Abstract
Studies have reported a greater blood flow response to muscle contractions when the limb is below the heart compared with above the heart, and these results have been interpreted as evidence for a skeletal muscle pump contribution to exercise hyperemia. If limb position affects the blood flow response to other vascular challenges such as reactive hyperemia, this interpretation may not be correct. We hypothesized that the magnitude of reactive hyperemia would be greater with the limb below the heart. Brachial artery blood flow (Doppler ultrasound) and blood pressure (finger-cuff plethysmography) were measured in 10 healthy volunteers. Subjects lay supine with one arm supported in two different positions: above or below the heart. Reactive hyperemia was produced by occlusion of arterial inflow for varying durations: 0.5 min, 1 min, 2 min, or 5 min in randomized order. Peak increases in blood flow were 77 ± 11, 178 ± 24, 291 ± 25, and 398 ± 33 ml/min above the heart and 96 ± 19, 279 ± 62, 550 ± 60, and 711 ± 69 ml/min below the heart (P < 0.05). Thus a standard stimulus (vascular occlusion) elicited different responses depending on limb position. To determine whether these differences were due to mechanisms intrinsic to the arterial wall, a second set of experiments was performed in which acute intraluminal pressure reduction for 0.5 min, 1 min, 2 min, or 5 min was performed in isolated rat soleus feed arteries (n = 12). The magnitude of dilation upon pressure restoration was greater when acute pressure reduction occurred from 85 mmHg (mimicking pressure in the arm below the heart; 28.3 ± 7.9, 37.5 ± 5.9, 55.1 ± 9.9, and 68.9 ± 8.6% dilation) than from 48 mmHg (mimicking pressure in the arm above the heart; 20.8 ± 4.8, 22.6 ± 4.4, 31.2 ± 5.8, and 49.2 ± 7.1% dilation). These data support the hypothesis that arm position differences in reactive hyperemia are at least partially mediated by mechanisms intrinsic to the arterial wall. Overall, these results suggest the need to reevaluate studies employing positional changes to examine muscle pump influences on exercise hyperemia.
Keywords: muscle blood flow, muscle contraction, skeletal muscle pump, functional hyperemia
at the onset of dynamic exercise, there is a rapid increase in blood flow [30, 31, 41, and see Fig. 2 in Clifford and Hellsten (5)]. In fact, even a single muscle contraction produces a prompt increase in blood flow (1, 7, 14, 26, 36, 39). There has been a debate over the last few decades about whether this rapid hyperemia is due to the muscle pump mechanism or to rapid vasodilation (5, 35).
Fig. 2.
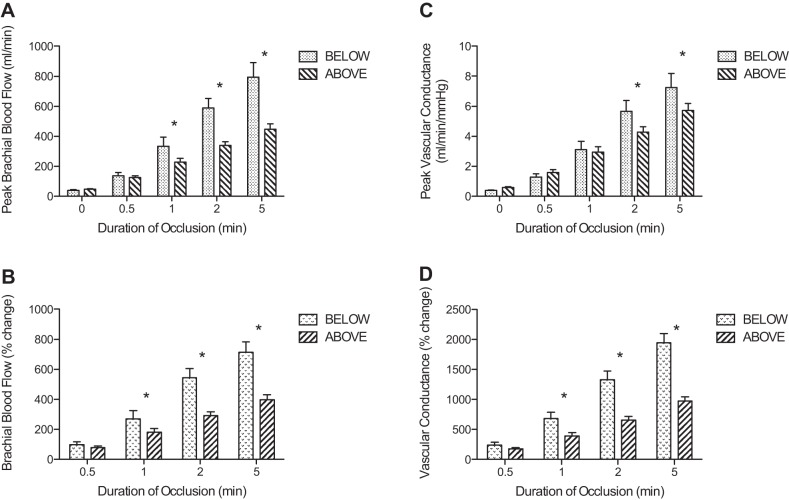
Peak brachial blood flow (A), peak vascular conductance (C), percentage change in peak brachial blood flow (B), and percentage change in vascular conductance (D) following release of cuff pressure after varying periods of occlusion. In A and C, 0 represents the values prior to occlusion. The graph compares the responses when the experimental arm was positioned above the heart vs. below the heart. *Significant differences between above and below (P < 0.05).
The muscle pump, as described by Laughlin (20), depends on an increased pressure gradient across the skeletal muscle vascular bed. Muscle contraction compresses the veins within the contracting muscle, expelling blood from the veins. Upon muscle relaxation, venous pressure is lower than it was prior to contraction (possibly even negative), increasing the pressure gradient across the muscle. This mechanism could increase blood flow without any change in vessel diameter (20). The best evidence supporting the muscle pump is the greater blood flow response to muscle contractions when the limb is below the heart, when veins should be full due to gravity, compared with the above heart position when veins are empty (e.g., Refs. 12, 22, 25, 31, 36).
