Abstract
Exercise can have anti-inflammatory effects in obesity, but the optimal type and intensity of exercise are not clear. This study compared short-term high-intensity interval training (HIIT) with moderate-intensity continuous training (MICT) in terms of improvement in cardiorespiratory fitness, markers of inflammation, and glucose control in previously inactive adults at elevated risk of developing type 2 diabetes. Thirty-nine inactive, overweight/obese adults (32 women) were randomly assigned to 10 sessions over 2 wk of progressive HIIT (n = 20, four to ten 1-min sessions at ∼90% peak heart rate, 1-min rest periods) or MICT (n = 19, 20-50 min at ∼65% peak heart rate). Before and 3 days after training, participants performed a peak O2 uptake test, and fasting blood samples were obtained. Both HIIT (1.8 ± 0.4 vs. 1.9 ± 0.4 l/min, pre vs. post) and MICT (1.8 ± 0.5 vs. 1.9 ± 0.5 l/min, pre vs. post) improved peak O2 uptake (P < 0.001) and lowered plasma fructosamine (P < 0.05). Toll-like receptor (TLR) 4 (TLR4) expression was reduced on lymphocytes and monocytes after both HIIT and MICT (P < 0.05) and on neutrophils after MICT (P < 0.01). TLR2 on lymphocytes was reduced after HIIT and MICT (P < 0.05). Plasma inflammatory cytokines were unchanged after training in both groups, but MICT led to a reduction in fasting plasma glucose (P < 0.05, 5.9 ± 1.0 vs. 5.6 ± 1.0 mmol/l, pre vs. post). Ten days of either HIIT or MICT can improve cardiorespiratory fitness and glucose control and lead to reductions in TLR2 and TLR4 expression. MICT, which involved a longer duration of exercise, may be superior for reducing fasting glucose.
Keywords: high-intensity interval training, prediabetes, aerobic exercise, glucose control, chronic inflammation
chronic low-grade inflammation, characterized by an increase in basal circulating proinflammatory cytokines and/or acute-phase reactants (9), is implicated in the pathogenesis of obesity, insulin resistance, and type 2 diabetes (T2D) (27). While the underlying cause of inflammation has not been fully elucidated, metabolic disruptions associated with insulin resistance have been shown to directly trigger innate immune responses. For example, hyperglycemia and elevated free fatty acids are linked with increased activation of immune cells, including increased surface protein expression of Toll-like receptors (TLRs) (11) and augmented release of proinflammatory cytokines (27). Studies have also reported elevated CD14+ monocyte TLR expression in patients with T2D compared with age-matched normoglycemic controls (10). TLRs are conserved pattern-recognition receptors that recognize a variety of exogenous and endogenous pathogens to coordinate innate immune responses (2). Increased TLR2 and TLR4 expression and the resulting proinflammatory environment are associated with a cluster of cardiometabolic risk factors, including insulin resistance, T2D, and atherosclerosis (10).
Exercise improves metabolic health and decreases the risk of T2D in individuals with prediabetes (8). One potent systemic benefit of regular exercise is thought to be its anti-inflammatory effects (33). Some of the anti-inflammatory effects of regular exercise are likely attributable to a reduction of adipose tissue (13), but there is also growing evidence that exercise, in the absence of weight loss, can directly impact immune cell phenotype and alter systemic inflammatory mediators (for review see Ref. 17). The ability of exercise to reduce monocyte TLRs is one hypothesized mechanism through which this anti-inflammatory effect may occur (38).
Multiple studies have shown reduced monocyte TLR4 expression after both acute exercise and training interventions (15, 38), but the impact of exercise on monocyte TLR2 is less clear (38). The influence of aerobic exercise on TLR expression on other distinct immune cells has not been adequately studied. One study examining TLR2 and TLR4 in mixed peripheral blood mononuclear cells (PBMCs) reported no effects after 15 days of aerobic exercise training (34). As PBMCs represent mostly lymphocytes and some monocytes, TLR expression on isolated cell types cannot be quantified using this technique.
Despite evidence for the anti-inflammatory impact of exercise, it is unclear what type or intensity of exercise is most effective (17). Recently, high-intensity interval training (HIIT) has gained attention as a time-efficient exercise strategy, providing a unique physiological stimulus compared with traditionally prescribed moderate-intensity continuous training (MICT) (45). A recent meta-analysis of 10 studies reported greater improvements in cardiorespiratory fitness following HIIT (average increase of 19.4%) compared with MICT (average increase of 10.3%) in patients with cardiometabolic disease (45). Studies have also reported greater improvements in endothelial function (40) and glucose control (25) after HIIT than traditional MICT in overweight and/or obese individuals. On the basis of the findings that HIIT promotes superior gains in cardiometabolic health, it is possible that HIIT may also have greater anti-inflammatory effects than MICT, but this hypothesis has not been tested.
The purpose of this study was to examine the impact of HIIT and MICT on markers of inflammation and cardiometabolic health in individuals at elevated risk of T2D. We examined 1) circulating pro- and anti-inflammatory cytokines, 2) leukocyte TLR2 and TLR4 expression, 3) ex vivo cytokine secretion in whole blood cultures, and 4) standard cardiometabolic health markers, before and after 2 wk of HIIT or MICT, in inactive, overweight/obese adults. We employed short-term training modeled after previous research (26) to minimize any changes in body composition to isolate the direct effects of HIIT and MICT on inflammatory parameters. We hypothesized that HIIT would result in greater improvements in cardiometabolic health than MICT. Because of the links between improved cardiorespiratory fitness (15, 29) and metabolic health (10) with reduced inflammation, we also tested the hypothesis that HIIT would lead to greater reductions in markers of inflammation than MICT.
METHODS AND MATERIALS
Participants
Participants recruited were considered to have prediabetes based on glycated hemoglobin (HbA1c; Bayer A1C Now) values between 5.7 and 6.4% (3) and/or a Canadian Diabetes Risk (CanRISK) assessment questionnaire score >21, along with body mass index (BMI) >24 kg/m2. Additionally, to be eligible, participants had to be inactive (assessed by standard 7-day physical activity recall interview conducted during screening and defined as completing fewer than two 30-min bouts of moderate-to-vigorous physical activity per week) and cleared for participation in vigorous activity as determined by the Canadian Society for Exercise Physiology (CSEP) Physical Activity Readiness Questionnaire-Plus (PAR-Q+) administered by a CSEP-certified exercise physiologist. Exclusion criteria included diagnosed diabetes, glucose-lowering medications, uncontrolled hypertension (blood pressure >160/90 mmHg), history of heart disease, myocardial infarction or stroke, and any other contraindications to exercise. All subjects provided written informed consent. The study was approved by the University of British Columbia Clinical Research Ethics Board. A total of 62 participants were screened, and 39 were eligible for the study and enrolled. Demographics and baseline characteristics are provided in Table 1.
