Abstract
The development and refinement of noninvasive techniques for imaging neural activity is of paramount importance for human neuroscience. Currently, the most accessible and popular technique is electroencephalography (EEG). However, nearly all of what we know about the neural events that underlie EEG signals is based on inference, because of the dearth of studies that have simultaneously paired EEG recordings with direct recordings of single neurons. From the perspective of electrophysiologists there is growing interest in understanding how spiking activity coordinates with large-scale cortical networks. Evidence from recordings at both scales highlights that sensory neurons operate in very distinct states during spontaneous and visually evoked activity, which appear to form extremes in a continuum of coordination in neural networks. We hypothesized that individual neurons have idiosyncratic relationships to large-scale network activity indexed by EEG signals, owing to the neurons' distinct computational roles within the local circuitry. We tested this by recording neuronal populations in visual area V4 of rhesus macaques while we simultaneously recorded EEG. We found substantial heterogeneity in the timing and strength of spike-EEG relationships and that these relationships became more diverse during visual stimulation compared with the spontaneous state. The visual stimulus apparently shifts V4 neurons from a state in which they are relatively uniformly embedded in large-scale network activity to a state in which their distinct roles within the local population are more prominent, suggesting that the specific way in which individual neurons relate to EEG signals may hold clues regarding their computational roles.
Keywords: EEG, local field potential, spiking activity
electroencephalography (EEG) has been a boon to human neuroscience. Because EEG is noninvasive, it is one of the few methods that can be used to measure human brain activity in both health and disease. Moreover, EEG has advantages over other neuroimaging methods. The cost is relatively low, compared with approaches such as functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG); there is no ionizing radiation, as with positron emission tomography (PET); the materials are portable (even usable with actively moving subjects; De Sanctis et al. 2012; Makeig et al. 2009), unlike nearly all alternatives; and the temporal resolution is exquisitely fine, unlike functional near-infrared spectroscopy (fNIRS) and fMRI. EEG covers a wide range of neurophysiological applications, from diagnosis of brain stem lesions to basic research on complex cognition.
The principal limitation of EEG is spatial resolution. Typically, EEG is useful for implicating activity in parts of the brain on the order of a cubic centimeter (Sejnowski et al. 2014). The computational machinations of the neurons within that volume that give rise to the particular overlying EEG signal are too minute to be probed by EEG. For example, a study comparing event-related potentials (ERPs) in response to symmetric and asymmetric visual stimuli found that lateral occipital areas generate a symmetry-related component starting ∼250 ms after stimulus onset (Snyder et al. 2012). These results clearly implicate a time and place in the brain that is involved in the processing of visual symmetry but cannot elucidate the nature of that ERP component in computational terms. Similarly, shifts in the amplitude of EEG oscillations can be monitored to detect shifts of attention (Banerjee et al. 2011; Belyusar et al. 2013; Foxe and Snyder 2011; Snyder and Foxe 2010), but the mechanisms that manifest and modulate these oscillations and their attendant effects on stimulus processing are not well understood. The situation is made more complicated by the realization that the relationship between neurocomputational processes and the corresponding EEG signals is unlikely to be static but rather is dependent on the brain areas involved and the nature of the computational state.
Neuronal populations do indeed appear to transition between distinct computational states (Kohn et al. 2009). Spontaneous activity exemplifies one such state, in which each neuron tends to be highly correlated with its neighbors and the population as a whole is strongly modulated by global network fluctuations. In contrast, visual stimulation transitions the population into a state in which neurons operate more independently (Kelly et al. 2010; Smith and Kohn 2008; Smith and Sommer 2013). Subtler computational states exist between these quite distinct modes of neural activity, and modulation in the degree of coordination between individual neurons and their surrounding populations has been reported in a variety of contexts, such as attention (Cohen and Maunsell 2009; Mitchell et al. 2009), learning (Gu et al. 2011; Jeanne et al. 2013), and contextual modulation (Snyder et al. 2014). At the level of the EEG, such state transitions are extremely salient—the earliest reported hallmark of the neural basis of the EEG signal was the large change in alpha oscillations with the eyes closed vs. open (Berger 1929). Changes in the character of neuronal population activity between spontaneous and evoked states, coupled with the large changes in the EEG under the same conditions, led us to reason that the relationship between individual neuron activity and the large-scale network activity indexed by EEG signals would vary substantially across behavioral contexts. We tested this by explicitly measuring the relationships between the spiking of many single neurons in visual area V4 and the overlying EEG signals in both an unstimulated (hereafter referred to as “spontaneous”) and a visually stimulated (hereafter referred to as “evoked”) state. We predicted specifically that the individual neurons would be less consistently aligned to the phase of the EEG signal (i.e., the neurons are more independent) in the evoked state compared with the spontaneous state.
Although researchers have been interested in the origins of EEG signals since the development of the technique in the early twentieth century, inquiry has focused on the physical current source for the EEG signals rather than on the originating computational processes. Early electrophysiologists investigated the nature of the EEG by recording it along with spiking activity, primarily from anesthetized cats (Buchwald et al. 1965; Fromm and Bond 1964; Li and Jasper 1953). It should be noted that the term “EEG” was used at that time to refer to low-frequency potentials regardless of the recording site, including signals that contemporary electrophysiologists would distinguish as local field potentials (LFPs), that is, potentials recorded from a microelectrode inserted into the parenchyma of the brain (e.g., Buchwald et al. 1965) or electrocorticography (ECoG) from the brain's surface (e.g., Li and Jasper 1953). These researchers reported that EEG signals were unlikely to be direct reflections of local spiking activity. However, a careful inspection of these early reports shows that spiking activity did exhibit general tendencies to occur during particular features of the low-frequency EEG oscillations (e.g., Fig. 10 in Li and Jasper 1953). Over time a consensus emerged that the EEG was generated by postsynaptic potentials, in particular those across the radially aligned apical dendrites of pyramidal cells (see Logothetis 2003 for review). Unfortunately, without access to digital computers to aid in time-frequency analysis, a more detailed understanding of what Li and Jasper (1953) described as the “ill-defined conditions” that linked EEG oscillations to spiking activity remained beyond the reach of these early investigations.
Fig. 10.
SFC for LFP. A: solid lines: grand-averaged SFC over all neurons (spontaneous: n = 1,220 neurons; evoked: n = 1,326 neurons; shading represents ±1 SE). Dashed lines: SFC for trial-shuffled data, capturing measurement bias (shading represents 90% confidence interval). Underlining indicates statistical significance at each frequency (blue and red underlines: 1-sample t-test for spontaneous and evoked SFC, respectively, against a null hypothesis of 0; black underline: independent-samples t-test for the difference between conditions). B: same as in A but with the trial-shuffled values subtracted from the raw values (i.e., ΔSFC), correcting for measurement bias. C: proportion of neurons at each frequency with significant SFC (by permutation test using trial-shuffled data). Dashed line reflects the false-positive rate of P = 0.05.
Later research aimed to identify the generators of the various peaks and troughs of voltage that characterize ERPs, which are frequently measured by EEG. For example, a series of related experiments by Schroeder and colleagues (Givre et al. 1994; Schroeder et al. 1991; Simpson et al. 1995) employed ECoG at the cortical surface to measure stimulus-evoked potentials in monkeys and then subsequently used multicontact depth electrodes to infer the relative contributions of LFPs and multiunit activity (MUA) in the different cortical lamina and subcortical structures to the componentry of those evoked potentials. In another study, Reinhardt et al. (2012) combined ERPs at the cranial surface with intracranial LFPs to identify the brain regions contributing to the monkey homolog of the “contralateral delay activity” ERP component in a visual working memory task. These efforts revealed important information regarding when and where, on average, groups of neurons respond to stimuli and other events. However, the question as to how exactly the identified spatiotemporal activation profiles in a brief evoked response translate to ongoing neural activity and the underlying neural computation remains unanswered.
Recently studies have focused on how networks of cells contribute to EEG or LFP signals, such as the generation of sleep spindles in the EEG by thalamocortical interactions (Steriade et al. 1993). While this approach has been enormously fruitful for processes such as sleep, relating spiking activity to the slow cortical potentials during complex cognition has proved more elusive. In one example, Fries et al. (2001) found increased alignment of spiking activity to the phase of gamma-band oscillations in the LFP with selective attention. A later study extended this result, finding increased phase-locking of spikes not only within sensory cortex but also between sensory and frontal areas hypothesized to be integral to attentional control (Gregoriou et al. 2009). However, Juergens et al. (1999) estimated that gamma-band LFP signals must be coherent on the scale of 3–6 cm2 to produce detectable gamma-band EEG signals. Thus it is not straightforward to generalize LFP results to scalp EEG studies in healthy humans. The current status is that we have excellent understanding of the electromagnetic origins of EEG signals but very little understanding of their neurocomputational origins.
Because computation is carried out by populations of spiking neurons, investigating the neurocomputational origin of EEG signals requires the simultaneous recording of EEG at the scalp and neuronal population activity intracranially. Few researchers have attempted this. For one such effort, Whittingstall and Logothetis (2009) recorded MUA in primary visual cortex from single microelectrodes threaded through a ring-shaped EEG electrode on the cranium. They found that the rate of MUA was negatively correlated with the voltage on the EEG electrode (referenced to a frontal site). In another case, Musall et al. (2014) used the same methodology as Whittingstall and Logothetis (2009), but with three penetrating microelectrodes rather than one. This enabled them to test the effect of the coherence of LFPs across pairs of microelectrodes on the magnitude of EEG signals. They reported that periods of high spatial coherence led to greater-amplitude EEG signals, presumably due to constructive summation of the local fields.
Here we add to this nascent body of knowledge by recording population spiking activity in V4 concurrently with EEG from eight scalp electrodes. This enabled us to assess the topographical distribution of the EEG signals related to spiking activity, an advance over previous EEG-spiking studies. Our work is the first to measure the relationship between many single neurons (in addition to combined MUA and LFPs) and the EEG. Our guiding thesis is that in the absence of visual stimulation V4 neurons receive relatively strong drive from internal signals that are common across the population, but when stimulated the neurons express idiosyncratic relationships to the large-scale network activity by virtue of their particular computational roles in the population. The first part of this claim is motivated by the observation that correlated variability between pairs of V4 neurons is relatively stronger during the spontaneous state (Smith and Sommer 2013), indicating shared endogenous drive. We predict that this shared internal drive not only underlies correlated firing rate variability between pairs of neurons but also creates relatively strong and consistent coherence between spiking activity and the overlying EEG signals. When visually stimulated, the neurons shift from the internally driven state to a state determined by the relationship between the stimulus properties and the neurons' tuning preferences. This transition is evidenced by a decrease in correlated variability between pairs of neurons during visual stimulation (Smith and Sommer 2013) and an increase in the signal reliability of individual neurons' responses (Churchland et al. 2010; Cohen and Maunsell 2009). We predict that this state transition during visual stimulation would also lead to weaker and less consistent coherence between spiking activity and the overlying EEG signals. Identifying the distinct ways in which individual neurons are linked to scalp EEG signals may provide clues about the distinct computational roles of different neurons within the population circuitry.