On the other hand, a number of studies over the last decade have provided evidence that muscle contraction causes rapid vasodilation. Experiments performed using the hamster cremaster (2, 24) or retractor muscles (39) demonstrate that muscle contraction causes vasodilation of arterioles within 2-3 s of the onset of muscle contraction. Experiments performed in the human forearm (34, 36) and dog hindlimb (26) indicate that the muscle pump cannot explain the entire hyperemic response to muscle contraction. Additionally, experiments measuring blood flow to the canine hindlimbs (14, 15) indicate that vasodilation is necessary for the immediate hyperemia at the onset of muscle contraction. Studies published over the last decade have provided evidence for the involvement of adenosine (2), potassium (8, 9), and vascular compression (6, 16, 37) in rapid vasodilation of the skeletal muscle resistance vasculature.
Thus the published literature regarding the mechanism for immediate exercise onset hyperemia is inconclusive. The classic study by Tschakovsky et al. (36) suggested that a combination of rapid vasodilation and the muscle pump cause exercise hyperemia. In this study, a single handgrip contraction with the arm above the heart caused a rapid increase in blood flow following the contraction, suggesting that rapid vasodilation was responsible. When a single handgrip of the same intensity was performed with the arm below the heart, the rapid increase in blood flow was about twice the magnitude of the previous trial. The authors interpreted this increased hyperemia to be evidence for involvement of the muscle pump (36).
From our perspective, a crucial piece of evidence has been lacking. Since the strongest evidence in support of the muscle pump theory comes from experiments in which limb position has been manipulated, it is essential to determine whether vascular challenges other than muscle contraction also produce different responses when limb position is altered. Previous experiments in humans in which changes in body position were used to alter femoral artery perfusion pressure during passive leg movement (13, 33) indicate that altered perfusion pressure changes both leg blood flow and vascular conductance independent of muscle contraction. Additionally, changing arm position during muscle contractions caused a change in forearm vascular conductance that was attributed to vascular smooth muscle myogenic responses because it was independent of changes in muscle contraction or metabolism (40). Thus, the primary goal of the current experiments was to apply a standardized vascular occlusion stimulus (to elicit reactive hyperemia) with the arm positioned above or below the heart. We hypothesized that the magnitude of reactive hyperemia would be greater with the arm below the heart.
We also performed a second set of experiments to determine whether positional differences in reactive hyperemia were due to mechanisms that are intrinsic to the vascular wall. Koller and Bagi (17, 18) previously demonstrated that brief periods of pressure reduction in isolated arterioles cause dilation. Since vessel diameter can be directly monitored in isolated vessels and because no parenchymal tissue is present in this model, we employed acute pressure changes in isolated vessels to mimic the pressure changes and durations that occurred in the forearm reactive hyperemia experiments. We hypothesized that the magnitude of reactive hyperemia would be greater when intraluminal pressure was reduced from a higher pressure (mimicking intra-arterial pressure with the arm below the heart) than when intraluminal pressure was reduced from a lower pressure (mimicking intra-arterial pressure with the arm above the heart).
METHODS
Protocol 1.
After being informed of the procedures and risks associated with the study, 10 healthy, recreationally active adult volunteers (6 males, 4 females) agreed to participate and signed informed consent. The experimental protocol was approved by the ethics committee at the University of Western Ontario where all human experiments were performed. The average age of the participants was 36.2 ± 3.7 years, height 173.1 ± 3.6 cm, and weight 68.0 ± 4.0 kg. The subjects reported to the laboratory after fasting for 8 h and abstaining from exercise and caffeine for 24 h.
Subjects lay supine in a quiet room with temperature controlled at ∼22 °C. A blood pressure cuff was placed on the upper arm of the experimental limb. This arm was supported in two different positions: extended ∼50° above the heart or 50° below the heart. On average, the difference in height of the midpoint of the forearm was 38.3 ± 1.0 cm. Reactive hyperemia was elicited by occlusion of arterial inflow with a blood pressure cuff rapidly inflated to 200 mmHg (Hokanson cuff inflator, Bellevue, WA) for varying durations presented in random order: 0.5 min, 1 min, 2 min, 5 min. No measurements were made until the arm was in the selected position for at least 5 min. This allowed adequate time between cuff inflations for blood flow to return to baseline levels (see Table 2). The cuff was inflated while the arm was in the above or below heart position, and the arm was not moved between cuff inflation and blood flow measurements.
Table 2.
Baseline brachial artery blood flow before reactive hyperemia
| 0.5 Min | 1 Min | 2 Min | 5 Min | |
|---|---|---|---|---|
| Arm above heart | 46.9 ± 3.7 | 48.0 ± 3.8 | 47.2 ± 3.5 | 48.6 ± 4.1 |
| Arm below heart | 39.8 ± 3.7* | 41.2 ± 3.0* | 43.1 ± 4.3 | 38.9 ± 3.4* |
Values are means ± SE in ml/min.
