Abstract
The discovery of carotid bodies as sensory receptors for detecting arterial blood oxygen levels, and the identification and elucidation of the roles of hypoxia-inducible factors (HIFs) in oxygen homeostasis have propelled the field of oxygen biology. This review highlights the gas-messenger signaling mechanisms associated with oxygen sensing, as well as transcriptional and non-transcriptional mechanisms underlying the maintenance of oxygen homeostasis by HIFs and their relevance to physiology and pathology.
As early as 1868, Pflüger reported that hypoxia stimulates breathing (58), a finding that spurred investigation to identify the structures that “sense” systemic O2 levels and trigger physiological responses. Nearly 50 years later, Fernando de Castro, along with Jean-Francois Heymans and Corneille Heymans, independently identified carotid bodies as the “sensory organs” for monitoring arterial blood O2 levels (10, 24). Heymans et al. further demonstrated that hypoxia-induced stimulation of breathing is due to a chemosensory reflex arising from the carotid body (24). A later study by von Euler et al. (89) firmly established the sensory nature of the carotid body by directly recording the sensory nerve responses to arterial hypoxemia (i.e., decreased arterial blood O2 levels). Since identification of the carotid body as an O2-sensing organ, much attention has been focused on delineating the mechanisms underlying the transduction process. Given that the carotid body responds to hypoxemia within a few seconds, it is likely that the transduction process involves changes in existing proteins rather than de novo protein synthesis (37).
Prolonged hypoxia initiates a series of physiological responses over a time scale of hours to days to maintain O2 homeostasis. Examples include increased red blood cell production, formation of new blood vessels, and metabolic re-programming of cells, to name a few (72). The increased number of red blood cells improves O2 carrying capacity, whereas angiogenesis facilitates the transport of oxygenated blood to the tissues, and re-organization of metabolic machinery optimizes O2 utilization under prolonged O2 deprivation. While all these biological consequences of prolonged hypoxia have been well documented for over a century (91), the underlying molecular mechanisms remained elusive until the last quarter century. The discovery of hypoxia-inducible factor 1 (HIF-1) in 1992 (76) and the subsequent identification of other members of the HIF family of transcriptional activators revolutionized the current understanding of the molecular mechanisms underlying O2 homeostasis (63).
O2 sensing by the carotid body impacts systemic cardiovascular and respiratory homeostasis in contrast to local effects, such as pulmonary vasoconstriction in response to alveolar hypoxia. The goal of this brief review is to highlight recently identified mechanisms of O2 sensing by the carotid body and maintenance of O2 homeostasis by HIF-dependent transcriptional programming during continuous exposure to hypoxia. The role of the carotid body and HIFs in the pathological response to intermittent hypoxia has been reviewed recently (70) and will not be discussed here.
O2 Sensing
Sensing Elements and Characteristics of the Sensory Response to Hypoxia
The carotid bodies are located at the bifurcation of the common carotid artery into external and internal carotid arteries. The carotid bodies receive the highest blood flow per tissue weight of any organ in the body (1, 8, 9). The sensory innervation to the chemoreceptor tissue is provided by a branch of the glossopharyngeal nerve called the carotid sinus nerve (CSN). Under normoxia (arterial Po2 of ∼100 Torr), the CSN activity (i.e., the frequency of nerve impulses) is low but increases dramatically even with a modest drop in arterial Po2 (e.g., from 100 to 60-80 Torr) (4, 14, 27, 88). The response is remarkably fast, occurring within 0.2–0.4 s after onset of the stimulus in intact animals (5). The chemoreceptor tissue is a conglomeration of two cell types: type I or glomus cells, which are of neuronal phenotype, and the glia-like type II or sustentacular cells. A substantial body of evidence indicates that type I cells are the primary site of O2 sensing and that they work in concert with the nearby afferent nerve ending as a sensory unit (37).
Signaling Mechanisms
Sensory receptors detecting diverse external stimuli such as tactile, thermal, visual, olfactory, and gustatory sensations utilize two categories of signaling molecules to initiate the sensory transduction. Ion channels initiate the transduction of touch and temperature, whereas G-protein-coupled receptors (GPCRs) trigger the transduction cascade at visual, olfactory, and taste receptors (32). In contrast, emerging evidence suggests that hypoxic sensing by the carotid body chemoreceptor utilizes biochemical mechanisms involving O2-dependent interplay between two gaseous messengers: carbon monoxide (CO) and hydrogen sulfide (H2S).
O2-dependent CO generation by heme oxygenase 2.
CO is generated during the enzymatic degradation of heme by heme oxygenase 1 (HO-1) and HO-2, which require O2 as a reaction substrate (42). Although HO-1 is an inducible isoform, HO-2 is constitutively expressed in various tissues, including carotid body type I cells of cat, rat (60), mouse (52), and humans (49). HO-2 is the primary enzyme contributing to CO generation in the carotid body (95). Hypoxia leads to a graded reduction of CO levels in the carotid body (57), and CO is absent in HO-2-null carotid bodies (95). The O2-dependent modulation of CO generation was also observed in human embryonic kidney (HEK)-293 cells with forced expression of HO-2 (95). These findings demonstrate that CO generation from HO-2 is sensitive to changes in O2.
Mechanisms of O2 sensitivity of HO-2.