METHODS
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. A separate analysis of these data was published previously (Snyder et al. 2015).
Subjects.
We implanted a 100-electrode “Utah” array (Blackrock Microsystems) in area V4 of the right hemisphere in each of two adult male rhesus macaques (Macaca mulatta). The basic surgical procedures have been described previously (Smith and Sommer 2013) and were conducted in aseptic conditions under isoflurane anesthesia. In addition to the microelectrode arrays, the animals were implanted with a titanium head post to immobilize the head during experiments. We recorded neurons with receptive fields (RFs) centered around 3.16° or 5.66° from the fovea in the lower visual field of each animal.
Behavioral tasks.
The subjects performed two delayed-saccade tasks. We presented visual stimuli with custom software written in MATLAB (The MathWorks) using the Psychophysics Toolbox extensions (Brainard 1997; Kleiner et al. 2007; Pelli 1997). We trained the subjects to maintain fixation on a 0.6° blue dot at the center of a flat-screen cathode ray tube monitor positioned 36 cm from their eyes. The background of the display was 50% gray. We measured the monitor luminance gamma functions with a photometer and linearized the relationship between input voltage and output luminance with lookup tables. In the “spontaneous” task, subjects were trained to maintain fixation on the central dot for 2 s, at which time the fixation point was moved 11.6° in a random direction and the animal received a liquid reinforcement for making a saccade to the new location. No other stimuli were presented for the spontaneous task. The “evoked” task was identical, except we presented a drifting sinusoidal grating (100% contrast, maximum luminance of 145 cd/m2) in the aggregate RF area of the neurons recorded on the microelectrode arrays during the 2-s fixation interval. Both subjects completed the spontaneous task (monkey B: 14 sessions spanning 22 calendar days; monkey W: 10 sessions spanning 57 calendar days) prior to performing the evoked task (monkey B: 10 sessions spanning 12 calendar days; monkey W: 8 sessions spanning 32 calendar days). This was necessary to maximize the number of trials in each session but left as a possible confound that neuronal relationships with the EEG changed over time. To mitigate this potential confound, we performed a within-session trial-shuffling procedure to identify and correct for potential biases in our analyses introduced by changes in recording quality over time (see Trial-shuffling correction).
Microelectrode array recordings.
Signals from the microelectrode arrays were band-pass filtered (0.3–7,500 Hz), digitized at 30 kHz, and amplified by a Grapevine system (Ripple). Data were then band-pass filtered into separate LFP (0.3–250 Hz) and spiking (250–7,500 Hz) signals. The online recording reference for the array was a wire inserted subdurally, tangent to the cortical surface. According to the array manufacturer, the impedance of the electrodes was 38–54 kΩ prior to implantation [sputtered iridium oxide film (SIROF) arrays]. The input impedance to the preamplifier of the Grapevine system was ∼450 MΩ. Spike signals crossing a threshold [adjusted daily using a multiple of the root-mean-squared (RMS) noise for each channel] were stored for off-line analysis. Note that variation in electrode impedance across sessions is not likely to meaningfully affect our results, as intracranial signals (Nelson and Pouget 2010) and scalp EEG (Kappenman and Luck 2010) have been shown to be impacted negligibly by electrode impedance if the input impedance to the amplifier is much greater, as it was in our case. These waveform segments were sorted with an automated clustering algorithm (Shoham et al. 2003) followed by manual refinement with custom MATLAB software (Kelly et al. 2007; available at http://www.smithlab.net/spikesort.html), taking into account the waveform shapes and interspike interval distributions. After sorting, we calculated the signal-to-noise ratio (SNR) of each candidate unit as the ratio of the average waveform amplitude to the standard deviation of the waveform noise (Kelly et al. 2007). Candidates with an SNR below 2.5 were discarded. We treated the neurons recorded by the arrays to be independent from session to session. This resulted in 1,293 single neurons sampled for the spontaneous condition and 1,482 single neurons sampled for the stimulus-evoked condition.
Receptive field mapping and tuning curves.
Prior to beginning the experiments, we mapped the RF areas of the units recorded on our arrays by presenting small (∼1°) sinusoidal gratings at four orientations positioned one at a time on the vertices of a lattice in the likely RF area per the anatomical location of the implant. After inspecting the responses of the units to these small-probe stimuli, we picked a stimulus size and position to roughly cover the aggregate RF area. For monkey B this was 5.87° diameter centered 4.00° below and 4.00° to the left of fixation, and for monkey W this was 4.70° diameter centered 1.18° below and 2.94° to the left of fixation. Throughout this report we use the term “RF” to refer to this aggregate area. We next measured tuning curves for the recorded units by presenting sinusoidal gratings to the RF area in four orientations and at a variety of spatial and temporal frequencies. For each subject we chose a compromise temporal and spatial frequency (3.00 Hz and 0.85 cycles/° for monkey B; 3.25 Hz and 0.40 cycles/° for monkey W) that evoked the maximum response from the array as a whole. Although the stimulus was not optimized for any one neuron in our sample, we nonetheless observed a robust and sustained firing rate increase during visual stimulation (100-1,900 ms after stimulus onset) compared with the 100 ms preceding stimulus onset (stimulus evoked: 4.43 ± 0.19 Hz, prestimulus: 2.31 ± 0.12 Hz; paired-samples t-test, t2962 = 16.47, P < 0.001, for all neurons and MUA recorded). Note that these firing rate values exclude the initial transient response to the visual stimulus, since we specifically excluded the transient response from our subsequent analyses. Despite this firing rate increase from baseline within the sessions with visual stimulation, we did not find a significant difference between stimulus-evoked and spontaneous conditions during the fixation period of the task (evoked: 4.43 ± 0.19 Hz, spontaneous: 4.88 ± 0.17 Hz; independent-samples t-test, t2773 = 1.80, P = 0.072). The lack of a difference is most likely because we collected the data for the two conditions in different sessions and happened to sample neurons with greater baseline firing rates during the sessions when the spontaneous condition was collected. As a consequence, our data were effectively controlled for any potential influence of firing rate on the other dependent variables we describe below.
EEG recordings.
We recorded EEG from eight Ag/AgCl electrodes (Grass Technologies) adhered to the scalp with conductive paste (see Fig. 1 for positioning). Signals were referenced online to the head post, digitized at 1 kHz, and amplified by a Grapevine system (Ripple) and band-pass filtered online at 0.3–250 Hz. At the conclusion of the experiment, we measured the EEG electrode impedance to be ∼100–500 Ω. The input impedance to the preamplifier of the Grapevine system was ∼450 MΩ. As mentioned above, variation in scalp electrode impedance is negligible when several orders of magnitude less than preamplifier impedance (Kappenman and Luck 2010), as was the case for our recordings. We analyzed data from 100 ms to 1,900 ms after the subject achieved fixation. We rejected epochs containing artifacts with two automated methods. First, we excluded epochs when the variance on any EEG channel was greater than 4 standard deviations above the mean variance over all channels and trials within the session. Second, we excluded epochs when any EEG channel spanned a range of >200 μV. After artifact rejection, we rereferenced the data to the common average of all channels. We baseline corrected each epoch by subtracting the best-fitting linear trend. EEG data were analyzed with FieldTrip (Oostenveld et al. 2011) for MATLAB and custom MATLAB routines. Unless otherwise specified, EEG data from the right parietal electrode (Fig. 1A) are depicted in subsequent figures.
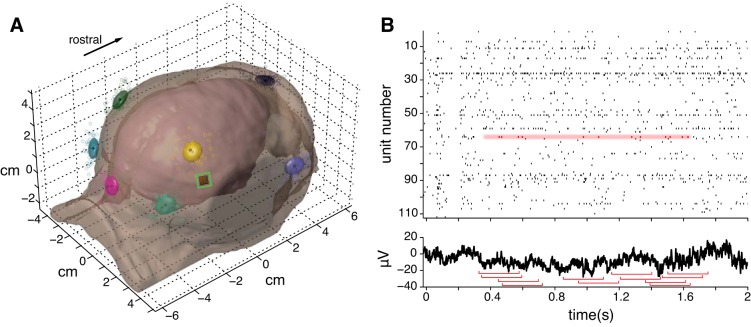
Fig. 1.
A: model of a representative subject's head derived from an MRI with coregistered electrode positions (colored scatter points). Each color represents a different position, and each marker of a given color represents a separate recording session. The corresponding ellipsoids each subtend ±1 SD of the position (∼5 mm, which was the electrode diameter) along each of the 3 principal axes of the position distribution. Electrode position could vary based on the experimenters' judgment of anatomical landmarks when applying the electrodes and noise in the position digitizer system. The points by the left lateral canthus are occluded in this view. Unless otherwise specified, EEG data from the right parietal electrode (yellow) are depicted in subsequent figures. The microelectrode array is shown to scale positioned in right V4 outlined by the green square. This image is reproduced from Fig. 1b in Snyder et al. (2015) with permission. B: example raw data from a single trial, illustrating the sampling procedure for spike-triggered averaging. Top: we excluded spikes from analysis that occurred near the start or end of a trial to avoid contamination by transient events around trial boundaries; the valid time points for an example neuron (unit 64) are highlighted in red in the raster. Bottom: we took a 500-ms window of EEG data (red brackets) centered at the time of each valid spike and averaged together all the windows from all trials to derive the spike-triggered average (STA).
Eye tracking.
We tracked the gaze of the subjects with an infrared eye tracking system (EyeLink 1000; SR Research). Gaze was monitored online by the experimental control software to ensure that subjects maintained fixation within 1.17° of the central fixation point during each trial.
Spike-triggered averaging.
We took 500-ms epochs of EEG data centered at the time of each spike that occurred during the fixation period. This excluded spikes that occurred within 350 ms from the start or end of the fixation period to reduce the impact of transient events in the EEG around trial boundaries. Neurons that fired a total of <200 spikes were excluded from the analysis (73 of 1,293 neurons excluded from the spontaneous condition; 156 of 1,482 neurons excluded from the stimulus-evoked condition).