P < 0.05.
In the experimental limb, brachial blood flow was measured beat by beat using Doppler ultrasound (GE Vingmed, System Five, Horten, Norway) and a hand-held linear array transducer operating at 5 MHz with a <60° insonation angle. The transducer was held in position by an investigator following application of a small amount of acoustic couplant gel. Arterial pressure was measured continuously by finger-cuff plethysmography (Finometer, Ohmeda, Louisville, CO) on the middle finger of the contralateral arm which was maintained at heart level. All analog signals were recorded in real time at 100 Hz (PowerLab, AD Instruments, Colorado Springs, CO) and stored on a computer for subsequent analysis. Beat-by-beat forearm blood flow was calculated as the product of mean blood velocity and the vessel cross-sectional area (πr2), where r is the vessel radius. Brachial artery diameter was measured with the arm above and below the heart. After the arm was in the selected position for at least 5 min, triplicate brachial artery diameter measurements were made in duplex mode at end diastole and averaged. Blood flow was monitored for 1 min before occlusion (baseline) and 5 min after release of the cuff. The data were analyzed in three ways. The peak blood flow response was determined as the peak blood flow after release of the cuff. The percent change in blood flow was calculated for each individual as [(peak blood flow − the baseline blood flow)/baseline blood flow] × 100. Total hyperemia was calculated by integrating the blood flow response for 1 min following cuff release. Vascular conductance was calculated as brachial blood flow divided by the calculated mean arterial pressure at the midpoint of the forearm.
Differences in responses across time for all variables measured (peak blood flow, percent change in blood flow, total hyperemia, mean arterial pressure, and vascular conductance) were determined by a repeated measures two-way ANOVA. An α level ≤ 0.05 was used to determine statistical significance in all cases. Data are presented as means ± SE.
Protocol 2.
Male Sprague-Dawley (300–400 g) rats were obtained (Charles River, Wilmington, MA) and housed two animals per cage with food and water provided ad libitum. Temperature was controlled at 23°C and the room was lit for 12 h/day. All animal experiments were performed at Pepperdine University, and all experimental procedures were approved by the Pepperdine University Institutional Animal Care and Use Committee. Experimental procedures were consistent with the principles established in the Guide for the Care and Use of Animals.
Rats were anesthetized with sodium pentobarbital (50.0 mg/kg) and the calf muscle group was removed and transferred to a dissection chamber filled with cold (4°C) physiological saline solution (PSS), as described previously (6). PSS contained (in mM) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 sodium pyruvate, 0.02 EDTA, and 3.0 MOPS (pH 7.4). Soleus feed arteries were carefully dissected, removed, and placed in a Lucite vessel chamber filled with PSS. The arteries were cannulated on one end with a glass micropipette and secured to the pipette using 11-0 opthalmic suture. Blood was flushed from the vessel lumen using PSS-albumin (1 g/100 ml) and the other end was secured to a second micropipette. The vessel chamber was then transferred to the stage of an inverted microscope (UNICO, Dayton, NJ) coupled with a video camera (Pulnix, San Jose, CA), monitor (Sony, San Jose, CA), and video micrometer (Colorado Video, Longmont, CO). Luminal diameter was continuously monitored throughout the experiment and recorded on a computer using a data acquisition system (PowerLab, AD Instruments). The vessel bath was gradually warmed and temperature was maintained at 37°C for the duration of the experiment. The micropipettes were connected to independent reservoirs, and the vessels were pressurized by raising the reservoirs to the specified height. Intraluminal pressure was initially set at 44 mmHg and raised to 66 mmHg after 30 min. This pressure was selected based on in vivo rat soleus feed artery pressures measured by Williams and Segal (42). There was no flow through the lumen at any time during the experiments, to obviate potential effects of shear stress. The vessel bath solution was replaced with new PSS every 15 min during the 1-h equilibration period. All vessels developed spontaneous tone during the equilibration period.
At the end of the equilibration period, acute reductions in pressure were accomplished by rapidly switching between a fluid reservoir set at a pressure of either 48 or 85 mmHg and a fluid reservoir set at 10 mmHg. These pressure differences were intended to mimic the in vivo reactive hyperemia protocols and were chosen based on the intravascular pressure differences between arm positions reported by Tschakovsky et al. (36). Pressure was reduced to 10 mmHg for periods of 0.5 min, 1 min, 2 min, or 5 min, the same time periods used in the in vivo experiments, and then returned to the previous baseline pressure while luminal diameter was continuously recorded (see Fig. 4). Each feed artery underwent acute pressure reductions for each time period from each baseline intraluminal pressure (48 and 85 mmHg) in randomized order. A recovery period of at least 5 min was allowed between pressure reductions.
Fig. 4.