The inherent O2 sensitivity of HO-2 requires the presence of two cysteine residues (Cys265 and Cys282) in the heme regulatory motif, and substitution of alanine for Cys265 and Cys282 prevents the inhibition of CO generation under hypoxic conditions (95). With intact Cys265 and Cys282 residues, HO-2 exhibits low affinity for O2, with an apparent Km = 65 ± 5 Torr, whereas mutation of Cys265 and Cys282 dramatically increases O2 affinity (apparent Km = 25 ± 3 Torr). Physiological studies showed that CO signaling during normoxia inhibits CSN activity (57, 60). Under hypoxic conditions, CO-dependent inhibition is released, leading to carotid body activation. Emerging evidence indicates that CO mediates carotid body activation indirectly by regulating the synthesis of H2S, another gaseous messenger.
H2S synthesis by cystathionine-γ-lyase mediates carotid body activation.
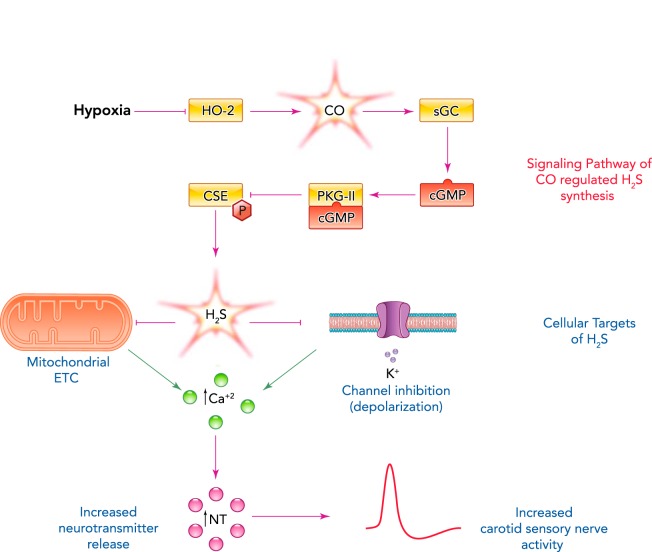
Type I cells express cystathionine-γ-lyase (CSE), an enzyme that catalyzes H2S generation (40, 56). Hypoxia increases H2S generation in the carotid body in a graded manner, and this response is markedly attenuated or absent following pharmacological blockade or genetic ablation of CSE activity (56). However, the increased H2S generation is not due to a direct effect of hypoxia on CSE, because in HEK-293 cells with forced expression of CSE, H2S generation was unaffected by low O2 (95). Recent studies have demonstrated that CO suppresses H2S levels by inhibiting CSE activity in the carotid body (56, 57) as well as in HEK-293 cells engineered to express both HO-2 and CSE (95). CO inhibits CSE by stimulating soluble guanylate cyclase, which produces cyclic GMP that activates protein kinase G (PKG). PKG-dependent phosphorylation of CSE at serine 377 renders the enzyme inactive, resulting in reduced H2S generation (95) (FIGURE 1).
FIGURE 1.
The O2-sensing and -signaling pathway in the carotid body
Schematic presentation of CO-regulated H2S generation in type I cells of the carotid body and its impact on sensory nerve activity. HO-2, heme oxygenase 2; sGC, soluble guanylate cyclase; cGMP, cyclic guanosine monophosphate; PKG- II, protein kinase G-II; CSE, cystathionine-ϒ-lyase; ETC, electron transport chain; NT, neurotransmitter.
The following findings demonstrate that CSE-derived H2S mediates increased CSN activity in response to hypoxia: 1) CSE-deficient mice exhibit a remarkable absence of CSN excitation by hypoxia and near absence of stimulation of breathing by low O2, a hallmark of the carotid body chemosensory reflex (56); 2) a similar reduction or absence of CSN responses to hypoxia was also seen following pharmacological inhibition of CSE (40, 56); and 3) exogenous application of H2S donors, like hypoxia, stimulate the CSN activity of several mammalian species, including mice, rats, rabbits, and cats (33, 40, 56).
Cellular basis of H2S-dependent activation of the carotid body by hypoxia.
The current consensus regarding the cellular basis of carotid body O2 sensing is that hypoxia depolarizes type I cells leading to Ca2+-dependent release of excitatory neurotransmitters, which, by stimulating the afferent nerve ending, increase CSN activity (15, 20, 37, 51, 62). Two theories have been proposed to explain how hypoxia facilitates Ca2+-dependent neurotransmitter release from type I cells. According to the membrane hypothesis, low O2 depolarizes type I cells by inhibiting K+ conductance, causing an influx of Ca2+ through voltage-gated Ca2+ channels, leading to neurotransmitter release. The metabolic or mitochondrial hypothesis proposes that hypoxia, by reducing a mitochondrial cytochrome, leads to depolarization of mitochondria that initiate sensory transduction (37).
The following findings suggest that H2S affects ion channel conductance in type I cells. First, H2S, like hypoxia, inhibits Ca2+-activated K+ channel activity (40, 85), as well as TASK-like K+ conductance, and depolarizes type I cells (6). Second, H2S increases [Ca2+]i in type I cells, and this response is not observed in the absence of extracellular Ca2+ (6, 43). Third, preventing the depolarization by voltage-clamping the type I cell at the resting membrane potential also abolishes H2S-induced [Ca2+]i elevation (6). Fourth, nifedipine, a blocker of L-type voltage-gated Ca2+ channels, as well as TTA-2, a T-type voltage-gated Ca2+ channel blocker, prevent H2S-mediated [Ca2+]i elevation in hypoxic type I cells (43, 44). Fifth, perhaps most important is the finding that CSE-null type I cells exhibit near absence of hypoxia-induced [Ca2+]i elevation and transmitter release (43).