Table 1.
Baseline characteristics of the participants before starting the 2-wk training
| HIIT (n = 20) | MICT (n = 18) | |
|---|---|---|
| %Female | 85 | 79 |
| Age, yr | 52 (10) | 52 (10) |
| CanRISK score | 29 (10) | 34 (11) |
| HbA1c, % | 6.0 (0.5) | 5.5 (0.4) |
| Blood pressure, mmHg | ||
| Systolic | 133 (17) | 131 (8) |
| Diastolic | 83 (7) | 82 (8) |
| V̇o2peak, ml·kg−1·min−1 | 20.4 (3.4) | 20.6 (4.9) |
| BMI, kg/m2 | 32.9 (6.6) | 31.4 (4.1) |
| Percent overweight (BMI 25–29.9 kg/m2) | 30 | 22 |
| Percent obese (BMI >30 kg/m2) | 65 | 73 |
| Medication, n | ||
| Antidepressants | 5 | 7 |
| Antihypertensive | 1 | 3 |
| Thyroid | 1 | 2 |
Values are means (SD). CanRISK, Canadian Diabetes Risk Assessment; HbA1c, glycated hemoglobin; V̇o2peak, peak O2 uptake; BMI, body mass index. Independent t-tests showed no differences between the 2 randomized groups at baseline (all P > 0.05).
Experimental Protocol
After they were screened, eligible participants were randomized to HIIT (n = 20: 3 men and 17 women) or MICT (n = 19: 4 men and 15 women). Both groups completed the same experimental protocol, which consisted of baseline (pre) testing, a 10-session exercise-training intervention over a 2-wk period, and posttesting conducted 48–72 h following the final training bout. Fasting blood samples were collected 48–72 h following the final training bout to avoid confounding influence from the last training bout (12).
Pretesting
Pretesting was conducted ≥7 days prior to the training program start date when participants had no current or recent infection symptoms (assessed through self-report). On the morning after an overnight (>8-h) fast, manual blood pressure was measured using Canadian Hypertension Education Program guidelines, and a blood sample was obtained from an antecubital vein by venipuncture. Body mass and height were assessed. Participants consumed a light snack prior to completing a continuous incremental ramp maximal exercise test on an electronically braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) to determine peak O2 uptake (V̇o2peak), peak heart rate (HRpeak), and peak power output (Wpeak). The test started at 50 W and increased by 15 W/min until volitional exhaustion and/or revolutions per minute fell below 50. Continual measures of O2 uptake and CO2 output were made by a metabolic cart (TrueOne 2400, Parvomedics, Salt Lake City, UT), which was calibrated with standard medical-grade gases and a 3.0-liter syringe before every test. V̇o2peak was defined as the highest 30-s average for O2 uptake (l/min and ml·kg−1·min−1). HRpeak and Wpeak were defined as the highest value achieved.
Training Intervention
The training program comprised 10 progressive sessions of exercise performed over a 2-wk period. Exercise sessions for HIIT and MICT were designed to be matched for external work based on calculations of %Wpeak obtained on the V̇o2peak test. Specifically, individuals randomized to HIIT began with four 1-min intervals at ∼85–90% Wpeak (eliciting ∼85–90% HRpeak) and increased to ten 1-min intervals by day 10. The interval protocol had a work-to-rest ratio of 1:1, with 1-min recovery intervals completed at 20% of Wpeak, and included a 3-min warm-up and cooldown at 32.5% Wpeak. This HIIT protocol was modeled after previous studies that indicate improvements in cardiometabolic health in individuals with, and at risk for, T2D (25, 26). Individuals randomized to MICT began with 20 min of continuous activity at ∼32.5% Wpeak (eliciting ∼60–65% HRpeak) (25) and gradually increased at the same percent increase in estimated total work up to 50 min by day 10. Participants completed the first and last sessions on a cycle ergometer at these prescribed intensities but were allowed to choose among treadmill walking, outdoor walking, elliptical training, and cycle ergometry for other sessions. During the intervention, participants wore downloadable heart rate monitors (FT7, Polar, Kempele, Finland) to ensure that training elicited the prescribed heart rate. Two trained research assistants supervised participants for 7 of the 10 exercise bouts in the lab. Three bouts (1 in week 1 and 2 in week 2) were performed independently as outdoor walking by participants at their home, with heart rate monitors used to ensure compliance.
Posttesting
At ∼48–72 h after the last training session, participants returned to the lab for posttesting, which was conducted in a manner identical to baseline testing.
Blood Measures
Metabolic markers.
Venous blood samples were collected into sodium heparin tubes (BD Vacutainer) by venipuncture using a 21-gauge needle (BD Eclipse). A portion of the sample was centrifuged at 1,200 g to obtain plasma and used for analyses. Fasting glucose was measured by the hexokinase method, fasting nonesterified fatty acids (NEFAs) were assessed by colorimetric assay (Wako Chemicals), and fasting insulin was measured by ELISA (Mercodia, Uppsala, Sweden) according to the manufacturer's instructions using a clinical chemistry analyzer (Chemwell 2910, Awareness Technologies), all in duplicate, with an average coefficient of variation (CV) <4% between duplicates. Homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) and HOMA β-cell function (HOMA-β) were calculated using the calculator provided by the University of Oxford Diabetes Trial Unit (www.dtu.ox.ac.uk/homacalculator). Plasma fructosamine was assessed by automated commercial assay (DZ112B-K, Diazyme, Poway, CA) using the aforementioned clinical chemistry analyzer. The CV of duplicates was <10%.
TLRs.