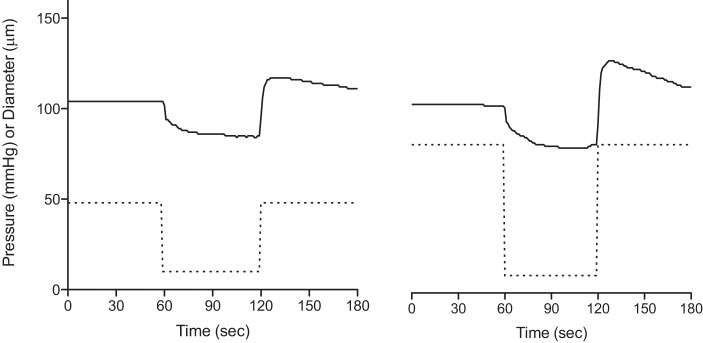
Raw data tracings of a single soleus feed artery illustrating the changes in diameter during 1 min exposure to low pressure (10 mmHg) starting at baseline pressures of 48 mmHg (leftl) and 85 mmHg (right). Solid lines, diameter; dotted lines, pressure.
Data are presented as change in diameter (in μm) from the original baseline or as percent change in diameter, calculated as [(DP − DB)/DB] × 100, where DP is diameter post-pressure restoration and DB is baseline diameter prior to the pressure reduction. Differences in responses across time and between pressures were determined by a repeated measures two-way ANOVA. An α level ≤ 0.05 was used to determine statistical significance in all cases. Data are presented as means ± SE.
RESULTS
Protocol 1.
Blood pressures at baseline and at peak hyperemia are shown in Table 1. There were no significant changes in blood pressure from baseline to peak hyperemia under any experimental conditions. As shown in Table 2, baseline blood flows were generally higher when the arm was above the heart.
Table 1.
Blood pressure measurements
| 0.5 Min | 1 Min | 2 Min | 5 Min | |
|---|---|---|---|---|
| Arm Above Heart | ||||
| Baseline | 93.9 ± 1.7 | 93.6 ± 1.7 | 95.1 ± 1.5 | 94.7 ± 1.7 |
| Peak hyperemia | 92.7 ± 1.8 | 90.7 ± 1.5 | 92.7 ± 1.3 | 92.1 ± 1.6 |
| Arm Below Heart | ||||
| Baseline | 92.4 ± 2.1 | 95.4 ± 3.4 | 93.3 ± 2.5 | 95.6 ± 2.4 |
| Peak hyperemia | 95.2 ± 3.2 | 94.5 ± 2.7 | 91.3 ± 2.7 | 96.1 ± 2.5 |
Values are means ± SE in mmHg.
Ensemble averages of the continuous brachial blood flow measurements are displayed in Fig. 1. The figure shows that the increases in blood flow following release of the cuff were graded, with longer durations of occlusion evoking larger increases in flow. In addition, the curves show blood flow responses were generally greater when the arm was below the heart than when the arm was above the heart.
Fig. 1.
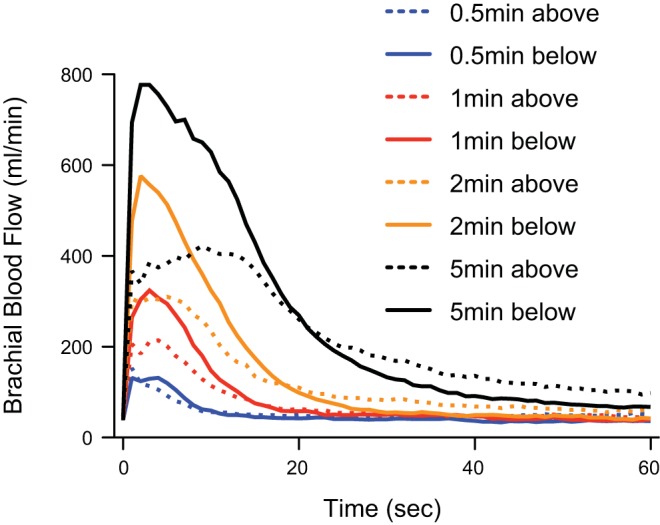
Ensemble average of individual subjects' brachial blood flow responses to release of cuff pressure after occlusion of varying periods of time. The experimental arm was positioned either above the heart or below the heart. Point zero represents a 30-s mean of baseline data.
Figure 2A displays the peak blood flows achieved following release of occlusion. After 0.5 min occlusions, there were no significant differences in the peak blood flow with the arm below vs. above the heart. However, for occlusions of 1, 2, and 5 min, the peak blood flows were significantly higher with the arm below the heart compared with the arm above the heart. The peak vascular conductance (Fig. 2C) was significantly higher with the arm below the heart for occlusions of 2 and 5 min. The percent change in blood flow (Fig. 2B), vascular conductance (Fig. 2D), and the total hyperemia (Fig. 3) were significantly greater with the arm below the heart compared with the arm above the heart at occlusion durations greater than 0.5 min. The time to peak brachial blood flow was not different between arm positions (between 2.5 and 3.9 s), except for the 5 min occlusion where the peak was substantially delayed when the arm was positioned above the heart (2.6 ± 0.2 s below vs. 10.2 ± 0.9 s above).
