Abstract
Extensive research over the past decades about the multifaceted roles of brain astrocytes led to the suggestion that the signals observed with functional neuroimaging might primarily reflect astrocytic rather than neuronal activity. The basis for this paradigm-shifting concept was the evidence for an involvement of astrocytes in the control of local cerebral blood flow through intracellular Ca2+ signaling. In this Neuro Forum, I discuss new important experimental findings obtained by Jego et al. (Jego P, Pacheco-Torres J, Araque A, Canals S. J Cereb Blood Flow Metab 34: 1599–1603, 2014) as well as other closely related studies published recently, prompting a dismissal of substantial astrocytic contribution in functional neuroimaging.
Keywords: neurovascular coupling, astrocytic Ca2+ signaling
the brain is one of the most perfused organs in the body. In the human brain cerebral blood flow (CBF) averages 50 ml·100 g−1·min−1, or ∼15% of adult cardiac output at rest. During basal conditions the metabolic rate of glucose and oxygen represents about 80% of that observed under intense sensory stimulation, consistent with the high signaling level of the resting brain (Shulman et al. 2014). Neural activity, metabolism, and CBF are all substantially elevated in the resting state. Furthermore, neurotransmission and oxidative metabolism are linearly coupled, from isoelectric to the awake status (Shulman et al. 2014). Although it is not directly possible to establish a corresponding coupling pattern between metabolism and CBF (anesthetics generally impair the autoregulatory vascular response), current literature suggests that neurometabolic and neurovascular coupling are always operational. This notion is important because CBF not only is regulated during functional hyperemia (the localized CBF increases occurring during stimulation on top of the baseline), but it is also modulated during basal conditions. The changes in CBF relative to an arbitrary baseline (typically identified by resting awake conditions in human studies, but more commonly by anesthetized conditions in animal studies) are those routinely measured by functional neuroimaging techniques based on neurovascular coupling.
The signals generated by neuroimaging techniques largely reflect the vascular properties underlying hemodynamic response to neuronal activity. These vascular properties in turn depend on many variables, including the frequency, amplitude, and duration of the stimuli, the activated populations of responding brain cells (including neurons and glia), the corresponding excitatory/inhibitory as well as presynaptic/postsynaptic balances, the region of the brain and perhaps the cortical layer(s) being stimulated, and also the baseline itself (Shulman et al. 2014). Understanding these dependencies in detail is critical for a correct interpretation of functional images.
New findings by Jego et al. and their relevance to functional neuroimaging.
Functional neuroimaging, by definition, is assumed to be a surrogate of neuronal activity. Nevertheless, a number of results obtained in the last decades have suggested that neurovascular coupling is largely a prerogative of non-neuronal cells, especially astrocytes (Wang et al. 2009). These cells have been proposed to couple neuronal activity with vascular response in a calcium (Ca2+)-mediated manner (reviewed by Howarth 2014). Recent experimental findings have put this notion into question. In particular, mice lacking inositol 1,4,5-trisphosphate (InsP3) type-2 receptor (InsP3R2), the principal enzyme isoform mediating Ca2+ increase in astrocytes, have been found to exhibit normal stimulus-evoked functional hyperemia (Nizar et al. 2013; Takata et al. 2013). In agreement with these studies, it has been reported that electrophysiological activity and functional neuroimaging signals based on blood oxygenation do occur independently of astrocytic Ca2+ signaling in InsP3R2 knockout mice (Jego et al. 2014). Jego et al. conducted their experiments recording both electrophysiological and blood oxygenation level-dependent (BOLD) signals from the cornu ammonis 1 (CA1)/dentage gyrus (DG) fields in the hippocampus of mice lacking InsP3R2. Following electrical stimulation of the perforant pathway, wild-type and InsP3R2 knockout animals exhibited virtually identical electrophysiological (during isoflurane anesthesia) as well as BOLD (during dexmedetomidine anesthesia) responses. These findings should be regarded as supportive of the argument that functional images really reflect neuronal activity. Moreover, they challenge the view that the coupling between neuronal activity and vascular response is provided by InsP3-mediated Ca2+ changes in astrocytes, although the authors did not measure astrocytic Ca2+ changes directly.
