Abstract
Muscle sympathetic nerve activity is increased during normotensive pregnancy while mean arterial pressure is maintained or reduced, suggesting baroreflex resetting. We hypothesized spontaneous sympathetic baroreflex gain would be reduced in normotensive pregnant women relative to nonpregnant matched controls. Integrated muscle sympathetic burst incidence and total sympathetic activity (microneurography), blood pressure (Finometer), and R-R interval (ECG) were assessed at rest in 11 pregnant women (33 ± 1 wk gestation, 31 ± 1 yr, prepregnancy BMI: 23.5 ± 0.9 kg/m2) and 11 nonpregnant controls (29 ± 1 yr; BMI: 25.2 ± 1.7 kg/m2). Pregnant women had elevated baseline sympathetic burst incidence (43 ± 2 vs. 33 ± 2 bursts/100 heart beats, P = 0.01) and total sympathetic activity (1,811 ± 148 vs. 1,140 ± 55 au, P < 0.01) relative to controls. Both mean (88 ± 3 vs. 91 ± 2 mmHg, P = 0.4) and diastolic (DBP) (72 ± 3 vs. 73 ± 2 mmHg, P = 0.7) pressures were similar between pregnant and nonpregnant women, respectively, indicating an upward resetting of the baroreflex set point with pregnancy. Baroreflex gain, calculated as the linear relationship between sympathetic burst incidence and DBP, was reduced in pregnant women relative to controls (−3.7 ± 0.5 vs. −5.4 ± 0.5 bursts·100 heart beats−1·mmHg−1, P = 0.03), as was baroreflex gain calculated with total sympathetic activity (−294 ± 24 vs. −210 ± 24 au·100 heart beats−1·mmHg−1; P = 0.03). Cardiovagal baroreflex gain (sequence method) was not different between nonpregnant controls and pregnant women (49 ± 8 vs. 36 ± 8 ms/mmHg; P = 0.2). However, sympathetic (burst incidence) and cardiovagal gains were negatively correlated in pregnant women (R = −0.7; P = 0.02). Together, these data indicate that the influence of the sympathetic nervous system over arterial blood pressure is reduced in normotensive pregnancy, in terms of both long-term and beat-to-beat regulation of arterial pressure, likely through a baroreceptor-dependent mechanism.
Keywords: pregnancy, baroreflex control, blood pressure, sympathetic nerve activity
pregnancy is a major physiological stressor, necessitating significant cardiovascular adaptations to support the healthy development of fetus and mother. Hemodynamic adaptations that occur during normotensive pregnancies are characterized by systemic vasodilation concomitant with elevations in cardiac output and blood volume, resulting in a curvilinear decrease in mean arterial pressure (MAP) (6). This drop in mean arterial pressure has been linked to elevated risk of syncope and presyncope in pregnant women relative to nonpregnant women (15, 25). Conversely, up to 8% of all pregnancies result in the de novo development of acute hypertension, including gestational hypertension and preeclampsia (48). These disorders are associated with significant increases in maternal-fetal morbidity and mortality (43, 53, 57), and moreover, women who develop maternal hypertensive disorders have an elevated lifelong risk of developing cardiovascular disease (53). These pregnancy-specific morbidities are suggestive of pregnancy-induced changes to the sympathetic regulation of arterial blood pressure in both normotensive and hypertensive women. However, information regarding the sympathetic control of blood pressure in pregnancy is lacking, and to date there have been no systematic evaluations of acute baroreflex control of sympathetic nervous system activity during normotensive pregnancy in humans (18).
Although regulation of arterial blood pressure is multifaceted, sympathetic nervous system regulation of the peripheral vasculature plays a major role. Sympathetic nerve activity contributes to long-term blood pressure regulation such that elevations in resting muscle sympathetic nerve activity (MSNA) have been associated with chronic hypertension and other cardiovascular diseases (27, 32). Moreover, acute (beat-to-beat) control of blood pressure is also strongly influenced by baroreflex regulation of sympathetic activity in nonpregnant populations, correcting acute falls in blood pressure (11, 52). However, the regulation of MSNA during pregnancy has only recently begun to be elucidated (17, 19, 21, 25, 38).
Contrary to expectation given a reduction in vascular resistance and blood pressure (6), MSNA is modestly elevated during normotensive pregnancy. This increase in sympathetic activity manifests within the first trimester (25) and persists throughout gestation (17, 19, 38). The disparity between reduced vascular resistance and blood pressure despite elevated sympathetic activity suggests a decreased contribution of the sympathetic nervous system to overall blood pressure regulation. Previous studies have evoked generalized baroreceptor unloading (drops in arterial pressure associated with head-up tilt or Valsalva's maneuver) to examine the reflex sympathetic responses which are enacted to maintain or restore arterial pressure (25, 42). These studies have indicated that sympathetic responses are similar between normotensive pregnant women and nonpregnant controls. However, these previous assessments did not control the magnitude of the stimulus (i.e., change in blood pressure) between groups or include specific quantifications of sympathetic baroreflex gain (the slope of the relationship between arterial pressure and sympathetic nerve activity). Therefore, it remains unknown whether the acute (i.e., beat-by-beat) regulation of blood pressure, via the sympathetic baroreflex, is depressed during pregnancy in humans.