Fig. 3.
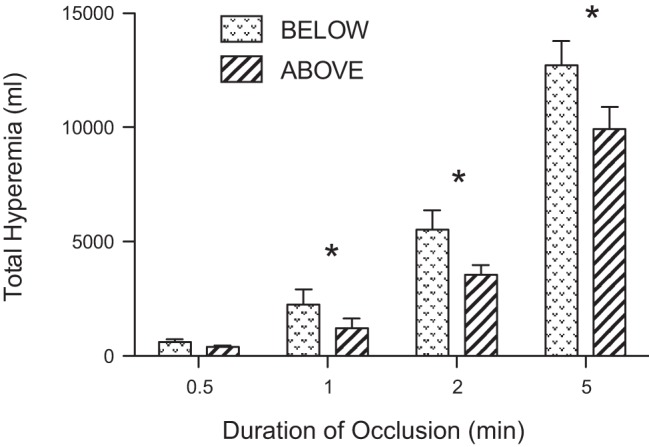
Total hyperemia following release of cuff pressure after varying periods of occlusion. The graph compares the responses when the experimental arm was positioned above the heart vs. below the heart. *Significant differences between above and below (P < 0.05).
Protocol 2.
Soleus feed artery maximal diameter was 185.3 ± 8.2 μm (n = 12). Feed arteries developed an average spontaneous tone of 42.4 ± 3.3% and had similar starting diameters prior to each pressure manipulation (Table 3). When intraluminal pressure was restored after pressure reduction, all feed arteries dilated to a diameter larger than the initial baseline diameter (Fig. 4). As shown in Fig. 5, increasing the duration of pressure reduction produced a larger increase in diameter (Fig. 5A) and a larger percent dilation (Fig. 5B). Importantly, feed arteries dilated more at the higher pressure than at the lower pressure following pressure reduction periods >0.5 min (Fig. 5).
Table 3.
Soleus feed artery diameters at each baseline pressure prior to pressure manipulations
| 0.5 Min | 1 Min | 2 Min | 5 Min | |
|---|---|---|---|---|
| 48 mmHg | 94.5 ± 10.8 | 93.0 ± 10.4 | 100.3 ± 11.6 | 87.2 ± 7.5 |
| 85 mmHg | 97.5 ± 8.4 | 91.3 ± 8.5 | 95.3 ± 8.8 | 86.3 ± 9.3 |
Values are means ± SE in μm.
Fig. 5.
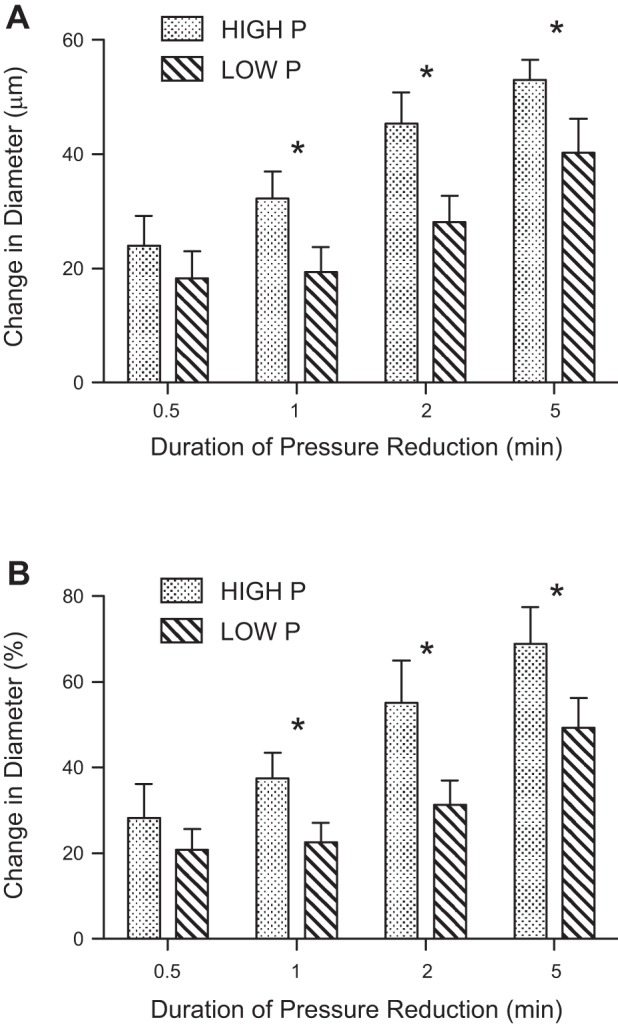
Absolute (A) and percentage (B) change in vessel diameter following restoration of pressure after varying periods of low pressure. The graph compares the responses to low pressure exposure when the vessels started and ended at baseline pressures of 48 mmHg vs. 85 mmHg. *Significant differences between high and low baseline pressures (P < 0.05).