The study by Jego et al. (2014) is arguably highly relevant to the functional imaging field. Given that astrocytes are mandatory partners of neurons in almost every aspect of neural activity, it was well expected to know the real contribution of these cells in the production of functional brain images. Yet, to put the above-mentioned results in the correct perspective, it is important to examine to what extent and under what circumstances they actually rule out a significant involvement of astrocytes and astrocytic Ca2+ signaling in the control of CBF.
InsP3 pathway does not exhaust the CBF regulatory competence of astrocytes via the same downstream signaling cascade.
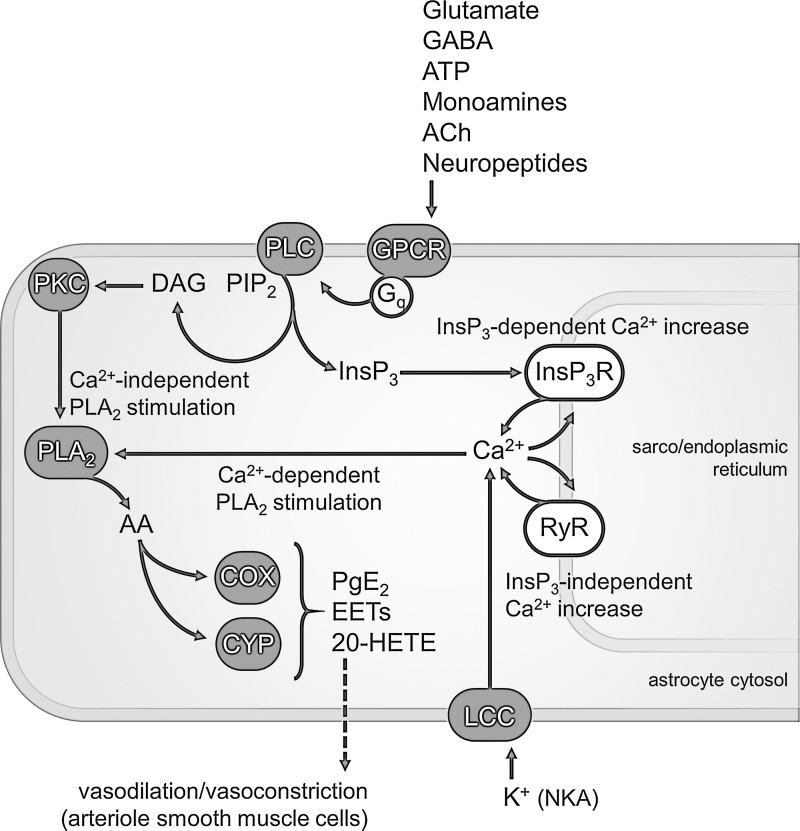
Anatomically, specialized fine astrocytic processes ensheathe neuronal elements as well as cerebral endothelium. Functionally, many neurotransmitters, including glutamate, γ-aminobutyric acid (GABA), adenosine triphosphate (ATP), monoamines, acetylcholine (ACh), and neuropeptides, acting on astrocytic Gq protein-coupled receptors (GPCRs) activate the Ca2+ signaling machinery in astrocytes via the phospholipase C (PLC) pathway (Fig. 1). Briefly, PLC cleaves the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and InsP3. The latter binds to InsP3 receptors located on the sarco(endo)plasmic reticulum, thereby causing the release of Ca2+ from these large internal organelles. The biochemical cascade underlying Ca2+-induced CBF response is a second-messenger signaling system, and as such it involves considerable amplification from receptors to the downstream targets. The first recipients of the effects of increased astrocytic Ca2+ are the lipolytic enzymes of the phospholipase A2 (PLA2) superfamily, which hydrolyze cellular phospholipids to yield (mainly) arachidonic acid (AA). In turn, AA can be brought to the cyclooxygenase (COX) pathway or metabolized by the cytochrome P-450 (CYP) enzymes, including epoxygenases and ω-hydroxylases. COX produce vasodilator prostaglandin E2 (PgE2), whereas CYP produce either the vasodilators epoxyeicosatrienoic acids (EETs) or the vasoconstrictor 20-hydroxyeicosatetraenoic acid (20-HETE). Being vasoactive agents, these molecules have to 1) be released by astrocytes in perivascular space and 2) diffuse to smooth muscle cells surrounding cerebral arterioles. Importantly, other mechanisms (not discussed in this article) are implicated in CBF regulation, such as relaxation of smooth muscle cells induced by astrocytic K+ release at perivascular space or by the activity-dependent synthesis and release of diffusible nitric oxide (NO) directly by activated neurons or endothelial cells (Howarth 2014). These mechanisms bidirectionally interact with the above-mentioned Ca2+ signaling cascade. For example, NO inactivates CYP enzymes and thus decreases production of both EETs and 20-HETE.