Based on spectral analysis, it was proposed that carotid bodies express a unique cytochrome with low affinity for O2, which is half-reduced at Po2 levels of 60–80 Torr (39, 81). It is quite possible that the spectral changes in the carotid body previously attributed to a low-affinity cytochrome might in fact be arising from the heme-HO-2 complex because it exhibits similar redox-dependent spectral changes (47). Furthermore, the apparent Km of HO-2 for O2 is 65 ± 5 Torr (95). The finding that H2S increases NADH autofluorescence in type I cells (6) suggests that H2S mediates its actions in part by affecting the mitochondrial electron transport chain (ETC). Taken together, these findings suggest that H2S transduces the hypoxic stimulus by altering ion channel function, and perhaps also mitochondrial electron transport chain activity, in type I cells to elicit sensory nerve excitation.
O2 sensing in HO-2-null mice: evidence for a backup signaling mechanism.
The inherent O2 sensitivity of HO-2 and the ability of CO to regulate generation of the effector molecule H2S suggests that HO-2-dependent CO generation is obligatory for carotid body O2 sensing. Consistent with this possibility, HO-2-null mice exhibited increased basal CSN activity and H2S levels (95). However, hypoxia still stimulated the carotid body and increased H2S levels. Further analysis revealed that loss of HO-2 in type I cells resulted in a compensatory increase of another O2-sensitive gaseous messenger-generating enzyme, neuronal nitric oxide synthase (nNOS), which catalyzes generation of nitric oxide (NO). Remarkably, inhibition of nNOS eliminated sensory nerve excitation and H2S generation in response to hypoxia in HO-2-null carotid bodies (95). The O2 affinity of nNOS is much lower (apparent Km = 250 Torr) (95) than HO-2 (apparent Km = 65 Torr), suggesting that even a modest reduction in Po2 will lead to decreased NO production by nNOS. Furthermore, NO, like CO, inhibits CSN activity (50, 61) by binding to soluble guanylate cyclase and stimulating PKG-dependent phosphorylation of CSE at Ser377 (95). These findings suggest that, in the absence of HO-2 activity, the nNOS/NO system provides an alternative mechanism by which H2S generation can be regulated according to O2 availability, thereby providing an important fail-safe redundancy for a vital homeostatic process (FIGURE 1).
Physiological implications of gaseous messenger-mediated carotid body O2 sensing.
The chemosensory reflex is a critical regulator of autonomic functions including breathing, sympathetic tone, and blood pressure (16, 37). However, the chemosensory reflex exhibits substantial variation in healthy human subjects (90) and in rodents (25, 82). For instance, the hypoxic ventilatory response (HVR), a hallmark of the chemosensory reflex, is augmented in spontaneous hypertensive (SH) rats and reduced in Brown Norway (BN) rats compared with Sprague-Dawley (SD) rats (23, 25, 79).
SH rats, even before development of hypertension, exhibited heightened carotid body responses to hypoxia, and this effect was associated with increased basal and hypoxia-induced H2S levels and reduced CO levels in the chemoreceptor tissue compared with SD rats (57). It was further shown that the altered gas messenger levels were not due to changes in CSE or HO-2 protein levels. Instead, they appear due in part to decreased affinity of HO-2 for its substrate hemin, resulting in reduced CO generation and impaired inhibition of CSE, leading to high levels of H2S. Treatment with L-propargylglycine (L-PAG), an inhibitor of CSE, or with CORM-2, a CO donor, eliminated hypersensitivity of the SH carotid body to hypoxia (57).
An exaggerated carotid body chemosensory reflex has been implicated in the development of essential hypertension in SH rats (55, 64, 86). Five-week-old SH rats were treated with L-PAG every day for 5 wk. L-PAG-treated rats exhibited a pronounced reduction in blood pressure compared with vehicle-treated controls. Ablation of the carotid bodies from 5-wk-old SH rats also attenuated age-dependent hypertension to the same extent as L-PAG treatment (57). However, treating carotid body-ablated rats with L-PAG caused no further decline in blood pressure. These findings indicate that elevated H2S signaling in the carotid body contributes to the pathogenesis of hypertension in SH rats.
In striking contrast to SH rats, BN rats exhibited a severely impaired carotid body response to hypoxia, which was associated with elevated CO and reduced H2S levels compared with SD rats. The chemosensory reflex was nearly absent in BN rats, as evidenced by the lack of ventilatory and sympathetic nerve stimulation by low O2. Chronic exposure to high-altitude hypoxia leads to a carotid body-mediated increase in breathing, a phenomenon known as ventilatory adaptation to hypoxia (VAH) (11). Insufficient VAH leads to pulmonary edema (22, 26, 46). BN rats exposed to hypobaric hypoxia (0.35 atm; simulating 8,500-m altitude) for 16 h exhibited an absence of VAH and developed pulmonary edema, as evidenced by elevated protein levels in bronchoalveolar lavage fluid and an increased wet-to-dry lung weight ratio compared with SD rats (57). Treating BN rats with an HO inhibitor, which lowers CO levels, restored the carotid body response to hypoxia comparable to that seen in SD rats, improved VAH, and prevented pulmonary edema in response to hypobaric hypoxia (57). Taken together, these findings suggest that dysregulation of CO-H2S signaling in the carotid body leads to pathological consequences.
