Abstract
Cortical glutamatergic projections are extensively studied in behavioral neuroscience, whereas cortical GABAergic projections to downstream structures have been overlooked. A recent study by Lee and colleagues (Lee AT, Vogt D, Rubenstein JL, Sohal VS. J Neurosci 34: 11519–11525, 2014) used optogenetic and electrophysiological techniques to characterize a behavioral role for long-projecting GABAergic neurons in the medial prefrontal cortex. In this Neuro Forum, we discuss the potential implications of this study in several learning and memory models.
Keywords: avoidance, aversion, striatum, amygdala
it has been a long-standing belief that behavior is modulated by cortical control over subcortical structures exclusively through excitatory glutamatergic projections. There has been a general consensus that inhibitory GABAergic neurons in the cortex participate mainly in local microcircuits. For example, GABAergic interneurons in the medial prefrontal cortex (mPFC) compose local microcircuits that shape prefrontal coding of fear expression (Courtin et al. 2014). Generally, models of top-down cortical control involve excitatory projections to downstream regions. In such cases, behavioral regulation depends on whether mPFC fibers selectively target excitatory or inhibitory neurons. This is the case of the mPFC-amygdala model proposed for fear regulation: the prelimbic prefrontal cortex (PL) drives fear by its excitatory projections to excitatory neurons in the basolateral amygdala (BLA), whereas the infralimbic prefrontal cortex (IL) inhibits fear by its excitatory projections to GABAergic intercalated cells (ITCs) in the amygdala (Sotres-Bayon and Quirk 2010).
Other models involve mPFC projections to distinct subregions that compete for behavioral control. In the reward system, PL excitatory neurons drive reward seeking through the nucleus accumbens (NAcc) core, whereas IL excitatory neurons inhibit reward seeking through its connections with the NAcc shell (Peters et al. 2009). However, until now, there was no functional evidence of prefrontal inhibitory projections to downstream regions that could directly influence behavior. A recent study by Lee et al. (2014) elegantly characterized long-projecting GABAergic neurons in the mPFC and tested whether these projections to the NAcc can modulate behavior.
The authors identified cortical GABAergic projections by infusing a viral vector containing channelrhodopsin (AAV-DIO-ChR2-EYFP) into the mPFC of Dlxi12b-Cre mice to selectively target GABAergic neurons. Labeling of GABAergic mPFC fibers was detected at several downstream regions including NAcc and BLA. Using whole cell recordings in NAcc, the authors found that optogenetic activation of mPFC ChR2-containing terminals in NAcc elicited inhibitory postsynaptic currents (IPSCs) in NAcc neurons. Blocking GABAA, but not glutamate, receptors in NAcc abolished the IPSCs, indicating that these mPFC long-range projections are GABAergic. Previous studies have shown that activation of GABAergic projections from subcortical regions to NAcc elicit aversion. Thus the authors tested whether cortical GABAergic projections could also mediate aversive responses. Indeed, they found that mice refrained from entering a chamber paired with stimulation of GABAergic mPFC fibers in NAcc, suggesting that long-range GABAergic neurons in mPFC can drive aversion through its projections to NAcc.
These findings are timely given that many research groups have investigated how cortical glutamatergic projections modulate behavior, overlooking a potential role for cortical GABAergic projections. All previous behavioral studies on cortical GABAergic neurons have focused exclusively on local inhibitory circuits. The study by Lee et al. (2014) is the first to demonstrate that cortical GABAergic projections to downstream targets can modulate aversive responses. Going forward, future studies need to characterize the potential role of cortical GABAergic projections in the neuropathology of mental illness.
GABAergic neurons in aversive behavior: cortical vs. subcortical projections.
Previous studies have demonstrated that subcortical GABAergic projections play a critical role in control of behavior. For example, long-range GABAergic projections from the ventral tegmental area (VTA) can elicit aversion by inhibiting NAcc (Creed et al. 2014). Also, long-range GABAergic projections from the rostromedial tegmentum drive aversion by indirectly inhibiting NAcc (Lammel et al. 2012). Furthermore, the central amygdala (CeA) can drive aversive behavior through long-range GABAergic projections to the midbrain periaqueductal grey matter (Penzo et al. 2014).