Fig. 1.
Schematic of the Ca2+ signaling pathways in astrocytes underlying cerebral blood flow (CBF) regulation. Virtually all transmitter molecules can activate Gq protein-coupled receptors (GPCRs) located on astrocytic plasma membrane. Synaptically released glutamate and GABA diffuse to astrocytes through synaptic spillover (essentially due to the lack of presynaptic reuptake systems), whereas the others diffuse to astrocytes after corelease (neuropeptides) or autocrine (ATP) as well as paracrine (ACh, monoamines) neurotransmission. GPCR-mediated Gq signaling involves cleavage of the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (InsP3; liberated into the cytosol) and diacylglycerol (DAG; remaining bound to the membrane) by PLC. Soluble InsP3 activates InsP3 type-2 receptor (InsP3R2)/Ca2+ channels on the sarco(endo)plasmic reticulum, resulting in increase of cytosolic Ca2+. The latter can also occur independently of InsP3 activation. Perhaps the main route for extracellular Ca2+ entry into astrocytes (see text for other possible options) involves the rise in extracellular K+ during neuronal activity. Most of the neuronally released K+ during action and synaptic potentials is taken up by astrocytes, and this causes membrane depolarization and opening of L-type Ca2+ channels (LCC). Release of Ca2+ from intracellular stores may arise via ryanodine receptors (RyR)/Ca2+ channels, as well. Increased cytosolic Ca2+ stimulate PLA2, which produces arachidonic acid (AA). The same outcome is brought about by Ca2+-independent PLA2 activated by the DAG-mediated PKC pathway. Finally, the signaling cascade leads to the production of vasoactive prostaglandin E2 (PgE2), epoxyeicosatrienoic acids (EETs), and/or 20-HETE by cyclooxygenase (COX) and cytochrome P-450 (CYP). NKA, Na+/K+-activated adenosine triphosphatase.
That the astrocytic Ca2+ signaling is amplificatory in nature also means that the downstream effects of Ca2+ become independent of what actually happens to the Ca2+ level that initiated the cascade. In other words, the concentration of any of the downstream molecules, including AA, might remain temporally elevated and spatially widespread even though that of Ca2+ declines rapidly in time and space. Interestingly, improvement of Ca2+ signal detection recently led to the demonstration that astrocytes exhibit a possibly small (i.e., under the detection limit of Ca2+ imaging), fast, and brief (time to peak ∼100 ms, latency 50 ms, decay time 40 ms) component of the stimulation-induced cytosolic Ca2+ increase, along with the commonly observed slow and sustained (time to peak 1–5 s, latency >500 ms) Ca2+ response (Lind et al. 2013). Since this fast Ca2+ increase in astrocytes matched neuronal activity, it is likely that it is mediated by rapid ion fluxes. Astrocytic K+ uptake is a reasonable candidate, as evidenced by the sensitivity for extracellular K+ of the astrocytic Na+/K+-activated adenosine triphosphatase (NKA), which causes the membrane potential of astrocytes to closely follow the rise in extracellular K+ concentration. As a consequence, Ca2+ can flow into astrocytes through L-type voltage-gated Ca2+ channels (LCC) after enhanced NKA activity.