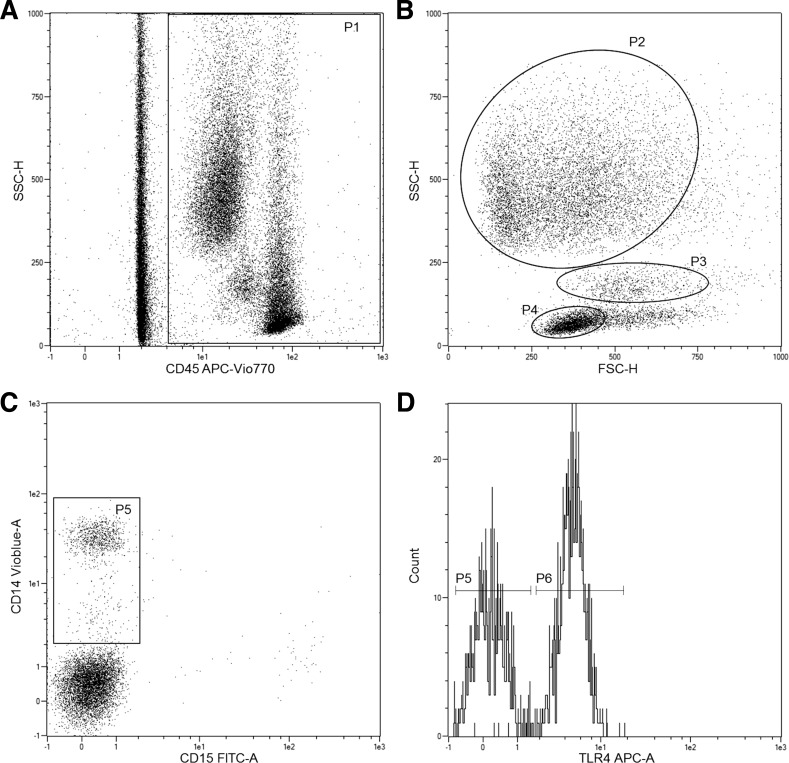
Whole blood was analyzed using flow cytometry to determine TLR2 and TLR4 expression on CD14+ monocytes, CD15+ neutrophils, and lymphocytes. Blood was kept on ice and analyzed within 45 min of collection. Six microliters of FcR blocking reagent were added to 54 μl of whole blood and incubated in darkness for 10 min at 4°C. Blood was stained with 10 μl each of CD45-APC-Vio770, CD14-VioBlue, CD15-FITC, TLR2-PE, and TLR4-APC (all from Miltenyi Biotech, Bergisch Gladbach, Germany), resulting in a 1:11 dilution of all antibodies. Samples were again incubated at 4°C in darkness for 10 min. MACSQuant running buffer (1 ml; Miltenyi Biotech, Bergisch Gladbach, Germany) was added to each of the samples, which were subsequently analyzed on a Miltenyi MACSQuant Analyzer 10 flow cytometer using a no-wash, no-lyse protocol. The trigger was set on the CD45+ channel to capture leukocyte events, while red blood cells and debris were omitted on the basis of lack of CD45 expression and scatter profile. Monocyte, neutrophil, and lymphocyte gates were established on the basis of characteristic forward and side scatter, as well as positive staining for CD14 (monocytes) and CD15 (neutrophils). Expression of TLR2 and TLR4 on CD14+ monocytes, CD15+ neutrophils, and lymphocytes was assessed as median fluorescence intensity using MACSQuant software. Fluorescence-minus-one controls were used to identify positive TLR2 and TLR4 events. The gating strategy is shown in Fig. 1.
Fig. 1.
Flow cytometry gating strategy used to quantify toll-like receptors (TLRs) on immune cell subtypes. Flow cytometry plots show gating cells with CD45+ trigger (P1; A), identification of monocyte (P3), lymphocyte (P4), and neutrophil (P2) populations based on scatter (B), gate for CD45+/CD14+ monocytes (P5; C), and histogram of TLR4 expression on CD14+ monocytes (P6) with corresponding fluorescence-minus-one (FMO) control (D).
Whole blood cultures.
Whole blood cultures were prepared by diluting blood 10 times in serum-free RPMI medium (Sigma) supplemented with penicillin (50 U/ml) and streptomycin (50 μg/ml) containing 5 mM glucose and seeding cells in 24-well cell culture plates at 540 μl per well, as we described previously (44). Cultures were stimulated with 1) the TLR4 agonist bacterial lipopolysaccharide (LPS, from Escherichia coli 055:B5; L6529, Sigma) at 100 pg/ml and 2) the synthetic bacterial lipoprotein TLR2 agonist PamCSK4 (L2000, EMC Microcollections, Tuebingen, Germany) at 100 ng/ml. Unstimulated cultures served as a control to confirm cytokine induction with LPS and PamCSK4. After 24 h of incubation at 37°C in 5% CO2, blood culture plates were centrifuged at 2,000 g for 15 min at 4°C, and supernatants were analyzed for tumor necrosis factor-α, interleukin (IL)-1β, IL-6, and IL-10 using ELISA (DuoSet human cytokine ELISA, R & D Systems) according to the manufacturer's instructions. Absorbance was read at 450 nm using a FluoStar Omega plate reader (BMG Labtech, Ortenburg, Germany), and cytokine secretion was expressed per CD45+ leukocyte as determined by flow cytometry.
Plasma cytokines.
Fasting plasma concentrations of tumor necrosis factor-α, IL-1β, IL-6, and IL-10 were analyzed by multiplex immunoassay (Custom MILLIPLEX panel, Millipore) and read on a MAGPIX Bio-Plex reader (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. Plasma was centrifuged at 10,000 g for 10 min at 4°C to remove any debris and analyzed in duplicate. The average CV for duplicates was <6%. Results were analyzed using Bio-Plex Manager 6.1 software.
Statistical Analysis
Data were analyzed using SPSS Statistics (v22, 2013). Normality was assessed using Q-Q plots and the Shapiro-Wilk test. Nonnormal data were log-transformed for analyses. Comparisons of baseline values between the two groups were analyzed with unpaired t-test. A series of two-factor (group × time) repeated-measures ANOVA were used to examine changes in cardiometabolic variables, anthropometrics, and markers of inflammation. Fisher's least significant difference post hoc tests were used to probe significant interactions. Statistical significance was set at P ≤ 0.05. Effect sizes were calculated using Cohen's d.