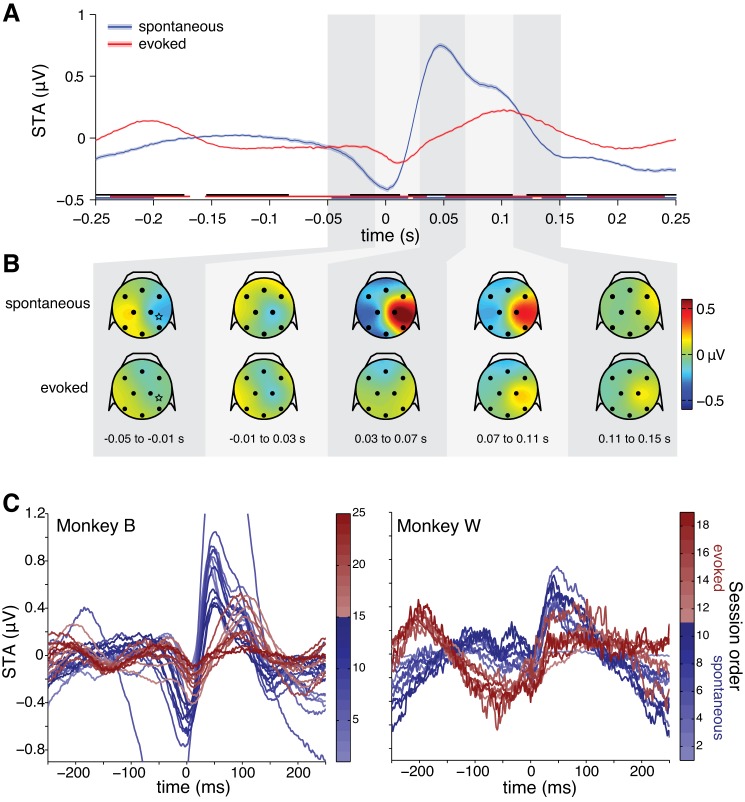
We calculated the spike-triggered average (STA) as the mean of all the EEG epochs centered on the spikes. We tested for statistical significance of each neuron's STA with a sample-by-sample Student's t-test. We performed this test for the STA measured at the right parietal EEG electrode, which showed the largest effect in the grand average (Fig. 2A). We compared the STA at each time point against a null hypothesis of 0 μV using a significance criterion of α = 0.05, Bonferroni corrected for the number of time points. We considered an STA to be significantly different from zero if at least one time point was significant.
Fig. 2.
Spike-triggered average EEG. A: grand-averaged STA over all neurons (spontaneous: n = 1,220 neurons; evoked: n = 1,326 neurons). Shading represents ±1 SE. Underlining represents significant time points (blue and red: 1-sample t-tests for spontaneous and evoked conditions, respectively; black: 2-sample t-test for difference between conditions), Bonferroni corrected for the number of time points. B: topographical scalp voltage maps for the STA in spontaneous and evoked conditions at selected time points. Black dots represent approximate EEG electrode locations. Stars represent the approximate array location projected onto the 2-dimensional scalp map. C: average STAs plotted for each individual recording session (darker colors denote later sessions). Note that variability between conditions is greater than within conditions; ordering of the recording sessions cannot account for differences between conditions.
Spike field coherence.
To calculate spike field coherence (SFC), we used the Chronux toolbox for MATLAB (Mitra and Bokil 2008). Chronux calculates the coherence between a spike train and a continuous signal, estimating the power spectrum of each signal with the multitaper method (Percival and Walden 1993). Chronux uses the ratio of the complex-valued cross-spectrum and the geometric mean of the two complex-valued autospectra: the magnitude and argument (polar angle) of this ratio are the SFC and preferred phase of coherence, respectively. We calculated a single value of SFC for each neuron, across all trials of a session, at each frequency within our resolution. As with the STA analysis, we restricted the spike trains and EEG data segments to the time period 100 ms to 1,900 ms after the start of the fixation period to avoid contamination by transient events around trial boundaries. We used default settings for Chronux; in particular, we used a set of five discrete prolate spheroidal sequences as tapers and a 1,025-point fast Fourier transform (FFT) for the spectral estimation.
STA and SFC for local field potentials.
For comparison purposes, we also calculated the STA and SFC for each neuron using two LFP measurements in the same way as described for the EEG signals above. First, we derived the STA and SFC using the LFP on the same channel that recorded the neuron in question. Second, we derived the STA and SFC using the summed LFP over all the channels on the multielectrode array except for the channel that recorded the neuron in question. We found that the STAs with these two methods were virtually identical, likely because of the relatively high degree of coherence between nearby LFP electrodes, except for the presence of a spike artifact when the same LFP channel that recorded the given neuron was used. In this report we analyze only the STAs without the spike artifact (i.e., excluding the LFP recording from the same electrode as the spikes), since such artifacts can “bleed through” to low frequencies and confound interpretation of the power spectra (Ray 2014). We excluded LFP epochs on the basis of EEG artifacts as described above, so that the same trials contributed to both EEG and LFP measures.
Trial-shuffling correction.
Because the data for the spontaneous and evoked conditions were collected in separate sessions and, more critically, because the spontaneous sessions always preceded the evoked sessions, the session order may confound our interpretation of the effects of visual stimulation. For example, the quality of recordings from implanted arrays gradually erodes over time (Barrese et al. 2013). If recording quality or other issues led to a systematic change in the power spectrum of either the spike trains or the EEG signals, a bias could have been introduced into our measurements of SFC between conditions. To minimize the influence of any such bias, we performed a statistical permutation procedure to estimate and subtract the baseline spike field relationship expected under the null hypothesis from each session's data.
Our procedure was as follows. For each neuron from each session, we randomly shuffled the order of trials for the EEG data while maintaining the original order of the trials for the spike trains and recalculated SFC for the shuffled data. By virtue of our randomization, these shuffled data theoretically have no correlation between the spike trains and the EEG signals. Thus any nonzero SFC measurements resulting from the trial-shuffled data reflect only measurement biases due to the properties of either the spike train or EEG signals themselves. For each neuron, we iterated the trial-shuffling procedure 100 times and averaged the SFC calculations across iterations to obtain a stable estimate of the bias. By subtracting this bias estimate from the raw SFC measurement for each neuron, we retained only the amount of correlation that exceeded what was accounted for by biases due to the properties of the two separate signals.
Phase consistency of STAs.
We wished to test how consistent the preferred EEG phases were across the population of neurons we recorded. For each neuron, we projected the preferred phase of coherence onto the complex unit circle. Then, for each session, we averaged these complex values across neurons and took the magnitude of the resultant vector as our measure of phase consistency. This quantity can range from a minimum of 0 when the phases are perfectly uniformly distributed to a maximum of 1 when the phases are perfectly aligned.
When coherence is weak the measurement of preferred phase is more subject to noise. That is, the preferred phase can be arbitrary if the preference is not strong. Thus a difference in coherence strength could lead to a spurious difference in phase consistency. To control for this, we binned neurons on the basis of their strength of coherence to the field potentials and calculated phase consistency within each bin. We binned using absolute values of coherence so that a coherence-matched comparison could be made between conditions. The bin values were based on the quintiles of our sample so that each bin contained roughly the same number of observations.
We tested for significant differences in phase consistency between conditions by performing independent-samples t-tests at each frequency, using each recording session as a degree of freedom, with a criterion of α = 0.05, Bonferroni corrected for the number of frequency points.
Multiunit activity.
We summed the spike trains of all the neurons (sorted as described above) across all the electrodes in the array within each session and smoothed the result with a 10-ms boxcar function to estimate time series of MUA. Then we calculated the Pearson product-moment correlation coefficient between the MUA and EEG time series on each trial between 0.5 s and 1.5 s after stimulus onset. We averaged correlation coefficients across trials within each session and then grand-averaged across sessions for each condition.
Local field potential correlates of EEG.
For this analysis, we first downsampled our data to 250 Hz. To ensure we had reliable estimates of correlation between our signals over trials, we excluded sessions with fewer than 500 trials (11 sessions excluded for spontaneous condition, 6 sessions excluded for stimulus-evoked condition). We also excluded LFP channels that did not record a spiking neuron above the SNR threshold, as these channels were typically noisy (mean 42.9 ± 3.9 channels excluded from each session). The results were qualitatively robust to changes in the specific values of these exclusion criteria. We multiplied each EEG and LFP data segment (fixation period) by a Hann function and applied the FFT to derive amplitude spectra. We also calculated magnitude-squared coherence on each trial between pairs of LFP electrodes. Because of the large number of observations, we randomly chose 500 pairs of LFP channels from the full set of possible pairings. We calculated magnitude-squared coherence by Welch's method with eight Hamming windows with 50% overlap and a 256-point FFT. For each frequency and for each EEG electrode, we calculated the partial Pearson correlation coefficients between EEG amplitude, average LFP amplitude across channels, and average coherence between EEG channels, using the MATLAB “partialcorr” function. The partial correlation between each pair of these three variables controlled for the third variable. Following Musall et al. (2014), we analyzed six canonical frequency bands of interest: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12), beta (12–24 Hz), low gamma (24–50 Hz), and high gamma (50–100 Hz). Values of amplitude and coherence were averaged across frequencies within each bin. We tested for significant nonzero correlations with two-tailed, one-sample Student's t-tests, with a criterion of α = 0.05, Bonferroni corrected for the six frequency bands of interest. We tested for differences between spontaneous and evoked conditions with independent-samples t-tests with the same criterion.
Induced Oscillatory Amplitudes
To derive the average amplitude spectra of induced EEG and LFP oscillations, we averaged the amplitude spectra that we calculated as described in Local field potential correlates of EEG. We also tested for statistical significance of the induced amplitude spectra in the six canonical frequency bands of interest with Student's t-tests as described above.
RESULTS
We recorded spontaneous and stimulus-evoked activity from macaque monkeys with scalp EEG electrodes and multielectrode arrays implanted in visual area V4. We studied the relationship between neuronal population activity and EEG signals with four dependent measures: 1) spike-triggered average EEG potential, 2) SFC, 3) broadband correlation between MUA and EEG, and 4) frequency-specific correlation between LFP amplitude/coherence and EEG amplitude. Our overarching goal was to establish the conditions present in intracranial signals that led to measurable effects in the EEG signal.
Spike-triggered average EEG.
In the spontaneous condition, the STA displayed clear componentry, characterized by a negative deflection starting ∼50 ms before the spike and a positive deflection after the spike that endured for ∼150 ms (Fig. 2A). The STA for the evoked condition showed a similar pattern, except with lesser amplitude and a relatively delayed time course (Fig. 2A). In both conditions, the topography of the STA showed a focal concentration at right parietal and occipital sites (Fig. 2B), which were closest to the neurons we recorded. We also measured large potentials at scalp sites diametrically opposite from the array location. Despite the large distance between recording sites, this latter result is consistent with the distant EEG electrode reflecting a current sink for a source near the array.
