Abstract
High temperature is one of the prominent environmental factors causing economic losses to the poultry industry as it negatively affects growth and production performance in broiler chickens. We used One Step TaqMan real time RT-PCR (reverse transcription polymerase chain reaction) technology to study the effects of chronic heat stress on the expression of genes codifying for the antioxidative enzymes superoxide dismutase (SOD), and catalase (CAT), as well as for heat shock protein (HSP) 70, HSP90, glucocorticoid receptor (NR3C1), and caspase 6 (CASP6) in the liver of two different broiler genetic strains: Red JA Cou Nu Hubbard (CN) and Ross 508 Aviagen (RO). CN is a naked neck slow growing broiler intended for the free range and/or organic markets, whereas RO is selected for fast growing. We also analysed the effect of chronic heat stress on productive performances, and plasma corticosterone levels as well as the association between transcriptomic response and specific SNPs (single nucleotide polymorphisms) in each genetic strain of broiler chickens. RO and CN broilers, 4 weeks of age, were maintained for 4 weeks at either 34 °C or 22 °C. The results demonstrated that there was a genotype and a temperature main effect on the broilers' growth from the 4th to the 8th week of age, but the interaction effect between genotype and temperature resulted not statistically significant. By considering the genotype effect, fast growing broilers (RO) grew more than the slow growing ones (CN), whereas by considering the temperature effect, broilers in unheated conditions grew more than the heat stressed ones. Corticosterone levels increased significantly in the blood of heat stressed broilers, due to the activation of the HPA (hypothalamic–pituitary–adrenocortical axis). Carcass yield at slaughter was of similar values in the 4 cohorts (genotype/temperature combinations or treatment groups), ranging from 86.5 to 88.6%, whereas carcass weight was negatively influenced by heat stress in both broiler strains. Heat stress affected gene expression by downregulating CASP6 and upregulating CAT transcript levels. HSPs, SOD and NR3C1 mRNA levels remained unaffected by heat stress. The differences found in the mRNA copies of CASP6 gene could be partly explained by SNPs.
Abbreviations: CASP6, caspase 6; CAT, catalase; cDNA, DNA complementary to RNA; CN, Red JA Cou Nu Hubbard; CORT, corticosterone; Ct, cycle threshold; GPX, glutathione peroxidase; HPA, hypothalamic–pituitary–adrenocortical axis; HSP, heat shock protein; kDa, kilodalton(s); NR3C1, glucocorticoid receptor: GR or nuclear receptor subfamily 3, group c, member 1; PCR, polymerase chain reaction; RO, Ross 508 Aviagen; rTH, reverse transcriptase; RT-PCR, reverse transcription PCR; SNP, single nucleotide polymorphism; SOD, superoxide dismutase
Keywords: Heat stress, Broiler, Corticosterone, Antioxidant enzymes, Real time PCR, Gene expression
Highlights
-
•
We examine the expression of six genes involved in the heat stress response.
-
•
We examine two broiler strains selected for fast and slow growing.
-
•
We explore the association between transcriptomic response and specific SNPs.
-
•
Heat stress affected gene expression and increased corticosterone in both strains.
-
•
The slow-growing line was more susceptible to high temperatures than fast growing.
1. Introduction
High environmental temperature is one of the most important stressors associated with economic losses to the poultry industry (Lin et al., 2006; Lu et al., 2007). It causes poor growth performance (Bottje and Harrison, 1985), immunosuppression (Young, 1990), and high mortality (Yahav et al., 1995), contributing thus to a decrease in productivity. Furthermore, heat stress deteriorates meat quality by accelerating postmortem glycolytic metabolism, resulting in pale and exudative meat characteristics in chicken (Sandercock et al., 2001; Hashizawa et al., 2013).
Although the responses to heat differ between chickens of different genetic backgrounds (Altan et al., 2003; Franco-Jimenez et al., 2007; Star et al., 2008; Felver–Gant et al., 2012), broilers are in general more sensitive to high environmental temperatures than other domestic animals (Geraert et al., 1993). Indeed, domestication and selective breeding are producing individuals that are more susceptible to stress rather than more resistant (Washburn et al., 1980; Cahaner et al., 1995). In particular, the resistance to heat stress of strains selected for rapid growth is significantly lower than that of slow-growing strains and the continuous selection for fast growth seems to be associated with increased susceptibility of broiler chicken to heat stress (Berrong and Washburn, 1998; Tan et al., 2010; Soleimani et al., 2011).
Different studies have demonstrated oxidative injury induced by high ambient temperatures in broiler chickens (Altan et al., 2003; Mujahid et al., 2007). Heat is a major source of oxidative stress since it causes a redox imbalance between the pro- and anti-oxidants in favour of prooxidants. However, several effective antioxidant systems prevent oxidative damage, including various antioxidant enzymes, such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPX) (Halliwell and Gutteridge, 1996). SOD catalyses dismutation of superoxide radicals to hydrogen peroxide and oxygen; CAT catalyses the breakdown of hydrogen peroxide to water and molecular oxygen, and GPX decomposes peroxides through other mechanisms (Halliwell, 2006). Several studies have clearly demonstrated that exposure to high ambient temperatures causes a compensatory increase in the activity of SOD, GSH-Px, and CAT in serum, liver, and muscle of broiler chickens (Tan et al., 2010; Azad et al., 2010).
Most organisms respond to heat stress by inducing the synthesis of a group of highly evolutionarily conserved stress-modulated proteins known as heat shock proteins (HSPs). Analogously to enzymatic oxygen scavengers, the expression of HSPs is upregulated in response to high temperatures, given that one of the most important functions of HSPs is to protect organisms from the toxic effects of heating (Barbe et al., 1988; Ganter et al., 2006; Staib et al., 2007;). Moreover, several HSPs function as intracellular chaperones for other proteins. They play an important role in protein assembling and disassembling (Pelham, 1985), protein folding and unfolding (Randall and Hardy, 1986), and protein translocation (Murakami et al., 1988). Of the many expressed HSPs, those with a molecular weight of approximately 70 kDa, named HSP70, have been extensively studied in chicken because they seem to best correlate with heat tolerance (Gabriel et al., 1996; Soleimani et al., 2011; Hasheimi et al., 2012; Gu et al., 2012;).
Rearing stress conditions and physical agents, like heat, can also activate apoptosis, which is triggered by caspases, a family of structurally related cysteine aspartases. It has been well documented that the expression of caspase genes may be influenced by persistent stress in broiler chicken and other vertebrates such as rainbow trout (Laing et al., 2001; Lin et al., 2004).
In chicken as in other vertebrates, stress activates the hypothalamus-pituitary-adrenocortical (HPA) axis, leading to a rapid release of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) from the cells located in the hypothalamus and pituitary, respectively. ACTH stimulates the synthesis and release of steroids from the adrenal cortex by promoting the uptake of cholesterol and its enzymatic conversion to the glucocorticoid hormone corticosterone (CORT) (Jones et al., 1988; Fraisse and Cockrem, 2006). CORT released into the circulatory system diffuse across the plasma membrane of the cells and binds to a high affinity cytosolic glucocorticoid receptor (NR3C1), which is mainly found in cytoplasm as a heterocomplex by coordinated associations with molecular chaperones, such as HSP40, HSP70 and HSP90 (Derijk et al., 2002; Marelli et al., 2010; Ramamoorthy and Cidlowski, 2013). Binding of CORT to NR3C1 induce the NR3C1 heterocomplex leading to NR3C1 homodimerization and nuclear translocation. Once inside the nucleus, NR3C1 can regulate gene transcription, through binding to a palindromic response element termed the glucocorticoid response element (GRE), which is located in the promoter regions of target genes (Yudt and Cidlowsky, 2001; Kwok et al., 2007). Binding to GRE induces conformational changes in NR3C1 leading to coordinated recruitment of coactivators and chromatin-remodelling complexes that influence the activity of polymerases and activate gene transcription (Kwok et al., 2007; Ramamoorthy and Cidlowski, 2013). However, according to recent discoveries, many NR3C1-binding sites are located far from the promoter proximal region of target genes and showed an unexpected difference between the activation and repressive functions of the NR3C1. What remains to be established, thus, is the functionality of these distant NR3C1-binding sites in relation to the transcription of genes or other undiscovered functions encoded in the NR3C1 protein (Ramamoorthy and Cidlowski, 2013).
Different authors have carried out studies in poultry utilizing molecular markers (including SNPs) (Sheng et al., 2013; Hoque et al., 2013). Currently, the search for SNP markers represents one of the favourite genotyping approaches because they are very abundant in the genome and amenable to high-throughput analysis (Yang et al., 2013).
Genetic variation influences gene expression as demonstrated by Stranger et al. (2007a, 2007b). The same authors, in a study carried out in human lymphoblastoid cell lines (Stranger et al., 2007a, 2007b), highlighted that SNPs captured 83.6 of the total detected genetic variation in gene expression. For these reasons, many SNPs in candidate genes seem to have an important role in regulating the expression level of these genes (Dixon et al., 2007).