Evidently, subcortical structures can mediate aversion through GABAergic projections, but it remained untested whether cortical GABAergic projection neurons could elicit aversive behavior as well. In this study, Lee et al. (2014) used the real-time place aversion test (RTPA) to demonstrate that indeed cortical GABAergic long-range projections can drive aversion through NAcc. Although the authors make an important insight about cortical GABAergic projections and acute aversion, further follow-up studies are necessary to determine the role of these projections in both avoidance and aversive memories. In RTPA, one side of a two-compartment box is paired with a stimulus that triggers an acute aversive response. However, it is important to note that RTPA is different from conditioned place aversion (CPA), in which rodents are brought back to the two-compartment box the following day for an aversive memory test. The RTPA also differs from conditioned avoidance paradigms, in which rodents learn to execute an instrumental response (e.g., shuttling or stepping onto a platform) to avoid a signaled footshock. Therefore, further studies using CPA and conditioned avoidance are required to test whether activation of mPFC GABAergic projections to NAcc can create an aversive memory as well as modulate conditioned avoidance responses.
Optogenetic activation can reveal if a region or projection may modulate a specific behavioral response. However, only silencing can determine if a region or projection is necessary to elicit such behavioral response. Therefore, silencing GABAergic mPFC efferents to NAcc is required to test whether this projection is essential for aversion coding. On another note, many studies have focused on the antagonistic roles PL and IL have on different behaviors. Although in the present study the authors did not dissociate PL from IL, it is likely that long-range GABAergic neurons in each structure mediate different behaviors.
The findings of Lee et al. (2014) emphasize the need to reevaluate optogenetic projection studies in the cortex, which focus mainly on glutamatergic neurons. As an example, it was previously shown that rodents would self-stimulate glutamatergic projections from BLA to NAcc, but not from mPFC to NAcc (Stuber et al. 2011). Could it be that GABAergic mPFC projections, rather than glutamatergic, would influence hedonic/aversive behaviors through NAcc? Another optogenetic study focused on glutamatergic projections from mPFC to the amygdala in the fear circuit (Cho et al. 2013). Could we be missing a key pathway in the fear circuit by omitting GABAergic mPFC projections to downstream regions? We now know that the field has overlooked a potentially critical mechanism for cortical control of behavior.
GABAergic mPFC neurons mediate aversion: a role in avoidance?
Avoidance is a core symptom of all anxiety disorders, and it can severely decrease a patient's quality of life by interfering with goal attainment. Moreover, avoidance impedes fear extinction by reducing patients' exposure to trauma reminders within a safe context. Elucidating the mechanisms underlying avoidance will help guide treatments for anxiety disorders. In rodents, pharmacological inactivation of either PL or NAcc impairs the expression of conditioned avoidance, suggesting that PL projections to NAcc mediate expression of conditioned avoidance (Bravo-Rivera et al. 2014). There is evidence that aversion coding from dopaminergic signaling in NAcc is necessary for conditioned avoidance learning (Darvas et al. 2011). Interestingly, Lee et al. (2014) showed that stimulating GABAergic mPFC projections to NAcc elicited aversion in RTPA. Although the authors did not test whether conditioned avoidance depends on PL GABAergic projections to NAcc, the aversion induced by activating these projections may contribute to the aversion necessary to learn conditioned avoidance (see Fig. 1A).
Fig. 1.