O2 Homeostasis
Maintenance of cellular O2 levels is critical because either insufficient or excess O2 leads to increased levels of reactive oxygen species (ROS), which oxidize lipids, proteins, and nucleic acids, leading to cell dysfunction and death. Thus both the delivery and the consumption of O2 are precisely regulated. Many different molecular mechanisms are utilized to maintain oxygen homeostasis. Here, we will focus on adaptive responses to continuous hypoxia that are mediated by HIFs.
HIFs
At the transcriptional level, HIFs function as master regulators of oxygen homeostasis by controlling O2 supply and demand. The HIFs are heterodimeric proteins that consist of an O2-regulated HIF-α subunit (HIF-1α, HIF-2α, or HIF-3α) and a constitutively expressed HIF-1 subunit. HIFs are subject to transcriptional, posttranscriptional, translational, and posttranslational regulation (63). In particular, the dioxygenases PHD2 and FIH-1 use O2 and α-ketoglutarate as substrates to hydroxylate proline and asparagine residues in the HIF-α subunits, which inhibit protein stability and transcriptional activity, respectively. Prolyl hydroxylation by PHD2 leads to binding of the VHL protein, which recruits an ubiquitin protein ligase that targets HIF-α subunits for proteasomal degradation, whereas asparagine hydroxylation by FIH-1 blocks binding of the co-activator protein p300 (63). Under hypoxic conditions, dioxygenase activity is inhibited, leading to HIF-α protein accumulation and induction of transcriptional activity, providing a molecular mechanism for the transduction of changes in O2 availability to changes in gene expression (FIGURE 2).
FIGURE 2.
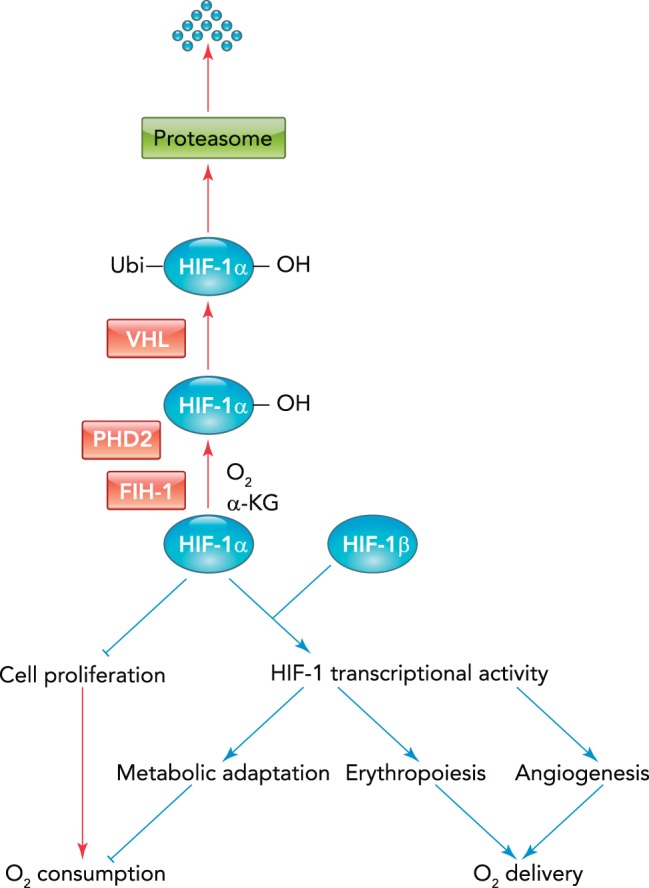
HIF-1 regulates O2 supply and demand
Pathways that are active under hypoxic and nonhypoxic conditions are indicated by blue and red arrows, respectively.
Over 1,500 target genes are known to be transactivated by binding of HIFs to a cis-acting hypoxia response element (HRE) containing the sequence 5′-RCGTG-3′ (R = A or G), located either within the target gene or its flanking sequences (75). Within any given cell, hypoxia increases the expression of hundreds of mRNAs and decreases the expression of a similar number of genes in a HIF-dependent manner (45). HIFs indirectly repress gene expression by transactivation of genes encoding transcriptional repressors and micro-RNAs. HIFs also have global epigenetic effects on transcription by transactivating genes encoding chromatin-modifying enzymes, such as histone demethylases and histone deacetylases (41, 74, 92, 93).
In addition to their canonical role in the regulation of gene transcription as components of heterodimeric DNA-binding proteins, isolated HIF-α subunits also play critical roles in hypoxic adaptation that are independent of HIF-1β. The HIF-1α subunit interacts with other DNA-binding transcription factors to function as either a co-activator or a co-repressor (74). HIF-1α and HIF-2α also regulate responses to hypoxia via non-transcriptional mechanisms: HIF-1α interacts with protein components of the pre-replication complex in hypoxic cells to regulate DNA synthesis (28, 29), and HIF-2α interacts with translation initiation factors to regulate protein synthesis under hypoxic conditions (87). Thus HIFs contribute to oxygen homeostasis by multiple molecular mechanisms and regulate the synthesis of DNA, mRNA, microRNA, and protein. The utilization of these molecular mechanisms results in physiological responses to continuous hypoxia that either increase O2 delivery or decrease O2 consumption, as illustrated by several examples below (FIGURE 2).