In view of these considerations, the first aim of our study was to investigate the effects of chronic heat exposure on the expression of genes codifying for the antioxidative enzymes SOD and CAT as well as for HSP70, HSP90, NR3C1, and caspase 6 (CASP6) in the liver of two different broiler hybrid strains: Red JA Cou Nu Hubbard (CN) and Ross 508 Aviagen (RO). CN is a naked neck slow growing broiler strain intended for the free range and/or organic markets, whereas RO is a strain selected for fast growing (Castellini et al., 2002). The effect of chronic heat stress on broilers was also studied by checking their productive performance, and plasma corticosterone levels. Another aim was to analyse a possible association between transcriptomic response and specific SNPs in each strain of broiler chickens.
2. Materials and methods
2.1. Animals, experimental design, and sample collection
RO and CN birds (average weight ± SE: RO = 98.48 ± 1.70 g; CN = 94.09 ± 1.67 g), were obtained from commercial hatcheries and raised for 4 weeks under standard conditions. As the chickens grew, the RT was gradually decreased from 35 °C to 22 °C and maintained by controlled ventilation and heating until day 28. At the age of 4 weeks, 120 RO and 120 CN broilers (sex ratio 1/1) were randomly divided into 4 cohorts (genotype/temperature combinations) of 60 animals which were then reared for 4 weeks at two different environmental temperatures: 60 RO and 60 CN were housed in separate pens situated in a room at 34 °C (high temperature, HT) and other 60 RO and 60 CN were housed (in separate pens) in another room and maintained at 22 °C (control temperature, CT). All birds were kept under standard conditions (wood-shaving litter, wire pens), and fed the same standard commercial diet (metabolizable energy: 11.8 MJ/kg; crude protein: 18%). Birds were given ad libitum access to feed and water throughout the experiment and were weighed weekly from the 1st week of age on. During the experiment, birds were under careful veterinarian examination; the underlying health status was good and no mortality was recorded. At the age of 8 weeks (end of the trial), all birds were individually weighted and then placed into coops and transported to an authorized commercial processing plant very close to the experimental farm. At the processing plant, birds were manually removed from coops, hung on shackles, and then electrically stunned by using two-stage electrical stunner (214 V, pulsed direct current at approximately 500 Hz for 18 s, followed by 14 V, 60 Hz alternate current for 9 s). After stunning, birds were jugulated by using a conventional unilateral neck cut to severe the carotid artery and jugular vein, bled for 140 s, and then eviscerated. Liver of 10 randomly selected birds/cohort (40 livers in total), were dissected out, immediately frozen in liquid N2, and then stored at − 80 °C until RNA analysis. Carcasses (bled, defeathered, and eviscerated) of 10 birds/cohort (40 carcasses in total) were weighed after 18 h of refrigeration at 4 °C. After recording carcass weight (CW), we calculated carcass yield (CY) by using the following formula: CY = CW after refrigeration/dock weight*100.
2.2. Ethics statement
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Experimental Animals of the University of Milan, Italy. The protocol was approved by the Ethics of Animal Experimentation Committee of the same University. All efforts were made to minimize birds suffering.
2.3. Blood sampling
Blood samples for CORT measurements and SNP genotyping were drawn from the right ulnar vein of 10 birds/cohort into sterile K3 EDTA 2.5 ml PiC Solution® type syringes (needle 22 gauge, 0.7 × 30 mm). The drawn time was 30–50 s. Blood was collected in polypropylene test tubes (vol. 5 ml — Ø 13 × 75 mm, Nuova Aptaca, Italy) with separating silicone gel. Each of the forty blood aliquots taken was immediately subdivided into two aliquots: the first one of whole blood that was stored at minus 80 °C until DNA extraction, and the second one that was immediately centrifuged (1800 × g for 5 min) to take the plasma, which was then stored at − 70 °C until CORT analysis. CORT concentration was measured using the Corticosterone HS EIA® (Immunodiagnostic Systems Limited, United Kingdom) kit, following the manufacturer protocol. This is a high sensitivity (0.17 ng/ml) competitive (EIA) kit, designed to work with very low levels of CORT. The Corticosterone HS kit is validated for chicken. It assures good accuracy and precision (intra-assay < 10%, inter-assay < 15%), and fast turnaround time (results in 5 h with 30 min hands-on time).
2.4. Total RNA isolation and first-strand (cDNA) synthesis
We extracted total RNA from 300 mg of each liver sample using the PureYield™ RNA Midiprep System (Promega, Italy). We then used NanoDrop2000 spectrophotometer (Thermo Scientific, Italy) at an absorbance of 260 nm to calculate the quantity of the extracted RNA, and electrophoresis to assess the integrity of RNA. After extraction, total RNA was reverse transcribed into cDNA using M-MLV Reverse Transcriptase kit (Invitrogen, Italy).
2.5. One-step TaqMan® real-time RT-PCR analysis of target genes transcript copies
We quantified the mRNA copies of HSP70, HSP90, CAT, SOD, NR3C1, and CASP6 by One Step TaqMan® real time RT-PCR (reverse transcription polymerase chain reaction) using the standard curve (absolute) method (please refer to Terova et al., 2011) for details on this method). The nucleotide sequences of all primers used in this study are reported in Table 1. We used Promega RiboProbe® In Vitro Transcription System kit for the in vitro transcription of the mRNAs of each target gene and the synthetic mRNAs produced in this way were then used as quantitative standards in the real time RT-PCR analysis of the experimental samples. Real time analysis was performed in duplicate per each sample using One-Step TaqMan® EZ RT-PCR Core Reagents kit (Life Technologies, Italy) and the following real time run conditions: 2 min at 50 °C, 30 min at 60 °C, and 5 min at 95 °C, followed by 40 cycles consisting of 20 s at 92 °C, and 1 min at 62 °C. Real-time Assays-by-DesignSM PCR primers (Table 2) and gene-specific fluorogenic probes were manufactured by Life Technologies, Italy. We used StepOne Real Time PCR System to perform TaqMan® PCR reactions and collected TaqMan® PCR runs data using StepOne™ Software 2.0. Cycle threshold (Ct) values obtained by each standard mRNA amplification were used to create a standard curve for each target gene. This curve served as a basis for calculating the unknown mRNA copies of each gene present in total RNA extracted from each liver sample.
Table 1.
Sequences of primers (F, forward; R, reverse) used to synthesize in vitro standard mRNAs.
| Gene name | Symbol | Accession number | Primer sequence (5′-3′) |
|---|---|---|---|
| Heat shock protein 90 | HSP90 | NM_206959 | F: CAATTAACCCTCACTAAAGGGAGATCGCGCAGCTCATGTC R: CACCAGGTAGGCGGAGTAG |
| Heat shock protein 70 | HSP70 | EU747335 | F: CAATTAACCCTCACTAAAGGGGTGGCCTTCACCGATACAGA R: GCATCACGTTAAGGCCAGTG |
| Catalase | CAT | NM_001031215 | F: CAATTAACCCTCACTAAAGGGAGGCTCTCAGAAGCCAGATG R: TCCCACAAGATCCCAGTTACC |
| Cu/Zn superoxide dismutase | SOD | NM_205064 | F: CAATTAACCCTCACTAAAGGGGGTCATCCACTTCCAGCAG R: GTACGGCCAATGATGCAGTGT |
| Caspase 6 | CASP6 | NM_204726 | F: CAATTAACCCTCACTAAAGGGTGGACAGCAAACCTACACCA R: TGGGCATCATATGCGTAGACA |
| Glucocorticoid receptor | NR3C1 | DQ227738 | F: CAATTAACCCTCACTAAAGGGCCCTGGACTGAGATCAGATG R: TTCGAGGTTCATGCCTGC |
Table 2.
Primers (F, forward; R, reverse) and TaqMan® probes (P) for quantitative real-time PCR.
| Gene | Symbol | Nucleotide sequence (5′-3′) |
|---|---|---|
| Heat shock protein 90 | HSP90 | F: TCATCAACACGTTCTACTCCAACAAG R: CGGAGGCGTTGGAGATGAG P: CCGCAGGAAGATCT |
| Heat shock protein 70 | HSP70 | F: CCAAGAACCAAGTGGCAATGAA R: CATACTTGCGGCCGATGAGA P: CCCCACCAACACCATC |
| Catalase | CAT | F: ACTGGTGCTGGCAACCC R: ACGTGGCCCAACTGTCAT P: AATAGGAGATAAGCTGAATATT |
| Cu/Zn Superoxide dismutase | SOD | F: CGGGCCAGTAAAGGTTACTGGAA R: TGTTGTCTCCAAATTCATGCACATG P: CAGACAAGCCGGTAATTT |
| Caspase 6 | CASP6 | F: CGTTAATCTTCAATCACGAGCACTT R: GCGTTTCAGATTGTTTCTGTCTGC P: CTGGCAGCCTTAAATG |
| Glucocorticoid receptor | NR3C1 | F: GCAGCTGCAAAGTGTTCTTCAAAA R: GTTCCTTCCAGCGCAGAGATAG P: TTGTGCTGCCCTTCCAC |
2.6. DNA preparation and SNP genotyping
Genomic DNA was isolated using the GenElute Blood Genomic DNA kit (Sigma Aldrich, USA). The SNPs were genotyped by LGC Genomics (UK) using KASPar technology. Then, 10% of the samples were genotyped in duplicates to assess genotyping accuracy.