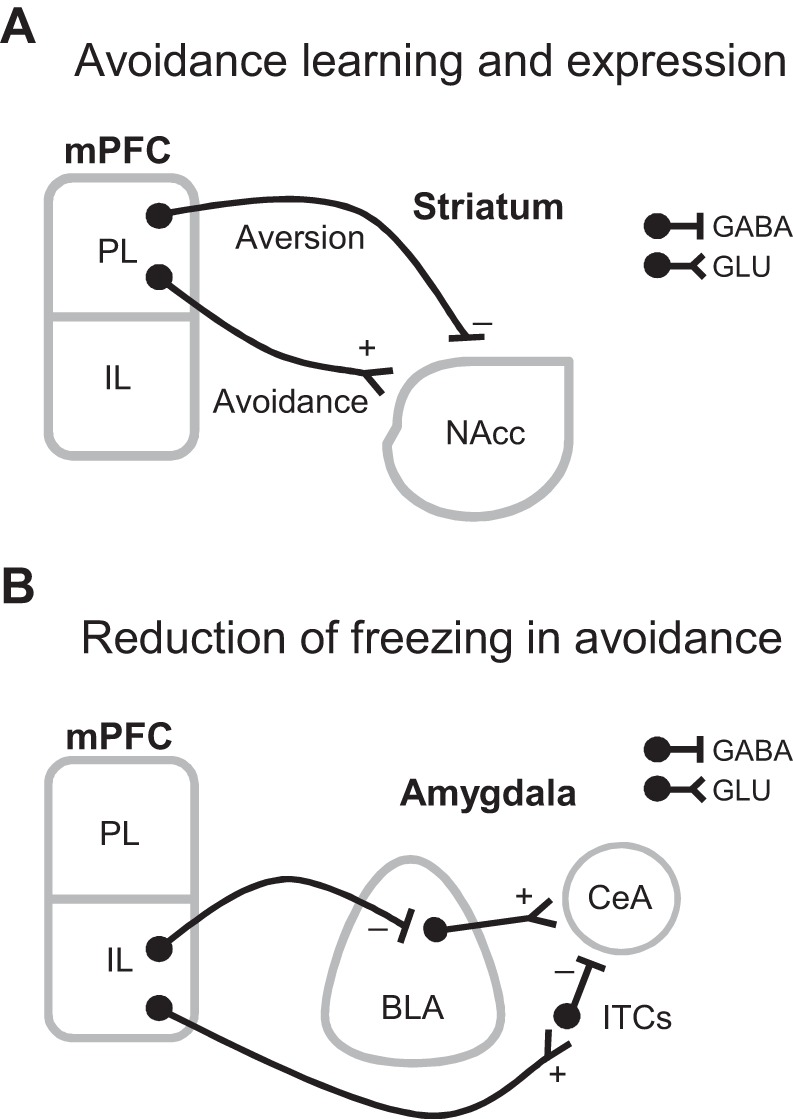
Possible roles of GABAergic projections from medial prefrontal cortex (mPFC) to subcortical structures in conditioned avoidance. A: prelimbic cortex (PL) GABAergic projections to nucleus accumbens (NAcc) could drive aversion necessary for conditioned avoidance learning, whereas PL glutamatergic projections to NAcc could drive expression of conditioned avoidance. GABA, GABAergic projection, GLU, glutamatergic projection. B: infralimbic cortex (IL) glutamatergic projections to intercalated cells of the amygdala (ITCs) could decrease freezing by inhibiting the central nucleus of the amygdala (CeA), whereas IL GABAergic projections to the basolateral nucleus of the amygdala (BLA) could decrease freezing by directly inhibiting CeA-projecting glutamatergic neurons in BLA.
In conditioned avoidance, rodents suppress freezing to avoid, and recent studies have shown that IL is necessary for freezing suppression in conditioned avoidance (Bravo-Rivera et al. 2014; Moscarello and LeDoux 2013). A proposed model of IL-mediated reduction of fear suggests that IL glutamatergic projections activate ITC neurons, which in turn inhibit CeA output that drives freezing (Sotres-Bayon and Quirk 2010). However, an alternative is that long-range GABAergic neurons in IL decrease fear expression by directly inhibiting BLA neurons that drive freezing through CeA (see Fig. 1B).
Cortical long-projecting GABAergic neurons: a role in pathology?
Given that long-projecting GABAergic neurons in the cortex can gate behavior through subcortical structures, there is a need to reevaluate faulty circuits in psychiatric disorders. Several studies have suggested that abnormalities in cortical GABAergic neurons are characteristic of several psychiatric disorders, including anxiety, drug abuse, schizophrenia, and autism. For example, a recent study reported that transgenic mice deficient in cortical GABAergic neurons showed impaired fear extinction and attention (Bissonette et al. 2014). Another study demonstrated that activating GABAergic neurons in PL decrease reward seeking in rodents (Sparta et al. 2014), suggesting that enhancing GABAergic activity in mPFC could be a target for treating addiction. All these studies focused on local GABAergic neurons in the cortex, but they overlooked existing long-projecting GABAergic neurons. Further studies characterizing the role of such cortical projections in behavioral regulation may contribute to the understanding and treatment of many mental illnesses.