Regulation of O2 Delivery
Erythropoiesis.
Erythropoietin is a glycoprotein hormone produced by the kidney that binds to receptors on erythroid progenitor cells in the bone marrow and stimulates their survival, proliferation, and differentiation (30, 36, 80). EPO levels increase in response to anemia or systemic hypoxia, leading to increased blood O2-carrying capacity. HIF-1 was originally identified as a nuclear DNA-binding protein that was induced by hypoxia and bound to an HRE in the human EPO gene, which encodes erythropoietin (76). Subsequent studies have revealed that HIF-2 plays a critical role in regulating erythropoiesis by activating transcription of EPO and genes encoding proteins that are required for the absorption and delivery of iron to the bone marrow, such as ceruloplasmin, transferrin, transferrin receptor, and the iron membrane transport protein DMT1 (46, 53, 69, 74, 77, 94). Individuals with hereditary erythrocytosis (also known as congenital polycythemia) have excess red blood cell production due to missense mutations in the gene encoding PHD2, VHL, or HIF-2α, providing genetic proof for the key role of the HIF pathway in regulating erythropoiesis in humans (73, 94). EPO production is impaired in patients with chronic renal failure who are currently treated by injections of recombinant human erythropoietin (13). Prolyl hydroxylase inhibitors, which induce HIF activity, provide an alternative pharmacological approach to stimulating erythropoiesis by induction of hepatic EPO production (3, 12, 48, 67, 83).
Angiogenesis.
Whereas anemia results in systemic hypoxia, vascular disease results in local tissue hypoxia in the distribution of the affected vessel in the heart or limb muscle of patients with coronary or peripheral vascular disease, respectively. A further difference is that anemia results in reduced O2 availability (i.e., hypoxia), whereas vascular disease results in ischemia, a condition in which reduced tissue perfusion leads to reduced availability of O2 and energy substrates (e.g., glucose) as well as increased levels of toxic metabolites (e.g., CO2, H+, K+, and NH3). Remarkably, however, hypoxia appears to represent the critical stimulus that evokes an adaptive response to ischemia, principally through the HIF-dependent production of multiple angiogenic cytokines and growth factors, including vascular endothelial growth factor, angiopoietins, placental growth factor, platelet-derived growth factor B, stem cell factor, and stromal-derived factor 1, which stimulate angiogenesis, which is the formation of new capillaries, as well as arteriogenesis, which is the remodeling of existing vessels to increase luminal diameter and blood flow (66). Aging impairs vascular responses to ischemia by inhibiting HIF activity and thereby inhibiting the production of angiogenic factors as well as the ability of bone marrow-derived angiogenic cells to contribute to vascular responses (65, 66).
Regulation of O2 Consumption
Metabolic re-programming.
The erythropoietic and angiogenic responses to hypoxia do not increase O2 delivery immediately, since it may take days to weeks to increase red blood cell counts or generate new blood vessels that are sufficient to improve tissue oxygenation. In contrast, responses designed to reduce O2 consumption occur over the course of hours to days. The vast majority of O2 is consumed by the mitochondria during oxidative phosphorylation, which utilizes reducing equivalents generated during the oxidation of acetyl coenzyme A (AcCoA) to form an electrochemical gradient that is used to synthesize ATP. The electron transport chain (ETC), which is responsible for forming the gradient, is designed to operate most efficiently at physiological Po2, and deviations above or below result in premature loss of electrons, leading to reactive oxygen species formation. Thus the principal metabolic response to hypoxia is a switch from oxidative to glycolytic metabolism.
HIF-1 controls the expression of multiple gene products that mediate the metabolic switch. First, HIF-1 activates transcription of the PDK1 gene, encoding pyruvate dehydrogenase (PDH) kinase 1, which phosphorylates and inactivates the catalytic subunit of PDH, the enzyme that converts glucose-derived pyruvate into AcCoA for entry into the mitochondrial tricarboxylic acid (TCA) cycle (33, 54). Second, HIF-1 transactivates the LDHA gene, which encodes lactate dehydrogenase, the enzyme that converts pyruvate to lactate (74). Third, HIF-1 mediates a subunit switch in cytochrome c oxidase (ETC complex IV) by transactivation of the LONP gene, which encodes a protease that degrades COX4-1 and the COX4I2 gene, which encodes the alternative COX4-2 subunit (17). Under hypoxic conditions, yeast cells undergo a homologous subunit switch, which serves to increase the efficiency of electron transfer (38). However, yeast lack HIF-1, and the subunit switch occurs by a different molecular mechanism, suggesting that subunit switching arose independently but represents a universal response of unicellular and multicellular eukaryotes to hypoxia. Fourth, HIF-1 mediates the suppression of acyl CoA dehydrogenases that generate AcCoA by oxidation of fatty acids (96). Fifth, HIF-1 mediates the expression of miR-210, a micro-RNA that inhibits the expression of ISCU1 and ISCU2, which are iron-sulfur complex assembly factors that are required for activity of the TCA enzyme aconitase and ETC complex I (7). Sixth, HIF-1 activates expression of BNIP3 (96) and BNIP3L (2), which are mitochondrial proteins that trigger mitochondrial-selective autophagy, thereby reducing cellular oxidation of both glucose and fatty acids. Thus multiple mechanisms are available to modulate metabolism, with different tissues using different strategies to maintain redox homeostasis during hypoxia (17).
Cell cycle arrest.