We chose two genes for SNP analysis: HSP70 (6 different SNPs) and CASP6 (4 SNPs). The list of these SNPs is reported in Table 3. After the analysis, five SNPs were rejected (two from HSP70, and three from CASP6) because they were found to be monomorphic in the studied breeds.
Table 3.
The studied SNPs.
| Gene | SNP name | Accession nr. | SNP | Supplementary information |
|---|---|---|---|---|
| HSP70 | HSP70-3 | AY178441-3 | C > T | |
| HSP70 | HSP70-4 | A > G | ||
| HSP70 | HSP70-5 | A > G | Monomorphic | |
| HSP70 | HSP70-6 | A > G | Monomorphic | |
| HSP70 | HSP70-7 | G > T | ||
| HSP70 | HSP70-9 | C > T | ||
| CASP6 | CASP6-3 | AF212219⁎ | C > G | Monomorphic |
| CASP6 | CASP6-9 | A > G | ||
| CASP6 | CASP6-10 | C > T | Monomorphic | |
| CASP6 | CASP6-18 | C > T | Monomorphic |
>: The “greater than” sign indicates the point of mutation for each studied SNP. The nomenclature for SNPs can be confusing: several variations can exist for an individual SNP and consensus has not yet been achieved. One approach is to write SNPs with a prefix, period and “greater than” sign showing the wild-type and altered nucleotide; for example, A > T.
Not available in chicken. The accession number is relative to Oncorhynchus mykiss.
2.7. Statistical analysis
Live weights (LW), carcass weight (CW), carcass yield (CY), CORT concentration and gene expression data were analysed by GLM procedure using the SAS software (SAS Institute Inc., SAS, Version 8, Cary, NC: SAS Institute Inc., 2000).
We considered genetic strain, environmental temperature, and their interaction as sources of variation according to following linear model:
where
- yijk
dependent variable (LW,CW,CY,CORT, GENE EXPRESSION);
- μ
overall mean;
- Si
fixed effect of the strain (i = 1,2);
- Tj
fixed effect of the temperature (j = 1,2);
- (S ∗ T)ij
interaction between strain and temperature effects;
- eijk
residual random effect of each observation.
Significance level was set at P < 0.05. The genotypic frequencies of SNPs were calculated by using the SAS software and allele frequencies were estimated as suggested by Rodriguez et al. (2009).
3. Results
3.1. Productive performance
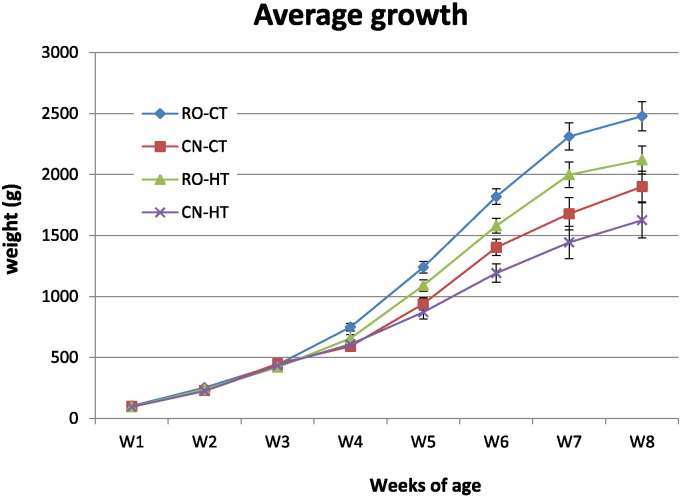
The mean live weights of the four broiler cohorts during the experiment are reported in Fig. 1. The results showed that there was a temperature and a genotype effect on the broilers' growth, from the 5th until the 8th week of age. However, the interaction effect between genotype and temperature was not significant; therefore, we considered the main effect of strain and temperature. The results of the statistical analysis considering the main effect of genotype (Table 4) showed that the RO broilers grew more than the CN broilers from the 4th until the 8th week of age. Indeed, RO broilers reared at either 22 °C or 34 °C had an average weight significantly higher than CN broilers through weeks 4, 5, 6, 7 and 8. By considering the main effect of temperature (Table 4), the unstressed broilers, either RO or CN, grew more than the heat stressed from the 5th until the 8th week of age and at the end of the trial (8th week of age), broilers of the control group (CT) weighted 317.1 g more than those of the stressed group (HT).
Fig. 1.
Effect of chronic heat stress on growth of two broiler chicken genetic strains. Broilers of two different hybrid strains, Ross 308 (RO), Red JA Cou Nu (CN), were maintained from the 4th to the 8th week of age at two different environmental temperatures: 60 RO and 60 CN (in separate pens), at 34 °C (high temperature, HT), and other 60 RO and 60 CN (in separate pens), at 22 °C (control temperature, CT). The mean body weight of 60 animals/strain/temperature is shown at each time point. Bars indicate standard error of the mean. For statistical differences, please refer to Table 4.
Table 4.
Weights LSMEANS (g) observed in the two breeds and in the two experimental temperature conditions at different ages (week).
| Strain |
Temperature |
|||
|---|---|---|---|---|
| Week | CN | RO | CT | HT |
| 1 | 97.5 ± 2.0 | 99.0 ± 1.7 | 101.5 ± 1.8A | 94.9 ± 1.9B |
| 2 | 226.7 ± 6.6a | 246.1 ± 5.5b | 240.8 ± 61 | 232.0 ± 6.1 |
| 3 | 441.1 ± 13.6 | 429.9 ± 10.9 | 444.5 ± 12.6 | 426.4 ± 12.1 |
| 4 | 599.4 ± 25.3A | 702.5 ± 21.9B | 669.8 ± 23.1 | 632.1 ± 24.3 |
| 5 | 905.6 ± 38.0A | 1164.833.7B | 1089.5 ± 34.6a | 980.9 ± 37.1b |
| 6 | 1297.0 ± 50.7A | 1698.7 ± 44.0B | 1610.4 ± 46.2A | 1385.3 ± 48.7B |
| 7 | 1560.6 ± 93.4A | 2155.0 ± 76.4B | 1994.8 ± 86.1a | 1720.7 ± 84.5b |
| 8 | 1762.4 ± 95.4A | 2299.1 ± 82.6B | 2189.3 ± 87.0a | 1872.2 ± 91.5b |
Different small, capital and capital bold letters in the same line (for each main effect) indicate significant differences at P ≤ 0.05, P ≤ 0.01 and P ≤ 0.0001, respectively.
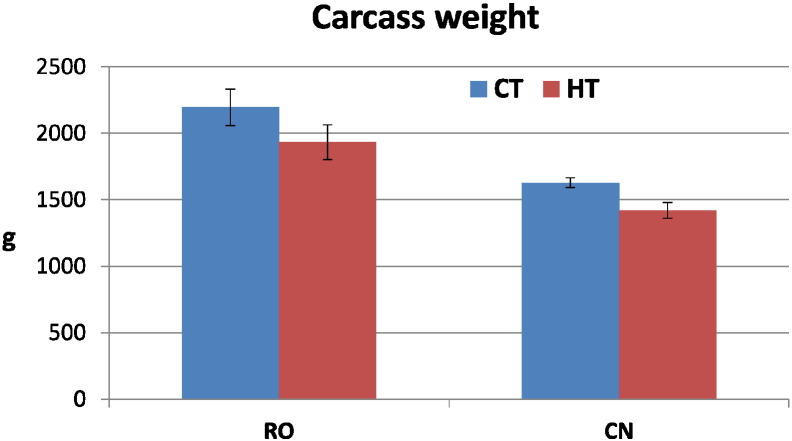
Carcass weight (Fig. 2) reached the highest value (2156.7 g) in RO broilers reared at 22 °C and the lowest (1422.17 g) in CN broilers reared at 34 °C. Since the interaction between temperature and genotype was not significant for this trait, we considered the main effects. The results of the statistical analysis (Table 5) considering the main effect of genotype showed that carcass weight was significantly higher in RO than in CN broiler, whereas by considering the main effect of temperature (Table 5), surprisingly, the carcasses of the unheated group (CT) resulted heavier than those of the heat stressed group. Indeed, although the difference in carcass weight between CT and HT was more than 200 g, we did not have enough evidence to be able to conclude that the difference was not due to chance.
Fig. 2.