An improved understanding of blood pressure regulation during normotensive pregnancies is important for understanding syncope and presyncope in these women. They also serve as a critical bench mark needed to identify potential mechanisms which contribute to the development of hypertension in some pregnancies. Therefore, the purpose of this study was to quantify the role of the sympathetic nervous system in the acute regulation of blood pressure in normotensive pregnant women in the third trimester of pregnancy relative to nonpregnant, similarly aged women. We hypothesized that pregnancy would be associated with reduced muscle sympathetic baroreflex gain (i.e., blunted sympathetic responses to spontaneous fluctuations in blood pressure) in conjunction with an upward resetting of the baroreflex set point (i.e., a higher basal sympathetic activity for a given basal pressure).
MATERIALS AND METHODS
Participants.
Data from 11 healthy pregnant women and 11 healthy nonpregnant controls who had volunteered to participate in larger studies examining neurovascular regulation were selected for analysis (see Table 1 for participant characteristics). These data have not been published previously. All women participated after providing written, informed consent. All test protocols performed in these studies were approved by the Health Research Ethics Board at the University of Alberta and conformed to the standards set by the latest revision of the Declaration of Helsinki.
Table 1.
Participant anthropometrics and baseline characteristics
| Nonpregnant | Pregnant | P | |
|---|---|---|---|
| n | 11 | 11 | |
| Gestation, wk | NA | 33 ± 1 | |
| Age, yr | 29 ± 1 | 31 ± 1 | 0.1 |
| Height, cm | 165 ± 1 | 166 ± 2 | 0.6 |
| Pregnant BMI, kg/m2 | NA | 27.5 ± 1.2 | |
| Non/prepregnant BMI, kg/m2 | 25.2 ± 1.7 | 23.4 ± 0.9 | 0.4 |
| Mean arterial pressure, mmHg | 91 ± 2 | 88 ± 3 | 0.4 |
| Systolic blood pressure, mmHg | 118 ± 2 | 112 ± 3 | 0.3 |
| Diastolic blood pressure, mmHg | 73 ± 2 | 72 ± 3 | 0.7 |
| Heart rate, bpm | 71 ± 2 | 83 ± 3 | 0.002 |
| R-R interval, ms | 861 ± 19 | 736 ± 26 | 0.002 |
| Respiration rate, breaths/min | 15.5 ± 0.9 | 17.9 ± 1.2 | 0.02 |
| Sympathetic burst frequency, bursts/min | 26 ± 2 | 39 ± 3 | 0.001 |
| Sympathetic burst incidence, bursts/100 hb | 33 ± 2 | 43 ± 2 | 0.01 |
| Total sympathetic activity, au | 1,140 ± 55 | 1,811 ± 148 | <0.001 |
NA, not applicable; BMI, body mass index.
All participants were normotensive nonsmokers, free of respiratory, cardiovascular, and neurological diseases. All pregnant women had singleton pregnancies and were tested in the third trimester. None of the participants reported a history of gestational diabetes, gestational hypertension, or preeclampsia. Nonpregnant participants were selected based on similar age and body mass index (BMI) (prepregnancy BMI) as the pregnant participants; height and weight were measured in all participants, while prepregnancy weight was self-reported. Four nonpregnant women were using hormonal contraceptives which inhibited monthly menses (Mirena intrauterine device, n = 3; Micronor oral contraceptive, n = 1). Because of the lack of menses in these participants, women taking hormonal contraceptives were tested at their convenience during the high exogenous (low endogenous) hormone phase of contraceptive use. Women not taking hormonal contraceptives were tested in the early follicular (low endogenous hormone) phase of the menstrual cycle.
Experimental protocol.
All measurements were collected a minimum of 1 h following a light standardized meal, and after participants had abstained from caffeine, alcohol, and strenuous exercise for 12 h. Women were seated in a semirecumbent position in a dentist-style chair in a quiet laboratory. Following study-specific instrumentation, all participants relaxed for an initial baseline period of not less than 10 min. For the purpose of this analysis, a minimum of 5 min of baseline data were extracted (range = 6–17 min), which were collected prior to the completion of any physiological perturbations specific to the individual studies in which the women were participating.
Measures.