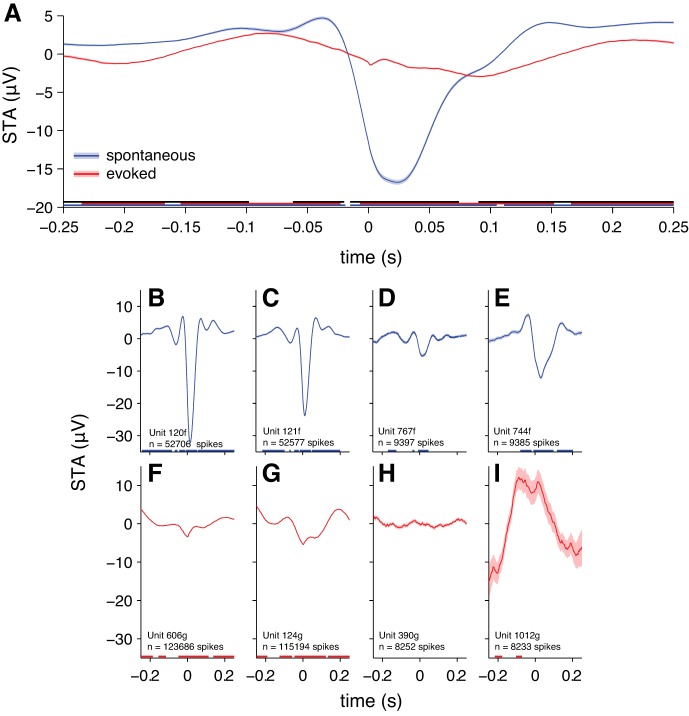
Examination of STAs for individual neurons revealed heterogeneous patterns that were not evident from the grand average (Fig. 3). While the grand average displayed a relatively slow positive component following the spike, some individual-neuron STAs displayed higher-frequency componentry during this period (e.g., Fig. 3, A and B), whereas other individual-neuron STAs more closely resembled the grand average (Fig. 3D). Some individual STAs showed clear positive components preceding the spike (Fig. 3, A–C) or robust oscillations at ∼5–7 Hz (Fig. 3, E and F). Other neurons did not appear to have a consistent relationship to the EEG (Fig. 3, G and H). These were the minority, however. We found that 67.1% of neurons that we sampled had significantly nonzero STAs.
Fig. 3.
Spike-triggered average EEG for example individual neurons. Shading represents ±1 SE. Underlining represents significant time points (1-sample t-test, α = 0.05, Bonferroni corrected for the number of time points). A–D: neurons recorded during the spontaneous condition. E–H: neurons recorded during the stimulus-evoked condition. Neurons in A, B, E, and F are the neurons with the greatest observed spike counts. Neurons in C, D, G, and H are neurons with spike counts around the 75th percentile for the sample.
Spike field coherence (EEG).
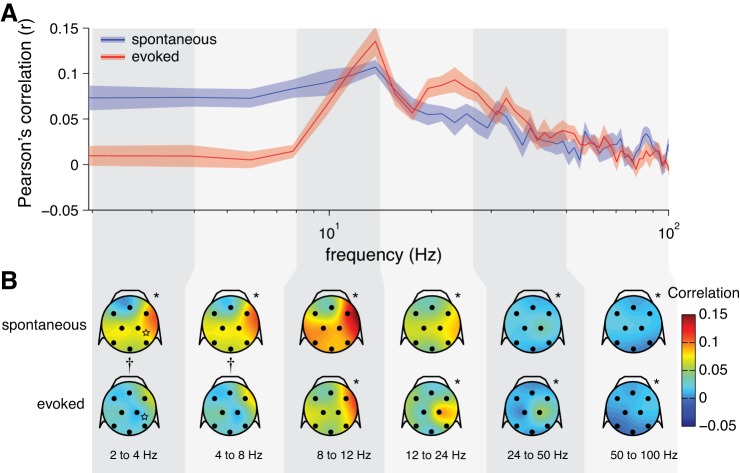
We measured SFC as the magnitude-squared coherence between each neuron's spike train and the scalp EEG signals with the Chronux toolbox (Mitra and Bokil 2008). The SFC reflects the degree to which a spike is phase-aligned to each frequency of the EEG signal. For both spontaneous and stimulus-evoked activity, we found a peak in SFC around the theta band (5–8 Hz) that decreased through the beta band (12–24 Hz) and then flattened out in the higher frequencies (Fig. 4). After correcting for measurement bias with trial shuffling, we found that SFC was significantly lesser in the stimulus-evoked condition below 30 Hz compared with the spontaneous condition (Fig. 4B).
Fig. 4.
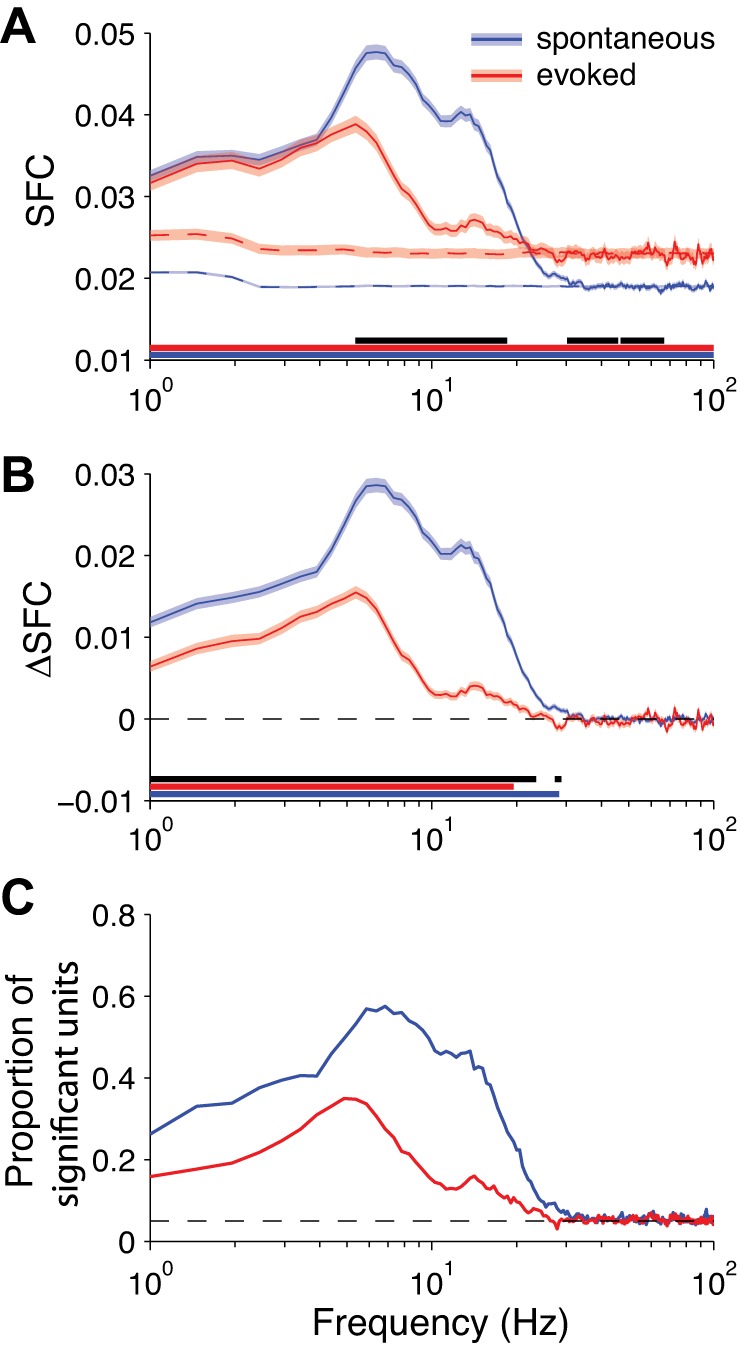
Spike field coherence (SFC) for EEG. A: solid lines: grand-averaged SFC over all neurons (spontaneous: n = 1,220 neurons; evoked: n = 1,326 neurons; shading represents ±1 SE). Dashed lines: SFC for trial-shuffled data, capturing measurement bias (shading represents 90% confidence interval). Underlining indicates statistical significance at each frequency (blue and red underlines: 1-sample t-test for spontaneous and evoked SFC, respectively, against a null hypothesis of 0; black underline: independent-samples t-test for the difference between conditions). B: same as in A but with the trial-shuffled values subtracted from the raw values (i.e., ΔSFC), correcting for measurement bias. C: proportion of neurons at each frequency with significant SFC (by permutation test using trial-shuffled data). Dashed line reflects the false-positive rate of P = 0.05.
Effect of visual stimulation on preferred EEG phase of firing.
Our finding that the magnitude of the grand-mean STA decreased with visual stimulation indicated that the spike-EEG relationships became less consistent in that condition. This change in the strength of spike-EEG relationships was reflected in the average within-neuron decrease in SFC with stimulation (Fig. 4), indicating that individual neurons tended to become less coherent with the EEG signal. However, the spike-EEG relationships could become less consistent not only at the individual-neuron level but also at the level of the population. That is, individual neurons could be strongly related to the EEG, but in different ways (e.g., Fig. 5), thereby leading to a flatter grand-mean STA. We confirmed this by measuring phase consistency while controlling for the strength of coherence, and we found that phases were more consistent across the population for spontaneous compared with stimulus-evoked activity when coherence strength was matched (Fig. 6). This difference was strongest in a range of frequencies centered around 10 Hz.
Fig. 5.
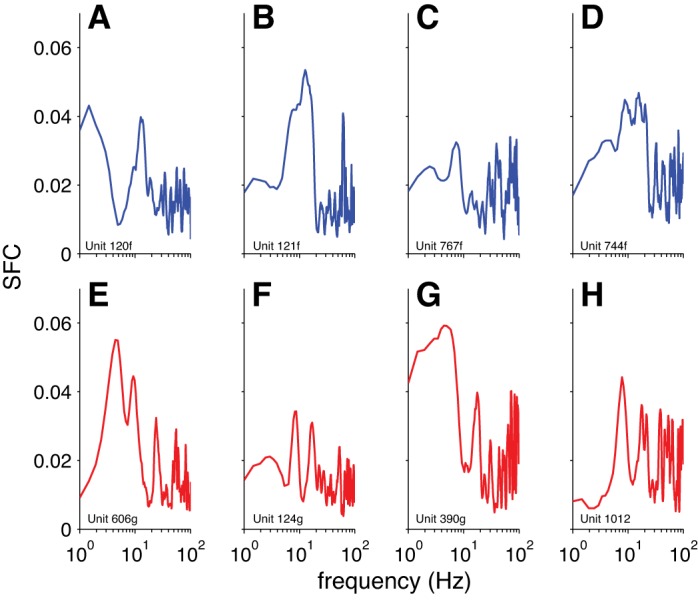
SFC for EEG for example individual neurons. A–D: neurons recorded during the spontaneous condition. E–H: neurons recorded during the stimulus-evoked condition. The same neurons are depicted as in Fig. 3. Data were smoothed with a 5-sample boxcar for plotting.