Carcass weight at slaughter of two genetic strains of broiler chickens maintained under heat stress conditions. Broilers of two different hybrid strains, Ross 308 (RO), Red JA Cou Nu (CN), were maintained from the 4th to the 8th week of age at two different environmental temperatures: 60 RO and 60 CN (in separate pens), at 34 °C (high temperature, HT), and other 60 RO and 60 CN (in separate pens), at 22 °C (control temperature, CT). The means of 10 birds/strain/temperature are shown in each histogram. Bars indicate standard error of the mean. For statistical differences, please refer to Table 5.
Table 5.
LSMEANS of the slaughtering traits of broilers of two strains reared at two different temperature conditions.
| Strain |
Temperature |
|||
|---|---|---|---|---|
| Carcass | CN | RO | CT | HT |
| Weight (g) | 1525.4 ± 88.2A | 2045.2 ± 73.1B | 1892.7 ± 77.5 | 1678.0 ± 84.4 |
| Yield (%) | 86.6 ± 0.4 | 87.8 ± 0.4 | 86.8 ± 0.4a | 87.6 ± 0.5b |
Different capital bold and small letters in the same line (for each main effect) indicate significant differences at P ≤ 0.0001 and P ≤ 0.05, respectively.
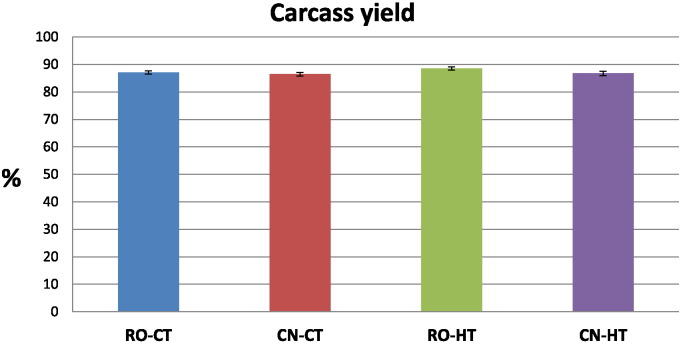
Carcass yield at slaughter (Fig. 3) reached similar values in the four groups ranging from 86.5 to 88.6%. The interaction effect genotype–temperature was not significant for carcass yield; therefore, we considered the strain and temperature main effects (Table 5). Although RO had a more favourable dressing percentage (87.8%), the statistical analysis of the main genotype effect showed that the difference in carcass yield between RO and CN was not significant. By considering the main temperature effect (Table 5), the heat stressed birds (HT) showed a significantly higher carcass yield than the controls (87.6% vs 86.8%).
Fig. 3.
Carcass yield at slaughter of broiler chickens of two genetic strains maintained under heat stress conditions. Broilers of two different hybrid strains, Ross 308 (RO), Red JA Cou Nu (CN), were maintained from the 4th to the 8th week of age at two different environmental temperatures: 60 RO and 60 CN (in separate pens), at 34 °C (high temperature, HT), and other 60 RO and 60 CN (in separate pens), at 22 °C (control temperature, CT). The means of 10 birds/strain/temperature are shown in each histogram. Bars indicate standard error of the mean.
3.2. Plasma CORT levels
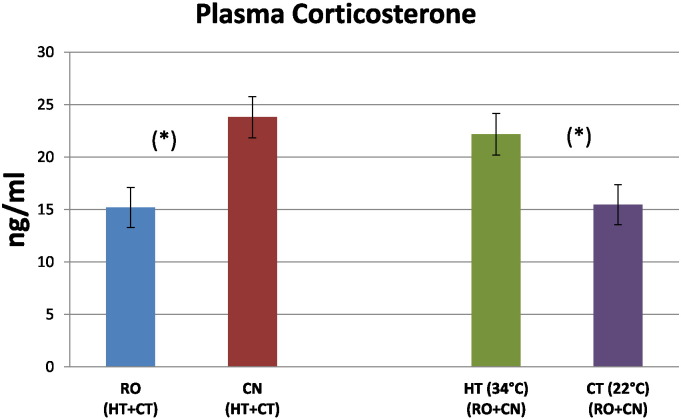
Statistical analysis by two-way ANOVA showed significant differences in plasma CORT concentration related to strain and temperature. However, the interaction effect between genotype and temperature was not significant for plasma CORT concentration. Data on the main effects of genetic strain and temperature on CORT plasma concentration are presented in Fig. 4. As shown, high temperature increased plasma CORT concentration in all birds regardless of breed. Indeed, the concentration of CORT in RO and CN broilers maintained at 34 °C (HT) was significantly higher than that of the same breeds maintained at 22 °C (CT). On the other hand, by considering the breed main effect, the CORT concentration in RO broilers maintained at either high or low temperature was significantly lower than that of the CN birds maintained at the same conditions.
Fig. 4.
Plasma corticosterone concentration in broiler chicken plasma. Broilers of two different hybrid strains, Ross 308 (RO), Red JA Cou Nu (CN), were maintained from the 4th to the 8th week of age at two different environmental temperatures: 60 RO and 60 CN (in separate pens), at 34 °C (high temperature, HT), and other 60 RO and 60 CN (in separate pens), at 22 °C (control temperature, CT). Each histogram represents the average level (means ± s.e.m. as error bar) of 40 broilers (20 birds/strain). (*) indicate significant differences (P < 0.05) between RO (HT + CT) and CN (HT + CT), and between HT (RO + CN) and CT (RO + CN).
3.3. Gene expression
The effects of 4-weeks heat exposure on the expression of six hepatic genes in RO and CN genetic strains are shown in Figs. 5–8.
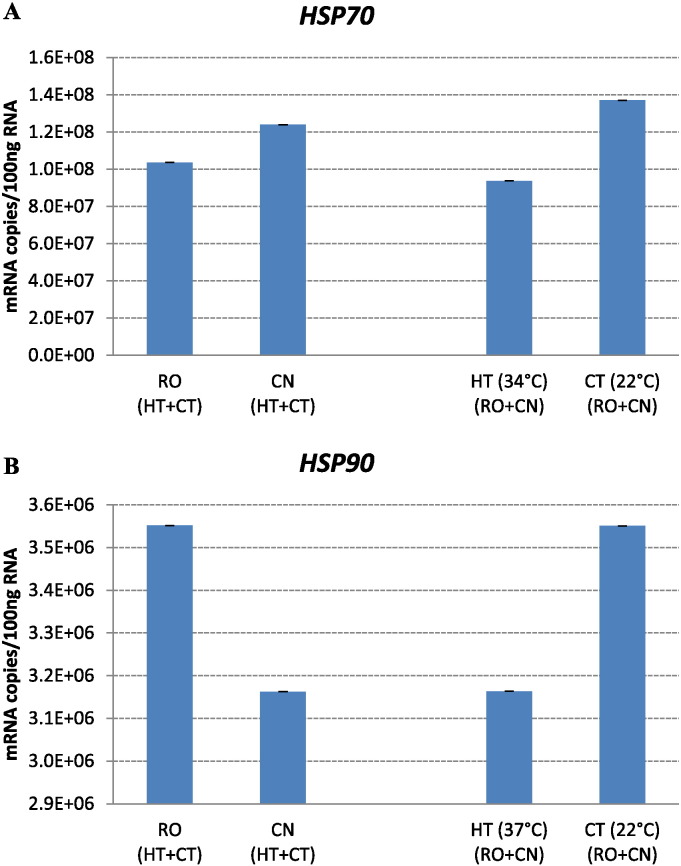
Fig. 5.
A, B. Effect of chronic heat stress on mRNA copies of HSP70 and HSP90 genes in broilers of two breeds. Ross 308 (RO), Red JA Cou Nu (CN) broiler genetic strains were maintained for 4 weeks (from the 4th to the 8th week of age) at temperatures of 34 °C (HT) and 22 °C (CT). Each histogram represents the average mRNA copies (means ± s.e.m. as error bar) of twenty broilers (10 birds/strain/temperature).
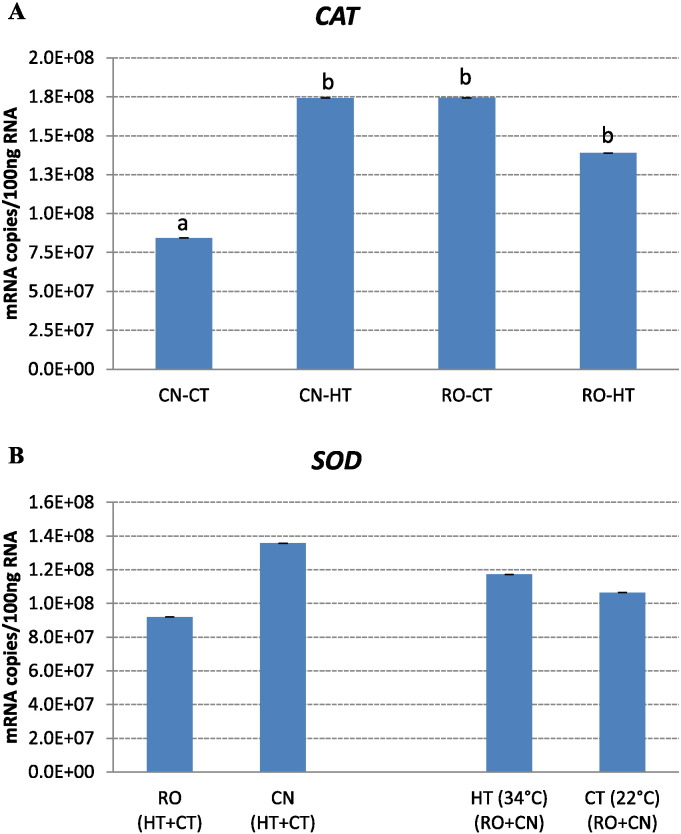
Fig. 6.