RESULTS
Of the 39 participants who were eligible and enrolled in the study, 20 were randomized to HIIT and 19 were randomized to MICT. All completed the 2-wk training period with no adverse effects; however, one female participant in MICT did not complete posttesting (no reason was provided: participant did not respond to phone calls). This participant was removed from analyses, resulting in final data available for 20 participants in HIIT and 18 in MICT. There were no significant differences between the two groups at baseline (Table 1). Expected workload intensity during training was confirmed, with the average %HRpeak elicited during HIIT being 82 ± 6% and MICT being 69 ± 7% (calculation included warm-up and cooldown). There was no difference in average %HRpeak between supervised sessions and home sessions (HIIT: 82 ± 4% vs. 82 ± 4%; MICT: 69 ± 6% vs. 69 ± 4%).
Anthropometrics
There was a significant effect of time on body mass (P = 0.029, Cohen's d = 0.11) and BMI (P = 0.025, Cohen's d = 0.08), with no difference between groups (group × time interaction, both P > 0.53). Body mass decreased on average by 0.54% (88.7 ± 20.1 vs. 88.0 ± 20.4 kg in HIIT, 88.0 ± 20.4 vs. 86.6 ± 13.5 kg in MICT). BMI decreased from 32.9 ± 6.6 to 32.6 ± 6.7 kg/m2 in HIIT and from 31.4 ± 4.1 to 31.3 ± 4.0 kg/m2 in MICT. There was a significant effect of time on mean arterial blood pressure (100 ± 9 vs. 98 ± 10 mmHg in HIIT, 98 ± 8 vs. 96 ± 7 mmHg in MICT, P = 0.010, Cohen's d = 0.22) but no differences between the groups.
Cardiorespiratory Fitness
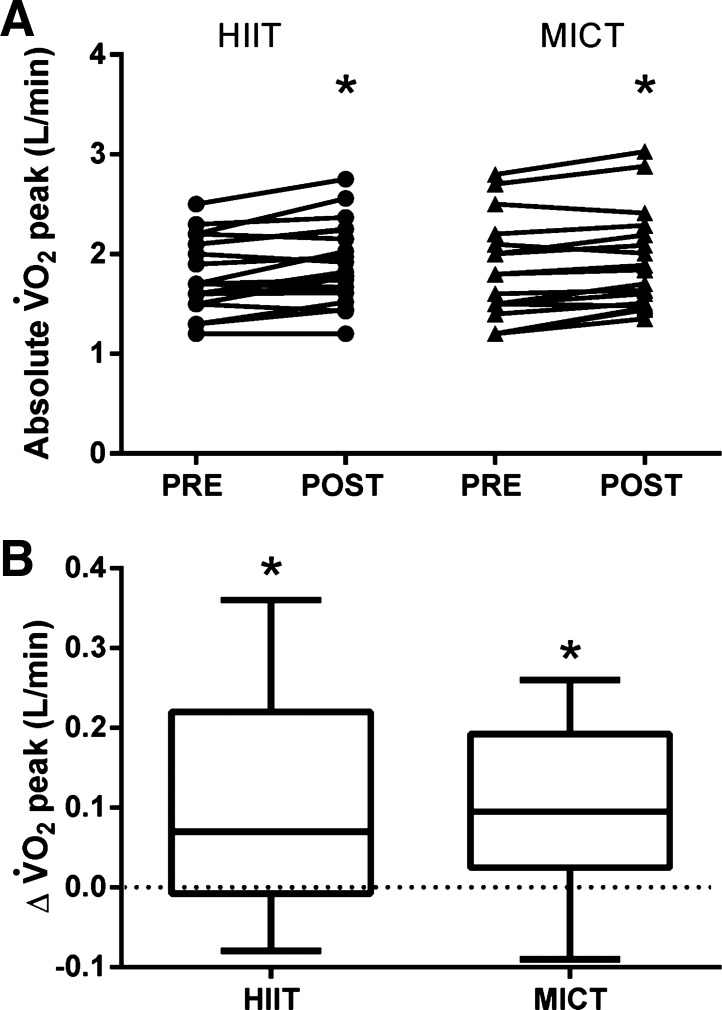
Changes in cardiorespiratory fitness over the 2-wk intervention are shown in Fig. 2. There was a significant main effect of time for absolute V̇o2peak (Fig. 2), relative V̇o2peak (20.4 ± 3.4 vs. 21.9 ± 4.0 ml·kg−1·min−1 in HIIT, 20.6 ± 4.9 vs. 22.1 ± 4.7 ml·kg−1·min−1 in MICT, P < 0.001, Cohen's d = 0.35), and Wpeak (152 ± 26 vs. 160 ± 29 W in HIIT, 153 ± 38 vs. 162 ± 36 W in MICT, P < 0.001, Cohen's d = 0.25), indicating that both HIIT and MICT improved fitness, with no difference between the two exercise conditions.
Fig. 2.
A 2-wk period of both high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) increases cardiorespiratory fitness [i.e., peak O2 uptake (V̇o2peak)]. A: individual changes. B: group change from pretraining. Main effect of time was significant (P < 0.05). *Significant increase from pretraining within group (P < 0.05).