Intracellular Ca2+ rise can also occur in other InsP3-independent manners. In addition to LCC, routes for plasmalemmal Ca2+ entry in astrocytes include transient receptor potential cation channels (TrpA1), store-operated channels (SOCs) and also ionotropic transmitter receptors expressed by these cells [e.g., glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-d-aspartic acid (NMDA) receptors as well as purinergic P2X-type receptors]. Rise in intracellular Ca2+ in astrocytes can also be transferred through the propagation of Ca2+ from adjacent, gap junction-coupled cells. Moreover, in principle, Ca2+-induced Ca2+ release can take place in the absence of InsP3 receptor-mediated signaling through ryanodine receptors (RyRs). Finally, the expression of Ca2+-independent PLA2 in astrocytes advances the possible existence of a purely astrocyte-mediated vascular responses that do not require Ca2+ signaling. In particular, Ca2+-independent PLA2 can be activated through phosphorylation via the DAG-mediated stimulation of protein kinase C (PKC). In summary, functional hyperemia is under the action of many effectors, including but not limited to InsP3-mediated Ca2+ signaling in astrocytes. Importantly, no exclusive mechanism has been demonstrated for the coupling between brain activity and local changes in blood flow, which is evidenced by the failure, without exception, to block functional hyperemia by pharmacologically inhibiting one single pathway. This argument has to be kept in mind when interpreting results of knockout experiments like that by Jego et al. (2014), discussed here, because developmental changes might act to compensate the constitutive genetic deletion of a specific regulatory mechanism in favor of the plethora of other available options, without any apparent behavioral alteration.
Astrocytic Ca2+ signaling can be important for the modulation of basal CBF during resting state and its aberration in pathology.
The potential implications of the results described previously (Jego et al. 2014; Nizar et al. 2013; Takata et al. 2013) for neuroimaging data interpretation are not restricted to functional hyperemia (i.e., neurovascular coupling). Simply put, these results suggest an uncoupling between astrocytic Ca2+ signaling and stimulation-induced functional hyperemia. Accordingly, the increase in neuroimaging signals due to enhanced neuronal activity could be observed without direct involvement of astrocytic Ca2+ signaling. Even when this conclusion is upheld (but see above), a critical point is that changes in neuroimaging signals may arise independently of neuronal activity due to intrinsic astrocytic Ca2+ signaling (Wang et al. 2009). Rhythmic slow astrocytic Ca2+ signaling occurs indeed spontaneously (i.e., in a non-receptor-mediated manner) and might well reflect the observed low-frequency, resting-state oscillations in CBF reported by functional neuroimaging, since the frequency range underlying both these types of oscillations largely overlaps (0.01–0.1 Hz). In fact, the physiological vasoregulatory mechanisms underlying resting-state activity could be different from those operating during evoked activation. As a direct proof to test this conjecture, it would be interesting to repeat InsP3R2 knockout experiments while monitoring changes in the slow CBF oscillations during the resting state.