Fig. 6.
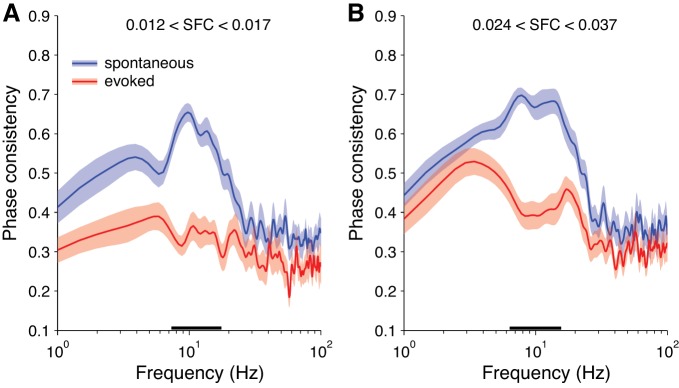
Consistency of preferred EEG phase across the sample of neurons. Shading represents ±1 SE (spontaneous: n = 24 sessions; evoked: n = 18 sessions). Underlining represents significant difference between conditions (2-sample t-test, α = 0.05, Bonferroni corrected for the number of frequency points). A: phase consistency for neurons with SFC between 0.012 and 0.017, which were around the 30th and 50th percentile values, respectively. B: phase consistency for neurons with SFC between 0.024 and 0.037, which were around the 70th and 90th percentile values, respectively.
Since we collected the data for the spontaneous condition before the evoked condition for both subjects, the order of the recording sessions could potentially confound our stimulation conditions. We asked directly how much variation in phase consistency could be accounted for by the date of the sessions as opposed to the stimulation condition, using a hierarchical multiple regression analysis. We looked specifically at mean phase consistency between 7 and 11 Hz, where we observed the greatest differences between conditions (Fig. 6). We found that the factor of “condition” accounted for 36.3% of the residual variance of phase consistency after controlling for the factors of “subject” and “session date,” which was a significant effect (F = 22.75, P < 0.001). In contrast, the factor “session date” accounted for only 1.5% of residual variance of phase consistency after controlling for the factors of “subject” and “condition,” which was not significant (F = 0.29, P = 0.751). This indicates that the absence or presence of visual stimulation was driving the differences in phase consistency between conditions, while session order made virtually no contribution.
Correlation between multiunit activity and broadband EEG.
We next asked how the EEG signal is related to the total spiking activity of the underlying population in aggregate. Given the diversity of ways that we found individual neurons to be related to the EEG as revealed by their STAs, and that pairs of V4 neurons can exhibit precise dependencies in their relative spike timing (Smith and Sommer 2013), it is not straightforward to predict what the relationship would be for the population as a whole. When we measured the correlation between the broadband EEG signal and the total MUA in V4, we found a topographic distribution of correlation, with negative values focused at the EEG electrode nearest the multielectrode array and positive values at opposing sites, consistent with a current source-sink pair (Fig. 7). This is in line with a prior report that MUA is greater when negative potentials are measured at the overlying scalp compared with when positive potentials are seen (Whittingstall and Logothetis 2009). We found a much weaker relationship, however (r = −0.02 compared with r = −0.12 for Whittingstall and Logothetis), which may reflect our different EEG electrode configuration (nearby for us vs. their ring EEG around the penetrating electrode) or recording area (V4 for us vs. V1 for their study).
Fig. 7.
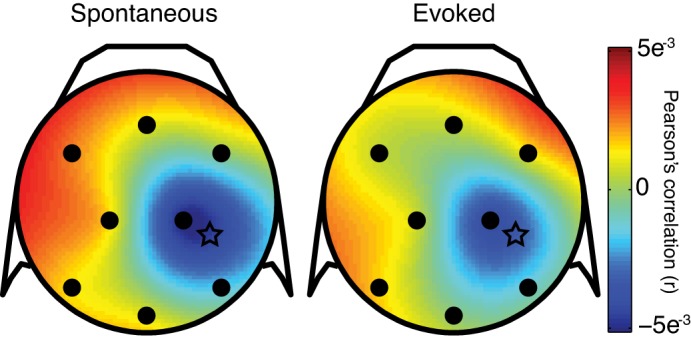
Spline-interpolated topographical map of correlation between scalp EEG and multiunit activity recorded from the array in right V4. Correlations between multiunit and broadband EEG were calculated on each trial, averaged over trials in each session, and then grand-averaged across session for each condition. Stars represent the approximate array location projected onto the 2-dimensional scalp map.
Correlation between LFP amplitude/coherence and EEG amplitude.
The above results relate spiking to the EEG at the scale of single neurons or MUA. Our microelectrode array simultaneously captured the LFP from each recording electrode along with spikes, providing an opportunity to relate this slightly larger-scale signal to the EEG. We sought to test the hypothesis that the amplitude of the EEG depends on both the amplitude and coherence of the underlying LFPs. To do this, we measured the correlations between LFP amplitude, LFP coherence, and EEG amplitude. We used partial correlation in each analysis to control for the interaction between LFP amplitude and coherence that was present in our data (partial Pearson's r ≈ 0.1–0.6 across the 6 frequency bands).
We found that LFP amplitude was significantly correlated with EEG amplitude across all frequency bands in the spontaneous condition (Fig. 8; delta through low gamma: all P < 0.001, high gamma: P = 0.003). For the stimulus-evoked condition, LFP amplitude was correlated with EEG amplitude only at and above the alpha band (alpha through low gamma: all P < 0.001, high gamma: P = 0.003). In contrast, LFP amplitude was not significantly correlated with EEG amplitude in the delta or theta band in the stimulus-evoked condition (both P > 0.145). This was a significant difference between conditions (both P < 0.001). The two conditions did not differ significantly at other frequency bands (all P > 0.174).
Fig. 8.
Relationship between local field potential (LFP) amplitude and EEG amplitude. The partial correlation coefficients control for the effect of LFP coherence (spontaneous: n = 13 sessions; evoked: n = 12 sessions). A: partial correlation between V4 LFP amplitude and right parietal EEG amplitude as a function of frequency. Shading represents ±1 SE. B: topographical maps of the correlation at selected frequencies. Stars represent the approximate array location projected onto the 2-dimensional scalp map. Each frequency band was tested for significance in 2 ways with t-tests: first to test for a difference from zero correlation, and second to test for a significant difference between conditions. *P < 0.05, Bonferroni corrected, for null hypothesis of no correlation. †P < 0.05, Bonferroni corrected, for null hypothesis of no difference between conditions.
Average LFP coherence was significantly correlated with EEG amplitude only in the stimulus-evoked condition and only at low frequencies (Fig. 9; delta: P = 0.006, theta: P < 0.001, alpha: P = 0.005, all other: P > 0.3677). While there was a trend for a significant correlation in the theta band for the spontaneous condition (P = 0.013), this was not robust to correction for multiple comparisons. No other frequency bands had a significant correlation in the spontaneous condition (all P > 0.197).
Fig. 9.
Effect of LFP coherence on EEG amplitude. The partial correlation coefficients control for the effect of LFP power (spontaneous: n = 13 sessions; evoked: n = 12 sessions). A: partial correlation between V4 LFP coherence and right parietal EEG amplitude as a function of frequency. Shading represents ±1 SE. B: topographical maps of the correlation at selected frequencies. Stars represent the approximate array location projected onto the 2-dimensional scalp map. *P < 0.05, Bonferroni corrected, for null hypothesis of no correlation.
Spike field coherence (LFP).
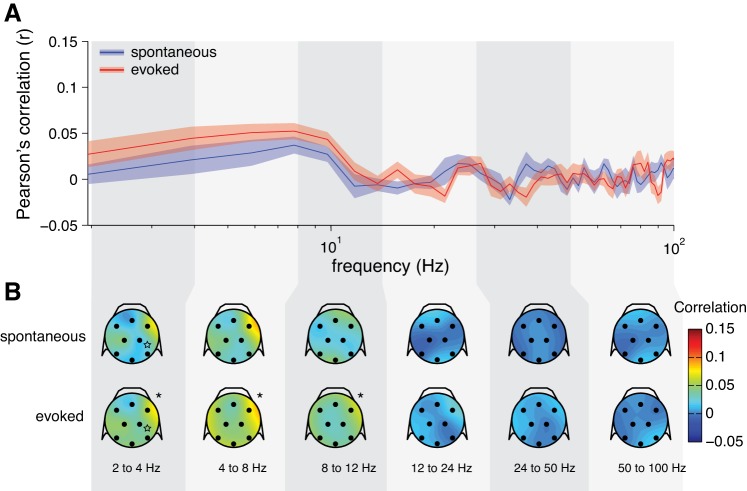
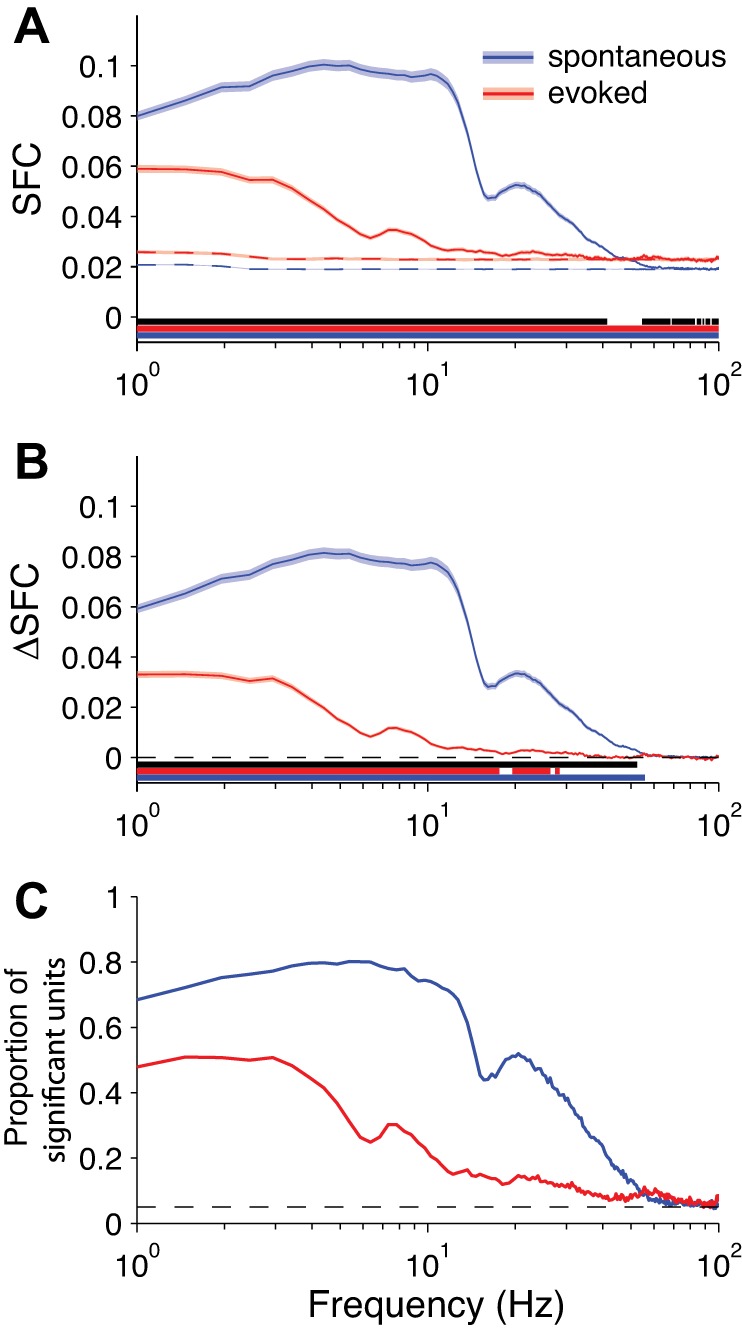
Previously, some researchers measuring SFC with LFPs have shown an increase in gamma-band phase-locking during visual stimulation (Fries et al. 2008b; Lima et al. 2010; Singer and Gray 1995). We did not find this to be the case for SFC with the EEG (Fig. 4). We wondered whether this was something peculiar to the EEG signals (e.g., lower SNR for EEG than LFP in the gamma band) or if this effect was absent in the SFC based on the LFPs that we recorded also. Thus we calculated the SFC based on the LFPs (Fig. 10). As with the SFC based on the EEG signals, we did not see a clear increase in the gamma band with visual stimulation for the SFC based on the LFPs. Rather, we found a decrease of SFC at lower frequencies with visual stimulation (Fig. 10B). Again, we found heterogeneous patterns of SFC across individual neurons (Fig. 11). Similarly to the results with the preferred EEG phase of firing, we found that the preferred LFP phase of firing was less consistent across the population during visual stimulation, compared with the spontaneous state, while controlling for the strength of SFC (Fig. 12).