A, B. Effect of chronic heat stress on mRNA copies of CAT and SOD genes in broilers of two breeds. Ross 308 (RO) and Red JA Cou Nu (CN) broiler genetic strains were maintained for 4 weeks (from the 4th to the 8th week of age) at temperatures of 34 °C (HT) and 22 °C (CT). Ten birds/strain/temperature were analysed and each histogram represents the average mRNA copies (means ± s.e.m. as error bar). Different small letters indicate significant differences at P ≤ 0.05.
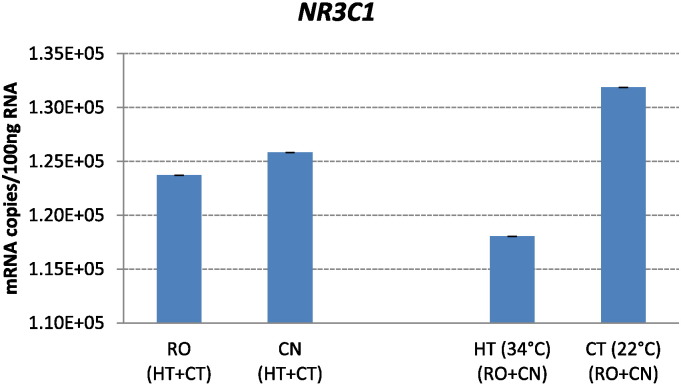
Fig. 7.
Effect of chronic heat stress on mRNA copies of NR3C1 genes in broilers of two breeds. Ross 308 (RO) and Red JA Cou Nu (CN) broiler genetic strains were maintained for 4 weeks (from the 4th to the 8th week of age) at temperatures of 34 °C (HT) and 22 °C (CT). Each histogram represents the average mRNA copies (means ± s.e.m. as error bar) of twenty broilers (10 birds/strain/temperature).
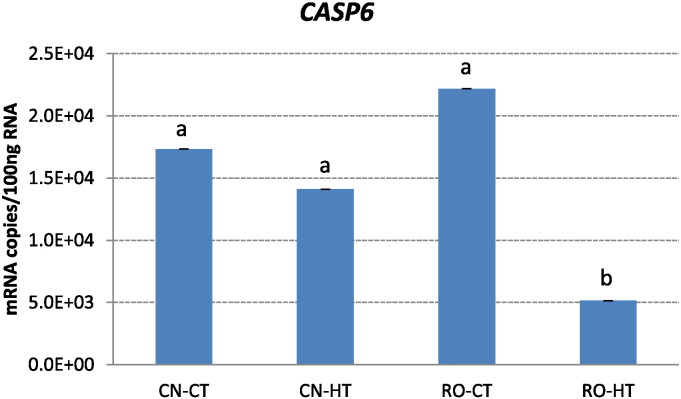
Fig. 8.
Effect of chronic heat stress on mRNA copies of CASP6 genes in broilers of two breeds. Ross 308 (RO) and Red JA Cou Nu (CN) broiler genetic strains were maintained for 4 weeks (from the 4th to the 8th week of age) at temperatures of 34 °C (HT) and 22 °C (CT). Each histogram represents the average mRNA copies (means ± s.e.m. as error bar) of ten broilers. Different small letters indicate significant differences at P ≤ 0.05.
With regard to HSP70 and HSP90 genes (Fig. 5A and B), the statistical analysis showed that there was not a significant interaction effect between temperature and genotype on the expression. Data on the main effects of genetic strain and temperature indicated that the differences in transcript levels of HSP genes were not significant between RO and CN and at different temperatures.
The response of genes codifying for antioxidant enzymes CAT and SOD diverged between the two broiler strains following high environmental temperature exposure (Fig. 6A and B). In particular, SOD transcript abundance in the liver of two strains was not influenced by heat stress (Fig. 6B), whereas in the case of CAT gene (Fig. 6A), we found an interaction genotype–temperature effect on the expression. Indeed, CAT mRNA copy number was significantly upregulated in the liver of CN broilers maintained at HT for 4 weeks in comparison to the mRNA levels found in broilers maintained at 22 °C. It is interesting to note that the expression of CAT in the liver of heat-stressed CN broilers was not statistically different from that of RO broilers maintained at either control or heat-stressed conditions (Fig. 6A).
No effect of chronic exposure to high ambient temperature was found in NR3C1 expression levels in either broiler strains (Fig. 7).
The interaction effect between genotype and temperature was significant for CASP6 gene expression. Likewise in CAT, heat stress influenced the expression of CASP6 in RO broilers (Fig. 8) by significantly downregulating it in heat-stressed RO broilers. In contrast, no significant variation in CASP6 expression was found in CN broilers exposed to chronic heat stress as compared to their respective controls (Fig. 8).
3.4. SNP analysis
Five out of ten studied SNPs, those of HSP70 and CASP6, were found to be monomorphic (only one genotype was found) (Table 3). In all cases the monomorphic status had the same genotype in both genetic strains. Therefore, these SNPs cannot modulate the gene expression level.
In SNP3 of HSP70 in CN (Table 6), the most frequently found genotype was “CT” (86%), whereas in RO the homozygous “TT” was the most often observed (60%). In SNP4 of the same gene, the heterozygous “AG” shows a twofold greater frequency in CN (80%) than in RO (40%).
Table 6.
Frequencies of SNPs in the studied genes.
| Gene | SNP | Breed⁎ | Genotype |
Allele |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | CC | GG | TT | AG | CT | GT | A | C | G | T | |||
| HSP70 | 3 | CN | 7 | 7 | 86 | 50 | 50 | ||||||
| RO | 7 | 60 | 33 | 24 | 76 | ||||||||
| 4 | CN | 7 | 13 | 80 | 47 | 53 | |||||||
| RO | 27 | 33 | 40 | 47 | 52 | ||||||||
| 7 | CN | 93 | 7 | 97 | 3 | ||||||||
| RO | 27 | 20 | 53 | 54 | 46 | ||||||||
| 9 | CN | 47 | 6 | 47 | 70 | 30 | |||||||
| RO | 67 | 33 | 17 | 83 | |||||||||
| CASP6 | 9 | CN | 100 | 100 | |||||||||
| RO | 53 | 47 | 76 | 24 | |||||||||
CN: Red JA Cou Nu; RO: Ross 308.
In SNP7 of the HSP70 the homozygote “GG” is almost monomorphic (93%) in CN, whereas in RO, the genotypic frequencies are more evenly distributed. In SNP9 of the same gene the genotype “CC” is missing in RO.
In RO strain of broilers, the genotype “AA” was the most represented one in SNP9 of CASP6 gene (53%), whereas in CN this SNP was monomorphic.
4. Discussion
High temperature is one of the prominent environmental factors causing economic losses to the poultry industry as it negatively affects growth and production performance in chickens. The majority of the available studies have focused their attention on the effect of acute (short-term, 1 week) heat stress on broilers growth performance. There have only been a few studies that have investigated the effect of chronic heat stress (> 3 weeks) on performance, physiological response, or stress related gene expression in broilers (Quinteiro–Filho et al., 2010; Willemsen et al., 2011; Zhang et al., 2012; Sohail et al., 2012).
Quinteiro–Filho et al. (2010) reported a decrease in body weight gain and feed intake in birds maintained at 31 °C. These authors applied two different heat stressors in their study: (31 ± 1 and 36 ± 1 °C/10 h per day) to broiler chickens from the 35th to the 42nd day of life. Activation of the stress-induced hypothalamic–pituitary–adrenocortical axis (HPA), leading to a rapid release of CORT from the adrenal cortex, was thought to be responsible for the negative effects observed in the broilers' performance in this trial. As known, plasma CORT level is an effective heat stress indicator in chicken (Freeman, 1983; Mc Farlane and Curtis, 1989; Delezie et al., 2007). In our study, plasma CORT concentrations were influenced by high environmental temperature. Indeed, chronic heat stress conditions caused a significant increase in CORT levels due to activation of the HPA axis in both genetic strains of broilers. This result is in agreement with the findings of Quinteiro–Filho et al. (2010), who reported the same effect.