Further studies.
The study by Lee et al. (2014) opened opportunities for exciting questions. Future studies could test whether activating mPFC GABAergic projections to NAcc can form an aversive memory. This could be achieved by optogenetically activating these projections in a RTPA paradigm and testing whether rodents avoid the stimulation-paired side on the following day. Also, it could be tested whether silencing mPFC GABAergic projections to NAcc impairs retrieval of a consolidated aversive memory.
Lee et al. (2014) did not distinguish between medial prefrontal subregions; evidence suggests that PL promotes fear, whereas IL inhibits fear. Thus differentiating PL and IL GABAergic projections might reveal opposing roles of these structures in aversion. The present findings may be also relevant for the fear conditioning field, in which the current model states that IL glutamatergic projections suppress fear by indirectly inhibiting CeA. Future experiments could assess whether optogenetically stimulating IL GABAergic projections suppress fear by directly inhibiting CeA-projecting glutamatergic neurons in BLA. This would suggest another mechanism of IL-mediated fear suppression.
Conclusion.
In summary, the study by Lee et al. (2014) not only characterized long-projecting GABAergic neurons in mPFC but also showed that these projections can mediate behavior downstream. The cortex is known to be critical for controlling behavior, and this study broadens our opportunities to understand the mechanisms underlying behavioral regulation. It also reminds us that technology optimization and cautious examination can change long-standing beliefs in neuroscience. Further studies evaluating the role of these cortical GABAergic projections in aversive and reward circuits will help us to understand top-down control of behavior.
GRANTS
This work was supported by National Institutes of Health Grants MH102968 (to C. Bravo-Rivera) MH105039 (to J. Rodriquez-Romaguera), MH106332 (to L. E. Rosas-Vidal), and University of Puerto Rico School of Medicine Research Initiative for Scientific Enhancement Fellowship GM061838 (to H. Bravo-Rivera and K. Quiñones-Laracuente).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B.-R., M.M.D., C.R.-O., J.R.-R., L.E.R.-V., H.B.-R., K.Q.-L., and F.H.D.-M. prepared figures; C.B.-R., M.M.D., C.R.-O., J.R.-R., L.E.R.-V., H.B.-R., K.Q.-L., and F.H.D.-M. drafted manuscript; C.B.-R., M.M.D., C.R.-O., J.R.-R., L.E.R.-V., H.B.-R., K.Q.-L., and F.H.D.-M. edited and revised manuscript; C.B.-R., M.M.D., C.R.-O., J.R.-R., L.E.R.-V., H.B.-R., K.Q.-L., and F.H.D.-M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Francisco Sotres-Bayon and Gregory J. Quirk for helpful comments on the manuscript.
REFERENCES
- Bissonette GB, Bae MH, Suresh T, Jaffe DE, Powell EM. Prefrontal cognitive deficits in mice with altered cerebral cortical GABAergic interneurons. Behav Brain Res 259: 143–151, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ. Neural structures mediating expression and extinction of platform-mediated avoidance. J Neurosci 34: 9736–9742, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron 80: 1491–1507, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505: 92–96, 2014. [DOI] [PubMed] [Google Scholar]
- Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci 8: 8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem 18: 136–143, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491: 212–217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Vogt D, Rubenstein JL, Sohal VS. A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci 34: 11519–11525, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscarello JM, LeDoux JE. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J Neurosci 33: 3815–3823, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzo MA, Robert V, Li B. Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J Neurosci 34: 2432–2437, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem 16: 279–288, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20: 231–235, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Hovelso N, Mason AO, Kantak PA, Ung RL, Decot HK, Stuber GD. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J Neurosci 34: 3699–3705, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475: 377–380, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