Baseline arterial blood pressure was assessed manually prior to the baseline data collection period. Blood pressure was determined with a mercury sphygmomanometer by trained observers. The average of three manual blood pressures was used later to calibrate the blood pressure values obtained by photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). Calibrated beat-by-beat pressure waveforms were analyzed to determine MAP, systolic (SBP), and diastolic (DBP) blood pressures. Heart rate was determined from the standard three-lead electrocardiogram. As a component of the larger studies from which these data were obtained, participants breathed through a mouthpiece which allowed for the recording of respiratory patterns. From these data we assessed respiration rate.
Postganglionic MSNA was assessed by microneurography at the peroneal (fibular) nerve (model 662C-3; Iowa University Bioengineering). A reference electrode was positioned subcutaneously 1–3 cm from the sympathetic recording site, while a tungsten microelectrode (35 mm in length, 200 μm in diameter, uninsulated 1–5 μm tip) was inserted transcutaneously into the nerve and manipulated into position within proximity of sympathetic neurons innervating vasculature within muscle. A muscle sympathetic site was determined based on the production of pulse-synchronous bursts of activity which increased in firing frequency during a voluntary apnea, but were unaffected by arousal to a loud noise (12). Neuronal recordings were amplified 1,000 times by a preamplifier and 10,000 times by a variable-gain, isolated amplifier before being bandpass filtered (700–2,000 Hz), rectified, and integrated (time constant 0.1 s).
Data analysis.
Bursts of sympathetic activity were identified by semiautomated peak detection by a trained observer. Sympathetic nerve activity was quantified as burst frequency (bursts/min), burst incidence (bursts/100 heart beats), as well as burst amplitude and total MSNA (amplitude × burst incidence). To account for interparticipant differences in burst amplitude which are indicative of the number (34) and size (49) of the neurons within the recording range of the electrode, and are therefore affected by electrode position, a normalization was applied to the burst amplitude data. Within each participant, all bursts were expressed relative to the largest burst observed at baseline, which was assigned a value of 100. The impact of pregnancy on the baroreflex control of sympathetic nerve activity was assessed by relating spontaneous burst incidence and total MSNA (22) to corresponding fluctuations in DBP values (52). Baroreflex set point was determined by comparing prevailing DBP and burst incidence and total MSNA between groups. That is, a difference in prevailing DBP for a given level of sympathetic nerve activity would indicate a lateral shift in the baroreflex set point, while a difference in sympathetic activity for a given DBP would indicate an upward/downward shift in the set point.
The slopes of the relationships between MSNA burst incidence/total MSNA and DBP were calculated to represent spontaneous sympathetic baroreflex gain (52). Briefly, MSNA data were shifted backward so that the peak of each sympathetic burst coincided with the diastolic period which initiated it. Burst amplitude data, as they corresponded to each DBP, were then extracted. Diastolic blood pressure data were then averaged into 2-mmHg bins. The percent occurrence of a sympathetic burst (ranging from 0 to 100%) within each DBP bin provided the values of sympathetic burst incidence. To determine the relationship between total MSNA and DBP, the sum of normalized burst amplitudes were determined within each DBP bin. This value was then divided by the number of bursts within each bin to calculate mean amplitude per burst, which was then multiplied by burst incidence of the corresponding DBP bin to generate a value of total MSNA for each DBP bin.
The spontaneous cardiovagal baroreflex control was determined by the sequence method (2, 39, 46). Briefly, regression analyses were performed on sequences of three or more consecutive cardiac cycles exhibiting concurrent changes in systolic blood pressure and R-R interval (both rising or both falling) (2, 50). Numbers of cardiac cycles and baroreflex sequences used for analysis were not different between groups. On average, the analysis was performed on 675 ± 236 cardiac cycles resulting in 89 ± 30 baroreflex sequences for analysis. We analyzed 11 of 22 data sets by using a lag 0 criterion (2), which is consistent with evidence that the R-R interval is generally modulated within the same cardiac cycle (40). However, seven data sets were analyzed by using a lag 1 criterion and four data sets were analyzed by using a lag 2 criterion which increased the number of baroreflex sequences (50). The mean slope of identified sequences was taken to represent cardiovagal baroreflex gain. Only regressions with an r2 > 0.85 were included in the analysis. The cardiovagal set point was determined as the quotient of the prevailing R-R interval and systolic blood pressure.
Because of the presence of sigmoidal curves in some participants, a two-step approach was used to isolate the linear portion of the baroreflex curves. First, three blinded, unbiased, independent reviewers visually inspected the data points of each participant's baroreflex curve to determine the upper and lower ends of the linear portion of each curve. The upper and lower plateaus of each curve, where they existed, were then removed from the analysis to isolate the linear portion of the curve. Consensus between two reviewers was required for removal of data. Next, the linear baroreflex data were fitted by using a linear regression to determine the slope in each participant.
Statistical analyses.