Inflammatory Markers
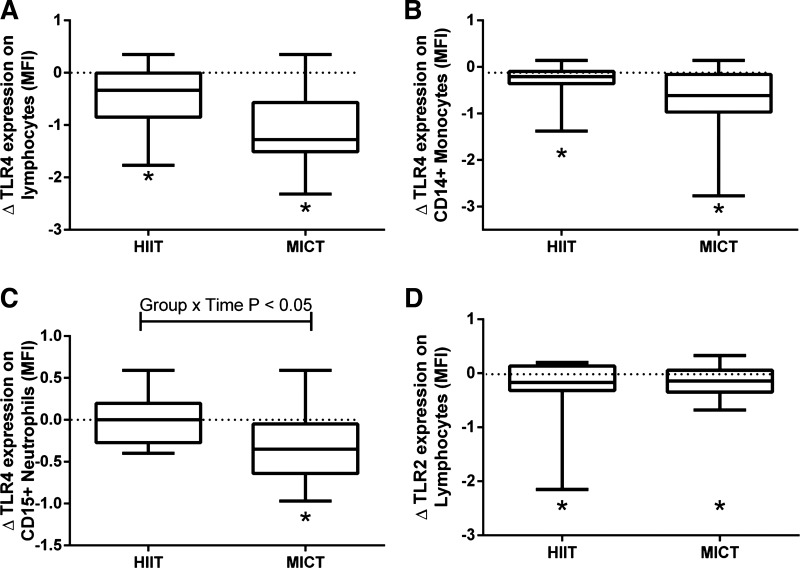
TLR4 expression on lymphocytes (Fig. 3A) and CD14+ monocytes (Fig. 3B) was reduced following training in both HIIT and MICT (main effects of time, both P < 0.05, Cohen's d = 1.54 and 0.68 respectively, reduced by ∼25% and ∼15%, respectively), with no difference between groups. There was a significant group × time interaction (P = 0.018) for TLR4 expression on CD15+ neutrophils (Fig. 3C), with post hoc tests revealing a reduction (∼15%) following MICT only (P = 0.003, Cohen's d = 1.10), with no change in HIIT. There was a significant main effect of time for lymphocyte TLR2 (P = 0.010, Cohen's d = 0.54), indicating a reduction with training (Fig. 3D; mean reduction of ∼5%). TLR2 on monocytes and neutrophils was not affected by training (both P > 0.20, data not shown). There were no significant changes in plasma cytokines (Table 2), except for an interaction effect on IL-10, with post hoc test showing a reduction after MICT (P < 0.05, Cohen's d = 0.11). The LPS and PamCSK4 stimulation led to significant induction of cytokines. As expected, cytokines were undetectable in unstimulated control cultures. There were no effects of training on cytokine induction with LPS or PamCSK4 stimulation in whole blood cultures (Table 2). Training had no effect on the concentration of blood monocytes, neutrophils, or lymphocytes (time main effects, all P > 0.20; Table 2).
Fig. 3.
A 2-wk period of both HIIT and MICT leads to reductions in immune cell TLR expression. TLR4 was measured on lymphocytes (A), CD14+ monocytes (B), CD15+ neutrophils (C), and TLR2 measured on lymphocytes (D) by flow cytometry before and after training. MFI, median fluorescence intensity. Main effects of time were significant for all (P < 0.05), with a significant group × time interaction for CD15+ neutrophils (P = 0.018). *Significant difference from pretraining within group. There were no significant changes in TLR2 on CD14+ monocytes or CD15+ neutrophils (data not shown).
Table 2.
Fasting plasma cytokines and LPS- and PamCSK4-induced cytokine release from whole blood cultures before and after 2 wk of HIIT or MICT
| HIIT |
MICT |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post | Time | Group | Interaction |
| Fasting plasma cytokines, pg/ml | |||||||
| TNF-α | 12.1 (3.4) | 13.3 (4.0) | 15.5 (5.2) | 15.1 (5.4) | 0.26 | 0.11 | 0.06 |
| IL-6 | 2.6 (1.4) | 2.8 (1.9) | 2.3 (1.5) | 2.0 (1.3) | 0.76 | 0.32 | 0.23 |
| IL-1β | 1.3 (1.6) | 1.2 (1.3) | 1.0 (1.6) | 1.0 (1.6) | 0.99 | 0.54 | 0.60 |
| IL-10 | 2.4 (2.9) | 2.3 (1.8) | 7.7 (11.6) | 6.4 (11.5) | 0.16 | 0.05 | 0.05 |
| LPS-induced cytokines, pg/CD45+ leukocyte x 104 | |||||||
| TNF-α | 3.3 (3.4) | 4.2 (4.2) | 5.1 (5.3) | 7.5 (5.8) | 0.13 | 0.26 | 0.47 |
| IL-6 | 8.7 (5.9) | 8.3 (5.5) | 7.0 (7.3) | 10.0 (5.9) | 0.40 | 0.99 | 0.26 |
| IL-1β | 1.3 (1.2) | 1.5 (1.6) | 1.1 (1.4) | 2.2 (1.0) | 0.22 | 0.66 | 0.40 |
| IL-10 | 1.1 (0.4) | 1.0 (0.3) | 0.9 (0.3) | 1.2 (0.4) | 0.18 | 0.84 | 0.10 |
| PamCSK4-induced cytokines, pg/CD45+ leukocyte x 104 | |||||||
| TNF-α | 2.3 (2.9) | 2.4 (1.5) | 2.0 (1.2) | 1.4 (1.0) | 0.74 | 0.43 | 0.52 |
| IL-6 | 8.4 (5.2) | 9.6 (4.8) | 8.7 (8.1) | 6.7 (7.6) | 0.66 | 0.70 | 0.06 |
| IL-1β | 0.2 (0.1) | 0.2 (0.2) | 0.3 (0.3) | 0.8 (1.4) | 0.23 | 0.25 | 0.36 |
| IL-10 | 1.3 (0.8) | 1.1 (0.2) | 1.4 (0.6) | 1.1 (0.6) | 0.17 | 0.92 | 0.88 |
| Immune cell number, x103 per μl/blood | |||||||
| Lymphocytes | 0.57 (0.2) | 0.51 (0.2) | 0.53 (0.2) | 0.55 (0.2) | 0.26 | 0.98 | 0.08 |
| CD14+ monocytes | 0.20 (0.07) | 0.19 (0.07) | 0.15 (0.05) | 0.16 (0.05) | 0.99 | 0.08 | 0.81 |
| CD15+ neutrophils | 2.5 (1.5) | 2.1 (0.7) | 2.1 (0.9) | 1.9 (0.5) | 0.20 | 0.30 | 0.87 |
Values are means (SD). HIIT, high-intensity interval training; MICT, moderate-intensity continuous training.
Metabolic Measures
Plasma fructosamine was reduced after training (main effect of time, P < 0.05, Cohen's d = 0.40; Table 3), with no difference between HIIT and MICT. There was a significant group × time interaction (P = 0.05; Table 3) for fasting plasma glucose, with post hoc tests revealing a reduction following MICT (P = 0.031, Cohen's d = 0.30) but no change in HIIT (P = 0.489). Neither HIIT nor MICT impacted fasting insulin, HOMA-IR, HOMA-β, or NEFA concentrations (Table 3).
Table 3.