Fig. 11.
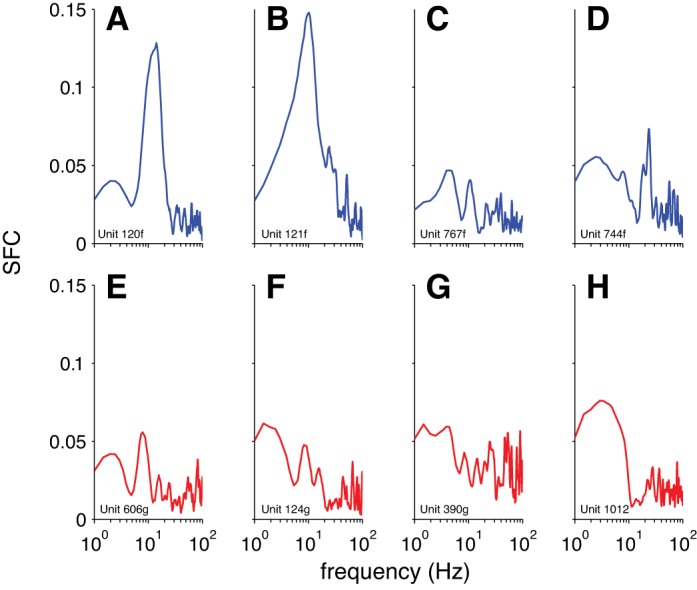
SFC for LFP for example individual neurons. A–D: neurons recorded during the spontaneous condition. E–H: neurons recorded during the stimulus-evoked condition. The same example neurons were depicted in previous figures of single-neuron data. Data were smoothed with a 5-sample boxcar for plotting.
Fig. 12.
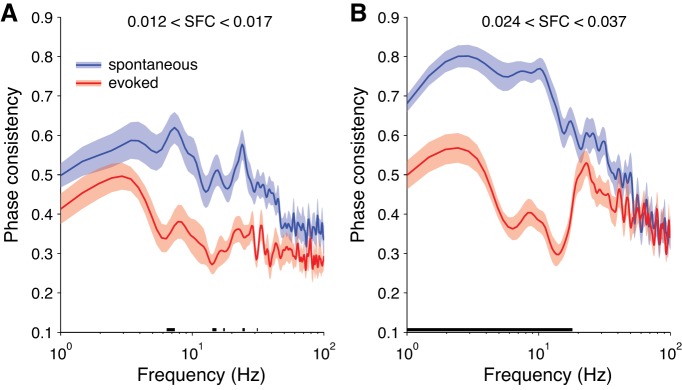
Consistency of preferred LFP phase across the sample of neurons. Shading represents ±1 SE (spontaneous: n = 24 sessions; evoked: n = 18 sessions). Underlining represents significant difference between conditions (2-sample t-test, α = 0.05, Bonferroni corrected for the number of frequency points). A: phase consistency for neurons with SFC between 0.012 and 0.017, which were around the 30th and 50th percentile values, respectively. B: phase consistency for neurons with SFC between 0.024 and 0.037, which were around the 70th and 90th percentile values, respectively.
Induced oscillations.
Another gamma-band finding that has been reported previously is that visual stimulation induces an increase in the amplitude of gamma-band oscillations in visual cortex. By “induces,” we mean specifically that the amplitude of gamma-band oscillations increases after visual stimulation although the phase of the oscillation need not be aligned to the stimulus (in other words, it is not a stimulus-evoked oscillation). This effect has been reported not only for LFPs recorded in monkeys (Bastos et al. 2014; Bosman et al. 2012; Burns et al. 2010; Fries et al. 2008a; Juergens et al. 1999) but also for EEG and MEG signals in humans (Fries et al. 2008a; Hoogenboom et al. 2006; but see Juergens et al. 1999; van Pelt et al. 2012). However, in our data we did not find an increase in the induced gamma amplitude for either the EEG or the LFPs (Fig. 13). If anything, induced amplitudes at and above the alpha band tended to decrease with visual stimulation. The most robust effect of visual stimulation on induced amplitudes was a decrease in the lower frequencies (delta and theta) of the EEG (Fig. 13A).
Fig. 13.
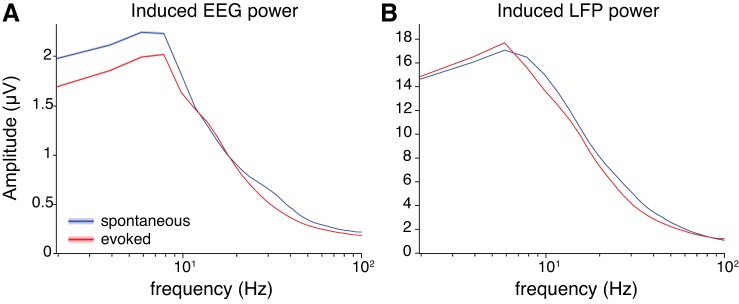
Average induced amplitude spectra. A: average induced EEG amplitudes at right parietal electrode (spontaneous: n = 14,337 trials; evoked: n = 13,774 trials). B: average induced amplitudes across all LFP channels (spontaneous: n = 14,337 trials; evoked: n = 13,774 trials). Shading represents ±1 SE, although SE may appear narrower than line width, especially at high frequencies.
Spike-triggered average LFP.
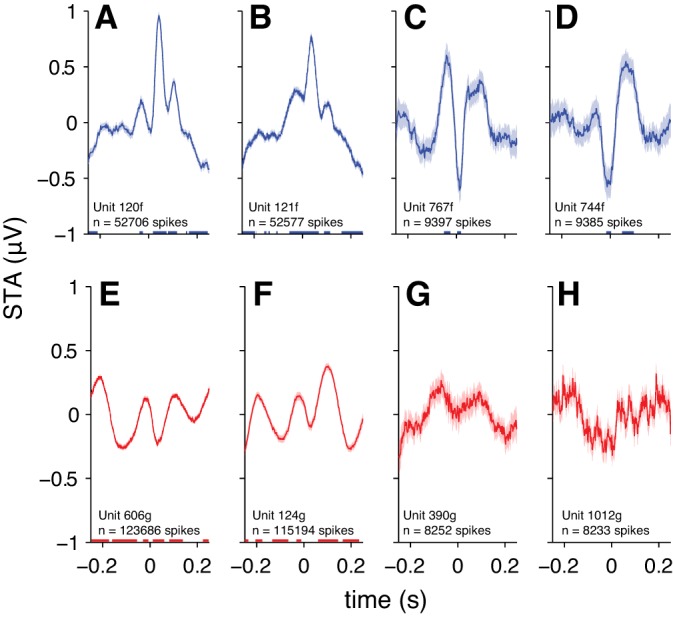
For comparison purposes, we also calculated the spike-triggered average LFP responses (Fig. 14). We found that 80.7% of neurons had significantly nonzero LFP STAs. This was a greater proportion than we observed for the EEG (67.1% of neurons), likely reflecting the greater SNR of LFP signals and proximity of the spiking neurons to the LFP sources. As with the spike-triggered average EEG, the LFP STA was lesser amplitude and delayed for the stimulus-evoked condition relative to the spontaneous condition. The STA was characterized by a negative deflection preceding a spike, which was also similar to what was observed with the EEG. Again, the individual neurons had quite heterogeneous responses (Fig. 14, B–I), and the phase consistency of the STAs was reduced with visual stimulation across all six frequency bands of interest (all P < 0.001). In contrast to the EEG STA, however, the LFP STA did not show a clear positive deflection following a spike.
Fig. 14.
Spike-triggered average LFP. A: grand-averaged STA over all neurons (spontaneous: n = 1,220 neurons; evoked: n = 1,326 neurons). Shading represents ±1 SE, although SE may appear narrower than line width at some points. B–I: spike-triggered average EEG for example individual neurons. B–E: neurons recorded during the spontaneous condition. F–I: neurons recorded during the stimulus-evoked condition. The same example neurons were depicted in previous figures of single-neuron data.