The possible regulation of CBF in the neocortex by astrocytes and astrocytic Ca2+ signaling cannot be ruled out only by looking at the site of increased cerebral perfusion. Since multiple subcortical structures, including brain stem and thalamic nuclei, participate in the cyclic modulation of cortical gross excitability, it is likely that they also play a role in the modulation of cortical CBF. These structures establish bidirectional communication with the neocortex so that localized intracortical Ca2+ signaling in astrocytes can originate from remote sites. For example, electrical activation of adrenergic locus coeruleus (which also receives direct projections from serotonergic raphe nuclei) has been found to trigger the elevation of intracellular Ca2+ in live mice cortical astrocytes (Bekar et al. 2008). Alternatively or in addition, subcortical astrocytes may actually transfer their oscillatory patterns to neocortical regions via neuron-mediated signaling. As an illustration, thalamic astrocytes exhibit spontaneous and rhythmic intracellular Ca2+ oscillations that eventually result in cyclic paroxysmal activity of thalamic neurons spreading to the neocortex (Lorincz et al. 2009). Several arguments to support a role for astrocytic Ca2+ signaling in the regulation of CBF are provided by pathological states. Common to many brain pathologies, including Alzheimer's disease and epilepsy, is the phenotypical change of astrocytes that ultimately leads to reactive astrocytosis, astrocytic proliferation, and neuronal cell death. Reactive astrocytes exhibit alterations in the expression of numerous proteins, including the Gq-coupled GPCR metabotropic glutamate receptor type 5 (mGluR5) and PLA2, which are involved in neuronal activity-mediated Ca2+ signaling and associated functional hyperemia (Howarth 2014). The disruption of normal coupling between astrocytic Ca2+ waves and neuronal activity has been reported in an amyloid precursor protein (APP) Alzheimer's transgenic mouse model (Kuchibhotla et al. 2009). Aberrations in the intracellular Ca2+ mobilization have also been observed in cultured astrocytes after the familiar Alzheimer's disease-linked mutation presenilin 1, which is known to perturb endoplasmic reticulum Ca2+ homeostasis (Johnston et al. 2006). The capacity of amyloid-β to impact directly on astrocytic Ca2+ waves has been recently demonstrated in purified cultures of isolated astrocytes (Chow et al. 2010). Since CBF is severely impaired during Alzheimer's disease, it is tempting to correlate CBF alterations to perturbed Ca2+ homeostasis in astrocytes, which is one of the prevailing hypotheses for the pathogenesis of the disease (see Chow et al. 2010 and references therein).
Conclusion.
In the present Neuro Forum, I have focused on the interpretation of the outcomes of the recent study by Jego et al. (2014), which questioned a role for Ca2+ signaling in the production of the functional neuroimaging signals used to monitor neuronal activity. This study in turn pursued two important findings obtained by Nizar et al. (2013) and Takata et al. (2013), who examined the contribution of astrocytic Ca2+ signaling in functional hyperemia. Overall, these results can be summarized as follows: large InsP3-mediated Ca2+ increases in astrocytes are not necessary for the CBF response to stimulation. While acknowledging the importance of these works, I argue that small non-InsP3-mediated astrocytic Ca2+ signaling should be considered before dismissing a role for astrocytes and astrocytic Ca2+ in the interpretation of the “simple pictures of complex biology” (Dienel and Cruz 2008) produced by functional neuroimaging.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.D. conception and design of research; M.D. prepared figures; M.D. drafted manuscript; M.D. edited and revised manuscript; M.D. approved final version of manuscript.
REFERENCES
- Bekar LK, He W, Nedergaard M. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex 18: 2789–2795, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SK, Yu D, Macdonald CL, Buibas M, Silva GA. Amyloid beta-peptide directly induces spontaneous calcium transients, delayed intercellular calcium waves and gliosis in rat cortical astrocytes. ASN Neuro 2: e00026, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Imaging brain activation: simple pictures of complex biology. Ann NY Acad Sci 1147: 139–170, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C. The contribution of astrocytes to the regulation of cerebral blood flow. Front Neurosci 8: 103, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego P, Pacheco-Torres J, Araque A, Canals S. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab 34: 1599–1603, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JM, Burnett P, Thomas AP, Tezapsidis N. Calcium oscillations in type-1 astrocytes, the effect of a presenilin 1 (PS1) mutation. Neurosci Lett 395: 159–164, 2006. [DOI] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323: 1211–1215, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA 110: E4678–E4687, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz ML, Geall F, Bao Y, Crunelli V, Hughes SW. ATP-dependent infra-slow (<0.1 Hz) oscillations in thalamic networks. PLoS One 4: e4447, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, Cui J, Gratiy SL, Sakadzic S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci 33: 8411–8422, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Insights from neuroenergetics into the interpretation of functional neuroimaging: an alternative empirical model for studying the brain's support of behavior. J Cereb Blood Flow Metab 34: 1721–1735, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLoS One 8: e66525, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Takano T, Nedergaard M. Astrocytic calcium signaling: mechanism and implications for functional brain imaging. Methods Mol Biol 489: 93–109, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]