Fasting plasma metabolic markers before and after 2 wk of HIIT or MICT
| HIIT |
MICT |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Variable | Pre | Post | Pre | Post | Time | Group | Interaction |
| Glucose, mmol/l | 5.6 (1.2) | 5.7 (1.1) | 5.9 (1.0) | 5.6* (1.0) | 0.34 | 0.78 | 0.05 |
| Fructosamine, μmol/l | 228 (93) | 177* (91) | 186 (97) | 161* (97) | 0.02 | 0.37 | 0.40 |
| Insulin, mU/l | 15.7 (11.4) | 15.1 (11.6) | 12.2 (3.5) | 13.0 (5.5) | 0.89 | 0.99 | 0.58 |
| HOMA-IR | 2.0 (1.4) | 2.0 (1.5) | 1.6 (0.5) | 1.7 (0.7) | 0.79 | 0.96 | 0.75 |
| HOMA-β | 112 (64) | 106 (58) | 94 (19) | 109 (31) | 0.37 | 0.69 | 0.07 |
| NEFA, mmol/l | 0.47 (0.24) | 0.49 (0.20) | 0.48 (0.16) | 0.48 (0.20) | 0.11 | 0.93 | 0.68 |
Values are means (SD). HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of β− cell function; NEFA, nonesterified fatty acid.
P < 0.05 vs. Pre within condition.
DISCUSSION
This study shows that, in previously inactive adults at an elevated risk of developing T2D, 2 wk of either HIIT or MICT significantly improved V̇o2peak and reduced TLR4 expression on monocytes and TLR2 and TLR4 expression on lymphocytes, with no differences between exercise conditions. MICT did appear superior to HIIT for reducing fasting plasma glucose and TLR4 expression on neutrophils. Both types of training improved glucose control assessed by plasma fructosamine. A small, but significant, reduction in body mass and BMI was found, but there were no significant changes in other markers of cardiometabolic health, including fasting plasma insulin, NEFA, or HOMA-IR.
Changes in TLR Expression
It is well accepted that exercise exerts an anti-inflammatory effect (17), and the significant effects of short-term exercise training on TLR2/4 shown here provide further evidence for this. Although previous studies have reported reduced monocyte TLR4 following exercise training (15, 38), increased monocyte TLR4 after 2 wk of HIIT has been reported (7). However, this is the first study, to our knowledge, to demonstrate reductions in TLR4 on neutrophils and TLR2 and TLR4 on lymphocytes. Both TLR2 and TLR4 have been implicated in the development of cardiometabolic disease, and both have been reported to be elevated on monocytes in conditions of high glucose or nutrient excess (10, 11). Agonism of both induces the release of cytokines to initiate an innate immune response. TLR2 responds primarily to gram-positive bacteria and bacterial lipoproteins, while TLR4 is the classic receptor for gram-negative cell wall components (i.e., LPS) (2). However, there are many reported endogenous and exogenous ligands for TLR2 and TLR4 that could perpetuate an inflammatory response in the context of obesity or metabolic disease (2, 10).
A large component of the innate immune response may have been missed by previous research in which only changes in monocyte TLR expression are measured, as monocytes only make up 5–15% of circulating immune cells (35). A previous study also employing short-term training showed no effect on TLRs measured in isolated PBMCs (containing lymphocytes and monocytes); however, the discrepancy may be explained by differences in training volume and technique of TLR measurement (Western blotting in PBMCs) compared with our study (34). The reduction in TLRs on multiple immune cell types highlights a direct and systemic effect that could partly explain the anti-inflammatory response to aerobic exercise. The relative importance of differences in TLR2 and TLR4 expression across different immune cells following exercise is not known. However, on the basis of our novel findings of changes in TLR2 on lymphocytes after both HIIT and MICT and TLR4 on neutrophils after MICT, it would seem that, in future research, lymphocytes and neutrophils should be examined, in addition to monocytes, when the cellular mechanisms underlying the anti-inflammatory effects of exercise are assessed.
Overall, both HIIT and MICT reduced cellular markers of inflammation, but MICT led to a greater reduction in TLR4 on CD15+ neutrophils. Neutrophil TLR4 is important in the innate immune response (19) and appears to regulate neutrophil survival and activation (36). The reduction in neutrophil TLR4 after MICT supports lower innate immune activation in this cell type and possible reductions in inflammation following training in MICT. The importance of altered neutrophil TLR4 following exercise requires further investigation. HIIT and MICT were not matched for exercise duration to maintain the time efficiency of HIIT in this study. As a result, individuals randomized to MICT spent approximately twice as much time exercising. It is possible that the longer-duration exercise and/or the moderate intensity of exercise had a greater impact on neutrophil TLR4.
Mechanisms Underlying the Reduction in Immune Cell TLR
The mechanisms behind exercise-induced TLR4 reductions are not fully understood. Reduced expression of TLR4 may occur as a result of low-dose exposure to exogenous ligands, including LPS, peptidoglycan, and double-stranded RNA, as well as heat shock protein (14), all of which may increase during each exercise bout throughout a training program. Exposure to these ligands may induce TLR4 tolerance, which can be measured as a decrease in expression (14). Additionally, recent research indicates that acute exercise-induced changes in anti-inflammatory gene expression may have the potential to modulate TLR expression and function (1). It is unclear how potential changes in these processes culminate or interact to alter TLR expression, highlighting the need for future mechanistic research, particularly with regard to individual sessions of HIIT or MICT within a training program.
As high glucose in vitro and hyperglycemia in vivo (10, 11) have been linked to elevated TLR4 surface expression on immune cells, exercise-mediated reductions in plasma glucose may be a potential mechanism leading to lower TLR4 surface protein expression. The reduction in plasma fructosamine, which reflects average blood glucose concentration over an ∼2-wk period (18), provides some support for the notion that improved glucose control may have contributed to lower TLRs. However, given that we found reduced fasting glucose after MICT only and reductions in TLR4 after both HIIT and MICT, reduced hyperglycemia does not seem to fully explain our findings.
Previous studies have indicated that the anti-inflammatory effects of exercise are primarily mediated by weight loss (17, 47). Using short-term training, we aimed to minimize reductions in fat mass to assess the direct effects of exercise on markers of inflammation. However, there was a significant main effect of time for body mass, BMI, and waist circumference, indicating that training did lead to a small, but statistically significant, reduction. Given that these reductions were minimal and had small effect sizes, we believe that reduction in immune cell TLRs was not related to changes in body mass.