Baseline hemodynamics, respiratory, and MSNA characteristics were compared between groups by using unpaired, equal variance, two-tailed t-tests; alpha was set at 0.05. Baroreflex slopes were calculated for each participant and were also compared between groups by using unpaired two-tailed t-tests. Associations between cardiovagal and sympathetic gains, as well as respiratory rates and sympathetic gains, were determined by linear regression analyses.
RESULTS
Baseline hemodynamic and muscle sympathetic characteristics are presented in Table 1. Heart rate and respiration rates were significantly elevated, and R-R interval was reduced, in pregnant women, whereas blood pressures were similar between pregnant and nonpregnant women. Sympathetic burst frequency, incidence, and total MSNA were greater in pregnant women relative to the nonpregnant controls (Fig. 1).
Fig. 1.
Sample microneurography recordings from a nonpregnant (A) and a pregnant woman (B). BMI, body mass index; DBP, diastolic blood pressure; hb, heartbeat.
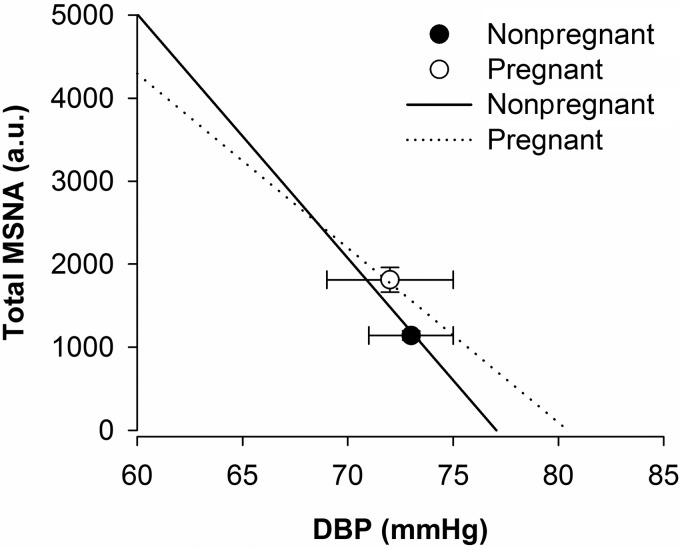
All linear regressions between DBP and both sympathetic burst incidence and total MSNA (i.e., sympathetic baroreflex gain) had R values greater than 0.8 (range: 0.82–0.99). The mean slope of the linear portion of the baroreflex curve was significantly steeper in nonpregnant women than in pregnant women, regardless of whether MSNA was quantified as burst incidence (−5.4 ± 0.5 vs. −3.7 ± 0.5 bursts·100 heart beats−1·mmHg; P = 0.03) (Fig. 2) or total MSNA (−294 ± 24 vs. −210 ± 24 au·100 heart beats−1·mmHg; P = 0.03) (Fig. 3).
Fig. 2.
Sympathetic burst incidence baroreflex gain in normotensive nonpregnant and pregnant women in the third trimester of gestation. A: muscle sympathetic nerve activity (MSNA) burst incidence was significantly elevated in normotensive pregnant women relative to nonpregnant controls. DBPs were similar between groups, indicating an upward resetting of the sympathetic baroreflex curve with pregnancy. The linear portion of the sympathetic baroreflex curves were less steep in pregnant women relative to the nonpregnant control participants (−3.7 ± 0.5 vs. −5.4 ± 0.5 bursts·100 heart beats−1·mmHg−1; P = 0.03), indicating a blunting of sympathetic baroreflex gain with pregnancy. B and C: sample sympathetic baroreflex curves in nonpregnant and pregnant women, respectively. BI, burst incidence. Data are means ± SE.
Fig. 3.
Total sympathetic activity baroreflex gain. The set point of the baroreflex was reset upward in normotensive pregnant women relative to nonpregnant controls. The linear portion of the total sympathetic baroreflex curve was less steep in pregnant women relative to nonpregnant women (−294 ± 24 vs. −210 ± 24 au·100 heart beats−1·mmHg; P = 0.03). Data are means ± SE.
Cardiovagal baroreflex gain was not different between nonpregnant controls and pregnant women (49 ± 8 vs. 36 ± 8 ms/mmHg; P = 0.2) (Fig. 4). In pregnant women, sympathetic burst incidence baroreflex gain was negatively associated with cardiovagal baroreflex gain (R = −0.7; P = 0.02). However, no relationship was observed between these slopes in the nonpregnant women (R = −0.2; P = 0.6).
Fig. 4.
Cardiovagal baroreflex gain. The set point of the cardiovagal baroreflex was reset downward in pregnant women relative to nonpregnant controls, favoring shorter R-R intervals without significant changes in systolic bloods pressure (SBP) during pregnancy. However, the slopes of the cardiovagal baroreflex curves were not significantly different between pregnant and nonpregnant women (36 ± 8 vs. 49 ± 8 ms/mmHg; P = 0.2). Data are means ± SE.