Just as more mitochondria within a single cell consume more O2, more cells within a tissue also consume more O2. Thus a fundamental physiological response to hypoxia is cell cycle arrest. HIF-1α is necessary for hypoxia-induced cell cycle arrest (19, 28, 29, 35, 84), and forced expression of HIF-1α is sufficient to induce G1-phase cell cycle arrest (21) due to inhibition of Myc activity (35) and direct interaction of HIF-1α with protein components of the pre-replication complex that blocks origin firing and DNA replication (28, 29).
Some cell types, such as endothelial cells stimulated by hypoxia-induced angiogenic growth factors, must proliferate under hypoxic conditions. But to do so, they must counteract the inhibitory effect of HIF-1α on DNA replication. The cyclin-dependent kinase CDK2, which is active from late G1 through S phase and G2, binds to HIF-1α and triggers lysosomal degradation of the protein by chaperone-mediated autophagy, whereas CDK1, which is active from late G2 through M phase, binds to HIF-1α and protects it from lysosomal degradation (28). Thus the cell cycle-specific regulation of HIF-1α by CDK1 and CDK2 allows HIF-1α to perform its role (with HIF-1β) as a transcription factor to mediate adaptive responses to hypoxia while preventing HIF-1α from performing its non-transcriptional role as an inhibitor of DNA replication.
Perspectives
O2 sensing by the carotid body and the chemosensory reflex play a critical role in regulation of breathing and blood pressure in various physiological conditions, including exercise, high altitude, and pregnancy, to name a few (37). How these physiological situations affect the gas-messenger signaling in the carotid body and the underlying mechanisms remains to be studied. Enhanced carotid body sensitivity to hypoxia has been implicated in a variety of cardiovascular diseases with increased sympathetic nerve activity, including sleep apnea and neurogenic hypertension (37, 59). Recent studies from Schultz and coworkers (68) have shown that disrupted gaseous messenger signaling also mediates carotid body hyperactivity, which contributes to increased sympathetic activation in an experimental model of congestive heart failure. Surgical ablation of the carotid body was proposed as a potential therapeutic intervention for reducing blood pressure in patients with essential hypertension (55). However, carotid body ablation would abolish adaptive systemic cardiovascular and ventilatory responses to hypoxia (57). Pharmacological manipulation of the gaseous messenger-signaling pathway may represent a safer and more effective approach for treating carotid body-related morbidity.
In addition to the essential involvement of HIFs in development and physiology, in many common disease states, HIFs play either protective roles (anemia, inflammatory bowel disease, ischemic cardiovascular disease) or pathogenic roles (cancer, ocular neovascularization, pulmonary hypertension, sleep apnea, transplant rejection) (18, 31, 63, 71, 78). In addition, the ventilatory acclimatization to high-altitude hypoxia, which is mediated by the carotid body, and responses of the isolated carotid body to acute hypoxia are also dependent on HIF-1α expression (34), providing a direct and unifying link between the systemic and molecular O2-sensing and response pathways.
Footnotes
The research in N.R.P.'s laboratory is supported by National Heart, Lung, and Blood Institute Grants PO1-HL-90554 and UH2-HL-123610. G.L.S. is the C. Michael Armstrong Professor at the Johns Hopkins University School of Medicine.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: N.R.P. and G.L.S. prepared figures; N.R.P. and G.L.S. drafted manuscript; N.R.P. and G.L.S. edited and revised manuscript; N.R.P. and G.L.S. approved final version of manuscript.
References
- 1.Barnett S, Mulligan E, Wagerle LC, Lahiri S. Measurement of carotid body blood flow in cats by use of radioactive microspheres. J Appl Physiol 65: 2484–2489, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouyssegur J, Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29: 2570–2581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhardt WM, Wiesener MS, Scigalla P, Chou J, Schmieder RE, Günzler V, Eckardt KU. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol 21: 2151–2156, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biscoe TJ, Bradley GW, Purves MJ. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol 208: 99–120, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black AM, McCloskey DI, Torrance RW. The responses of carotid body chemoreceptors in the cat to sudden changes of hypercapnic and hypoxic stimuli. Respir Physiol 13: 36–49, 1971. [DOI] [PubMed] [Google Scholar]
- 6.Buckler KJ. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflügers Arch 463: 743–754, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke JA, de Burgh Daly M, Ead HW. Dimensions and volume of the carotid body in the adult cat, and their relation to the specific blood flow through the organ. A histological and morphometric study. Acta Anat (Basel) 126: 84–86, 1986. [DOI] [PubMed] [Google Scholar]
- 9.De Burgh Daly M, Lambertsen CJ, Schweitzer A. Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol 125: 67–89, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Castro F. Sur la structure et l'innervation de la glande intercarotidienne (glomus caroticum) de l'homme et des mammiferes et sur un nouveau systeme de l'innervation autonome du nerf glossopharyngien. Trav Lab Rech Biol 24: 365–432, 1926. [Google Scholar]
- 11.Dempsey JA, Forster HV. Mediation of ventilatory adaptations. Physiol Rev 62: 262–346, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Duan LJ, Takeda K, Fong GH. Hematological, hepatic, and retinal phenotypes in mice deficient for prolyl hydroxylase domain proteins in the liver. Am J Pathol 184: 1240–1250, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med 316: 73–78, 1987. [DOI] [PubMed] [Google Scholar]