As for the body weight gain of broilers in our study, it almost appears that the slow-growing line was more susceptible to high temperatures in comparison to fast growing. Indeed, RO broilers, either stressed or unstressed grew more than CN ones, from the 4th till the 8th week of age. This was an unexpected result because, in addition to being slow growing, CN are also naked neck broilers and such reduction in feather coverage is known to affect their metabolism, so, they seemingly should have done better in the heat. Studies from Yahav et al. (1998), and from Deeb and Cahaner (1999) have shown that naked-neck broilers have a higher rate of heat dissipation and better thermoregulation in the heat in comparison to their normally feathered counterparts, resulting in higher growth rate and meat production at high ambient temperatures (Cahaner et al., 1993).
We also investigated the effect of chronic heat stress on the expression of some key genes involved in the heat-stress response in broiler chickens. Liver, the hub of metabolism, was chosen as the target tissue. During heat stress, both lipid and carbohydrate stores of liver can be mobilized to generate energy for attenuating the negative effects of stress (Manoli et al., 2007). In our study, broilers were maintained at high ambient temperature from week 4 to week 8 of age because, in general, the broilers have the most rapid growth in this phase of development and it is presumed that exposure to heat stress during this period is more likely to affect gene expression levels. Furthermore, from literature data results that high temperatures significantly affect broilers performance in particular, between the 4th and the 6th week of age (Geraert et al., 1996).
When animals are exposed to thermal stress, the synthesis of most proteins is usually retarded, unlike that of a group of highly conserved proteins such as HSPs, which are rapidly synthesized. HSPs are a large family of proteins that are synthetized in response to environmental stress, including heat stress. Therefore, an increased expression of HSP genes following a heat shock is expected. However, in our study, we did not detect any change in mRNA copies of HSP70 in response to 4 weeks of heat stress. These results are in agreement with what Felver–Gant et al. (2012) observed in laying hens. In that study, laying hens exposed to heat stress for one week exhibited higher HSP70 mRNA copies in liver than the controls, but the expression did not remain at the same high level at the end of the second week of exposure to high ambient temperature. The authors commented that laying hens might have adapted to maintain normal thermal homeostasis at the elevated temperature and thus did not show a high expression of the HSP70 gene. Having only data from the endpoint, we have not the evidence to state that the same explanation could be also applied to broilers of our study that were kept at high temperatures for four weeks. However, a similar adaptation of HSP70 mRNAs could have been possible in our broilers, too. In addition, in the study of Gu et al. (2012), in which an acute heat stress was applied, the HSP70 expression was significantly higher in stressed broiler chickens at 2 and 3 h of heat stress but then returned to control values 5 h later.
Like HSP70, the HSP90 gene is usually expressed at low levels under normal condition, but increases when stress is experienced. However, in the present study, no variations in this gene mRNA copies were found in the liver of chronically heat-stressed broilers. In agreement with our results, Lei et al. (2009) found no differences in HSP90 mRNA copies between heat-stressed and control broilers. Indeed, in that study, HSP90 protein expression increased in the liver of all heat-treated broilers after 2 h of acute heat stress, and then showed a continuous drop after 3, 5, and 10 h of heat stress. Already at 3 h of heat stress, no significant differences were recorded in comparison to control broilers. One of the hypotheses put forth by Lei et al. (2009) was that the high HSP90 expression at the initial stage of heat stress could enhance the survival ability of cells in disadvantageous environments. Long-term and/or excessive stress results in a decline in the expression levels of the protein due to cell lesion development (Lei et al., 2009). Furthermore, the pattern of HSP90 mRNA transcription has been demonstrated to be closely correlated to protein expression (Miller and Qureshi, 1992).
As known, heat can be an environmental factor responsible for oxidative stress and damage. In broiler chickens, the oxidative injury induced by high ambient temperatures has been demonstrated in several studies (Altan et al., 2003; Mujahid et al., 2005; Tan et al., 2010; Azad et al., 2010). The antioxidative enzyme system, (comprising SOD and CAT) acts as the first line of antioxidant defence. Our results showed that four weeks of chronic stress from high environmental temperature exposure caused an increased expression of CAT gene in CN broilers. Such an increase in transcripts levels probably implies an increase in CAT enzyme activity since enzyme activity is often paralleled by the increase in mRNA copy number. An increase in CAT enzyme activity has been considered a protective response to oxidative stress in human (Devi et al., 2000; Thomas, 2000).
The high temperature either in RO or in CN broiler chickens did not influence SOD expression levels. This finding is consistent with the results of Willemsen et al. (2011), who reported that chronic heat stress in broiler chickens did not change plasma SOD activity. Otherwise, chronic heat stress enhanced the activity of SOD in skeletal muscle of broilers exposed to a constant temperature of 34 °C for 2 weeks, but not to cyclic (32 to 24 to 32 °C: 32 °C for 8 h/d) heat exposure in the study of Azad et al. (2010).
Several mechanisms might explain these results. The most promising is the involvement of a nonenzymatic defence system. Willemsen and co-workers found significantly increased plasma uric acid levels in chickens exposed to high temperatures, and uric acid is considered a potent scavenger of free radicals in mammals and particularly in birds (Simoyi et al., 2002).
An unexpected result in our study was that the expression levels of CASP6 gene decreased in RO broilers exposed to heat stress. CASP6 is considered to be one of the genes involved in apoptosis, acting downstream of the apoptotic initiators such as caspase 2, 8, 9, and 10 (Wolf and Green, 1999). Apoptosis has an important role in maintaining tissue homeostasis in cellular stress responses, such as oxidative stress and inflammation (Gorman et al., 1996). It is interesting to note that in the same group of broilers we found the lowest expression of the HSP90 gene (data not shown). Recently, a study on rat pheochromocytoma (PC12) cells reported that the silencing of HSP90 gene induced cytoprotective pathways that could protect neurons against apoptosis (Alani et al., 2014). Alani and co-workers demonstrated for the first time that HSP90 gene knockdown was associated with a decrease in apoptosis level, and this result was confirmed by the decreased levels of caspase-3 protein. The results of Alani et al., suggested that the downregulation of HSP90 could reduce cell vulnerability to stress-induced death by suppressing pro-apoptotic pathways. It has been previously reported that in normal conditions, HSP90 binds to the inactive transcription factor heat shock factor-1 (HSF-1). However, in response to oxidative stress or to HSP90 inhibitors, HSF-1 dissociates from the HSP90 complex, trimerizes, and translocates to the nucleus to activate transcription of HSP70, which prevents the immediate apoptosis of cells and allows cellular adaptation to ensure cell survival (Kitamei et al., 2007).
With regard to the glucocorticoid receptor, despite the increase in plasma CORT levels observed in heat-stressed CN and RO, NR3C1 mRNA levels were not influenced by chronic heat stress in any of the two broiler strains. These results are in disagreement with what we observed in a previous study conducted in three Italian chicken breeds (Valdarnese Bianca, Robusta Maculata, and Bionda Piemontese) which were reared under stress conditions from high stocking density (Marelli et al., 2010). The Valdarnese Bianca is a breed of large white chicken, which is raised principally for its firm and tasty meat that is notably different from that produced by intensive farming methods. Hens are poor layers and tend to become broody (Gualtieri, 2006). The Robusta Maculata and Bionda Piemontese are dual-purpose breeds of chicken. The first one was created between 1959 and 1965, by crossbreeding Buff Orpingtons with the commercial strain known in Italy as “White America” (queryACB, 2012a), whereas the second one originates in the Piemonte region of northwestern Italy (ACB, 2012b). In that study (Marelli et al., 2010), we found a negative correlation between NR3C1 transcripts in the liver and plasma CORT concentration, i.e., NR3C1 gene expression was down regulated by CORT.
The differences found in this study in the expression of CASP6 gene could partly be due to SNPs polymorphisms found in this gene. In particular, we found a SNP polymorphism in the CASP6 gene (CASP6-9) with different allelic frequencies between the two target breeds. In addition three SNPs for the HSP70 (HSP70-3, -7, -9) were detected even if in this case they were not associated to significant differences in expression levels between the two broiler breeds. We hypothesize that these SNPs could have affected the transcription level of genes, as it was demonstrated by Rajib et al. (2013) who investigated in cattle the effect of SNPs on HSP family gene expression levels.
Based on these findings, we can reasonably conclude that heat stress activated the HPA axis in broilers, increasing CORT plasma levels and consequently decreasing body weight gain in the CN broilers. Gene expression was influenced by heat stress showing a downregulation of CASP6 transcripts levels in RO and an upregulation of CAT mRNAs in CN, whereas HSPs, SOD and NR3C1 transcripts levels remained unaffected by heat stress in both target breeds. The differences found in the transcripts level of CASP6 gene between the two strains, could be partly explained by different SNPs found. Additional research is necessary to confirm the relation between the polymorphisms and gene expression response to heat-stress conditions in broilers. However, the evidence given in this study, in terms of gene expression and genome polymorphisms could be useful in the identification of molecular genetic markers to assist in selecting broilers that are more tolerant to heat.
Acknowledgements
This work was funded under the PRIN 2008 project protocol N° 2008FN93B3: “Animal Welfare, product quality and environmental impact in chicken production under heat stress: a gene expression panel.”