DISCUSSION
This investigation employed two complimentary protocols to investigate the influence of limb position on blood flow responses to vascular challenge. The salient results are as follows. First, a standard stimulus vascular occlusion elicits different reactive hyperemia responses depending on limb position, with the increase in blood flow being greater with the arm below the heart compared with the arm above the heart. Second, reducing intraluminal pressure for a brief time (mimicking vascular occlusion) in isolated vessels reveals that the magnitude of vasodilation is dependent on the value to which intraluminal pressure is restored. In light of these findings, there is a need to reevaluate studies employing positional changes to examine muscle pump influences on exercise hyperemia. Position-related differences in blood flow responses to contraction cannot be used as ipso facto evidence for the existence of a muscle pump effect.
The rationale for this study was that the primary evidence for a muscle pump effect in exercise hyperemia comes from results of experiments which have demonstrated that limb position influences the blood flow response to muscle contraction (12, 22, 25, 31, 36). We reasoned that a critical control experiment has been lacking, i.e., testing whether the response to vascular challenges other than contraction elicit positional differences in blood flow. Thus our primary intention with these experiments was to apply a standardized stimulus to the resistance vasculature of the forearm at different limb positions. We chose to employ a widely used and reproducible stimulus, i.e., vascular occlusion (28). The increase in blood flow following vascular occlusion is termed reactive hyperemia and is thought to occur primarily as a result of metabolites that accumulate in the ischemic tissues, although there is some evidence that other mechanisms may contribute (see later).
The results of the current study show that the increase in blood flow and vascular conductance following release of occlusion is strongly dependent on limb position. One previous study examined the effects of limb position on reactive hyperemia. With the use of strain gauge plethysmography, Krishnan et al. (19) observed that peak blood flow was highest with the limbs down or horizontal and lowest with the limbs up. These authors provided little mechanistic explanation for their results, attributing them to position-dependent differences in “vascular transmural pressure.” Our in vivo data suggest that driving pressure after cuff release and vascular dilation are both key factors determining the blood flow response, because a greater increase in blood flow was associated with a higher driving pressure and a higher vascular conductance (Fig. 2).
The rationale for using isolated soleus feed arteries was to determine whether the different dilation responses observed in humans with varying arm position could result from inherent properties of the arterial wall. Therefore, we mimicked the human experiments as closely as possible by choosing periods of pressure reduction identical to the time periods employed in the human protocols (0.5 min, 1 min, 2 min, and 5 min). When intraluminal pressure was restored back to the prior baseline pressure, arteries dilated to a diameter greater than the prior baseline diameter. The magnitude of reactive dilation increased as the duration of the pressure reduction period increased (Fig. 5). Similar to our findings, Koller and Bagi previously found that isolated rat gracilis (17) or coronary (18) arterioles exhibited reactive dilation to brief periods (30 s, 1 min, and 2 min) of pressure reduction and that the magnitude of dilation was duration dependent. It is interesting to note that these studies and our study demonstrate reactive dilation in isolated vessels in the absence of any parenchymal tissue. Therefore, the dilation results from mechanisms in the vascular wall and not from metabolic factors released from parenchymal tissue. The new finding in the current study is that the magnitude of dilation was greater when the acute pressure reduction returns to a higher baseline pressure (mimicking the arm below the heart) than to a lower baseline pressure (mimicking the arm above the heart; Fig. 5).
Together, the in vivo and in vitro data from the current study indicate that the enhanced blood flow response with the arm below the heart is due to a combination of the higher driving pressure coupled with greater dilation in the resistance vessels. An effect of body position on both perfusion pressure and dilation has been noted previously in a different model (13, 33). In these experiments, alterations in body position (supine vs. seated) were used to alter hemodynamic responses to passive leg movement. The authors found that femoral perfusion pressure was higher in the seated position at rest, and that perfusion pressure, leg blood flow, and leg vascular conductance were all higher in the seated position during passive leg movement. Our results using arm occlusion agree with the results of Groot et al. (13) and Trinity et al. (33), in that the below heart arm position, which increases perfusion pressure (36), also increased arm vascular conductance during reactive hyperemia (Fig. 2). Walker et al. (40), using a forearm occlusion model, found that changing arm position from the above heart to below heart position during isometric handgrip contraction rapidly increased both perfusion pressure and forearm vascular conductance. Taken together, the results of these studies and the current study indicate that changes in body position which alter perfusion pressure also alter limb vascular conductance.