DISCUSSION
The notion of cortical neurons operating in different states, one characterized by highly correlated activity and coordination with global network fluctuations and another in which neurons operate more independently, is well established in the literature (Kohn et al. 2009). Highly structured spontaneous activity appears to be an extreme example of one state and has been observed with methods ranging from intracellular recordings (Poulet and Petersen 2008) to optical imaging (Kenet et al. 2003; Tsodyks et al. 1999) to MRI (Nir et al. 2008). While the present study and numerous previous results (Churchland et al. 2010; Smith and Kohn 2008; Smith and Sommer 2013) point to a distinct change in state that occurs when an external stimulus perturbs the network, more subtle changes can occur because of shifts in attention (Cohen and Maunsell 2009; Mitchell et al. 2009), learning over time (Gu et al. 2011; Jeanne et al. 2013), and contextual modulation (Snyder et al. 2014). A pair of recent studies have highlighted the diversity of how neurons couple with populations (Okun et al. 2015) and the dynamic changes in that coupling over time (Scholvinck et al. 2015). Our results fit solidly into the context of this literature, demonstrating that the presence of a stimulus changes how neurons link to the population and affects the diversity of their coupling to global oscillations as measured by the EEG or LFP. Taken together, this body of research points to the importance of understanding the coupling of individual neurons to the network in processes ranging from low-level stimulus responses to higher-order phenomena such as spatial attention and learning.
We found that most neurons had significant interactions between spiking activity and the EEG. These interactions were predominantly confined to lower frequency bands (<30 Hz) and were topographically focused at scalp locations closest to the neuronal populations we recorded (Fig. 2). When we presented a visual stimulus, the relationship between spiking activity and the EEG changed. In particular, spikes aligned to more diverse phases of the EEG (Fig. 6). This was due not only to a reduction of the magnitude of the spike-EEG relationship for individual neurons but also to more diverse patterns of spike-EEG relationships across the population (after controlling for changes in the strength of coherence). These results suggest that the large-scale networks that generate the EEG signals are driving V4 neurons relatively uniformly in the spontaneous state. When a visual stimulus is presented, the individual neurons shift to adopt idiosyncratic relationships to those large-scale networks. These idiosyncrasies likely reflect distinct functional roles of individual cells within the neural circuitry.
We also found that low-frequency components of the LFP were most correlated with the strength of the EEG signal (Figs. 8 and 9), consistent with the spiking results. In the spontaneous condition, the amplitude of the LFP was most strongly related to the strength of the EEG signal across all frequencies. During visual stimulation the coherence of the LFP, but not the amplitude, was related to the strength of the EEG at low frequencies. In other words, visual stimulation leads to a shift from a state in which the low-frequency EEG is mostly determined by the amplitude of the underlying LFPs to a state in which the spatial coherence of those local fields plays a larger role. This is consistent with a paradigm in which the evoked and spontaneous states are characterized by fundamentally different levels of spatial coordination. In the evoked state, increases in LFP amplitude among independently operating sites would destructively interfere in the EEG, whereas increases in LFP coherence could constructively sum to impact the EEG. In the spontaneous state, associated with more homogeneous LFP activity, amplitude increases can directly impact the EEG. This parallels our spiking results, for which visual stimulation was associated with relatively more heterogeneous spike-EEG relationships.
EEG is the most popular means of gaining access to the activity of large groups of neurons—the presence of strong electrical signals at the scalp implies the coordination of large groups of neurons in the brain. Despite this popularity, few researchers have recorded spiking or LFPs intracranially alongside EEG at the scalp in order to characterize how EEG signals relate to ongoing neural activity. Whittingstall and Logothetis (2009) found that the broadband EEG potential is negatively correlated with the amount of underlying MUA, which was also true for our data (Fig. 7). They moreover reported that multiunit firing rates were modulated by the phase of delta oscillations, which is consistent with our observation of high SFC in the delta range (Fig. 4). Musall et al. (2014) recorded simultaneous EEG and LFP during visual stimulation with three penetrating electrodes, which enabled them to assay the contribution of spatial coherence of the LFP signals to the amplitude of the EEG. They found, as we did (Figs. 8 and 9), that amplitude and coherence of the LFPs are each separately correlated with the magnitude of the EEG signal. However, while Musall et al. (2014) found significant partial correlations for both amplitude and coherence at all six frequency bands of interest (except for the alpha band, for which coherence was not significantly correlated), we found that LFP amplitude (Fig. 8), but not LFP coherence (Fig. 9), was correlated with EEG amplitude at low frequencies. Nevertheless, our findings are largely in accord with these two previous studies, while our topographically distributed EEG correlates and spontaneous/evoked dynamics extend their results.
One issue potentially affecting the generalizability of studies measuring simultaneous intracranial (i.e., spiking and LFP) and extracranial (i.e., EEG at the skull or scalp) signals is the need to perforate the skull. This craniotomy changes the properties of the volume conductor, and therefore may affect the propagation of electrical signals. If these effects are substantial, then the results of simultaneous intra-/extracranial recording studies may not generalize to the vast majority of human EEG research, since most human subjects do not have artificial craniotomies. However, a few studies have reported negligible effects of craniotomies on EEG signals. For example, in human subjects with severe epilepsy small amounts of electric current have been injected via implanted depth electrodes (Cohen et al. 1990; Cuffin et al. 1991, 2001; Krings et al. 1999; van Burik and Peters 1999). With the use of inverse source estimation algorithms to estimate the source of the injected current (with known true positions), localization accuracy was quite good: ∼1–2 cm, even when using anatomically inaccurate spherical head models that did not take into account the craniotomies (Cohen et al. 1990; Cuffin et al. 1991, 2001; Krings et al. 1999). Another recent study specifically examined the effect of craniotomies on EEG signals in macaque monkeys by recording somatosensory evoked potentials before and after a craniotomy (Gindrat et al. 2015). They found that evoked potentials after the surgery did not significantly differ in magnitude, time course, or topography from evoked potentials recorded before the surgery. Together these reports give us confidence that our results are generalizable to other EEG studies of subjects with intact skulls.
We found the clearest relationships between EEG and spiking/LFP activity at low frequencies, particularly the delta, theta, and alpha bands. This is evidenced by the low-frequency peak in SFC (Fig. 4) and by the finding that LFP coherence/power are most strongly correlated with EEG amplitude in this frequency range. The observation that spiking activity seems to be embedded in a context of low-frequency oscillations fits well with the finding that there are functional interactions between neurons reflected in substantial correlated firing rate variability on a timescale of many tens to hundreds of milliseconds (Cohen and Kohn 2011; Kohn and Smith 2005; Smith and Kohn 2008; Smith and Sommer 2013). This correlated spiking variability spans a relatively large spatial scale of up to 1 cm or more (Smith and Kohn 2008), in line with a source that would be detectable with EEG (Nunez and Srinivasan 2006). Together these observations suggest that the EEG signal is tapping into the same low-frequency oscillations that generate correlated spiking variability over large distances, and raise the possibility that concurrent EEG recordings can add substantial information about global context to local population recordings of neuronal spiking.
Previous researchers reported effects of visual stimulation in the gamma band (Bastos et al. 2014; Bosman et al. 2012; Burns et al. 2010; Fries et al. 2008a, 2008b; Hoogenboom et al. 2006; Juergens et al. 1999; Lima et al. 2010; Singer and Gray 1995; van Pelt et al. 2012) that we did not find in this experiment. Specifically, we did not find an increase in either gamma-band SFC or induced gamma-band amplitude with stimulation. We are not alone in this negative result. While stimulus-related gamma-band effects have often been reported, such effects are highly sensitive to experimental parameters such as the exact size, orientation, and spectral content of the visual stimulus (Bartolo et al. 2011; Berens et al. 2008; Gieselmann and Thiele 2008; Hermes et al. 2014; Jia et al. 2011; Lima et al. 2010). Our visual stimuli were chosen to best drive the spiking response of the population as a whole without concern for gamma-band effects and therefore might not have triggered an increase in gamma-band SFC or induced gamma amplitude. Moreover, different visual areas may have stimulus-related gamma-band effects in opposite directions (Chalk et al. 2010), which interact at the level of the EEG signal to reduce the measured effect size. Our multielectrode array was implanted in area V4, while most of the above-cited recordings were made from area V1. However, this latter interpretation is unlikely since we did not see gamma-band effects of visual stimulation at the level of the LFPs either (Figs. 9 and 10). Gamma-band LFP effects of visual stimulation have also been shown to depend on which cortical layer is observed, with superficial layers showing a more prominent band-limited peak of induced power in the gamma range compared with deep layers (Buffalo et al. 2011; Smith et al. 2013). Our 1-mm-long electrodes were likely located near the middle layers of V4 or slightly deeper (Yoshioka et al. 1992), which could account for why we did not find clear LFP effects in the gamma band. However, this cannot account for the negative EEG results in the gamma band, since the EEG signal is sensitive to current sources from as far away as the opposite side of the head (e.g., Fig. 2B) and therefore also probes the entire thickness of cortex. Notwithstanding the cause, our lack of a significant result in the gamma band suggests that gamma-band SFC and induced power increases are not unwavering hallmarks of visual processing generally but are rather more likely confined to specific sets of experimental circumstances.
To summarize, we found that the spiking activity of most V4 neurons is structured within the context of low-frequency EEG oscillations. This structure is relatively uniform across the population in the spontaneous state but becomes much more diverse with visual stimulation. This diversity provides a potential new avenue for studying how individual neurons interact with large-scale networks. It may be the case that different spike-EEG relationships correspond to different functional roles in the network. Moreover, this observation cautions us that the spiking correlates of EEG signals may vary across contexts, so care is necessary in generalizing the functional interpretations of EEG signals across different experimental contexts.
GRANTS
A. C. Snyder was supported by a National Institutes of Health (NIH) fellowship (F32 EY-023456). M. A. Smith was supported by NIH Grants R00 EY-018894, R01 EY-022928, and P30 EY-008098, a career development grant, and an unrestricted award from Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ENDNOTE
At the request of the authors, readers are herein alerted to the fact that additional materials related to this manuscript may be found at the institutional website of one of the authors, which at the time of publication they indicate is: http://www.smithlab.net/spikesort.html. These materials are not a part of this manuscript, and have not undergone peer review by the American Physiological Society (APS). APS and the journal editors take no responsibility for these materials, for the website address, or for any links to or from it.
AUTHOR CONTRIBUTIONS
Author contributions: A.C.S. and M.A.S. conception and design of research; A.C.S. performed experiments; A.C.S. analyzed data; A.C.S. and M.A.S. interpreted results of experiments; A.C.S. prepared figures; A.C.S. drafted manuscript; A.C.S. and M.A.S. edited and revised manuscript; A.C.S. and M.A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Cory Willis for help with data collection.