References
- ACB (a) Atlas of chicken breeds — Italian breeds: Robusta Maculata (in Italian) 2012. http://www.agraria.org/polli/robustamaculata.htm Accessed January 2012. Website address:
- ACB (b) Atlas of chicken breeds — Italian breeds: Bionda Piemontese. 2012. http://www.agraria.org/polli/biancadisaluzzo.htm Accessed January 2012. Website address:
- Alani B., Salehi R., Sadeghi P., Zare M., Khodagholi F., Arefian E., Hakemi M.G., Digaleh H. Silencing of Hsp90 chaperone expression protects against 6-hydroxydopamine toxicity in PC12. J. Mol. Neurosci. 2014;52:392–402. doi: 10.1007/s12031-013-0163-9. [DOI] [PubMed] [Google Scholar]
- Altan O., Pabuccuoğlu A., Altan A., Konyalioğlu S., Bayraktar H. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 2003;44:545–550. doi: 10.1080/00071660310001618334. [DOI] [PubMed] [Google Scholar]
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Barbe M.F., Tytell M., Gower D.J., Welch W.J. Hyperthermia protects against light damage in the rat retina. Science. 1988;241:1817–1820. doi: 10.1126/science.3175623. [DOI] [PubMed] [Google Scholar]
- Berrong S.L., Washburn K.W. Effects of genetic variation on total plasma protein, body weight gains, and body temperature responses to heat stress. Poult. Sci. 1998;77:379–385. doi: 10.1093/ps/77.3.379. [DOI] [PubMed] [Google Scholar]
- Bottje W.G., Harrison P.C. Effect of carbonated water on growth performance of cockerels subjected to constant and cyclic heat stress temperatures. Poult. Sci. 1985;64:1285–1292. doi: 10.3382/ps.0641285. [DOI] [PubMed] [Google Scholar]
- Cahaner A., Deeb N., Gutman M. Effects of the plumage-reducing naked-neck (Na) gene on the performance of fast-growing broilers at normal and high ambient temperatures. Poult. Sci. 1993;72:767–775. [Google Scholar]
- Cahaner A., Pinchasov Y., Nir I., Nitsan Z. Effects of dietary protein under high ambient temperature on body weight, breast meat yield, and abdominal fat deposition of broiler stocks differing in growth rate and fatness. Poult. Sci. 1995;74:968–975. doi: 10.3382/ps.0740968. [DOI] [PubMed] [Google Scholar]
- Castellini C., Dal Bosco A., Mugnai C., Bernardini M. Performance and behaviour of chickens with different growing rate reared according to the organic system. Ital. J. Anim. Sci. 2002;291-300 [Google Scholar]
- Deeb N., Cahaner A. The effect of naked-neck genotypes, ambient temperature, feeding status and their interactions on body temperature and performance of broilers. Poult. Sci. 1999;78:1341–1346. doi: 10.1093/ps/78.10.1341. [DOI] [PubMed] [Google Scholar]
- Delezie E., Swennen Q., Buyse J., Decuypere E. The effect of feed withdrawal and crating density in transit on metabolism and meat quality of broiler at slaughter weight. Poult. Sci. 2007;86:1414–1423. doi: 10.1093/ps/86.7.1414. [DOI] [PubMed] [Google Scholar]
- Derijk R.H., Schaaf M., De Kloet E.R. Glucocorticoids receptor variants: clinical implications. J. Steroid Biochem. Mol. Biol. 2002;81:103–122. doi: 10.1016/s0960-0760(02)00062-6. [DOI] [PubMed] [Google Scholar]
- Devi G.S., Prasad M.H., Saraswathi I., Raghu D., Rao D.N., Reddy P.P. Free radicals antioxidant enzymes and lipid peroxidation in different types of leukemias. Clin. Chim. Acta. 2000;293:53–62. doi: 10.1016/s0009-8981(99)00222-3. [DOI] [PubMed] [Google Scholar]
- Dixon A.L., Liang L., Moffatt M.F., Chen W., Heath S., Wong K.C.C., Taylor J., Burnett E., Gut I., Farrall M., Lathrop G.M., Abecasis G.R., Cookson W.O.C. A genome-wide association study of global gene expression. Nat. Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- Felver–Gant J.N., Mack L.A., Dennis R.L., Eicher S.D., Cheng H.W. Genetic variations alter physiological responses following heat stress in 2 strains of laying hens. Poult. Sci. 2012;91:1542–1551. doi: 10.3382/ps.2011-01988. [DOI] [PubMed] [Google Scholar]
- Fraisse F., Cockrem J.F. Corticosterone and fear behaviour in white and brown caged laying hens. Br. Poult. Sci. 2006;47:110–119. doi: 10.1080/00071660600610534. [DOI] [PubMed] [Google Scholar]
- Freeman B.M. Adrenal glands in physiology and biochemistry of domestic fowl. Vol. 4. Academic Press; London: 1983. pp. 191–209. (eds.) [Google Scholar]
- Gabriel J.E., Ferro J.A., Stefani R.M.P., Ferro M.I.T., Gomes S.L., Macari M. Effect of acute heat stress on heat shock protein 70 messenger RNA and on heat shock protein expression in the liver of broilers. Br. Poult. Sci. 1996;37:443–449. doi: 10.1080/00071669608417875. [DOI] [PubMed] [Google Scholar]
- Ganter M.T., Ware L.B., Howard M., Roux J., Gartland B., Matthay M.A., Fleshner M., Pittet J. Extracellular heat shock protein 72 is a marker of the stress protein response in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:354–361. doi: 10.1152/ajplung.00405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraert P.A., Guillaumin S., Leclercq B. Are genetically lean broilers more resistant to hot climate? Br. Poult. Sci. 1993;34:643–653. doi: 10.1080/00071669308417623. [DOI] [PubMed] [Google Scholar]
- Geraert P.A., Padilha J.C., Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- Gorman A.M., McGowan A., O'Neill C., Cotter T. Oxidative stress and apoptosis in neurodegeneration. J. Neurol. Sci. 1996;139:45–52. doi: 10.1016/0022-510x(96)00097-4. [DOI] [PubMed] [Google Scholar]
- Gualtieri M. L'allevamento della Valdarnese bianca (in Italian) In: Gualtieri M., editor. “Raising the white Valdarnese”. ARSIA: Agenzia Regionale per lo Sviluppo e l'Innovazione nel Settore Agricolo-Forestale. Press Service srl; Sesto Fiorentino (Firenze): 2006. (Website address: http://www.pollodelvaldarno.it/pollodelvaldarno.pdf) [Google Scholar]
- Franco-Jimenez D.J., Scheideler S.E., Kittok R.J., Brown–Brandl T.M., Robeson L.R., Taira H., Beck M.M. Differential effects of heat stress in three strains of laying hens. J. Appl. Poult. Res. 2007;16:628–634. [Google Scholar]
- Gu X.H., Hao Y., Wang X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 2. Intestinal oxidative stress. Poult. Sci. 2012;91:790–799. doi: 10.3382/ps.2011-01628. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. Clarendon Press; Oxford: 1996. Lipid peroxidation: a radical chain reaction; pp. 188–266. [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasheimi S.R., Zulkifli I., Somchit M.N., Zunita Z., Loh T.C., Soleimani A.F., Tang S.C. Dietary supplementation of Zingiber officinale and Zingiber zerumbet to heat-stressed broiler chickens and its effect on heat shock protein 70 expression, blood parameters and body temperature. J. Anim. Physiol. Anim. Nutr. 2012;97:632–638. doi: 10.1111/j.1439-0396.2012.01302.x. [DOI] [PubMed] [Google Scholar]
- Hashizawa Y., Kubota M., Kadowaki M., Fujimura S. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions. J. Anim. Sci. 2013;84:732–736. doi: 10.1111/asj.12079. [DOI] [PubMed] [Google Scholar]
- Hoque M.R., Jin S., Heo K.N., Kang B.S., Jo C., Lee J.H. Investigation of MC1R SNPs and their relationships with plumage colours in Korean native chicken. Asian–Australasian. J. Anim. Sci. 2013;26:625–629. doi: 10.5713/ajas.2012.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.B., Beuving G., Blokhuis H.J. Tonic immobility and heterophil/lymphocyte responses of the domestic fowl to corticosterone infusion. Physiol. Behav. 1988;42:249–253. doi: 10.1016/0031-9384(88)90078-9. [DOI] [PubMed] [Google Scholar]
- Kitamei H., Kitaichi N., Yoshida K., Nakai A., Fujimoto M., Kitamura M., Iwabuchi K., Miyazaki A., Namba K., Ohno S., Onoé K. Association of heat shock protein 70 induction and the amelioration of experimental autoimmune uveoretinitis in mice. Immunobiology. 2007;212:11–18. doi: 10.1016/j.imbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Kwok A.H.Y., Wang Y., Wang C.Y., Leung F.C. Cloning of chicken glucocorticoid receptor (GR) and characterization of its expression in pituitary and extra pituitary tissues. Poult. Sci. 2007;86:423–430. doi: 10.1093/ps/86.2.423. [DOI] [PubMed] [Google Scholar]
- Laing K.J., Holland J., Bonilla S., Cunningham C., Secombes C.J. Cloning and sequencing of caspase 6 in rainbow trout, Oncorhynchus mykiss, and analysis of its expression under conditions known to induce apoptosis. Dev. Comp. Immunol. 2001;25:303–312. doi: 10.1016/s0145-305x(00)00061-6. [DOI] [PubMed] [Google Scholar]
