nuclei. Clin Exp Pharmacol Physiol 32: 450–456, 2005. [DOI] [PubMed] [Google Scholar]
- 126.Saha S, Batten TF, Henderson Z. A GABAergic projection from the central nucleus of the amygdala to the nucleus of the solitary tract: a combined anterograde tracing and electron microscopic immunohistochemical study. Neuroscience 99: 613–626, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Saha S, Drinkhill MJ, Moore JP, Batten TF. Central nucleus of amygdala projections to rostral ventrolateral medulla neurones activated by decreased blood pressure. Eur J Neurosci 21: 1921–1930, 2005. [DOI] [PubMed] [Google Scholar]
- 128.Saha S, Henderson Z, Batten TF. Somatostatin immunoreactivity in axon terminals in rat nucleus tractus solitarii arising from central nucleus of amygdala: coexistence with GABA and postsynaptic expression of sst2A receptor. J Chem Neuroanat 24: 1–13, 2002. [DOI] [PubMed] [Google Scholar]
- 129.Samson RD, Duvarci S, Pare D. Synaptic plasticity in the central nucleus of the amygdala. Rev Neurosci 16: 287–302, 2005. [DOI] [PubMed] [Google Scholar]
- 130.Samson RD, Paré D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J Neurosci 25: 1847–1855, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sananes CB, Campbell BA. Role of the central nucleus of the amygdala in olfactory heart rate conditioning. Behav Neurosci 103: 519–525, 1989. [PubMed] [Google Scholar]
- 132.Sawchenko PE, Swanson LW. Localization, colocalization, and plasticity of corticotropin-releasing factor immunoreactivity in rat brain. Fed Proc 44: 221–227, 1985. [PubMed] [Google Scholar]
- 133.Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci 2: 1424–1438, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shepard JD, Barron KW, Myers DA. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res 861: 288–295, 2000. [DOI] [PubMed] [Google Scholar]
- 135.Shi CJ, Cassell MD. Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 399: 440–468, 1998. [DOI] [PubMed] [Google Scholar]
- 136.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35: 169–191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V, Canteras NS, Ragozzino D, Gross CT. Independent hypothalamic circuits for social and predator fear. Nat Neurosci 16: 1731–1733, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol 342: 232–248, 1994. [DOI] [PubMed] [Google Scholar]
- 139.St-Pierre F, Marshall JD, Yang Y, Gong Y, Schnitzer MJ, Lin MZ. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci 17: 884–889, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Steckler T. Peptide receptor ligands to treat anxiety disorders. In: Handbook of Anxiety and Fear, edited by Blanchard DC, Griebel G, Nutt DJ. Amsterdam: Elsevier, 2008, p. 157–221. [Google Scholar]
- 141.Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol 330: 381–404, 1993. [DOI] [PubMed] [Google Scholar]
- 142.Sun N, Roberts L, Cassell MD. Rat central amygdaloid nucleus projections to the bed nucleus of the stria terminalis. Brain Res Bull 27: 651–662, 1991. [DOI] [PubMed] [Google Scholar]
- 143.Sun N, Yi H, Cassell MD. Evidence for a GABAergic interface between cortical afferents and brainstem projection neurons in the rat central extended amygdala. J Comp Neurol 340: 43–64, 1994. [DOI] [PubMed] [Google Scholar]
- 144.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194: 555–570, 1980. [DOI] [PubMed] [Google Scholar]
- 145.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–186, 1983. [DOI] [PubMed] [Google Scholar]
- 146.Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: a behavioral and neural analysis of predator odor-induced fear. Neurosci Biobehav Rev 29: 1157–1167, 2005. [DOI] [PubMed] [Google Scholar]
- 147.Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol 313: 295–325, 1991. [DOI] [PubMed] [Google Scholar]
- 148.Ursin H, Kaada BR. Functional localization within the amygdaloid complex in the cat. Electroencephalogr Clin Neurophysiol 12: 1–20, 1960. [DOI] [PubMed] [Google Scholar]
- 149.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428: 191–212, 2000. [DOI] [PubMed] [Google Scholar]
- 150.Veening JG, Swanson LW, Sawchenko PE. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res 303: 337–357, 1984. [DOI] [PubMed] [Google Scholar]
- 151.Veinante P, Yalcin I, Barrot M. The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1: 9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51: 32–58, 2004. [DOI] [PubMed] [Google Scholar]
- 153.Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508: 212–237, 2008. [DOI] [PubMed] [Google Scholar]
- 154.Viviani D, Charlet A, van den Burg E, Robinet C, Hurni N, Abatis M, Magara F, Stoop R. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333: 104–107, 2011. [DOI] [PubMed] [Google Scholar]
- 155.Viviani D, Stoop R. Opposite effects of oxytocin and vasopressin on the emotional expression of the fear response. Prog Brain Res 170: 207–218, 2008. [DOI] [PubMed] [Google Scholar]
- 156.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Walker P, Carrive P. Role of ventrolateral periaqueductal gray neurons in the behavioral and cardiovascular responses to contextual conditioned fear and poststress recovery. Neuroscience 116: 897–912, 2003. [DOI] [PubMed] [Google Scholar]
- 158.Werka T, Skar J, Ursin H. Exploration and avoidance in rats with lesions in amygdala and piriform cortex. J Comp Physiol Psychol 92: 672–681, 1978. [DOI] [PubMed] [Google Scholar]
- 159.Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26: 12387–12396, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr Comp Biol 42: 541–551, 2002. [DOI] [PubMed] [Google Scholar]
- 161.Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci 31: 1664–1670, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn Mem 14: 634–644, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]