REFERENCES
- Banerjee S, Snyder AC, Molholm S, Foxe JJ. Oscillatory alpha-band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: supramodal or sensory-specific control mechanisms? J Neurosci 31: 9923–9932, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, Donoghue JP. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng 10: 066014, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolo MJ, Gieselmann MA, Vuksanovic V, Hunter D, Sun L, Chen X, Delicato LS, Thiele A. Stimulus-induced dissociation of neuronal firing rates and local field potential gamma power and its relationship to the resonance blood oxygen level-dependent signal in macaque primary visual cortex. Eur J Neurosci 34: 1857–1870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, Usrey WM. Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for gamma-band oscillations. J Neurosci 34: 7639–7644, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyusar D, Snyder AC, Frey HP, Harwood MR, Wallman J, Foxe JJ. Oscillatory alpha-band suppression mechanisms during the rapid attentional shifts required to perform an anti-saccade task. Neuroimage 65: 395–407, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P, Keliris GA, Ecker AS, Logothetis NK, Tolias AS. Comparing the feature selectivity of the gamma-band of the local field potential and the underlying spiking activity in primate visual cortex. Front Syst Neurosci 2: 2, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. Über das Elektrenkephalogramm des Menschen. Eur Arch Psychiatry Clin Neurosci 87: 527–570, 1929. [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron 75: 875–888, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Buchwald JS, Halas ES, Schramm S. Comparison of multiple-unit and electroencephalogram activity recorded from the same brain sites during behavioral conditioning. Nature 205: 1012–1014, 1965. [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA 108: 11262–11267, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns SP, Xing D, Shapley RM. Comparisons of the dynamics of local field potential and multiunit activity signals in macaque visual cortex. J Neurosci 30: 13739–13749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron 66: 114–125, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Yu BM, Cunningham JP, Sugrue LP, Cohen MR, Corrado GS, Newsome WT, Clark AM, Hosseini P, Scott BB, Bradley DC, Smith MA, Kohn A, Movshon JA, Armstrong KM, Moore T, Chang SW, Snyder LH, Lisberger S, Priebe NJ, Finn IM, Ferster D, Ryu SI, Santhanam G, Sahani M, Shenoy KV. Stimulus onset quenches neural variability: a widespread cortical phenomenon. Nat Neurosci 13: 369–378, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy JG, Schomer DL. MEG versus EEG localization test using implanted sources in the human brain. Ann Neurol 28: 811–817, 1990. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Kohn A. Measuring and interpreting neuronal correlations. Nat Neurosci 14: 811–820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12: 1594–1600, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffin BN, Cohen D, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy J, Schomer D. Tests of EEG localization accuracy using implanted sources in the human brain. Ann Neurol 29: 132–138, 1991. [DOI] [PubMed] [Google Scholar]
- Cuffin BN, Schomer DL, Ives JR, Blume H. Experimental tests of EEG source localization accuracy in spherical head models. Clin Neurophysiol 112: 46–51, 2001. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Butler JS, Green JM, Snyder AC, Foxe JJ. Mobile brain/body imaging (MoBI): high-density electrical mapping of inhibitory processes during walking. Conf Proc IEEE Eng Med Biol Soc 2012: 1542–1545, 2012. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The role of alpha-band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2: 154, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001. [DOI] [PubMed] [Google Scholar]
- Fries P, Scheeringa R, Oostenveld R. Finding gamma. Neuron 58: 303–305, 2008a. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci 28: 4823–4835, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm GH, Bond HW. Slow changes in the electrocorticogram and the activity of cortical neurons. Electroencephalogr Clin Neurophysiol 17: 520–523, 1964. [DOI] [PubMed] [Google Scholar]
- Gieselmann MA, Thiele A. Comparison of spatial integration and surround suppression characteristics in spiking activity and the local field potential in macaque V1. Eur J Neurosci 28: 447–459, 2008. [DOI] [PubMed] [Google Scholar]
- Gindrat AD, Quairiaux C, Britz J, Brunet D, Lanz F, Michel CM, Rouiller EM. Whole-scalp EEG mapping of somatosensory evoked potentials in macaque monkeys. Brain Struct Funct 220: 2121–2142, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givre SJ, Schroeder CE, Arezzo JC. Contribution of extrastriate area V4 to the surface-recorded flash VEP in the awake macaque. Vision Res 34: 415–428, 1994. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324: 1207–1210, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Liu S, Fetsch CR, Yang Y, Fok S, Sunkara A, DeAngelis GC, Angelaki DE. Perceptual learning reduces interneuronal correlations in macaque visual cortex. Neuron 71: 750–761, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Wandell BA, Winawer J. Stimulus dependence of gamma oscillations in human visual cortex. Cereb Cortex (May 22, 2014). doi: 10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. Neuroimage 29: 764–773, 2006. [DOI] [PubMed] [Google Scholar]
- Jeanne JM, Sharpee TO, Gentner TQ. Associative learning enhances population coding by inverting interneuronal correlation patterns. Neuron 78: 352–363, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Smith MA, Kohn A. Stimulus selectivity and spatial coherence of gamma components of the local field potential. J Neurosci 31: 9390–9403, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens E, Guettler A, Eckhorn R. Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical- and EEG-potentials, but not in human EEG. Exp Brain Res 129: 247–259, 1999. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology 47: 888–904, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RC, Smith MA, Kass RE, Lee TS. Local field potentials indicate network state and account for neuronal response variability. J Comput Neurosci 29: 567–579, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RC, Smith MA, Samonds JM, Kohn A, Bonds AB, Movshon JA, Lee TS. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J Neurosci 27: 261–264, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 425: 954–956, 2003. [DOI] [PubMed] [Google Scholar]
- Kleiner M, Brainard DH, Pelli D. What's new in Psychtoolbox-3? Perception 36: 2007. [Google Scholar]
- Kohn A, Smith MA. Stimulus dependence of neuronal correlation in primary visual cortex of the macaque. J Neurosci 25: 3661–3673, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A, Zandvakili A, Smith MA. Correlations and brain states: from electrophysiology to functional imaging. Curr Opin Neurobiol 19: 434–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krings T, Chiappa KH, Cuffin BN, Cochius JI, Connolly S, Cosgrove GR. Accuracy of EEG dipole source localization using implanted sources in the human brain. Clin Neurophysiol 110: 106–114, 1999. [DOI] [PubMed] [Google Scholar]
- Li CL, Jasper H. Microelectrode studies of the electrical activity of the cerebral cortex in the cat. J Physiol 121: 117–140, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima B, Singer W, Chen NH, Neuenschwander S. Synchronization dynamics in response to plaid stimuli in monkey V1. Cereb Cortex 20: 1556–1573, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23: 3963–3971, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Gramann K, Jung TP, Sejnowski TJ, Poizner H. Linking brain, mind and behavior. Int J Psychophysiol 73: 95–100, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron 63: 879–888, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Bokil H. Observed Brain Dynamics. Oxford, UK: Oxford Univ. Press, 2008. [Google Scholar]
- Musall S, von Pfostl V, Rauch A, Logothetis NK, Whittingstall K. Effects of neural synchrony on surface EEG. Cereb Cortex 24: 1045–1053, 2014. [DOI] [PubMed] [Google Scholar]
- Nelson MJ, Pouget P. Do electrode properties create a problem in interpreting local field potential recordings? J Neurophysiol 103: 2315–2317, 2010. [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Arieli A, Fried I, Malach R. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci 11: 1100–1108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: the Neurophysics of EEG. Oxford, UK: Oxford Univ. Press, 2006. [Google Scholar]
- Okun M, Steinmetz NA, Cossell L, Iacaruso MF, Ko H, Bartho P, Moore T, Hofer SB, Mrsic-Flogel TD, Carandini M, Harris KD. Diverse coupling of neurons to populations in sensory cortex. Nature 521: 511–515, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral Analysis for Physical Applications: Multitaper and Conventional Univariate Techniques. Cambridge, UK: Cambridge Univ. Press, 1993. [Google Scholar]
- Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature 454: 881–885, 2008. [DOI] [PubMed] [Google Scholar]
- Ray S. Challenges in the quantification and interpretation of spike-LFP relationships. Curr Opin Neurobiol 31C: 111–118, 2014. [DOI] [PubMed] [Google Scholar]
- Reinhart RM, Heitz RP, Purcell BA, Weigand PK, Schall JD, Woodman GF. Homologous mechanisms of visuospatial working memory maintenance in macaque and human: properties and sources. J Neurosci 32: 7711–7722, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Saleem AB, Benucci A, Harris KD, Carandini M. Cortical state determines global variability and correlations in visual cortex. J Neurosci 35: 170–178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Tenke CE, Givre SJ, Arezzo JC, Vaughan HG Jr. Striate cortical contribution to the surface-recorded pattern-reversal VEP in the alert monkey. Vision Res 31: 1143–1157, 1991. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ, Churchland PS, Movshon JA. Putting big data to good use in neuroscience. Nat Neurosci 17: 1440–1441, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Fellows MR, Normann RA. Robust, automatic spike sorting using mixtures of multivariate t-distributions. J Neurosci Methods 127: 111–122, 2003. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Foxe JJ, Vaughan HG Jr, Mehta AD, Schroeder CE. Integration of electrophysiological source analyses, MRI and animal models in the study of visual processing and attention. Electroencephalogr Clin Neurophysiol Suppl 44: 76–92, 1995. [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 18: 555–586, 1995. [DOI] [PubMed] [Google Scholar]
- Smith MA, Jia X, Zandvakili A, Kohn A. Laminar dependence of neuronal correlations in visual cortex. J Neurophysiol 109: 940–947, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Kohn A. Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci 28: 12591–12603, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Sommer MA. Spatial and temporal scales of neuronal correlation in visual area V4. J Neurosci 33: 5422–5432, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: a high-density electrical mapping study. J Neurosci 30: 4024–4032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Morais MJ, Kohn A, Smith MA. Correlations in V1 are reduced by stimulation outside the receptive field. J Neurosci 34: 11222–11227, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Morais MJ, Willis CM, Smith MA. Global network influences on local functional connectivity. Nat Neurosci 18: 736–743, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Shpaner M, Molholm S, Foxe JJ. Visual object processing as a function of stimulus energy, retinal eccentricity and Gestalt configuration: a high-density electrical mapping study. Neuroscience 221: 1–11, 2012. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685, 1993. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286: 1943–1946, 1999. [DOI] [PubMed] [Google Scholar]
- van Burik M, Peters M. EEG and implanted sources in the brain. Arch Physiol Biochem 107: 367–375, 1999. [DOI] [PubMed] [Google Scholar]
- van Pelt S, Boomsma DI, Fries P. Magnetoencephalography in twins reveals a strong genetic determination of the peak frequency of visually induced gamma-band synchronization. J Neurosci 32: 3388–3392, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingstall K, Logothetis NK. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron 64: 281–289, 2009. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Levitt JB, Lund JS. Intrinsic lattice connections of macaque monkey visual cortical area V4. J Neurosci 12: 2785–2802, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]