Impact of HIIT on Inflammation
To our knowledge, this is the first short-term training study to compare the inflammatory profile after HIIT vs. MICT in previously inactive overweight or obese individuals at elevated risk for T2D. Our findings indicate that 2 wk of HIIT has an apparent anti-inflammatory effect as measured by a reduction in monocyte TLR4 and lymphocyte TLR2 and TLR4 expression. However, our hypothesis that HIIT would lead to greater reductions in inflammatory markers was not supported. If anything, there were greater anti-inflammatory effects of MICT as supported by larger reductions in neutrophil TLR4. These findings provide preliminary evidence that moderate-intensity exercise may lead to greater anti-inflammatory responses in inactive individuals who are overweight and/or obese.
Circulating Cytokines and Metabolic Markers
The significant reduction in immune cell TLR2 and TLR4 occurred without a change in basal circulating proinflammatory cytokines, in agreement with previous research (28). Plasma cytokines originate from spillover from various organs and tissues, such as adipose, skeletal muscle, liver, circulating immune cells (46), and blood vessels (27). Thus, despite potential anti-inflammatory effects detected at the cellular level, changes in inflammatory cytokines may not be detected in plasma. We believe that this highlights the additional insight provided by measuring the impact of exercise at the level of immune cells as opposed to solely assessing plasma cytokines.
Plasma IL-10, known to be anti-inflammatory (21), was decreased with MICT only. This finding was somewhat unexpected and may warrant further study. However, it is important to recognize that an increase in plasma IL-10 may be an early compensatory response to chronic low-grade inflammation (5); thus it could be speculated that reduced plasma IL-10 is indicative of lower, as opposed to greater, inflammation. Additionally, each exercise bout may result in a temporary increase in circulating IL-10 (32), resulting in the development of an anti-inflammatory environment after each training session.
The reduction of plasma fructosamine after both HIIT and MICT provides evidence that, over the course of the 2-wk training period, overall exposure to hyperglycemia was lower. Previous research has shown reduced hyperglycemia assessed by continuous glucose monitoring following an acute session of HIIT (25) and MICT (42) in individuals at elevated risk of T2D. Therefore, the reduction in fructosamine likely reflects the cumulative effect of glucose lowering following each bout of exercise throughout training. Despite equal effects on fructosamine, fasting plasma glucose was only reduced following MICT. This supports previous findings that indicate that duration of exercise may be the most important factor for improving glucoregulation in response to exercise (30). One explanation for this effect of exercise duration may be that when longer-duration exercise is prescribed, there are fewer hours in which sedentary behavior can occur. Alternatively, differences in fatty acid oxidation related to exercise intensity may have an important role in altering glucose homeostasis, possibly via reductions in ectopic lipid deposition (30). However, we did not see any changes in fasting plasma insulin or insulin resistance estimated by HOMA after training. The lack of change in HOMA is in agreement with some (37), but not all (20), short-term training studies. It could be that body composition changes are needed for exercise to improve insulin sensitivity (23).
LPS-Stimulated Whole Blood Cultures
Previous studies examining MICT and/or resistance training have shown that reduced TLR4 is accompanied by reduced induction of cytokines in whole blood stimulated with LPS (28, 39). In the current study, reductions in TLR4 expression were not accompanied by changes in cytokine secretion following LPS stimulation of whole blood cultures. This indicates that, despite a reduction in cellular markers of inflammation, innate immune function has been maintained. A longer training intervention may be required to detect changes in cytokine secretion (28). In addition, whole blood culture supernatants were only collected at 24 h of stimulation, which may not have been optimal for detection of differences in individual cytokines.
Limitations
It is possible that the predominance of women in this study may have influenced the findings, and this may explain the difference between our results and a previous study that showed an increase in monocyte TLR4 after 2 wk of HIIT in men (7). Previous studies have described differences in inflammatory response to LPS stimulation (4) and glucose control response to HIIT (16) between men and women. We did not have the statistical power to analyze potential sex differences, but when data from only the female participants were analyzed, the results remained the same as when data from men and women were combined.
Although participants were told not to alter their diet during the course of the study, we did not specifically control or measure dietary intake, so it is possible that alterations in diet may have contributed to the changes in body mass/BMI. However, the small changes in body mass were equal between groups and likely of little clinical significance.
Our results indicate that, over 2 wk, both HIIT and MICT may have anti-inflammatory effects; however, future studies are needed to determine how these exercise interventions compare over the long term. Changes to adipose tissue mass over prolonged (12–16 wk) training may contribute to greater anti-inflammatory effects, but this remains to be determined. The optimal exercise for fat loss is unclear, as some studies have indicated superior fat loss after HIIT (6, 41), whereas others report greater fat loss after MICT (24, 31) or comparable changes between these two types of training (43).
Conclusion
Short-term HIIT and MICT significantly improved V̇o2peak and reduced monocyte and lymphocyte TLR4 expression and lymphocyte TLR2 expression in a group of inactive adults at risk of developing T2D. These findings support the idea that reduction in TLRs on multiple immune cell types is a possible direct anti-inflammatory response to short-term exercise training at either high or moderate intensity. Both HIIT and MICT reduced plasma fructosamine, providing evidence of improved glucose control. However, MICT showed a significant reduction in fasting plasma glucose, which was not seen after HIIT, and a greater reduction in neutrophil TLR4, which may be attributable to longer-duration exercise. More research is warranted to determine whether the direct anti-inflammatory effects at the cellular level are related to improvement in cardiometabolic health and reduction of the risk for T2D over time.
GRANTS
This study was funded through an internal grant provided by the University of British Columbia to J. P. Little and M. E. Jung and a Natural Sciences and Engineering Research Council of Canada Discovery Grant awarded to J. P. Little.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.R., C.D., S.S., J.E.B., E.V., and J.P.L. performed the experiments; E.R., C.D., S.S., and J.P.L. analyzed the data; E.R., C.D., S.S., M.E.J., J.E.B., and J.P.L. interpreted the results of the experiments; E.R. and C.D. prepared the figures; E.R. and J.P.L. drafted the manuscript; E.R., C.D., M.E.J., J.E.B., E.V., and J.P.L. edited and revised the manuscript; E.R., C.D., S.S., M.E.J., J.E.B., E.V., and J.P.L. approved the final version of the manuscript; M.E.J. and J.P.L. developed the concept and designed the research.