To determine whether differences in respiration rates could account for the pregnancy-related differences in sympathetic baroreflex gain, baroreflex slopes were regressed against respiration rates in both groups of women. However, respiration rates were unrelated to either sympathetic or cardiovagal baroreflex gain.
DISCUSSION
This study is the first to directly examine spontaneous sympathetic baroreflex gain in pregnant women, and as such provides the first human evidence that sympathetic regulation of beat-to-beat blood pressure is reduced in normotensive pregnancy. We observed a significant association between cardiovagal and sympathetic baroreflex gains in the pregnant women, suggesting a common underlying mechanism, such as the baroreceptors themselves, in the etiology of this reduction in sympathetic baroreflex gain. These data also demonstrate an upward shift in the sympathetic baroreflex set point during pregnancy (i.e., an elevation in mean sympathetic nerve activity despite a maintenance of arterial pressure) relative to the nonpregnant state, further indicating a reduced role of the sympathetic nervous system in the long-term regulation of blood pressure during normotensive pregnancy.
Prior to this study, all research regarding sympathetic baroreflex sensitivity during pregnancy has been conducted with animal models. These studies indicate that baroreflex-mediated increases in renal sympathetic nerve activity during acute reductions in blood pressure are blunted in pregnant rabbits (5) and rats (33). Similar studies also indicate that the maximum gain of the sympathetic baroreflex is attenuated by pregnancy (5, 9), although not all studies have replicated this finding (33). While our study is the first to directly examine sympathetic baroreflex gain in pregnant women, previous studies have examined generalized sympathetic responses to acute changes in blood pressure. During head-up tilt (25) and Valsalva's maneuver (42), similar increases in MSNA have been documented in normotensive pregnant women and nonpregnant controls in the first (25) and third (42) trimesters of pregnancy, suggesting that absolute baroreflex-mediated sympathoexcitatory responses are not affected by pregnancy.
Several factors may contribute to an attenuation of sympathetic baroreflex function during pregnancy. First, respiration rate is increased during normal, healthy pregnancy. This induces more frequent swings in intrathoracic pressure, inducing fluctuations in blood pressure which might affect baroreflex gain. However, we failed to observe any association between respiration rate and either sympathetic or cardiovagal baroreflex slopes. Therefore, pregnancy-dependent differences in breathing patterns cannot explain the attenuation to sympathetic baroreflex gain. Alternatively, arterial distensibility may be increased in pregnant women (47), which would reduce baroreflex loading (or unloading) for any given change in blood pressure. Further, some evidence [but not all (28)] suggests that aortic baroreceptor afferent nerve responses are attenuated for a given distending pressure in pregnant rats relative to control animals (24). There is good [but not universal (29)] evidence that cardiovagal baroreflex regulation of heart rate is also impaired during normal pregnancy (3, 19, 30, 44) and further so during hypertensive pregnancy disorders (16, 44). These data support a mechanism specific to the baroreceptors themselves. To investigate this further, we compared cardiovagal baroreflex gain between the pregnant women and the nonpregnant controls. Although baseline cardiovagal baroreflex gain was not different between groups, we observed a significant, negative correlation between sympathetic burst incidence baroreflex gain and cardiovagal gain in the pregnant women only. These data indicate that the steeper (and more positive) the cardiovagal baroreflex slope, the steeper (and more negative) the sympathetic baroreflex slope. While it has been established that cardiovagal baroreflex sensitivity is not related to sympathetic baroreflex sensitivity in young, healthy humans (14), the association observed in the pregnant women is suggestive of a common, baroreceptor-dependent mechanism at play which contributes to the blunting of sympathetic gain in pregnant women relative to nonpregnant controls. Finally, central integration sites of sympathetic outflow also appear to be downregulated in pregnancy, as indicated by animal studies (10, 13). The etiology of these adaptations remains speculative, but the increases in circulating sex hormones (estradiol, progesterone, and testosterone) which occur during human pregnancy (37) have been suggested to contribute to these mechanisms (4, 18), as have changes in pressor and pituitary hormones (4). A recent review by Brooks and colleagues (4) has highlighted the possible roles of elevated circulating 3-alpha-hydroxy-dihydroprogesterone, a metabolite of progesterone, and reduced effects of circulating insulin, both of which may act to impair central baroreflex processing in the pregnant brain.