The underlying mechanism for the observed differences in hyperemia with different limb positions is uncertain, in part because the mechanism for reactive hyperemia itself is uncertain. Traditionally, the hyperemia has been attributed to the accumulation of metabolites released from ischemic skeletal muscle, although a myogenic component has been shown previously by Bjornberg et al. (3). Regardless of the initiating event, the relaxation of vascular smooth muscle in the resistance vessels appears to be due in part to activation of inwardly rectifying potassium channels (KIR) (9). The results of most (8, 11, 27, 32) but not all (10, 23) studies have found that endothelium signaling pathways make little or no contribution to peak reactive hyperemia in humans. In isolated vessels, endothelium removal or inhibition of nitric oxide synthase partially blocked both peak reactive dilation and the duration of dilation (17, 18). How can we best explain the differences in the magnitude of dilation with different arm positions? We speculate that, regardless of limb position, resistance vessels in the occluded arm lose smooth muscle tone (myogenic dilation) due to the low pressure in the arm during the occlusion period. Upon release of arm occlusion, the higher perfusion pressure when the arm is in the below heart position causes a greater distention of the resistance vessels, which results in a greater hyperemia. This could be described as the passive component of reactive hyperemia. There must also be an active component, because a longer duration of arm occlusion caused a greater hyperemic response. Although this is consistent with a metabolic mechanism, our in vitro data show that longer durations of reduced intraluminal pressure cause increased dilation, even when no parenchymal tissue is present. Thus the precise stimulus leading to activation of KIR channels is open to question.
The concept underlying the muscle pump hypothesis is that, during muscle contraction, the veins within the muscle are compressed and the venous blood is expelled. Relaxation of the muscle fibers (which are tethered to the walls of the veins) opens the lumen of the compliant veins and creates low pressure within them (20, 21). The reduction in venous pressure increases the pressure gradient across the muscle vascular bed and enhances muscle perfusion. Because of technical limitations, it has not been feasible to perform experiments to directly test this hypothesis, yet the idea has persisted despite the lack of direct confirmation. Data from studies showing a greater blood flow response to muscle contractions when the limb is in the dependent position (12, 22, 25, 31, 36) have been the primary evidence for a skeletal muscle pump contribution to exercise hyperemia. We have argued previously that the magnitude of contraction-elicited changes in blood flow is far greater than can be accounted for by putative changes in intravascular pressure (38) and that the time course of changes in blood flow does not correlate with that predicted from the muscle pump (4). Furthermore, the studies by Hamann et al. (14, 15) clearly show that, in the absence of vasodilation, muscle contractions do not evoke an increase in muscle blood flow. Despite these arguments, explanations for the rapid rise in blood flow at the onset of exercise often include the muscle pump theory. The findings of the current study provide an alternative interpretation to the observations of a greater blood flow response to muscle contractions with the limb in the dependent position. The enhanced blood flow response is likely to result from a higher driving pressure and greater dilation of the resistance vasculature rather than the muscle pump.
There are two experimental limitations that should be considered. First, vascular occlusion was employed as a standard stimulus to probe vascular function. Because the durations of occlusion were the same with the arm below the heart and above the heart, it is assumed that the metabolic stimulus was the same. Another approach that could have been used is intra-arterial administration of vasodilator drugs, but we chose to use a less invasive approach. A second limitation is that we used the initial baseline diameter to calculate blood flow and vascular conductance in the human forearm experiments rather than measuring diameter continuously. Because brachial artery diameter changes relatively slowly in response to shear stress (29), peak blood flow and vascular conductance (Fig. 2) should be unaffected, but the total hyperemia (Fig. 3) may be underestimated.
Using both in vivo and in vitro approaches, we attribute the positional variations in reactive hyperemia to differences in driving pressure coupled with differences in the dilation of resistance vessels in the forearm. The fact that the magnitude of reactive hyperemia is greater with the arm below the heart compared with above shows that position-related differences in blood flow responses are not restricted to muscle contraction. This raises questions about using position-related differences in blood flow responses to contraction as ipso facto evidence for the existence of a muscle pump effect. We conclude that there is a need to reevaluate studies employing positional changes to examine muscle pump influences on exercise hyperemia.
GRANTS
This project was supported by the Natural Sciences and Engineering Research Council of Canada (K. Shoemaker) and the Seaver Research Council of Pepperdine University (J. Jasperse).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.J., J.K.S., and P.S.C. conception and design of research; J.L.J., J.K.S., E.J.G., and P.S.C. performed experiments; J.L.J., E.J.G., and P.S.C. analyzed data; J.L.J., J.K.S., E.J.G., and P.S.C. interpreted results of experiments; J.L.J., E.J.G., and P.S.C. prepared figures; J.L.J. and P.S.C. drafted manuscript; J.L.J., J.K.S., and P.S.C. edited and revised manuscript; J.L.J., J.K.S., E.J.G., and P.S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors wish to thank Amanda Rothwell for technical assistance with the human subjects experiments.