REFERENCES
- 1.Abbasi A, Hauth M, Walter M, Hudemann J, Wank V, Niess AM, Northoff H. Exhaustive exercise modifies different gene expression profiles and pathways in LPS-stimulated and un-stimulated whole blood cultures. Brain Behav Immun 39: 130–141, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett 85: 85–95, 2003. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 36 Suppl 1: S67–S74, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 16: 340–343, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Avdiushko R, Hongo D, Lake-Bullock H, Kaplan A, Cohen D. IL-10 receptor dysfunction in macrophages during chronic inflammation. J Leukoc Biol 70: 624–632, 2001. [PubMed] [Google Scholar]
- 6.Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes 2011: 868305, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Child M, Leggate M, Gleeson M. Effects of two weeks of high-intensity interval training (HIIT) on monocyte TLR2 and TLR4 expression in high BMI sedentary men. Int J Exerc Sci 6: 10, 2013. [Google Scholar]
- 8.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B, American College of Sports Medicine, American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement executive summary. Diabetes Care 33: 2692–2696, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25: 4–7, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Dasu MR, Devaraj S, Park S, Jialal I. Increased Toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 33: 861–868, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces Toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57: 3090–3098, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durrer C, Robinson E, Wan Z, Martinez N, Hummel ML, Jenkins NT, Kilpatrick MW, Little JP. Differential impact of acute high-intensity exercise on circulating endothelial microparticles and insulin resistance between overweight/obese males and females. PLos One 10: e0115860, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher G, Hyatt TC, Hunter GR, Oster RA, Desmond RA, Gower BA. Effect of diet with and without exercise training on markers of inflammation and fat distribution in overweight women. Obesity (Silver Spring) 19: 1131–1136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn MG, McFarlin BK. Toll-like receptor 4: link to the anti-inflammatory effects of exercise? Exerc Sport Sci Rev 34: 176–181, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol 95: 1833–1842, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gibala MJ, Gillen JB, Percival ME. Physiological and health-related adaptations to low-volume interval training: influences of nutrition and sex. Sports Med 44: 127–137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol 11: 607–615, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM, Sacks DB. Tests of glycemia in diabetes. Diabetes Care 27: 1761–1773, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood 102: 2660–2669, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Hood MS, Little JP, Tarnopolsky MA, Myslik F, Gibala MJ. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Med Sci Sports Exerc 43: 1849–1856, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Joyce DA, Gibbons DP, Green P, Steer JH, Feldmann M, Brennan FM. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur J Immunol 24: 2699–2705, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol 19: 39–45, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Karstoft K, Winding K, Knudsen SH, Nielsen JS, Thomsen C, Pedersen BK, Solomon TP. The effects of free-living interval-walking training on glycemic control, body composition, and physical fitness in type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 36: 228–236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keating SE, Machan EA, O'Connor HT, Gerofi JA, Sainsbury A, Caterson ID, Johnson NA. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J Obes 2014: 834865, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little JP, Jung ME, Wright AE, Wright W, Manders RJ. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl Physiol Nutr Metab 39: 1–7, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Jung ME, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol 111: 1554–1560, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 121: 2111–2117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc 36: 1876–1883, 2004. [DOI] [PubMed] [Google Scholar]
- 29.McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, Timmerman KL, Coen PM. Physical activity status, but not age, influences inflammatory biomarkers and Toll-like receptor 4. J Gerontol A Biol Sci Med Sci 61: 388–393, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Newsom SA, Everett AC, Hinko A, Horowitz JF. A single session of low-intensity exercise is sufficient to enhance insulin sensitivity into the next day in obese adults. Diabetes Care 36: 2516–2522, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nybo L, Sundstrup E, Jakobsen MD, Mohr M, Hornstrup T, Simonsen L, Bülow J, Randers MB, Nielsen JJ, Aagaard P. High-intensity training versus traditional exercise interventions for promoting health. Med Sci Sports Exerc 42: 1951–1958, 2010. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Reyna SM, Tantiwong P, Cersosimo E, DeFronzo RA, Sriwijitkamol A, Musi N. Short-term exercise training improves insulin sensitivity but does not inhibit inflammatory pathways in immune cells from insulin-resistant subjects. J Diabetes Res 2013: 107805, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogacev KS, Ulrich C, Blomer L, Hornof F, Oster K, Ziegelin M, Cremers B, Grenner Y, Geisel J, Schlitt A, Kohler H, Fliser D, Girndt M, Heine GH. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur Heart J 31: 369–376, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Sabroe I, Prince LR, Jones EC, Horsburgh MJ, Foster SJ, Vogel SN, Dower SK, Whyte MK. Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 170: 5268–5275, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Skleryk J, Karagounis L, Hawley J, Sharman MJ, Laursen PB, Watson G. Two weeks of reduced-volume sprint interval or traditional exercise training does not improve metabolic functioning in sedentary obese men. Diabetes Obes Metab 15: 1146–1153, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. Influence of exercise training and age on CD14+ cell-surface expression of Toll-like receptor 2 and 4. Brain Behav Immun 19: 389–397, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? J Leukoc Biol 84: 1271–1278, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Tjonna AE, Lee SJ, Rognmo O, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slordahl SA, Kemi OJ, Najjar SM, Wisloff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118: 346–354, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trapp E, Chisholm D, Freund J, Boutcher S. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes 32: 684–691, 2008. [DOI] [PubMed] [Google Scholar]
- 42.van Dijk J, Manders R, Tummers K, Bonomi A, Stehouwer C, Hartgens F, van Loon L. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 55: 1273–1282, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallman K, Plant LA, Rakimov B, Maiorana AJ. The effects of two modes of exercise on aerobic fitness and fat mass in an overweight population. Res Sports Med 17: 156–170, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Wan Z, Durrer C, Mah D, Simtchouk S, Little JP. One-week high-fat diet leads to reduced Toll-like receptor 2 expression and function in young healthy men. Nutr Res 34: 1045–1051, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Weston KS, Wisloff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 48: 1227–1234, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Womack J, Tien PC, Feldman J, Shin JH, Fennie K, Anastos K, Cohen MH, Bacon MC, Minkoff H. Obesity and immune cell counts in women. Metab Clin Exp 56: 998–1004, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yudkin J. Inflammation, obesity, and the metabolic syndrome. Arterioscler Thromb Vasc Biol 39: 707–709, 2007. [DOI] [PubMed] [Google Scholar]