The secondary finding of our investigation was the upward shift in the sympathetic and downward shift in the cardiovagal baroreflex set point in pregnant women. Our participants were tested in the third trimester of pregnancy, during which blood pressure returns toward prepregnancy values (6), and as a result we observed blood pressures which were similar between the pregnant and nonpregnant women. However, this occurred within the context of a pregnancy-induced sympathoexcitation, reflected as increases in sympathetic burst frequency, incidence, and total sympathetic activity. These data imply that sympathetic neurovascular transduction, the translation of the sympathetic neural signal into a vascular outcome, is inhibited by pregnancy, a phenomenon which was documented in a recent study conducted in the first trimester of pregnancy (25). Increases in circulating nitric oxide have been hypothesized to oppose the vasoconstrictor influence of elevated sympathetic nerve activity (25, 45). Such increases might be elicited through an increase in shear stress on the vascular endothelium due to elevated cardiac output (7, 56), and/or an estradiol-mediated increase in nitric oxide synthase (51). Alternatively, the efferent sympathetic signal could be associated with reductions in neurotransmitter release with pregnancy, thereby reducing the vasoconstrictor effects of a given burst of sympathetic activity. Indeed, some studies support a lack of change in circulating norepinephrine during normotensive pregnancy (35, 36, 54), although other studies have documented elevated norephinephrine relative to nonpregnant controls (1, 8, 25, 58). However, a complete understanding of the mechanisms which contribute to changes in the relationships between blood pressure, sympathetic nerve activity, and vascular outcomes remain to be fully elucidated. Importantly, the changes in baroreflex function and neurovascular transduction which appear to occur during normotensive pregnancies may represent potential mechanisms explaining an increased incidence of orthostatic intolerance in normotensive pregnant women relative to nonpregnant controls (15, 25). The sympathetic nervous system plays an important role in regulation of peripheral blood flow, and increases in sympathetic nerve activity occur during acute reductions in blood pressure which, in turn, increase vascular resistance to favor the return of blood to the heart and a restoration of set point blood pressure (26, 41, 55). Accordingly, alterations to the gain of this loop would be associated with inappropriate sympathetic responses to acute perturbations in blood pressure. Indeed, our finding of reduced baroreflex gain in normotensive pregnant women indicates that sympathetic responses to acute falls in blood pressure may be insufficient to properly restore blood pressure on a beat-by-beat basis, despite the maintenance of normal blood pressure when averaged over time. Furthermore, if decreased sympathetic baroreflex regulation of blood pressure is considered a normative adaptation during normotensive pregnancy, failure of this adaptation to occur may contribute to increased blood pressure in hypertensive pregnancies. Indeed, hypertensive pregnancies are associated with exaggerated increases in basal sympathetic activity relative to normotensive pregnancies (19, 20, 20, 21, 42).
Limitations.
In this study, we utilized the spontaneous method for calculating sympathetic baroreflex function, as opposed to the “gold standard” for baroreflex assessment, the modified Oxford method. The modified Oxford method reveals sympathetic responses to a wide range of blood pressures, as hypotension and hypertension are elicited through pharmacological means. However, a pharmacological approach is not ideally suited to pregnant women (15, 25). On the other hand, spontaneous measures of baroreflex sensitivity have been validated against modified Oxford methodologies (23) and are representative of a physiological range of blood pressures.
This study made use of a cross-sectional design which compared women in the third trimester of gestation to similarly aged nonpregnant controls. Although less statistically robust than a longitudinal approach, this design allowed us to compare normotensive nonpregnant women to pregnant women in the third trimester of gestation, the period when these women are at greatest risk of developing pregnancy-induced hypertensive disorders. As such, these data were designed to create a point from which to compare future data collected in women with gestational hypertension and preeclampsia, disorders which develop after 20 wk of gestation (31).
Summary.
In the present study we observed that both the gain and set point of the sympathetic baroreflex are affected by pregnancy. These data suggest a mechanism by which pregnant women might be more prone to orthostatic hypotension than their nonpregnant counterparts (15, 25). Perhaps more importantly, these data provide an important benchmark level of knowledge regarding sympathetic regulation of blood pressure in pregnant women. These data are particularly relevant for the study of mechanisms leading to hypertensive pregnancy disorders which are associated with an increase in blood pressure in conjunction with greatly elevated sympathetic activity compared with normotensive pregnancy (19–21, 42).
GRANTS
Supported for this study was provided by the Women and Children's Health Research Institute, the Natural Sciences and Engineering Research Council of Canada, and the University of Alberta Human Performance and Scholarship Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.W.U., R.J.S., M.H.D., and C.D.S. performed experiments; C.W.U. analyzed data; C.W.U., M.H.D., and C.D.S. interpreted results of experiments; C.W.U. prepared figures; C.W.U. drafted manuscript; C.W.U., R.J.S., R.S.C., C.G.J., M.K.S., M.H.D., and C.D.S. edited and revised manuscript; C.W.U., R.J.S., R.S.C., C.G.J., M.K.S., M.H.D., and C.D.S. approved final version of manuscript; R.S.C., C.G.J., M.K.S., M.H.D., and C.D.S. conception and design of research.