REFERENCES
- 1.Anrep GV, von Saalfeld E. The blood flow through the skeletal muscle in relation to its contraction. J Physiol 85: 375–399, 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol 581: 841–852, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornberg J, Albert U, Mellander S. Resistance responses in proximal arterial vessels, arterioles and veins during reactive hyperaemia in skeletal muscle and their underlying regulatory mechanisms. Acta Physiol Scand 139: 535–550, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Clifford PS, Hamann JJ, Valic Z, Buckwalter JB. Counterpoint: the muscle pump is not an important determinant of muscle blood flow during exercise. J Appl Physiol 99: 371–375, 2005. [PubMed] [Google Scholar]
- 5.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in skeletal muscle vasculature. J Physiol 572: 561–567, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19: 142–146, 1964. [DOI] [PubMed] [Google Scholar]
- 8.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crecelius AR, Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Reactive hyperemia occurs via activation of inwardly rectifying potassium channels and Na+/K+-ATPase in humans. Circ Res 113: 1023–1032, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, Schenke W, Quyyumi AA. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension 32: 9–15, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Folkow B, Gaskell P, Waaler BA. Blood flow through limb muscles during heavy rhythmic exercise. Acta Physiol Scand 80: 61–72, 1970. [DOI] [PubMed] [Google Scholar]
- 13.Groot HJ, Trinity JD, Layec G, Rossman MJ, Ives SJ, Richardson RS. Perfusion pressure and movement-induced hyperemia: evidence of limited vascular function and vasodilatory reserve with age. Am J Physiol Heart Circ Physiol 304: H610–H619, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamann JJ, Buckwalter JB, Clifford PS. Vasodilatation is obligatory for contraction-induced hyperemia in canine skeletal muscle. J Physiol 557: 1013–1020, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamann JJ, Valic Z, Buckwalter JB, Clifford PS. Muscle pump does not enhance blood flow in exercising skeletal muscle. J Appl Physiol 94: 6–10, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller A, Bagi A. On the role of mechanosensitive mechanisms eliciting reactive hyperemia. Am J Physiol Heart Circ Physiol 283: H2250–H2259, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Koller A, Bagi A. Nitric oxide and H2O2 contribute to reactive dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 287: H2461–H2467, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan A, Lucassen EB, Hogeman C, Blaha C, Leuenberger UA. Effects of limb posture on reactive hyperemia. Eur J Appl Physiol 111: 1415–1420, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laughlin MH. Skeletal muscle blood flow capacity: role of muscle pump in exercise hyperemia. Am J Physiol Heart Circ Physiol 253: H993–H1004, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Laughlin MH, Schrage WG. Effects of muscle contractions on skeletal muscle blood flow: when is there a muscle pump? Med Sci Sports Exerc 31: 1027–1035, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Leyk D, Essfeld D, Baum K, Stegemann J. Early leg blood flow adjustment during dynamic foot plantar flexions in upright and supine body position. Int J Sports Med 15: 447–452, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol 270: H1435–H1440, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol 82: 282–287, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Nadland IH, Walloe L, Toska K. Effect of the leg muscle pump on the rise in muscle perfusion during muscle work in humans. Eur J Appl Physiol 105: 829–841, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol 87: 1741–1746, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Nugent AG, McGurk C, McAuley D, Maguire S, Silke B, Johnston GD. Forearm reactive hyperaemia is not mediated by nitric oxide in healthy volunteers. Br J Clin Pharmacol 48: 457–459, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson GC, Whelan RF. Reactive hyperemia in the human forearm. Clin Sci 14: 197–211, 1955. [PubMed] [Google Scholar]
- 29.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol 97: 499–508, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Sheriff DD, Rowell LB, Scher AM. Is rapid rise in vascular conductance at onset of dynamic exercise due to muscle pump? Am J Physiol Heart Circ Physiol 265: H1227–H1234, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Shoemaker JK, Tschakovsky ME, Hughson RL. Vasodilation contributes to the rapid hyperemia with rhythmic contractions in humans. Can J Physiol Pharmacol 76: 418–427, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90: 2285–2290, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MAH, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Tschakovsky ME, Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271: H1697–H1701, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Turturici M, Mohammed M, Roatta S. Evidence that the contraction-induced rapid hyperemia in rabbit masseter muscle is based on a mechanosensitive mechanism, not shared by cutaneous vascular beds. J Appl Physiol 113: 524–531, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J Appl Physiol 98: 72–76, 2005. [DOI] [PubMed] [Google Scholar]
- 39.VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol 290: H119–H127, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Walker KL, Saunders NR, Jensen D, Kuk JL, Wong SL, Pyke KE, Dwyer EM, Tschakovsky ME. Do vasoregulatory mechanisms in exercising human muscle compensate for changes in arterial pressure? Am J Physiol Heart Circ Physiol 293: H2928–H2936, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Walloe L, Wesche J. Time course and magnitude of blood flow changes in the human quadriceps muscles during and following rhythmic exercise. J Physiol 405: 257–273, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol 463: 631–646, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]