- 14.Eyzaguirre C, Lewin J. Effect of different oxygen tensions on the carotid body in vitro. J Physiol 159: 238–250, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fidone SJ, Gonzalez C. Initiation and control of chemoreceptor activity in the carotid body. In: Handbook of Physiology. The Respiratory System. Control of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. II, pt. 1, chapt. 9, p. 247–312. [Google Scholar]
- 16.Fitzgerald RS, Lahiri S. Reflex responses to carotid/aortic body stimulation. In: Handbook of Physiology The Respiratory System Control of Breathing. Bethesda, MD: American Physiological Society, 1986, sect. 3, vol. II, pt. 1, chapt. 10, p. 313–362. [Google Scholar]
- 17.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129: 111–122, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14: 430–439, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 23: 359–369, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Hackenbeck T, Knaup KX, Schietke R, Schödel J, Willam C, Wu X, Warnecke C, Eckardt KU, Wiesener MS. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle 8: 1386–1395, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Hackett PH, Roach RC, Schoene RB, Harrison GL, Mills WJ Jr. Abnormal control of ventilation in high-altitude pulmonary edema. J Appl Physiol 64: 1268–1272, 1988. [DOI] [PubMed] [Google Scholar]
- 23.Hayward LF, Castellanos M, Noah C. Cardiorespiratory variability following repeat acute hypoxia in the conscious SHR versus two normotensive rat strains. Auton Neurosci 171: 58–65, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Heymans J, Heymans C. Sur les modifications directes et sur la regulationreflexede l'activitie du centre respiratory de la tete isolee du chien. Arch Int Pharmacodyn Ther 33: 273–372, 1927. [Google Scholar]
- 25.Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol 93: 974–983, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Hohenhaus E, Paul A, McCullough RE, Kucherer H, Bartsch P. Ventilatory and pulmonary vascular response to hypoxia and susceptibility to high altitude pulmonary oedema. Eur Respir J 8: 1825–1833, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Hornbein TF, Griffo ZJ, Roos A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol 24: 561–568, 1961. [DOI] [PubMed] [Google Scholar]
- 28.Hubbi ME, Kshitiz, Gilkes DM, Rey S, Wong CC, Luo W, Kim DH, Dang CV, Levchenko A, Semenza GL. A nontranscriptional role for HIF-1α as a direct inhibitor of DNA replication. Sci Signal 6: ra10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbi ME, Luo W, Baek JH, Semenza GL. MCM proteins are negative regulators of hypoxia-inducible factor 1. Mol Cell 42: 700–712, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev 72: 449–489, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Sung YK, Tian W, Qian J, Semenza GL, Nicolls MR. Graft microvascular disease in solid organ transplantation. J Mol Med 92: 797–810, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julius D, Nathans J. Signaling by sensory receptors. Cold Spring Harb Perspect Biol 4: a005991, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 99: 821–826, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 23: 1949–1956, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koury MJ. Abnormal erythropoiesis and the pathophysiology of chronic anemia. Blood Rev 28: 49–66, 2014. [DOI] [PubMed] [Google Scholar]
- 37.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Comp Physiol 2: 141–219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwast KE, Burke PV, Poyton RO. Oxygen sensing and the transcriptional regulation of oxygen-responsive genes in yeast. J Exp Biol 201: 1177–1195, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Lahiri S, Ehleben W, Acker H. Chemoreceptor discharges and cytochrome redox changes of the rat carotid body: role of heme ligands. Proc Natl Acad Sci USA 96: 9427–9432, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Sun B, Wang X, Jin Z, Zhou Y, Dong L, Jiang LH, Rong W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal 12: 1179–1189, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Luo W, Chang R, Zhong J, Pandey A, Semenza GL. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci USA 109: 3367–3376, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Makarenko VV, Nanduri J, Raghuraman G, Fox AP, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am J Physiol Cell Physiol 303: C916–C923, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makarenko VV, Peng YJ, Yuan G, Fox AP, Kumar GK, Nanduri J, Prabhakar NR. CaV3.2 T-type Ca2+ channels in H2S-mediated hypoxic response of the carotid body. Am J Physiol Cell Physiol 308: C146–C154, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105: 659–669, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119: 1159–1166, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCoubrey WK, Huang TJ, Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem 272: 12568–12574, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Minamishima YA, Kaelin WG. Reactivation of hepatic EPO synthesis in mice after PHD loss. Science 329: 407, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mkrtchian S, Kahlin J, Ebberyd A, Gonzalez C, Sanchez D, Balbir A, Kostuk EW, Shirahata M, Fagerlund MJ, Eriksson LI. The human carotid body transcriptome with focus on oxygen sensing and inflammation: a comparative analysis. J Physiol 590: 3807–3819, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moya EA, Alcayaga J, Iturriaga R. NO modulation of carotid body chemoreception in health and disease. Respir Physiol Neurobiol 184: 158–164, 2012. [DOI] [PubMed] [Google Scholar]
- 51.Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol 95: 657–667, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Ortega-Saenz P, Pascual A, Gomez-Diaz R, Lopez-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. J Gen Physiol 128: 405–411, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paliege A, Rosenberger C, Bondke A, Sciesielski L, Shina A, Heyman SN, Flippin LA, Arend M, Klaus SJ, Bachmann S. Hypoxia-inducible factor-2alpha-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int 77: 312–318, 2010. [DOI] [PubMed] [Google Scholar]