ACKNOWLEDGMENTS
We thank Christina M. MacKay, Emily C. King, Sydney M. L. Schmidt, and Jeffrey A. Vela for their technical assistance.
REFERENCES
- 1.Barron WM, Mujais SK, Zinaman M, Bravo EL, Lindheimer MD. Plasma catecholamine responses to physiologic stimuli in normal human pregnancy. Am J Obstet Gynecol 154: 80–84, 1986. [DOI] [PubMed] [Google Scholar]
- 2.Blaber AP, Yamamoto Y, Hughson RL. Methodology of spontaneous baroreflex relationship assessed by surrogate data analysis. Am J Physiol Heart Circ Physiol 268: H1682–H1687, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Blake MJ, Martin A, Manktelow BN, Armstrong C, Halligan AW, Panerai RB, Potter JF. Changes in baroreceptor sensitivity for heart rate during normotensive pregnancy and the puerperium. Clin Sci (Lond) 98: 259–268, 2000. [PubMed] [Google Scholar]
- 4.Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 299: R439–R451, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks VL, Quesnell RR, Cumbee SR, Bishop VS. Pregnancy attenuates activity of the baroreceptor reflex. Clin Exp Pharmacol Physiol 22: 152–156, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int 54: 2056–2063, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Cockell AP, Poston L. Flow-mediated vasodilatation is enhanced in normal pregnancy but reduced in preeclampsia. Hypertension 30: 247–251, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Cohen WR, Galen LH, Vega-Rich M, Young JB. Cardiac sympathetic activity during rat pregnancy. Metabolism 37: 771–777, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Crandall ME, Heesch CM. Baroreflex control of sympathetic outflow in pregnant rats: effects of captopril. Am J Physiol Regul Integr Comp Physiol 258: R1417–R1423, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Curtis KS, Cunningham JT, Heesch CM. Fos expression in brain stem nuclei of pregnant rats after hydralazine-induced hypotension. Am J Physiol Regul Integr Comp Physiol 277: R532–R540, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. [DOI] [PubMed] [Google Scholar]
- 12.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972. [DOI] [PubMed] [Google Scholar]
- 13.Deng Y, Kaufman S. Effect of pregnancy on activation of central pathways following atrial distension. Am J Physiol Regul Integr Comp Physiol 269: R552–R556, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Dutoit AP, Hart EC, Charkoudian N, Wallin BG, Curry TB, Joyner MJ. Cardiac baroreflex sensitivity is not correlated to sympathetic baroreflex sensitivity within healthy, young humans. Hypertension 56: 1118–1123, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Easterling TR, Schmucker BC, Benedetti TJ. The hemodynamic effects of orthostatic stress during pregnancy. Obstet Gynecol 72: 550–552, 1988. [PubMed] [Google Scholar]
- 16.Faber R, Baumert M, Stepan H, Wessel N, Voss A, Walther T. Baroreflex sensitivity, heart rate, and blood pressure variability in hypertensive pregnancy disorders. J Hum Hypertens 18: 707–712, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Fischer T, Schobel HP, Frank H, Andreae M, Schneider KT, Heusser K. Pregnancy-induced sympathetic overactivity: a precursor of preeclampsia. Eur J Clin Invest 34: 443–448, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Semin Reprod Med 27: 330–337, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104: 2200–2204, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Greenwood JP, Scott EM, Walker JJ, Stoker JB, Mary DA. The magnitude of sympathetic hyperactivity in pregnancy-induced hypertension and preeclampsia. Am J Hypertens 16: 194–199, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Greenwood JP, Stoker JB, Walker JJ, Mary DA. Sympathetic nerve discharge in normal pregnancy and pregnancy-induced hypertension. J Hypertens 16: 617–624, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Halliwill JR. Segregated signal averaging of sympathetic baroreflex responses in humans. J Appl Physiol 88: 767–773, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hart EC, Joyner MJ, Wallin BG, Karlsson T, Curry TB, Charkoudian N. Baroreflex control of muscle sympathetic nerve activity: a nonpharmacological measure of baroreflex sensitivity. Am J Physiol Heart Circ Physiol 298: H816–H822, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hines T. Baroreceptor afferent discharge in the pregnant rat. Am J Physiol Regul Integr Comp Physiol 278: R1433–R1440, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD, Fu Q. Sympathetic activation during early pregnancy in humans. J Physiol 590: 3535–3543, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res 34: 515–524, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol 93: 715–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laiprasert JD, Hamlin RL, Heesch CM. Afferent baroreceptor discharge in pregnant rats. Am J Physiol Heart Circ Physiol 281: H2456–H2462, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Leduc L, Wasserstrum N, Spillman T, Cotton DB. Baroreflex function in normal pregnancy. Am J Obstet Gynecol 165: 