- Lei L., Yu J., Bao E. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br. Poult. Sci. 2009;50:504–511. doi: 10.1080/00071660903110851. [DOI] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus): 2. Short-term effect. Comp. Biochem. Physiol. 2004;139:745–751. doi: 10.1016/j.cbpc.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Lin H., Jiao H.C., Buyse J., Decuypere E. Strategies for preventing heat stress in poultry. Worlds Poult. Sci. J. 2006;62:71–86. [Google Scholar]
- Lu Q., Wen J., Zhang H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007;86:1059–1064. doi: 10.1093/ps/86.6.1059. [DOI] [PubMed] [Google Scholar]
- Manoli I., Alesci S., Blackman M.R., Su Y.A., Rennert O.M., Chrousos G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 2007;18:190–198. doi: 10.1016/j.tem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Marelli S.P., Terova G., Cozzi M.C., Lasagna E., Sarti F.M., Guidobono C.L. Gene expression of hepatic glucocorticoid receptor NR3C1 and correlation with plasmatic corticosterone in Italian chickens. Anim. Biotechnol. 2010;21:140–148. doi: 10.1080/10495391003608621. [DOI] [PubMed] [Google Scholar]
- Mc Farlane J.M., Curtis S.E. Multiple concurrent stressors in chicks. 3. Effect on plasma corticosterone and the heterophil:lymphocyte ratio. Poult. Sci. 1989;68:522–527. doi: 10.3382/ps.0680522. [DOI] [PubMed] [Google Scholar]
- Miller L., Qureshi M.A. Molecular changes associated with heat shock treatment in avian mononuclear and lymphoid lineage cells. Poult. Sci. 1992;71:473–481. doi: 10.3382/ps.0710473. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Pumford N.R., Bottje W., Nakagawa K., Miyazawa T., Akiba Y., Toyomizu M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. J. Poult. Sci. 2007;44:439–445. [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., Toyomizu M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005;84:307–314. doi: 10.1093/ps/84.2.307. [DOI] [PubMed] [Google Scholar]
- Murakami H.K., Pain D., Blobel G. 70–kDa heat shock–related protein is one of at least two distinct cytosolic factors stimulating protein import into mitochondria. J. Cell Biol. 1988;107:2051–2057. doi: 10.1083/jcb.107.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H.R.B. Activation of heat-shock genes in eukaryotes. Trends Genet. 1985;1:31–35. [Google Scholar]
- Quinteiro–Filho W.M., Ribeiro A., Ferraz–de–Paula V., Pinheiro M.L., Sakai M., Sá L.R.M., A JP F., Palermo–Neto J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult. Sci. 2010;89:1905–1914. doi: 10.3382/ps.2010-00812. [DOI] [PubMed] [Google Scholar]
- Rajib D., Sajjanar B., Singh U., Kumar S., Brahamane M.P., Singh R., Sengar G., Sharma A. Promoter variants at AP2 box region of Hsp70.1 affect thermal stress response and milk production traits in Frieswal cross bred cattle. Gene. 2013;532:230–235. doi: 10.1016/j.gene.2013.09.037. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S., Cidlowski J.A. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr. Dev. 2013;24:41–56. doi: 10.1159/000342502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L.L., Hardy S.J.S. Correlation of competence for export with lack of tertiary structure of the mature species: a study in vivo of maltose-binding protein in E. coli. Cell. 1986;46:921–928. doi: 10.1016/0092-8674(86)90074-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez S., Gaunt T.R., Day I.N.M. Hardy–Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am. J. Epidemiol. 2009;169:505–514. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandercock D.A., Hunter R.R., Nute G.R., Mitchell M.A., Hocking P.M. Acute heat stress-induced alterations in blood acid–base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult. Sci. 2001;80:418–425. doi: 10.1093/ps/80.4.418. [DOI] [PubMed] [Google Scholar]
- Sheng Q., Cao D., Zhou Y., Lei Q., Han H., Li F., Lu Y., Wang C. Detection of SNPs in the cathepsin D gene and their association with yolk traits in chickens. PLoS ONE. 2013;8:e56656. doi: 10.1371/journal.pone.0056656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoyi M.F., Van Dyke K., Klandorf H. Manipulation of plasma uric acid in broiler chicks and its effect on leukocyte oxidative activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:791–796. doi: 10.1152/ajpregu.00437.2001. [DOI] [PubMed] [Google Scholar]
- Sohail M.U., Hume M.E., Byrd J.A., Nisbet D.J., Ijaz A., Sohail A., Shabbir M.Z., Rehman H. Effect of supplementation of prebiotic mannan–oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult. Sci. 2012;91:2235–2240. doi: 10.3382/ps.2012-02182. [DOI] [PubMed] [Google Scholar]
- Soleimani A.F., Zulkifli I., Omar A.R., Raha A.R. Physiological responses of 3 chicken breeds to acute heat stress. Poult. Sci. 2011;90:1435–1440. doi: 10.3382/ps.2011-01381. [DOI] [PubMed] [Google Scholar]
- Staib J.L., Quindry J.C., French J.P., Criswell D.S., Powers S.K. Increased temperature, not cardiac load, activates heat shock transcription factor 1 and heat shock protein 72 expression in the heart. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:432–439. doi: 10.1152/ajpregu.00895.2005. [DOI] [PubMed] [Google Scholar]
- Star L., Decuypere E., Parmentier H.K., Kemp B. Effect of single or combined climatic and hygienic stress in four layer lines: 2. Endocrine and oxidative stress responses. Poult. Sci. 2008;87:1031–1038. doi: 10.3382/ps.2007-00143. [DOI] [PubMed] [Google Scholar]
- Stranger B.E., Forrest M.S., Dunning M., Ingle C.E., Beazley C., Thorne N., Redon R., Bird C.P., de Grassi A., Lee C., Tyler–Smith C., Carter N., Scherer S.W., Tavaré S., Deloukas P., Hurles M.E., Dermitzakis E.T. Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007;315:848–853. doi: 10.1126/science.1136678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger B.E., Nica A.C., Forrest M.S., Dimas A., Bird C.P., Beazley C., Ingle C.E., Dunning M., Flicek P., Koller D., Montgomery S., Tavaré S., Deloukas P., Dermitzakis E.T. Population genomics of human gene expression. Nat. Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Terova G., Cattaneo A.G., Preziosa E., Bernardini G., Saroglia M. Impact of acute stress on antimicrobial polypeptides mRNA copy number in several tissues of marine sea bass (Dicentrarchus labrax) BMC Immunol. 2011;12:69. doi: 10.1186/1471-2172-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M.J. The role of free radicals and antioxidants. J. Nutr. 2000;16:716–718. doi: 10.1016/s0899-9007(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Washburn K.W., Peavey R., Renwick G.M. Relationship of strain variation and feed restriction to variation in blood pressure and response to heat stress. Poult. Sci. 1980;59:2586–2588. doi: 10.3382/ps.0592586. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Swennen Q., Everaert N., Geraert P.A., Mercier Y., Stinckens A., Decuypere E., Buyse J. Effects of dietary supplementation of methionine and its hydroxy analog dl-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011;90:2311–2320. doi: 10.3382/ps.2011-01353. [DOI] [PubMed] [Google Scholar]
- Wolf B.B., Green D.R. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J. Biol. Chem. 1999;274:20,049–20,052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- Yahav S., Goldfeld S., Plavnik I., Hurwitz S. Physiological responses of chickens and turkeys to relative humidity during exposure to high ambient temperature. J. Therm. Biol. 1995;20:245–253. [Google Scholar]
- Yang W., Kang X., Yang Q., Lin Y., Fang M. Review on the development of genotyping methods for assessing farm animal diversity. J. Anim. Sci. Biotechnol. 2013;4:2. doi: 10.1186/2049-1891-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahav S., Luger D., Cahaner A., Dotan M., Rusal M., Hurwitz S. Thermoregulation in naked-neck chickens subjected to different ambient temperatures. Br. Poult. Sci. 1998;39:133–138. doi: 10.1080/00071669889510. [DOI] [PubMed] [Google Scholar]
- Young R.A. Stress proteins and immunology. Immunol. Annu. Rev. Immunol. 1990;8:401–420. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- Yudt M.R., Cidlowsky J.A. Molecular identification and characterization of a and b forms of the glucocorticoid receptor. Mol. Endocrinol. 2001;15:1093–1103. doi: 10.1210/mend.15.7.0667. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]