- 54.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 3: 187–197, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61: 5–13, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA 107: 10719–10724, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc Natl Acad Sci USA 111: 1174–1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pflüger E. Ueber die Ursache der Athembewegungen, sowie der Dyspnoë und Apnoë. Pflügers Arch Gesamte Physiol Meschen Tiere 1: 61–106, 1868. [Google Scholar]
- 59.Prabhakar NR. Sensing hypoxia: physiology, genetics and epigenetics. J Physiol 591: 2245–2257, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci USA 92: 1994–1997, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA. Nitric oxide in the sensory function of the carotid body. Brain Res 625: 16–22, 1993. [DOI] [PubMed] [Google Scholar]
- 62.Prabhakar NR, Peers C. Gasotransmitter regulation of ion channels: a key step in O2 sensing by the carotid body. Physiology 29: 49–57, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92: 967–1003, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Przybylski J. Do arterial chemoreceptors play a role in the pathogenesis of hypertension? Med Hypotheses 7: 127–131, 1981. [DOI] [PubMed] [Google Scholar]
- 65.Rey S, Luo W, Shimoda LA, Semenza GL. Metabolic reprogramming by HIF-1 promotes the survival of bone marrow-derived angiogenic cells in ischemic tissue. Blood 117: 4988–4998, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86: 236–242, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safran M, Kim WY, O'Connell F, Flippin L, Günzler V, Horner JW, Depinho RA, Kaelin WG. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA 103: 105–110, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schultz HD, Li YL. Carotid body function in heart failure. Respir Physiol Neurobiol 157: 171–185, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood 105: 3133–3140, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Semenza G, Prabhakar N. Neural regulation of hypoxia-inducible factors and redox state drives the pathogenesis of hypertension in a rodent model of sleep apnea. J Appl Physiol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76: 39–56, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood 114: 2015–2019, 2009. [DOI] [PubMed] [Google Scholar]
- 74.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med 2: 336–361, 2010. [DOI] [PubMed] [Google Scholar]
- 75.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 12: 5447–5454, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab 9: 152–164, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimoda LA, Laurie SS. HIF and pulmonary vascular responses to hypoxia. J Appl Physiol 116: 867–874, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst 56: 184–190, 1996. [DOI] [PubMed] [Google Scholar]
- 80.Spivak JL. The biology and clinical applications of recombinant erythropoietin. Semin Oncol 25: 7–11, 1998. [PubMed] [Google Scholar]
- 81.Streller T, Huckstorf C, Pfeiffer C, Acker H. Unusual cytochrome a592 with low Po2 affinity correlates as putative oxygen sensor with rat carotid body chemoreceptor discharge. FASEB J 16: 1277–1279, 2002. [DOI] [PubMed] [Google Scholar]
- 82.Strohl KP, Thomas AJ, St Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol 82: 317–323, 1997. [DOI] [PubMed] [Google Scholar]
- 83.Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood 111: 3229–3235, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7: 391–402, 2010. [DOI] [PubMed] [Google Scholar]
- 85.Telezhkin V, Brazier SP, Cayzac SH, Wilkinson WJ, Riccardi D, Kemp PJ. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir Physiol Neurobiol 172: 169–178, 2010. [DOI] [PubMed] [Google Scholar]
- 86.Trzebski A, Tafil M, Zoltwski M, Przybylski J. Central and peripheral chemosensitivity in early essential hypertension in man. In: Central Neurone Environment and the Control Systems of Breathing and Circulation, edited by Schlaefke ME, Koepchen HP, See W. New York: Springer-Verlag, 1983, p. 204–213. [Google Scholar]
- 87.Uniacke J, Holterman CE, Lachance G, Franovic A, Jacob MD, Fabian MR, Payette J, Holcik M, Pause A, Lee S. An oxygen-regulated switch in the protein synthesis machinery. Nature 486: 126–129, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vidruk EH, Olson EB, Ling L, Mitchell GS. Responses of single-unit carotid body chemoreceptors in adult rats. J Physiol 531: 165–170, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Von Euler U, Liljestrand G, Zotterman Y. The excitation mechanism of the chemoreceptors of the carotid body. Scand Arch Physiol 83: 132–152, 1939. [Google Scholar]
- 90.Weil JV. Variation in human ventilatory control-genetic influence on the hypoxic ventilatory response. Respir Physiol Neurobiol 135: 239–246, 2003. [DOI] [PubMed] [Google Scholar]
- 91.West JB. Oxygen sensing. High Alt Med Biol 13: 67, 2012. [DOI] [PubMed] [Google Scholar]
- 92.Wu CY, Tsai YP, Wu MZ, Teng SC, Wu KJ. Epigenetic reprogramming and post-transcriptional regulation during the epithelial-mesenchymal transition. Trends Genet 28: 454–463, 2012. [DOI] [PubMed] [Google Scholar]
- 93.Xia X, Lemieux ME, Li W, Carroll JS, Brown M, Liu XS, Kung AL. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Proc Natl Acad Sci USA 106: 4260–4265, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoon D, Ponka P, Prchal JT. Hypoxia and hematopoiesis. Am J Physiol Cell Physiol 300: C1215–C1222, 2011. [DOI] [PubMed] [Google Scholar]
- 95.Yuan G, Vasavda C, Peng YJ, Makarenko VV, Raghuraman G, Nanduri J, Gadalla MM, Semenza GL, Kumar GK, Snyder SH, Prabhakar NR. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal 8: ra37, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]