886–890, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Lucini D, Strappazzon P, Dalla VL, Maggioni C, Pagani M. Cardiac autonomic adjustments to normal human pregnancy: insight from spectral analysis of R-R interval and systolic arterial pressure variability. J Hypertens 17: 1899–1904, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Magee LA, Helewa M, Moutquin JM, von Dadelszen P, Hypertension Guideline Committee, Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can 30: S1–S48, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Masilamani S, Heesch CM. Effects of pregnancy and progesterone metabolites on arterial baroreflex in conscious rats. Am J Physiol Regul Integr Comp Physiol 272: R924–R934, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Ninomiya I, Malpas SC, Matsukawa K, Shindo T, Akiyama T. The amplitude of synchronized cardiac sympathetic nerve activity reflects the number of activated pre- and postganglionic fibers in anesthetized cats. J Auton Nerv Syst 45: 139–147, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Nisell H, Hjemdahl P, Linde B. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol 5: 479–493, 1985. [DOI] [PubMed] [Google Scholar]
- 36.Nisell H, Hjemdahl P, Linde B, Lunell NO. Sympatho-adrenal and cardiovascular reactivity in pregnancy-induced hypertension. I. Responses to isometric exercise and a cold pressor test. Br J Obstet Gynaecol 92: 722–731, 1985. [DOI] [PubMed] [Google Scholar]
- 37.O'Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem 37: 667–672, 1991. [PubMed] [Google Scholar]
- 38.Okada Y, Best SA, Jarvis SS, Shibata S, Parker RS, Casey BM, Levine BD, Fu Q. Asian women have attenuated sympathetic activation but enhanced renal-adrenal responses during pregnancy than Caucasian women. J Physiol 593: 1159–1168, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension 25: 1058–1068, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Pickering TG, Davies J. Estimation of the conduction time of the baroreceptor-cardiac reflex in man. Cardiovasc Res 7: 213–219, 1973. [DOI] [PubMed] [Google Scholar]
- 41.Rowell LB. Human Cardiovascular Control. New York: Oxford University Press, 1993. [Google Scholar]
- 42.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia – a state of sympathetic overactivity. N Engl J Med 335: 1480–1485, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365: 785–799, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Silver HM, Tahvanainen KU, Kuusela TA, Eckberg DL. Comparison of vagal baroreflex function in nonpregnant women and in women with normal pregnancy, preeclampsia, or gestational hypertension. Am J Obstet Gynecol 184: 1189–1195, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol Regul Integr Comp Physiol 272: R441–R463, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Smyth HS, Sleight P, Pickering GW. Reflex regulation of arterial pressure during sleep in man: A quantitative method of assessing baroreflex sensitivity. Circ Res 24: 109–121, 1969. [DOI] [PubMed] [Google Scholar]
- 47.Spaanderman ME, Willekes C, Hoeks AP, Ekhart TH, Peeters LL. The effect of pregnancy on the compliance of large arteries and veins in healthy parous control subjects and women with a history of preeclampsia. Am J Obstet Gynecol 183: 1278–1286, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Steegers EA, von DP, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet 376: 631–644, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Steinback CD, Salmanpour A, Breskovic T, Dujic Z, Shoemaker JK. Sympathetic neural activation: an ordered affair. J Physiol 588: 4825–4836, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinback CD, Salzer D, Medeiros PJ, Kowalchuk J, Shoemaker JK. Hypercapnic vs. hypoxic control of cardiovascular, cardiovagal, and sympathetic function. Am J Physiol Regul Integr Comp Physiol 296: R402–R410, 2009. [DOI] [PubMed] [Google Scholar]
- 51.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Sundlof AG, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tooher J, Chiu CL, Yeung K, Lupton SJ, Thornton C, Makris A, O'Loughlin A, Hennessy A, Lind JM. High blood pressure during pregnancy is associated with future cardiovascular disease: an observational cohort study. BMJ Open 3: e002964, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tunbridge RD, Donnai P. Plasma noradrenaline in normal pregnancy and in hypertension of late pregnancy. Br J Obstet Gynaecol 88: 105–108, 1981. [DOI] [PubMed] [Google Scholar]
- 55.Victor RG, Leimbach WN Jr. Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol 63: 2558–2562, 1987. [DOI] [PubMed] [Google Scholar]
- 56.Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. Nitric oxide-mediated vasodilation in human pregnancy. Am J Physiol Heart Circ Physiol 272: H748–H752, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 22: 203–212, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Zuspan FP. Pregnancy induced hypertension. 1. Role of sympathetic nervous system and adrenal gland. Acta Obstet Gynecol Scand 56: 283–286, 1977. [DOI] [PubMed] [Google Scholar]